Novel observations in this report include the demonstration of collateral resistance as the primary limitation of hindlimb perfusion, elevated NADPH oxidase (Nox) expression in peripheral arteries, unimpaired monocyte mobilization and demargination, and reversal of suppressed principle collateral growth by Nox2 ablation/inhibition in a diet-induced obese mouse model of arterial occlusion.
Keywords: collateral resistance, arteriogenesis, NADPH oxidase, Nox2, vascular resistance
Abstract
The present study was undertaken to establish the role of NADPH oxidase (Nox) in impaired vascular compensation to arterial occlusion that occurs in the presence of risk factors associated with oxidative stress. Diet-induced obese (DIO) mice characterized by multiple comorbidities including diabetes and hyperlipidemia were used as a preclinical model. Arterial occlusion was induced by distal femoral artery ligation in lean and DIO mice. Proximal collateral arteries were identified as the site of major (∼70%) vascular resistance to calf perfusion by distal arterial pressures, which decreased from ∼80 to ∼30 mmHg with ligation in both lean and DIO mice. Two weeks after ligation, significant vascular compensation occurred in lean but not DIO mice as evidenced by increased perfusion (147 ± 48% vs. 49 ± 29%) and collateral diameter (151 ± 30% vs. 44 ± 17%). Vascular mRNA expression of p22phox, Nox2, Nox4, and p47phox were all increased in DIO mice. Treatment of DIO mice with either apocynin or Nox2ds-tat or with whole body ablation of either Nox2 or p47phox ameliorated the impairment in both collateral growth and hindlimb perfusion. Multiparametric flow cytometry analysis demonstrated elevated levels of circulating monocytes in DIO mice without impaired mobilization and demargination after femoral artery ligation. These results establish collateral resistance as the major limitation to calf perfusion in this preclinical model, demonstrate than monocyte mobilization and demarginatin is not suppressed, implicate Nox2-p47phox interactions in the impairment of vascular compensation to arterial occlusion in DIO mice, and suggest that selective Nox component suppression/inhibition may be effective as either primary or adjuvant therapy for claudicants.
NEW & NOTEWORTHY
Novel observations in this report include the demonstration of collateral resistance as the primary limitation of hindlimb perfusion, elevated NADPH oxidase (Nox) expression in peripheral arteries, unimpaired monocyte mobilization and demargination, and reversal of suppressed principle collateral growth by Nox2 ablation/inhibition in a diet-induced obese mouse model of arterial occlusion.
novel therapies to reverse or prevent disease progression and functional decline related to peripheral arterial occlusive disease (PAD) have been identified as a great unmet medical need (32, 58). Some individuals, especially the young, have a remarkable capacity to compensate for arterial occlusion through both large artery and microvascular adaptations (100). Yet those with vascular risk factors and comorbidities, such as hypercholesterolemia, diabetes, and advanced age, demonstrate very limited capacity for such adaptation (42, 100). In these individuals, arterial occlusion results in significant disability and even death. A recent clinical trial has demonstrated that the angiotensin-converting enzyme inhibitor Ramipril provides a substantially greater functional improvement in claudicants (2) than medications currently Federal Drug Agency approved for PAD-related walking disorders (58). While the mechanisms of action remain unknown, angiotensin-converting enzyme inhibitors such as Ramipril provide vascular protection by decreasing oxidative stress and improving endothelial function (63). Oxidative stress and endothelial dysfunction compromise perfusion and walking capacity in humans (11), likely through 1) impairment of vasodilatory capacity in resistance vessels and 2) suppression of flow-mediated outward remodeling, such as occurs in collateral growth in humans (90) and animals (79, 80) and that may result from reductions in bone marrow-derived cell (BMDC) function including mobilization and demargination (28, 40, 42, 93).
NADPH oxidases (Nox) are a major source of vascular ROS (13, 92) and exhibit increased expression and activation in the context of vascular risk factors and PAD (51, 52). A major source of endothelial oxidative stress results from ANG II-induced activation of Nox2 (20), and studies in both animals and humans have implicated a causal role of Nox2 in cardiovascular diseases and endothelial dysfunction (13, 23, 24, 51, 52, 75, 92). Loffredo et al. (51, 53) have provided evidence that Nox2 significantly contributes to the oxidative stress that is responsible for impaired endothelial dysfunction and impaired flow-mediated dilation in claudicants. Nox2 has been identified as a potential therapeutic target for vascular disease (13, 23, 24, 92), and PAD would be a logical application for such therapy. However, divergent effects of ROS on vascular compensation and on the BMDC subtypes believed to mediate it, including ROS derived from Nox and specifically Nox2 (22, 25, 34, 35, 67, 77, 78, 84, 85), have been reported. Consequently, a better understanding is needed for the role of Nox in vascular compensation in the presence of comorbidities typical of the human population with PAD, especially for the vascular adaptation(s) with the greatest capacity for improved perfusion.
The present study was undertaken to clarify the role of Nox, and specifically Nox2, in the suppression of the normal vascular compensation to arterial occlusion that occurs in the presence of multiple risk factors for cardiovascular disease. We used diet-induced obese (DIO) mice characterized by glucose intolerance (5, 38, 43, 57, 76), insulin resistance (5, 65, 76), hyperlipidemia/hypercholesterolemia (38, 65), chronic inflammation and oxidative stress (5, 38, 43, 44, 65), endothelial dysfunction (44, 57), and hypertension (64) as well as peripheral vascular insufficiency (6) and augmented hindlimb ischemic injury (3). Arterial occlusion was produced by distal femoral artery ligation at the most common site of clinical vascular occlusive disease (19). Distal arterial pressure measurement by a servo-null microelectrode method (7, 8), so as to not influence blood flow, and calculation of segmental vascular resistances from pressure measurements identified that the proximal collateral bypass arteries are the primary site of vascular resistance after occlusion in our animal model. These are the vessels that undergo enlargement to restore blood flow after occlusion (21). In DIO mice, vascular compensation was impaired, as assessed by both hindlimb perfusion and collateral enlargement, circulating monocytes in DIO were elevated at baseline, and mobilization and demargination were not suppressed after femoral artery ligation. The role of Nox in the impairment of vascular compensation was evaluated by vascular Nox expression as well as via pharmacological inhibition and genetic ablation of Nox. The results support the hypothesis that impaired compensation to focal arterial occlusion in the DIO mouse results from Nox2- and p47phox-mediated ROS production and the resultant suppression of preexisting collateral vessel enlargement through mechanisms other than impairment of BMDC mobilization/demargination.
MATERIALS AND METHODS
Animal groups and model.
The Institutional Animal Care and Use Committee of the Indiana University School of Medicine approved all procedures performed in this study. C57BL/6J, B6.129S-Cybbtm1Din/J (Nox2−/−), and B6 (Cg)-Ncf1m1J/J (p47phox−/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Experimental DIO mice were started on a high-fat diet (60% of kcal from fat, D12492, Research Diets, New Brunswick, NJ) at 6 wk of age and maintained on this diet until experimentation at ∼4 mo of age. This DIO model was selected as it has been characterized to have multiple risk factors, including three of the most important risk factors associated with PAD, as identified by Fowkes et al. (29). We performed preliminary experiments that confirmed that mice on this diet became obese, hyperglycemic under fasting conditions (mean blood glucose of 227 mg/dl), and hypercholesterolemic (average total cholesterol of 208 mg/dl). Control (lean) mice were fed standard chow (2018SX Teklad Global Rodent Diet, Harlan, Indianapolis, IN) for the duration of the experiments. Some groups of DIO mice were pretreated with the antioxidant apocynin (acetovanillone, Fisher Scientific, Pittsburg, PA, 3 mM in drinking water) (37) starting 3 days before femoral artery ligation and continued until the animal was euthanized on day 14 postligation. Other DIO mice were given the Nox2-specific inhibitor Nox2ds-tat (gp91ds-tat) (17, 66), which was custom synthesized (EZbiolabs, Westfield, IN) according to Rey et al. (66), and delivered at a dose of 10 mg·kg−1·day−1 via Alzet osmotic pumps (1002, Durect, Cupertino, CA). The osmotic pumps were aseptically implanted in a subcutaneous pocket in the nape of the neck at the time of femoral artery ligation and delivered Nox2ds-tat until final experimentation at 14 days. Previous studies have demonstrated the effectiveness of these concentrations/doses in normalizing arterial nitric oxide and ROS concentrations (98, 99), reducing oxidative stress (66, 75), and impacting compensatory collateral growth (22, 59).
