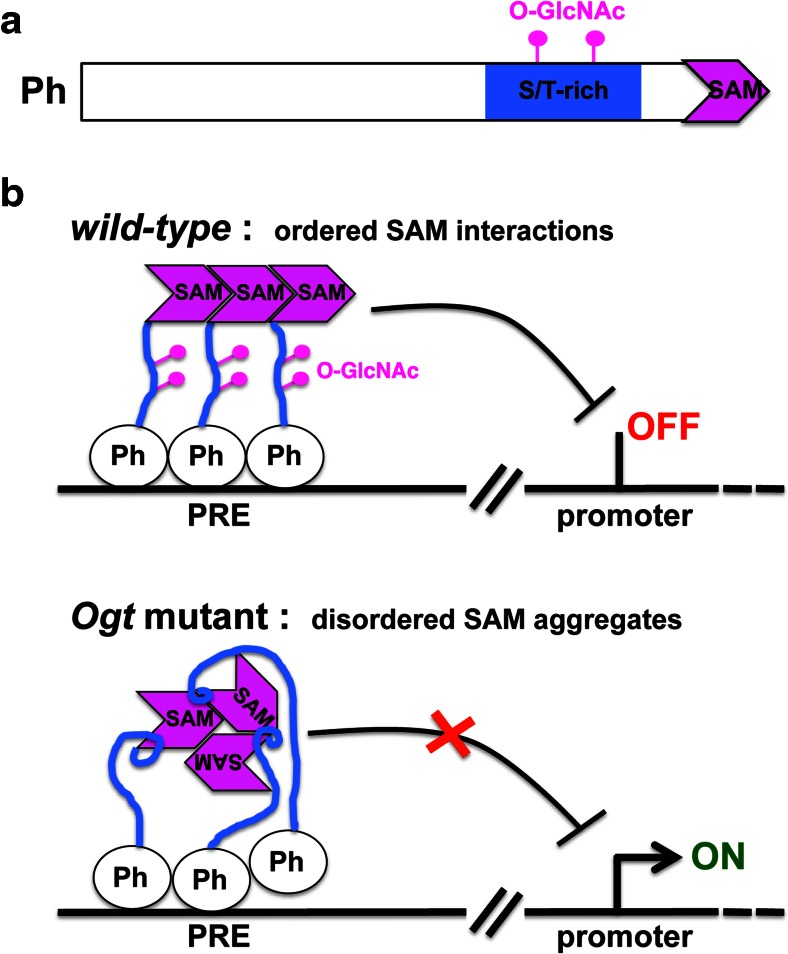
Fig. 3.
Model of O-GlcNAcylation function in Polycomb repression in Drosophila. a Schematic representation of the fly Ph protein that is O-GlcNAcylated on an S/T-rich region, located close to the C-terminal SAM domain. b Model illustrating how O-GlcNAcylation of Ph allows the formation of ordered SAM-SAM assemblies that are needed to silence Polycomb target genes (top). Ph is bound at PREs as part of the PRC1 complex (other PRC1 subunits are not shown) in both wild-type and Ogt mutant animals. In the absence of O-GlcNAcylation of the S/T stretch, Ph molecules aggregate through their SAM domains (Gambetta and Müller 2014) (bottom). The exact molecular mechanism through which O-GlcNAcylation of the S/T-rich stretch prevents Ph molecules from engaging in non-productive contacts with other SAM domains is not known, but it might involve intramolecular contacts (illustrated here as small loops) between the S/T stretch and the SAM domain that alter SAM conformation in a way that favors aggregation with other SAM domains in a similar conformation (Gambetta and Müller 2014)