The major finding of this study is that autonomic regulatory therapy, using vagus nerve stimulation, mitigates myocardial infarction-induced remodeling of the intrinsic cardiac nervous system, correspondingly preserving ventricular contractile function via both neural and cardiomyocyte-dependent actions.
Keywords: intrinsic cardiac nervous system, myocardial infarction, autonomic regulation therapy, vagus nerve stimulation, guinea pig
Abstract
This paper aims to determine whether chronic vagus nerve stimulation (VNS) mitigates myocardial infarction (MI)-induced remodeling of the intrinsic cardiac nervous system (ICNS), along with the cardiac tissue it regulates. Guinea pigs underwent VNS implantation on the right cervical vagus. Two weeks later, MI was produced by ligating the ventral descending coronary artery. VNS stimulation started 7 days post-MI (20 Hz, 0.9 ± 0.2 mA, 14 s on, 48 s off; VNS-MI, n = 7) and was compared with time-matched MI animals with sham VNS (MI n = 7) vs. untreated controls (n = 8). Echocardiograms were performed before and at 90 days post-MI. At termination, IC neuronal intracellular voltage recordings were obtained from whole-mount neuronal plexuses. MI increased left ventricular end systolic volume (LVESV) 30% (P = 0.027) and reduced LV ejection fraction (LVEF) 6.5% (P < 0.001) at 90 days post-MI compared with baseline. In the VNS-MI group, LVESV and LVEF did not differ from baseline. IC neurons showed depolarization of resting membrane potentials and increased input resistance in MI compared with VNS-MI and sham controls (P < 0.05). Neuronal excitability and sensitivity to norepinephrine increased in MI and VNS-MI groups compared with controls (P < 0.05). Synaptic efficacy, as determined by evoked responses to stimulating input axons, was reduced in VNS-MI compared with MI or controls (P < 0.05). VNS induced changes in myocytes, consistent with enhanced glycogenolysis, and blunted the MI-induced increase in the proapoptotic Bcl-2-associated X protein (P < 0.05). VNS mitigates MI-induced remodeling of the ICNS, correspondingly preserving ventricular function via both neural and cardiomyocyte-dependent actions.
NEW & NOTEWORTHY
The major finding of this study is that autonomic regulatory therapy, using vagus nerve stimulation, mitigates myocardial infarction-induced remodeling of the intrinsic cardiac nervous system, correspondingly preserving ventricular contractile function via both neural and cardiomyocyte-dependent actions.
autonomic control of the heart is mediated by complex interactions between peripheral and central aspects of the cardiac nervous system (4, 7, 75). These interactions are modulated by nested feedback loops to promote optimal cardiac output under a wide variety of environmental conditions and stresses (7). Myocardial infarction (MI) induces profound changes within this hierarchy, from the level of the heart to the central nervous system that regulates its performance (6, 11, 75). Neurohumoral interactions are likewise altered by MI, including upregulation of the renin-angiotensin-aldosterone system and circulating catecholamines, both of which contribute to the progression of heart failure (23, 32, 37). Cardiac myocyte structure and function are also affected by MI; such changes include apoptosis (17), changes in the collagen matrix (24), and induction of electromechanical dyssynchrony, which is associated with altered ventricular contractile function (3, 55).
Recent work from our laboratory demonstrated time-dependent changes in the intrinsic cardiac (IC) neurons after MI. Specifically, in the 1st wk post-MI, IC neurons demonstrate an increased sensitivity to norepinephrine (NE), reduced sensitivity to both the muscarinic agonist bethanechol and Ang II, and increased network synaptic efficacy (36). Longer term (2 mo) MI-induced changes within the IC neuronal plexus include a return of synaptic efficacy toward baseline values in association with increases in IC neuronal nitric oxide synthase, local mast cell density, and increased neuronal responsiveness to histamine (36, 38).
Whereas the cardiac nervous system is optimized to respond to everyday stressors (orthostatic, heat, exercise, emotion), it can be critically disrupted by the stress of myocardial ischemia/infarction (6, 47), sometimes with lethal effects (11, 13). As a consequence of MI, the IC nervous system (ICNS), the final convergence point for cardiac neural control, is subjected to altered afferent inputs, as well as changes in descending neuronal inputs that include reflex-induced sympatho-excitation and reduced central drive from the parasympathetic nervous system (7, 11, 22).
We have previously demonstrated that chronic MI alters IC neurons, thereby increasing IC neuronal plexus excitability leading to cardiac dysfunction (36, 38). It is known that beta-adrenergic blockade and pharmacological modulation of angiotensin AT1 and AT2 receptors can attenuate such remodeling (33, 37, 39). We have demonstrated further that neuromodulation therapy, using spinal cord stimulation, is effective in blunting ischemia-induced reflex excitation of extracardiac and IC neurons (5, 9), thereby mitigating the ischemia-induced arrhythmogenic substrate (16) and reducing cardiomyocyte cell death (63, 64). Vagus nerve stimulation (VNS) activates both ascending (afferent) and descending (efferent) axons that course in the vagus (12, 14), thereby impacting central and peripheral aspects of the cardiac nervous system. In either case, IC neurons represent the final convergence point for parasympathetic neural-cardiac control (7, 45).