Femoral artery ligation.
The femoral artery ligation model used in the present study was chosen because short-segment total occlusion of the distal superficial femoral artery is the most common lesion in patients with PAD (19). Briefly, mice were anesthetized via a 2.5% isoflurane and oxygen mixture, an ∼7-mm incision was aseptically made in the skin, the femoral artery was carefully teased away from the vein and nerve, a 6-0 sterilized silk suture was used to ligate the artery (care was taken to prevent tissue desiccation), and the skin was closed via a 5-0 sterile absorbable suture. Animals were then allowed to recover for 2 wk.
Distal limb pressure measurement and calculation of collateral and microvascular segmental resistances.
Using a servo-null micropressure system (Instrumentation For Physiology and Medicine model 5A) as previously described for small and low-pressure vessels (7, 81), arterial pressure was measured before (Pintact) and after acute (Pacute) distal femoral artery occlusion in the left limb and chronic femoral artery ligation (Pchronic) in the right limb. With the mouse anesthetized with isoflurane, the right and left saphenous arteries were exposed and continuously bathed with physiological saline solution. Each micropressure pipette was calibrated immediately before measurement. After reference zero pressure in the saline bath had been established, the sharpened micropipette (8- to 10-μm outer diameter to tolerate arterial puncture) was inserted into the lumen of the saphenous artery under microscopic observation, and a stable recording was obtained for at least 30 s. The point of micropressure pipette insertion was at a point distal to the gracilis arteries but proximal to the feed vessels to the microcirculation and thus provided perfusion pressure for the distal limb before and after arterial ligation. This permitted the segmental resistance of the collaterals (Rc) and distal microvasculature (Rmv) to be calculated as a percentage of total limb resistance as previously reported for other species (26, 49, 56, 69, 83, 94). The following equations were used: Rc (in %) = [(Pintact − P)/Pintact] × 100% and Rmv (in %) = (P/Pintact) × 100%, where P is Pacute or Pchronic.
Assessment of collateral vessel enlargement.
On day 14 postocclusion, animals were anesthetized via isoflurane, the abdominal cavity was opened, the infrarenal abdominal aorta was cannulated in the direction of flow, and the animal was perfused at ≥100-mmHg pressure with 10 ml of dilator (0.1 mM adenosine and 0.01 mM sodium nitroprusside in 0.9% saline) followed by 10 ml of 4% Zn-formalin. After fixation, ∼0.1 ml of yellow Microfil vascular casting compound (MV-122, Flow Tech, Carver, MA) was injected into the cannula while the ligated limb vasculature was visualized under the dissecting scope until filling of the saphenous artery was observed and the primary collateral path was noted. The casting agent was allowed to cure at 4°C overnight. In situ digital images of the collateral midzone region of the anterior and posterior gracilis arteries were then obtained as well as same animal matched controls, and vessel diameters were determined via ImageJ software (National Institutes of Health). We have previously shown these vessels to comprise the primary collaterals after surgical ligation of the distal femoral artery between the origins of the superficial epigastric artery and the geniculate/saphenous/popliteal arteries (21).
Laser-Doppler perfusion imaging.
Perfusion of the plantar surface of the hindpaw was measured via laser-Doppler perfusion imaging (model LDI2-IR, Moor Instruments, Devon, UK) on both ligated and control limbs on 1 and 14 days postocclusion. Animals under 2.5% isoflurane plus oxygen mixed anesthesia were placed on a 37°C heating pad and allowed to acclimate for 10 min before being scanned. Experimental limb perfusion data are expressed as a percentage of the control limb calculated from individual limb laser-Doppler flux values.
Real-time quantitative PCR.
Relative differences in iliac/femoral artery Nox subunit mRNA expression from lean and DIO mice were determined using reverse transcription real-time quantitative PCR as previously described in detail (22, 59). After perfusion with cold PBS and RNAlater (Ambion, Austin, TX) via the caudal aorta, the entire length from the bifurcation of the aorta to the distal end of the femoral arteries was isolated for RNA extraction (iliac-femoral). Purification of total RNA was completed, and aliquots of RNA (0.2 μg) were reverse transcribed into cDNA. For PCR, cDNA (5.0 μl, 1:50 dilution) was combined with primers and probes for Nox2, p47phox, Nox4, p22phox, Nox1, or β-actin endogenous control (TaqMan Gene Expression Assays, Applied Biosystems, Foster City, CA) in the presence of a PCR master mix (FastStart Universal Probe Master Mix, Roche Applied Science, Indianapolis, IN). Reactions were run in triplicate on an Applied Biosystems 7500 Real-Time PCR system using relative quantification (ddCt) with standard two-step 7500 PCR cycling conditions (40 cycles). Differences in PCR product yields between groups were determined by comparing the fold differences between target mRNA after normalization to β-actin.
Multiparametric fluorescence-activated cell sorting.
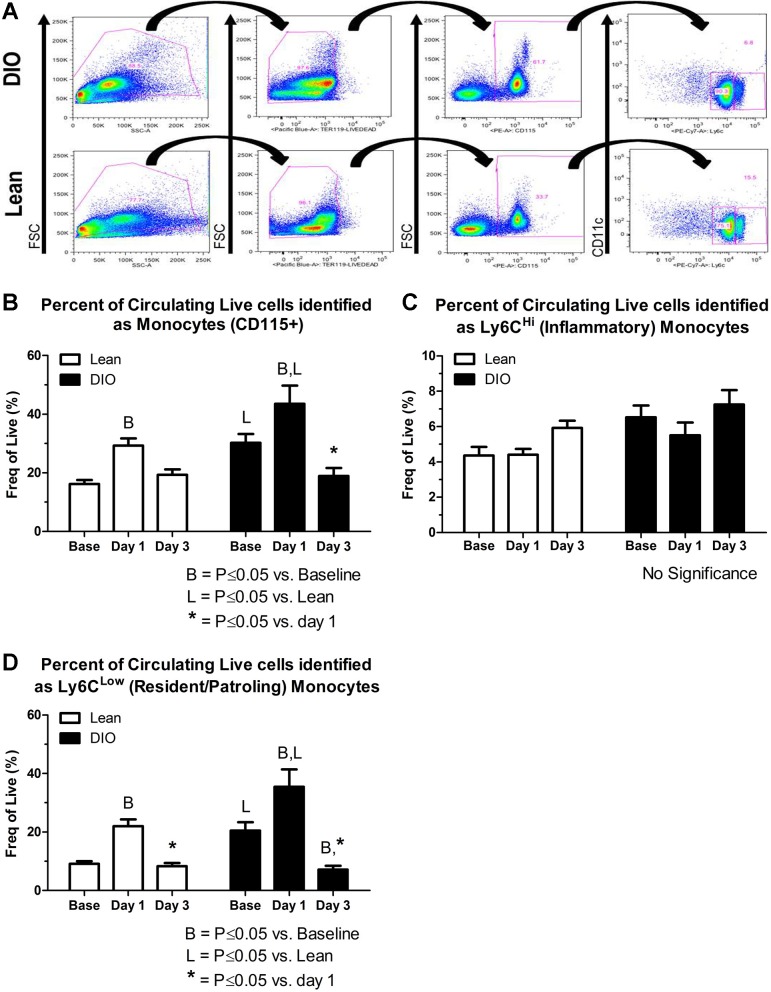
Multiparametric fluorescence-activated cell sorting (FACS) analysis was used to assess mobilization and demargination of circulating monocytes. Peripheral blood samples were collected into EDTA tubes (BD Biosciences, San Jose, CA) via a tail vein at baseline (before) and 1 and 3 days postligation and subjected to processing and staining for FACS analysis as previously described (74). Cells were washed in 2% FBS after red blood cell (RBC) lysis (Qiagen, Germantown, MD), centrifuged, resuspended in 2% FBS in PBS, and incubated for 10 min with murine Fc Blocking Reagent (Miltenyi Biotec, Cologne, Germany). Cells were then reacted for 30 min at 4°C in the dark with LIVE/DEAD violet fixable cell stain (Invitrogen, Grand Island, NY); CD115-PE and TER119-PacBlue (eBioscience, San Diego, CA); CD11c-PerCP-Cy5.5, Ly6C-PE-Cy7, and CD11b-APC-Cy7 (BD Biosciences); or F4/80-APC and F7/4-FITC (Serotech, Oxford, UK). True positive and negative events were determined using “fluorescence-minus-one” gating controls, and titration of antibodies for optimal staining was performed (27, 36). Data were acquired uncompensated on a BD LSRII flow cytometer, and at least 100,000 events were recorded per sample. FlowJo software (version 8.7.3, Tree Star, Ashland, OR) was used to first create a compensation matrix and then analyze the data. The gating strategy for the identification of circulating monocytes is shown in Fig. 5A and included 1) forward/side scatter to remove debris and cell doublets/triplets, 2) a viability stain in combination with the RBC marker Ter119 to eliminate RBCs and dead cells, 3) CD115 positivity to identify total monocytes, and 4) CD11c negativity as well as high versus low expression of Ly6C to identify total monocytes as either inflammatory (Ly6CHi) or resident/patrolling (Ly6CLow).
Fig. 5.
Multiparametric fluorescence activating cell sorting analysis was used to assess mobilization and demargination of circulating monocytes. A: representative dot plots from lean and DIO mice showing the gating strategy used to identify the circulating live monocyte subpopulations: total (CD115+), inflammatory (CD11c−, Ly6CHi), or resident/patrolling (CD11c−, Ly6CLow) monocytes. B–D: quantification of the percent circulating live total, inflammatory, and resident/patrolling monocytes in peripheral blood at baseline (Base) or 1 or 3 days postligation. B: there were significantly more total monocytes at baseline in DIO versus lean mice. The total monocyte percentage significantly increased from baseline to day 1 in both groups (mobilization) and then decreased from days 1 to 3 in both groups (demargination). C and D: the significant changes observed in the total monocyte population were mirrored in the Ly6CLow/patrolling monocyte population but not in the Ly6CHi/inflammatory monocyte subset.
Statistics.
Unless otherwise noted, parameters were evaluated with two-way repeated-measures ANOVA (SigmaPlot 12.5, Systat Software). Multiple pairwise comparisons were performed via the Holm-Sidak method when significance between groups was detected. P values of ≤0.05 were considered significant, and data are presented as group averages ± SE.
RESULTS
Body mass.
Average body mass for control and experimental DIO mice is shown in Table 1. DIO mice on the high-fat diet exhibited greater total body mass than control (lean) mice fed the standard chow.
Table 1.
Body masses
| Treatment Group | Mean Total Body Mass, g | Number of Mice |
|---|---|---|
| Untreated | ||
| Lean | 30.57 ± 0.78 | 7 |
| DIO | 42.20 ± 1.56 | 5 |
| DIO treatment/ablation | ||
| Apocynin | 36.17 ± 1.42 | 6 |
| Nox2ds-tat | 38.64 ± 1.36 | 11 |
| Nox2−/− | 37.00 ± 1.91 | 8 |
| p47phox−/− | 42.75 ± 2.09 | 8 |
Values are group averages ± SE.
DIO, diet-induced obese.
Hindlimb arterial pressures and segmental resistances in lean and DIO mice.
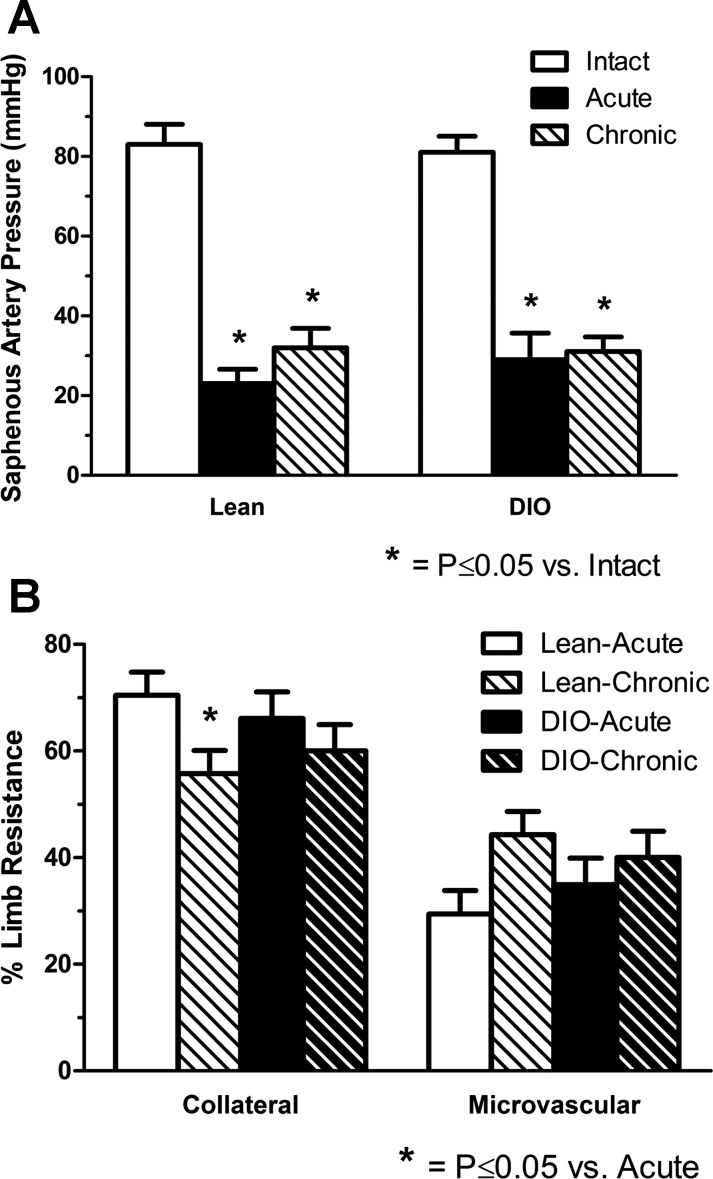
Saphenous artery pressures measured in lean and DIO mice are shown in Fig. 1A, and calculated segmental collateral and microvascular resistances are shown in Fig. 1B. In lean mice, saphenous artery pressure averaged 83 ± 5.1 mmHg in intact limbs without femoral artery occlusion. This pressure should very closely reflect mean arterial pressure and indicates that measurements were made under conditions in which cardiovascular system performance was well developed. With acute and chronic femoral artery ligation, saphenous artery pressure was significantly and substantially reduced by 72 ± 4.3% and 63 ± 5.0%, respectively. Similar results were observed in DIO mice, where the normal arterial pressure of 80 ± 4.0 mmHg was reduced by 65 ± 7.3% and 62 ± 3.1% with acute and chronic occlusion. When collateral and microvascular resistances were calculated from these pressure measurements, the upstream proximal collateral resistance was significantly greater than distal microvascular resistance in lean and DIO mice for both acute and chronic ligation (Fig. 1B). When within-animal comparisons were made of limbs with acute and chronic femoral ligation, a significant decrease in segmental collateral resistance was observed in lean but not DIO mice.
Fig. 1.
Hindlimb arterial pressure and segmental resistances in lean and diet-induced obese (DIO) mice. A: saphenous artery pressures measured by the servo-null technique in lean and obese mice. Saphenous artery pressure was significantly reduced in lean (23 ± 3.6 mmHg) and DIO mice with acute and chronic ligation (≥2 wk) of the distal femoral artery. B: segmental resistances were calculated for the collateral arteries and distal microcirculation. Relative collateral resistance was greater than microvascular resistance in both lean and DIO mice for both acute and chronic ligation. The only decrease in relative resistance from acute to chronic ligation occurred in lean mice and indicates collaterals as the primary site of compensation (n = 7 lean and DIO mice).
Apocynin prevented DIO-induced impairment of vascular compensation to femoral artery occlusion.
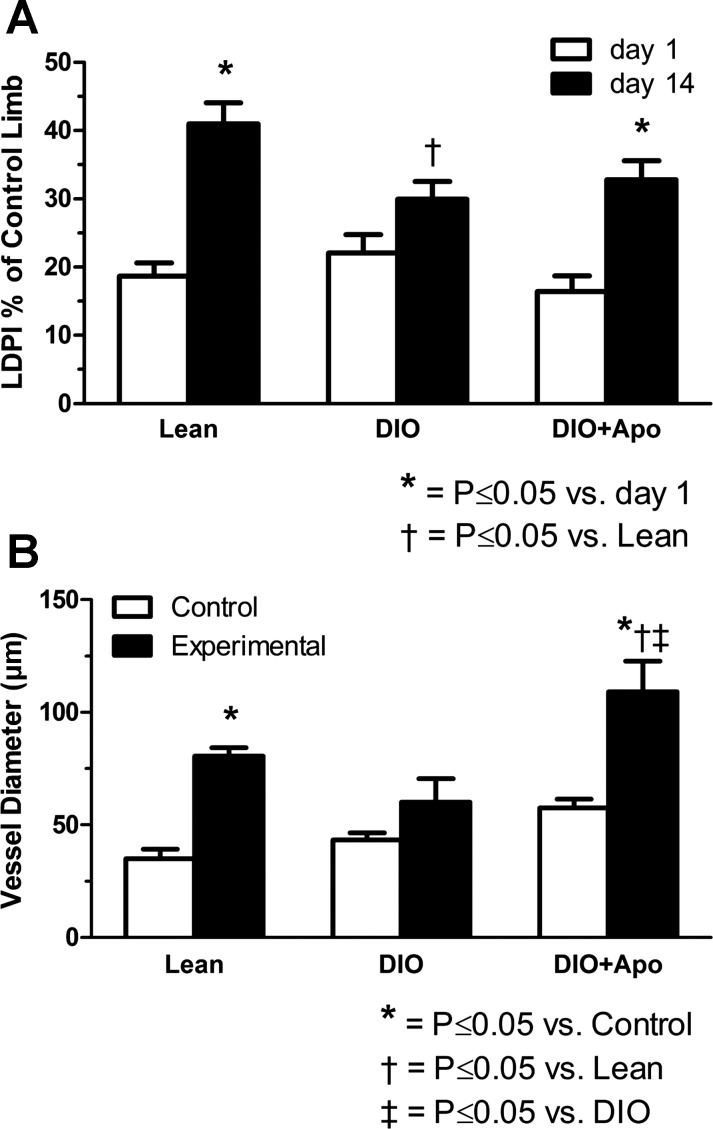
As shown in Fig. 2A, foot perfusion at 1 day after femoral artery ligation was reduced to a similar level in lean, DIO, and apocynin-treated DIO mice (19 ± 2.5% 22 ± 3.4%, and 16 ± 3.1% of the control limb, respectively). On day 14, perfusion was significantly increased in lean and apocynin-treated DIO groups but not in the DIO group. The increases in perfusion from days 1 to 14 averaged 147 ± 48% in lean mice and 113 ± 25% in apocynin-treated DIO mice versus 49 ± 29% in DIO mice. Figure 2B shows average diameters of anterior and posterior gracilis arteries from control and experimental limbs. Luminal diameters of the experimental (collateral) arteries in limbs with femoral artery ligation were significantly greater than the same arteries in control limbs in lean and apocynin-treated DIO mice but not in DIO mice (Fig. 2B). Compared with same-animal control limbs, diameters of the gracilis arteries were increased 151 ± 30% and 88 ± 17% in lean and apocynin-treated DIO mice versus 44 ± 17% in DIO mice.
Fig. 2.
Hindlimb perfusion and collateral vessel enlargement after femoral artery ligation in lean, DIO, and apocynin-treated DIO (DIO + Apo) mice. A: experimental limb foot perfusion, measured as a percentage of the control limb, increased significantly from postligation days 1 to 14 in both lean and DIO + Apo mice but did not change significantly in untreated DIO mice (n = 5–9). B: quantitation demonstrated a significant increase in the collateral vessel luminal diameter versus same animal controls in lean and DIO + Apo mice but not in DIO mice (n = 5–7). The n for lean diameters was 2 fewer than that for lean laser-Doppler perfusion imaging (LDPI) because 2 animals died during cannulation of the aorta at the time of death.
Increased vascular Nox subunit mRNA expression in DIO mice.
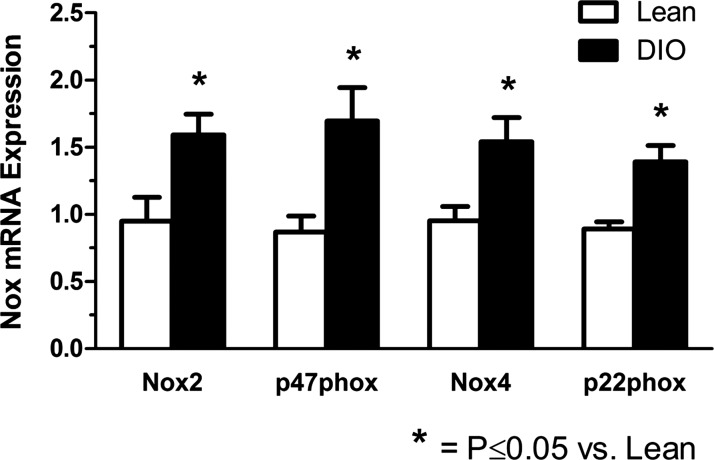
mRNA expression of the Nox components p22phox, Nox2, p47phox, and Nox4 in the peripheral vasculature (iliac-femoral arteries) was assessed by PCR. The results shown in Fig. 3 demonstrate that these were all significantly elevated in DIO versus lean mice, with the increase averaging 56%, 68%, 95%, and 62%, respectively, for p22phox, Nox2, p47phox, and Nox4. Nox1 appeared to be much less abundant, and no quantitation was possible because, under the same amplification conditions, ddCt did not rise above background fluorescence until the highest cycle numbers.
Fig. 3.
NADPH oxidase (Nox) expression in iliac/femoral arteries from lean and DIO mice. Changes in specific Nox subunit mRNA expression due to high-fat diet feeding were assessed using real-time quantitative PCR. Results are expressed as relative fold changes after normalization to β-actin (n = 3–6).
Nox2-p47phox inhibition/ablation prevents impaired vascular compensation in DIO mice.
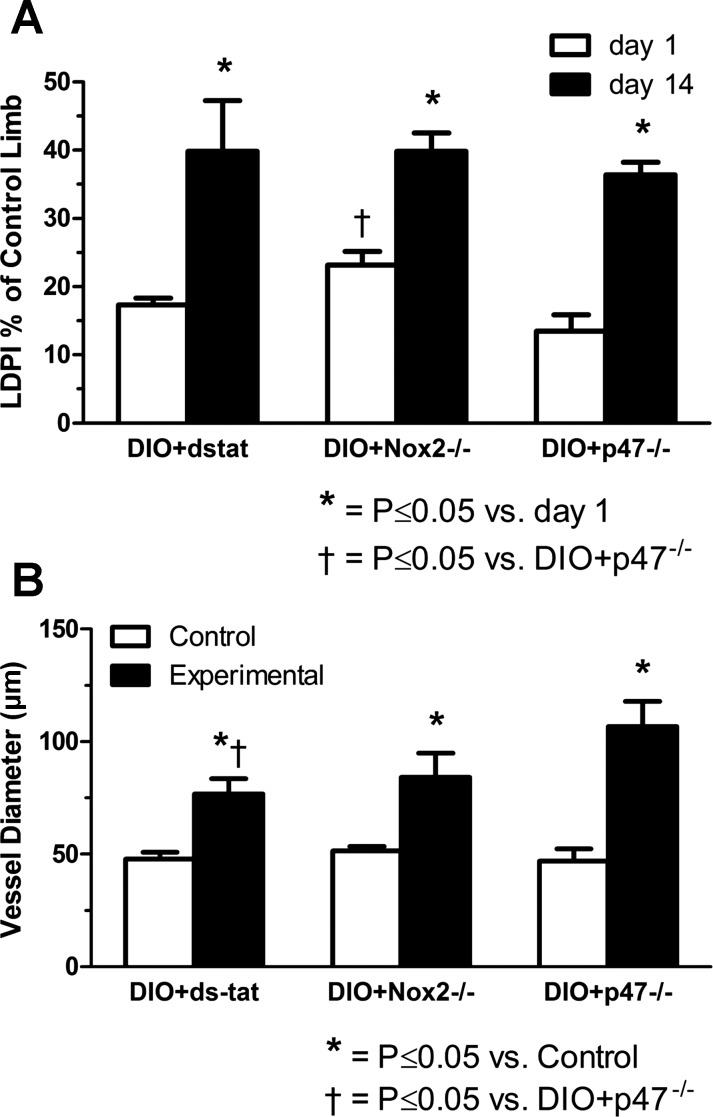
To assess the potential role of Nox2 and p47phox in the obesity-induced impairment of vascular compensation to arterial occlusion, we used a specific inhibitor of Nox2 (Nox2ds-tat) and Nox2−/− and p47phox−/− mice. As shown in Fig. 4, DIO mice in all these groups exhibited significantly increased foot perfusion and collateral artery enlargement after femoral artery ligation, unlike the untreated DIO mice shown in Fig. 2. Increases in perfusion from days 1 to 14 averaged 129 ± 29% in mice treated with Nox2-ds-tat, 80 ± 17% in Nox2−/− mice, and 207 ± 51% in p47phox −/− mice, respectively. Diameter increases in gracilis collateral arteries relative to same animal controls averaged 62 ± 10%, 68 ± 26%, and 132 ± 19%, respectively, for mice treated with Nox2ds-tat and Nox2−/− and p47phox−/− mice.
Fig. 4.
Assessment of Nox inhibition on hindlimb reperfusion and collateral vessel enlargement after femoral artery ligation in DIO mice. A: specific pharmacological inhibition of the Nox2-p47phox interaction (via Nox2ds-tat; DIO + dstat) and whole body ablation of either Nox2 or p47phox subunits (DIO + Nox2−/− and DIO + p47−/−, respectively) attenuated the impairment in foot perfusion seen in obese mice (refer to Fig. 2A), as evidenced by a significant increase in their ligated limb perfusion from days 1 to 14 after occlusion (n = 3–8; note that LDPI imaging was not available for most of the DIO + dstat mice). B: treatment of obese mice with Nox2ds-tat or ablation of Nox2 or p47phox also attenuated the impairment in collateral growth observed in high-fat diet-fed mice (refer to Fig. 2B), as evidenced by a significant increase in collateral vessel diameter versus same animal controls for each of these treatment groups (n = 7–11).
Collateral growth-associated inflammatory cell mobilization and demargination are not impaired in DIO mice.
Multiparametric FACS analysis was performed to determine if an impairment in mobilization and/or demargination of BMDCs contributes to the mechanism of collateral growth dysfunction observed in obesity. Quantification of the percent live circulating total monocytes demonstrated that there were significantly more total monocytes at baseline in DIO versus lean mice (P ≤ 0.05; Fig. 5B). Furthermore, this quantification demonstrated that the percentage of live circulating total monocytes in both groups significantly increased from baseline to day 1 postligation and that this percentage then decreased from days 1 to 3 postocclusion (Fig. 5B), indicating mobilization and demargination events, respectively. When inflammatory versus patrolling monocyte subsets were interrogated, no significant alterations were noted in the percentage of the inflammatory (Ly6CHi) monocyte subset; however, significant changes in the patrolling (Ly6CLow) monocyte subset were observed, which mirrored those of the total monocyte population (Fig. 5, C and D). These data suggest that the mobilization and demargination events observed in the total and patrolling monocyte populations as well as the relatively minor fluctuations in the inflammatory monocyte population were not impaired in DIO versus lean mice.
DISCUSSION
The results of this study demonstrate a profound impairment of vascular compensation to focal arterial occlusion in DIO mice that is mediated by Nox and involves the suppression of collateral artery luminal remodeling to a larger diameter. Distal arterial pressure measurements identified proximal collaterals as the site of primary vascular resistance after peripheral arterial occlusion, and in situ diameter measurements established that enlargement of gracilis arterial collaterals was impaired in DIO mice. The impairment in DIO mice of hindlimb perfusion and primary collateral vessel enlargement was associated with an elevation of circulating BMDCs and mobilization and demargination were not suppressed. Treatment with the antioxidant apocynin, Nox2ds-tat (a specific inhibitor of Nox2/p47phox interaction), or whole body ablation of Nox2 and p47phox restored the capacity for collateral growth in DIO mice. The significance of each of these novel observations is discussed below as well as potential implications for pharmacological and molecular therapies in PAD.
Importance of collaterals in terms of vascular resistance.
To our knowledge, this study is the first to provide segmental resistances in the mouse limb after femoral artery ligation, especially in the context of cardiovascular risk factors. Generally, ≥60% or more of mean arterial pressure reaches the terminal feed arteries that directly precede the microcirculation in skeletal muscle (30, 47, 48). After femoral artery occlusion in the mouse, pressure in the saphenous artery is decreased to ≤30 mmHg (Fig. 1A). This is less than the pressure normally present in medium to small arterioles (8, 30) and is approximately the pressure in normal skeletal muscle capillaries. Thus, in this mouse model with distal femoral artery ligation, there is a major shift in the site of primary resistance after major arterial occlusion such that ∼70% of the total vascular resistance for limb perfusion occurs proximal to the calf muscles and less than half of the normal distal arterial pressure is available to force the flow of blood from the saphenous artery through small arteries, skeletal muscle arterioles, capillaries, and venules and return blood to the heart. This means that compensation in the distal vasculature, such as increased arteriolar numbers or diameters, would have limited effect on perfusion unless accompanied by decreased collateral resistance. In effect, without the cooperation of collateral arteries to bridge blood flow around arterial occlusion sites, the distal microvascular adaptations in capillaries and arterioles would be of minor functional relevance.
In the mouse hindlimb, the collateral resistance as percentage of total hindlimb resistance decreased from ∼70 to 56% in lean mice but was unchanged in DIO mice (Fig. 1). The relative resistances determined for collateral and microcirculation vasculatures in the mouse hindlimb are similar to previous reports in preclinical models and humans. In the rat hindlimb under resting conditions, we have shown the percent collateral resistance to decrease from ∼70% initially after femoral artery ligation to ∼60% (83) and 50% (49) at 1 and 8 wk postligation, respectively. In the feline hindlimb under maximally dilated conditions, collateral resistance decreases from ∼80% with acute femoral artery ligation to ∼50% in 5 wk (69). Segmental resistances have also been reported for dogs [decrease from ∼50% to 25% in 250 days (26)] and monkeys [from ∼60% to 30% in 16 mo (55)]. In human patients with femoropopliteal arterial occlusion (an equivalent occlusion site compared with the mouse model used), Ludbrook (56) measured collateral resistance and observed it to be increased with disease progression. As a percentage of total vascular resistance, collateral resistance at rest and during peak flow conditions was, respectively, 23% and 73% in claudicants without rest pain and 52% and 89% in PAD patients with rest pain. Similar results were reported by Mundth et al. (62) for measurements made in patients undergoing femoral-popliteal bypass surgery: the percent collateral resistance was 46%, 63%, and 81% for patients with claudication, rest pain, and gangrene, respectively. It is thus important to include assessment of collateral growth and/or function in preclinical studies of vascular compensation to arterial occlusion.
Relevance of experimental model of impaired collateral compensation to peripheral arterial occlusion.
All of the studies in various species including humans in which pressures have been measured distal to the occlusion site indicate that the vascular segment of greatest resistance is the collaterals. In most organs, including skeletal muscle, the arterial-arteriolar tree is such that these collateral vessels will be small arteries (16, 33, 54, 70). The “collateral growth” or arteriogenesis that occurs in human legs is generally considered to involve preexisting small arteries (4, 70). The primary collateral pathway in humans with focal occlusion of the distal superficial femoral or proximal popliteal artery is typically the genicular arteries of 1- to 2-mm diameter (16, 70).
In the mouse hindlimb model with distal femoral artery ligation, the gracilis arteries form a collateral pathway commonly studied and we have shown this pathway from the profunda femoral through the gracilis arteries to the saphenous artery to be a major collateral circuit (21). The results shown in Fig. 2 demonstrated that both distal limb perfusion and collateral diameter enlargement were suppressed in DIO mice, which have multiple cardiovascular risk factors and are characterized by endothelial dysfunction and oxidative stress (44, 57). Endothelial dysfunction and oxidative stress not only compromise the vasodilatory capacity of resistance vessels but are also associated with suppression of compensatory flow-mediated remodeling and collateral enlargement in the context of arterial occlusion in humans (90, 95, 96) and animals (60, 79, 80, 87, 98, 99).
The major risk factors for PAD have been identified (29) to include advanced age, smoking, diabetes, hypertension, and hypercholesterolemia. Several of these factors as well as related endothelial dysfunction and oxidative stress are also associated with impaired compensation to arterial occlusion. Although obesity itself is not a major risk factor of arterial disease, it has a strong correlation with and contribution to the development of and progression of diabetes, hypercholesterolemia, and hypertension (50, 61). The murine model used in this study reflects major characteristics of the human condition of arterial disease, including multiple risk factors, endothelial dysfunction, oxidative stress associated with elevated Nox expression, and impaired compensation in collateral vessels, which comprise the primary site of resistance. While the DIO mouse hindlimb model used in the present study appears to mimic impaired compensation observed in humans with focal arterial occlusion, murine models of arterial occlusion have been criticized for their inability to predict clinical outcomes; thus, potential limitations of this model should be considered. The occlusive event was sudden, as can occur in humans with an atherosclerotic embolus, but was not associated with the decade-long presence of vascular disease, as is often the case clinically. The murine primary collateral vessels are much smaller (∼50 times) than in humans, have much thinner walls, and are located shorter distances from ischemic tissues (100). Consequently, the hemodynamic and metabolic stimuli that influence remodeling may be different. However, as mentioned above, the relative distribution of vascular resistances in mice is quite similar to those of large species and the relative changes in resistance after occlusions are of similar magnitude. Accordingly, the relevance of the mouse to large-mammal hemodynamics and compensatory mechanisms appear to provide valuable insights and facilitates the investigation of mechanisms using genetically modified animals.
Role of Nox2-p47phox in impaired compensation to arterial occlusion.
As shown in Fig. 3, the Nox subunits p22phox, Nox2, p47phox, and Nox4 all had significantly elevated expression in femoral/iliac arteries from DIO versus lean mice, whereas the expression of Nox1 was significantly lower. We initially attempted to quantify mRNA Nox levels in same animal collateral and control gracilis arteries. However, the low yields from these vessels would have required ∼15 mice per RNA replicate (18); thus, the more proximal peripheral arteries were used. It is important to note that mRNA expression may not reflect protein levels or Nox activity and that the expression present in iliac-femoral arteries may differ from smaller gracilis arteries. However, to our knowledge, this study is the first report of Nox expression in peripheral vessels smaller than the aorta in DIO mice. These increases observed in DIO mice are consistent with reports of Nox expression increases in other vascular tissues, cells, or plasma from experimental DIO and insulin resistance as well as available clinical studies. In the aorta of DIO mice, Du et al. (24) observed increased expression of Nox2 and p22phox protein but not Nox1 or Nox4. Pulmonary endothelial cells collected from insulin-resistant mice (75) showed elevated Nox2 and Nox4 expression but not Nox1. In humans, Silver et al. (72) showed increased p47phox expression in venous endothelial cells from overweight and obese humans, and elevated levels of soluble Nox2 have been reported for obese and hypercholesterolemic patients (52). It will be important for future studies to identify the specific vascular cells that have altered Nox expression in the presence of vascular risk factors. Based on studies of the aorta in DIO mice (24) and rats characterized by hypertension and metabolic disorders (92), we expect that expression of Nox2 would be limited to endothelial cells and fibroblasts. However, Nox2 expression has been reported in vascular smooth muscle cells cultured from rat mesenteric arteries (12). The improved compensation in DIO mice pretreated with apocynin (Fig. 2) is consistent with a major role for oxidative stress in the impairment of vascular compensation. Our results with the selective Nox2 inhibitor Nox2ds-tat and whole body ablation of Nox2 and p47phox (Fig. 4) provide evidence to support the role of Nox2-p47phox interactions in the impairment of hindlimb perfusion and primary collateral growth observed in DIO mice. This finding is consistent with reports that Nox2 mediates 1) endothelial dysfunction in DIO mice (24, 57); 2) impaired vascular compensation to arterial occlusion in the presence of risk factors including aging, smoking, hypercholesterolemia, and diabetes (25, 34, 35, 78); and 3) abnormal flow-mediated nitric oxide responses in collateral arteries of rat models of impaired collateral compensation (98., 99).
Potential mechanisms mediating impaired collateral growth.
While vascular risk factors such as age, hypercholesterolemia, diabetes, and smoking impair vascular compensation to arterial occlusion, the mechanisms responsible for impaired collateral compensation in the presence of cardiovascular risk factors have not been fully defined and may vary between risk factors (42). Much attention has been focused on the role of BMDCs in collateral growth, and impaired BMDC mobilization and recruitment represent potential primary mechanisms for impairment, especially in diabetic models (40–42). On the other hand, a preexisting, proinflammatory state is associated with impaired flow-mediated collateral dilation and outward remodeling (90). Monocytes have been suggested to be the most important BMDC in mediating arteriogenesis in response to arterial occlusion (31, 39, 41) with either Ly6CHi (14, 15) or Ly6CLow (86) monocyte subsets being of primary importance. Our results obtained with flow cytometry indicate that total monocytes are elevated in DIO mice at baseline and that their mobilization and demargination are not suppressed (Fig. 5). This baseline elevation is consistent with previous reports of a proinflammatory state in the DIO mouse (73, 88). Such low-grade chronic inflammation is associated with PAD (10, 71) and the impairment of flow mediated dilation and outward remodeling of collaterals (90). Our results also demonstrate there is no impairment in the ability of monocytes to mobilize into or demarginate from the circulation in response to femoral artery ligation in DIO mice, consistent with a report (3) of significantly increased numbers of recruited leukocytes in DIO mice after hindlimb ischemia-reperfusion injury. Furthermore, our data demonstrate that the Ly6CLow monocyte population was more significantly affected by femoral ligation than the Ly6CHi population (Fig. 5, C and D). Our results for mobilization of these monocyte subsets are consistent those of van den Hengel et al. (86) but not Cochain et al. (15) in young healthy mice. Possible reasons for the discrepancy between results include the degree of ischemia induced (femoral artery ligation vs. excision) and the methods used to identify inflammatory monocytes and assess collaterals (15). While our data demonstrate that impaired mobilization or demargination of monocytes does not occur in DIO mice and recruitment has been shown to be augmented (3), it is possible that a loss of angiogenic potential of monocytes occurs as proposed as a mechanism of impairment by Kinnaird et al. (42) and demonstrated for type 1 diabetic mice (25). However, our preliminary observations with injections of BMDCs from lean and DIO mice into DIO mice revealed no difference in the effect on collateral growth, consistent with what has been observed in aged mice with impaired vascular compensation when given BMDCs from young donors (91). In contrast, Ito et al. (41) recently reported impaired monocyte recruitment to mediate reduced collateral growth in the leptin receptor-deficient Zucker fatty rat model of type II diabetes but not in rats made hypertensive by renal clamping. Taken together, these results suggest that mechanisms other than those related to BMDC function may mediate the impaired compensation observed in the presence of some major vascular risk factors. Such mechanisms may include vascular-specific deficits (42) such as dysfunctional nitric oxide signaling (91) and abnormal redox-dependent regulation of gene expression (82). Appropriate antioxidant therapy might also mediate improvement through altered metabolic status (64); however, it has been shown in insulin resistance that vascular function is impaired by Nox2 independent of altered glucose status (75).
Potential for Nox-related therapies in PAD.
Vascular Nox has been identified as a therapeutic target to prevent and treat vascular endothelial dysfunction and disease, including PAD, based on numerous preclinical studies that have shown one or more of Nox isoforms 1, 2, and 4, with different cell-specific expression patterns and functions (9), to be the major source of vascular oxidative stress. The beneficial effects of Ramipril in claudicants (1) could be due to the effects of angiotensin-converting enzyme inhibitors on Nox activation (63). Nox2-derived ROS induce endothelial dysfunction and vascular disease (for reviews, see Refs. 9 and 68), whereas Nox 2 inhibition and ablation improve compensation in preclinical models of arterial occlusion (present study and Refs. 25, 34, 35, and 78). Clinical studies have demonstrated elevated serum soluble Nox2 associated with endothelial dysfunction (flow-mediated dilation) and increased oxidative stress in obese and hypercholesterolemic patients (52), nonsmoking claudicants (51), and reduced atherosclerotic burden in human carriers of Nox2 deficiency (89). Thus, there are compelling reasons for optimism for Nox2-related therapies for PAD.
However, more investigation is needed as divergent effects of Nox2-derived ROS on vascular compensation to arterial occlusion have been reported (22, 25, 34, 35, 77, 78). A major concern is that while some of the above studies demonstrated Nox2-inhibition and ablation to provide significant benefit in terms of vascular compensation, others reported suppressed compensation (13, 16, 56). Conversely, the beneficial effects of Nox2 inhibition/ablation were always observed in the presence of risk factors [advanced age (78), hypercholesterenemia (34), smoking (35), and type 1 diabetes (25)] and suppressed compensation was shown in the absence of known risk factors (13, 16, 56). This observation could relate to critical levels of ROS for compensation and pathology, as previously proposed (46, 97). The specific effect on compensation may also depend on the vascular site and level of ischemia (21, 77) as well as cell-specific Nox expression patterns (9).
Another concern is that Nox2-inhibition/ablation could have beneficial effects for one organ system but be harmful in another. For example, whereas Nox2 inhibition may restore vascular compensation after arterial occlusion, Nox2 activity in inflammatory cells could be required for the skeletal muscle repair and regeneration that occurs in the ischemic muscles (21, 25, 77). Because oxidative stress-related myopathy (45) as well as vasculopathy is observed in claudicants, additional preclinical studies are especially needed to address this issue.
Conclusions.
The present work establishes that collateral arteries provide the primary resistance to perfusion after distal femoral artery occlusion and that natural compensation by collaterals is impaired in DIO mice with multiple risk factors, as occurs in humans. This impairment does not involve the suppression of BMDC mobilization or demargination but is associated with a preexisting proinflammatory state. Nox2 and p47phox expression is elevated in DIO mice, as also shown for humans with vascular disease. Thus, this represents a relevant preclinical model to investigate mechanisms of compensation impairment as well as potential therapies. Nox2 and p47phox ablation/inhibition prevents the impaired compensation in DIO mice. Additional work is needed to determine if Nox-related therapies can reverse as well as prevent impaired collateral compensation to arterial occlusion and to establish potential mechanisms and effects of such therapies on PAD-related myopathy.
GRANTS
This work was supported by National Institutes of Health grants R01-HL-42898 (to J. L. Unthank) and R01-HL-092012 (to S. J. Miller) as well as T32-DK-007519 (to M. R. DiStasi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R.D., J.A.M., H.G.B., S.J.M., D.A.I., M.C.D., and J.L.U. conception and design of research; M.R.D., J.A.M., H.G.B., S.J.M., and J.L.U. performed experiments; M.R.D., J.A.M., H.G.B., S.J.M., and J.L.U. analyzed data; M.R.D., J.A.M., H.G.B., S.J.M., D.A.I., M.C.D., and J.L.U. interpreted results of experiments; M.R.D., J.A.M., S.J.M., and J.L.U. prepared figures; M.R.D., J.A.M., S.J.M., and J.L.U. drafted manuscript; M.R.D., J.A.M., H.G.B., S.J.M., and J.L.U. edited and revised manuscript; M.R.D., J.A.M., H.G.B., S.J.M., D.A.I., M.C.D., and J.L.U. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Todd Cook, Brian Johnstone, and the Cardiovascular Ischemia and Vasculogenesis Core at Indiana University School of Medicine for performing the laser-Doppler perfusion imaging and data acquisition. The authors also thank Ingram laboratory members Brandon D. Downing, Waylan K. Bessler, and Brian K. Stansfield for assistance with blood collection and sample processing for the flow cytometry analysis. In addition, the authors thank Melissa P. Swan of the Hickman laboratory and the Laboratory Animal Resource Center for serum chemistry analyses and Miquel Ortiz for excellent technical assistance.
REFERENCES
- 1.Ahimastos AA, Latouche C, Natoli AK, Reddy-luthmoodoo M, Golledge J, Kingwell BA. Potential vascular mechanisms of ramipril induced increases in walking ability in patients with intermittent claudication. Circ Res 114: 1144–1155, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Ahimastos AA, Walker PJ, Askew C, Leicht A, Pappas E, Blombery P, Reid CM, Golledge J, Kingwell BA. Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA 309: 453–460, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Albadawi H, Oklu R, Cormier NR, O'Keefe RM, Heaton JT, Kobler JB, Austen WG, Watkins MT. Hind limb ischemia-reperfusion injury in diet-induced obese mice. J Surg Res 190: 683–691, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10: 387–396, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi T, Higa JK, Aoyagi H, Yorichika N, Shimada B, Matsui T. Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 308: H1530–H1539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltgalvis KA, White K, Li W, Claypool MD, Lang W, Alcantara R, Singh BK, Friera AM, McLaughlin J, Hansen D, McCaughey K, Nguyen H, Smith IJ, Godinez G, Shaw SJ, Goff D, Singh R, Markovtsov V, Sun TQ, Jenkins Y, Uy G, Li Y, Pan A, Gururaja T, Lau D, Park G, Hitoshi Y, Payan DG, Kinsella TM. Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice. Am J Physiol Heart Circ Physiol 306: H1128–H1145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohlen HG. Determinants of resting and passive intestinal pressures in rat and rabbit. Am J Physiol Gastrointest Liver Physiol 253: G587–G595, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Bohlen HG, Gore RW, Hutchins PM. Comparison of microvascular pressures in normal and spontaneously hypertensive rats. Microvasc Res 13: 125–130, 1977. [DOI] [PubMed] [Google Scholar]
- 9.Brandes RP, Schroder K. Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol 19: 513–518, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation 122: 1862–1875, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 197: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood 108: 2438–2445, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochain C, Rodero MP, Vilar J, Récalde A, Richart AL, Loinard C, Zouggari Y, Guérin C, Duriez M, Combadière B, Poupel L, Lévy BI, Mallat Z, Combadière C, Silvestre JS. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc Res 88: 186–195, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Conley JE, Kennedy WF. Collateral arterial circulation in the legs. Arch Surg 81: 348–356, 1960. [DOI] [PubMed] [Google Scholar]
- 17.Csányi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egaña L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg 201: 115–131, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Distasi MR, Case J, Ziegler MA, Dinauer MC, Yoder MC, Haneline LS, Dalsing MC, Miller SJ, Labarrere CA, Murphy MP, Ingram DA, Unthank JL. Suppressed hindlimb perfusion in Rac2−/− and Nox2−/− mice does not result from impaired collateral growth. Am J Physiol Heart Circ Physiol 296: H877–H886, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distasi MR, Unthank JL, Miller SJ. Nox2 and p47phox modulate compensatory growth of primary collateral arteries. Am J Physiol Heart Circ Physiol 306: H1435–H1443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Fan LM, Mai A, Li JM. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptors and endothelium in dietary obesity of middle-aged mice. Br J Pharmacol 170: 1064–1077, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169: 719–728, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckstein RW, Gregg DE, Pritchard WH. The magnitude and time of development of the collateral circulation in occluded femoral, carotid, and coronary arteries. Am J Physiol 132: 351–361, 1941. [Google Scholar]
- 27.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. In: Current Protocols in Cytometry, edited by Robinson JP. New York: Wiley, 2010, 52: 9.33: 1–11. [DOI] [PubMed] [Google Scholar]
- 28.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 36: 943–949, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382: 1329–1340, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol 228: 791–796, 1975. [DOI] [PubMed] [Google Scholar]
- 31.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol 3: 353, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gornik HL. Rethinking the morbidity of peripheral arterial disease and the “normal” ankle-brachial index. J Am Coll Cardiol 53: 1063–1064, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Gray H. Anatomy of the Human Body, edited by Lewis WH. Philadelphia, PA: Lea & Febiger, 1918. [Google Scholar]
- 34.Haddad P, Dussault S, Groleau J, Turgeon J, Maingrette F, Rivard A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis 217: 340–349, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Ménard C, Perez G, Maingrette F, Rivard A. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol 29: 1522–1528, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol 7: 681–685, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Heyman L, Axling U, Blanco N, Sterner O, Holm C, Berger K. Evaluation of beneficial metabolic effects of berries in high-fat fed C57BL/6J mice. J Nutr Metab 2014: 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoefer IE, Grundmann S, van Royen N, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 181: 285–293, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol 34: 1126–1135, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Ito WD, Lund N, Sager H, Becker W, Wenzel U. Differential impact of diabetes mellitus type II and arterial hypertension on collateral artery growth and concomitant macrophage accumulation. Vasa 44: 31–41, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res 78: 257–264, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Kodama K, Toda K, Morinaga S, Yamada S, Butte AJ. Anti-CD44 antibody treatment lowers hyperglycemia and improves insulin resistance, adipose inflammation, and hepatic steatosis in diet-induced obese mice. Diabetes 64: 867–875, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol 295: H1514–H1521, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutakis P, Weiss DJ, Miserlis D, Shostrom VK, Papoutsi E, Ha DM, Carpenter LA, McComb RD, Casale GP, Pipinos II. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol 2: 921–928, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85: 753–766, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Lash JM. Arterial and arteriolar contributions to skeletal muscle functional hyperemia in spontaneously hypertensive rats. J Appl Physiol 78: 93–100, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. J Appl Physiol 76: 1512–1519, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Lash JM, Nixon JC, Unthank JL. Exercise training effects on collateral and microvascular resistances in rat model of arterial insufficiency. Am J Physiol Heart Circ Physiol 268: H125–H137, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 53: 1925–1932, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Loffredo L, Carnevale R, Cangemi R, Angelico F, Augelletti T, Di Santo S, Calabrese CM, Della Volpe L, Pignatelli P, Perri L, Basili S, Violi F. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int J Cardiol 165: 184–192, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Loffredo L, Martino F, Carnevale R, Pignatelli P, Catasca E, Perri L, Calabrese CM, Palumbo MM, Baratta F, Del Ben M, Angelico F, Violi F. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J Pediatr 161: 1004–1009, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Loffredo L, Pignatelli P, Cangemi R, Andreozzi P, Panico MA, Meloni V, Violi F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: effect of an antioxidant treatment. J Vasc Surg 44: 525–530, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Longland CJ. The collateral circulation of the limb. Ann R Coll Surg Engl 13: 161–176, 1953. [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez JA, Armstrong ML, Harrison DG, Piegors DJ, Heistad DD. Responsiveness of iliac collateral vessels to constrictor stimuli in atherosclerotic primates. Circ Res 63: 1020–1028, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Ludbrook J. Collateral artery resistance in the human lower limb. J Surg Res 6: 423–434, 1966. [DOI] [PubMed] [Google Scholar]
- 57.Lynch CM, Kinzenbaw DA, Chen X, Zhan S, Mezzetti E, Filosa J, Ergul A, Faulkner JL, Faraci FM, Didion SP. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke 44: 3195–3201, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDermott M. Medications for improving walking performance in peripheral artery disease: still miles to go. JAMA 309: 487–488, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Miller SJ, Coppinger BJ, Zhou X, Unthank JL. Antioxidants reverse age-related collateral growth impairment. J Vasc Res 47: 108–114, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat (SHR) mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol 292: H2523–H2531, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Morse SA, Gulati R, Reisin E. The obesity paradox and cardiovascular disease. Curr Hypertens Rep 12: 120–126, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Mundth ED, Darling RC, Moran JM, Buckley MJ, Linton RR, Austen WG. Quantitative correlation of distal arterial outflow and patency of femoropopliteal reversed saphenous vein grafts with intraoperative flow and pressure measurements. Surgery 65: 197–206, 1969. [PubMed] [Google Scholar]
- 63.Münzel T, Keaney JF. Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation 104: 1571–1574, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Mykkänen OT, Huotari A, Herzig KH, Dunlop TW, Mykkänen H, Kirjavainen PV. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLos One 9: e114790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLos One 7: e46057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Rocic P, Kolz C, Reed RE, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Rodino-Janeiro BK, Paradela-Dobarro B, Castineiras-Landeira MI, Raposeiras-Roubin S, Gonzalez-Juanatey JR, Alvarez E. Current status of NADPH oxidase research in cardiovascular pharmacology. Vasc Health Risk Manag 9: 401–428, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanne H, Sivertsson R. The effect of exercise on the development of collateral circulation after experimental occlusion of the femoral artery in the cat. Acta Physiol Scand 73: 257–263, 1968. [DOI] [PubMed] [Google Scholar]
- 70.Schoop W, Schaper W, Schaper J. Limb collaterals. In: Collateral Circulation: Heart, Brain, Kidneys, and Limbs. Boston, MA: Kluwer Academic, 1993, p. 317–327. [Google Scholar]
- 71.Shankar A, Li J, Nieto FJ, Klein BEK, Klein R. Association between C-reactive protein level and peripheral arterial disease among U.S. adults without cardiovascular disease, diabetes, or hypertension. Am Heart J 154: 495–501, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Singer K, DelProposto J, Lee Morris D, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Nien-Kai Lumeng C. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 3: 664–675, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing B, Li F, Sarchet KN, DiStasi MR, Conway SJ, Kapur R, Ingram DA. Heterozygous inactivation of the Nf1 gene in myeloid cells enhances neointima formation via a rosuvastatin-sensitive cellular pathway. Hum Mol Genet 22: 977–988, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sukumar P, Viswambharan H, Imrie H, Cubbon RM, Yuldasheva N, Gage M, Galloway S, Skromna A, Kandavelu P, Santos CX, Gatenby VK, Smith J, Beech DJ, Wheatcroft SB, Channon KM, Shah AM, Kearney MT. Nox2 NADPH Oxidase Has a Critical Role in Insulin Resistance-Related Endothelial Cell Dysfunction. Diabetes 62: 2130–2134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and AJ mice. Metabolism 44: 645–651, 1995. [DOI] [PubMed] [Google Scholar]
- 77.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Turgeon J, Haddad P, Dussault S, Groleau J, Maingrette F, Perez G, Rivard A. Protection against vascular aging in Nox2-deficient mice: Impact on endothelial progenitor cells and reparative neovascularization. Atherosclerosis 223: 122–129, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Tuttle JL, Hahn TL, Sanders BM, Witzmann FA, Miller SJ, Dalsing MC, Unthank JL. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol 283: H146–H155, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Herring BP, Dalsing MC, Unthank JL. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation 9: 343–351, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Unthank JL, Bohlen HG. Lymphatic pathways and the role of valves in lymph propulsion from small intestine. Am J Physiol Gastrointest Liver Physiol 254: G389–G398, 1988. [DOI] [PubMed] [Google Scholar]
- 82.Unthank JL, McClintick JN, Labarrere CA, Li L, DiStasi MR, Miller SJ. Molecular basis for impaired collateral artery growth in the spontaneously hypertensive rat: insight from microarray analysis. Physiol Rep 1: e0005, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol 79: 73–82, 1995. [DOI] [PubMed] [Google Scholar]
- 84.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urao N, McKinney RD, Fukai T, Ushio-Fukai M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: role in reparative mobilization of progenitor cells. Stem Cells 30: 923–934, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van den Hengel LG, Hellingman AA, Nossent AY, van Oeveren-Rietdijk AM, de Vries MR, Spek CA, van Zonneveld AJ, Reitsma PH, Hamming JF, de Boer HC, Versteeg HH, Quax PHA. Protease-activated receptor (PAR)2, but not PAR1, is involved in collateral formation and anti-inflammatory monocyte polarization in a mouse hind limb ischemia model. PLos One 8: e61923, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Weel V, de Vries M, Voshol PJ, Verloop RE, Eilers PHC, van Hinsbergh VWM, van Bockel JH, Quax PHA. hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler Thromb Vasc Biol 26: 1383–1390, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Varol C, Zvibel I, Spektor L, Mantelmacher FD, Vugman M, Thurm T, Khatib M, Elmaliah E, Halpern Z, Fishman S. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J Immunol 193: 4002–4009, 2014. [DOI] [PubMed] [Google Scholar]
- 89.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol 33: 406–412, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Vita JA, Holbrook M, Palmisano J, Shenouda SM, Chung WB, Hamburg NM, Eskenazi BR, Joseph L, Shapira OM. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation 117: 3126–3133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Peng X, Lassance-Soares R, Najafi A, Alderman L, Sood S, Xue Z, Chan R, Faber J, Epstein S, Burnett M. aging-induced collateral dysfunction: impaired responsiveness of collaterals and susceptibility to apoptosis via dysfunctional eNOS signaling. J Cardiovasc Transl Res 4: 779–789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AHS, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56: 490–497, 2010. [DOI] [PubMed] [Google Scholar]
- 93.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg 50: 1412–1422, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise training produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol Heart Circ Physiol 282: H301–H310, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord 27: 1541–1545, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Yilmaz MB, Caldir V, Guray Y, Guray U, Altay H, Demirkan B, Cay S, Kisacik HL, Korkmaz S. Relation of coronary collateral vessel development in patients with a totally occluded right coronary artery to the metabolic syndrome. Am J Cardiol 97: 636–639, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal 11: 1961–1974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol 295: H1008–H1016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol 297: H2227–H2233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, George Akingba A, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 17: 3–20, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]