Given the above, we hypothesize that restoration of an exogenous, biomimetic, “central” parasympathetic drive via cervical VNS will mitigate remodeling of IC neurons and render cardiac myocytes stress resistant. Together, these effects of VNS will mitigate progression of cardiac dysfunction post-MI. This hypothesis is further predicated on the antiadrenergic responses that are elicited by parasympathetic efferent neuronal inputs acting at processing nodes within the cardiac nervous system and at the efferent neural-myocyte interface (11, 50, 54).
To evaluate this hypothesis critically, we examined cardiac function in vivo via echocardiography in animals with chronic MI, either in the absence or presence of chronic VNS therapy, to determine the following: 1) how such therapy impacts IC neuronal transmembrane electrical properties, 2) their interactive synaptic efficacy in vitro, and 3) the capacity of such therapy to induce a cardioprotective state at the myocyte level. As such, we were able to assess the therapeutic benefits of VNS therapy, not only at the cardiomyocyte level but also how it impacts IC neurons that regulate cardiomyocyte behavior.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of East Tennessee State University and were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition, published by The National Academies Press.
Implantation of VNS systems.
Eighteen male Hartley guinea pigs (Charles River Laboratories, Wilmington, MA), weighing between 500 and 650 g (9 wk old), were implanted with a VNS system comprised of a pulse generator and bipolar lead. Under aseptic conditions, animals were pretreated with atropine (0.1 mg/kg sc) and ketamine (80 mg/kg ip). Thereafter, anesthesia was induced with 3% isoflurane via an induction chamber (VetEquip, Pleasanton, CA). Upon removal from the induction chamber, 2.5% isoflurane was delivered via a conical nose cone (VetEquip) until responses to hindlimb toe-pinch stimuli were absent. Following endotracheal intubation, mechanical ventilation was initiated and maintained with a positive pressure ventilator (SAR-830/P ventilator; IITC Life Science, Woodland Hills, CA) using 100% O2. Anesthesia was maintained with isoflurane (1–3%). Core body temperature was maintained at 38.5°C with a circulating water heating pad. Buprenorphine (0.05 mg/kg sc) was administered preoperatively.
Following anesthesia induction, a midline neck incision was made. The right cervical carotid and associated vagus nerve were isolated, and a bipolar VNS electrode (Cyberonics, Houston, TX) was positioned around that artery-nerve complex. The lead was secured in place and tunneled to a subcutaneous pocket created over the dorsal aspect of the back where the implantable VNS pulse generator (Demipulse, Model 103; Cyberonics) was positioned. Incisions were closed in layers. Postoperative care included buprenorphine (0.05 mg/kg sc), given as needed, and cefazolin (30 mg/kg im) for 7 days. The pulse generator was inactive for the recovery period (∼2 wk).
Surgical induction of heart disease.
Two weeks later, using techniques detailed previously (36, 38), MI was surgically induced by ligation of the ventral descending coronary artery and associated vein in all 18 guinea pigs equipped with a VNS system. Anesthesia and postoperative care were the same as defined above. Work from our laboratory (36, 38) and others (18) has shown that such methodology induces an ∼8% infarct. Of 18 animals, two died early on as a direct result of MI, and two were euthanized secondary to necrosis surrounding the pulse generator. Of the remaining 14 animals, following 1 wk of MI recovery, animals were randomized to groups that were either stimulated (VNS-MI group; n = 7) or not stimulated (MI group; n = 7). All VNS-MI animals were treated with chronic, intermittent (continuously cyclic), low-intensity right cervical VNS (20 Hz pulse frequency, 0.9 ± 0.2 mA pulse amplitude, 500 μs pulse duration, 14 s on time, 48 s off time for 80 days). Age-matched animals without surgery were used as concurrent controls (control group, n = 8).
VNS stimulation parameters were chosen to be close to the neural fulcrum, where any effects on heart rate were minimized by the relative effects of VNS on afferent- and efferent-dependent responses (45). One-third of the animals demonstrated a 5% evoked bradycardia during active-phase VNS. Fifty percent of animals exhibited no significant change in heart rate with VNS. Two animals exhibited a slight tachycardia during active-phase VNS. VNS intensity levels are limited in the guinea pig by effects on water and food intake. In the animals that did not exhibit bradycardia, attempts to increase stimulus intensity further resulted in loss of body weight.
Echocardiography.
Following sedation with isoflurane (1–2% via nodose cone), short-axis and long-axis echocardiograms were used to determine the left ventricular end systolic volume (LVESV) and the LV ejection fraction (LVEF) for each animal. These measurements were acquired before VNS implant surgery and at 90 days post-MI, just before the terminal experiment.
Terminal experiments.
Following echocardiography, animals were euthanized by CO2 inhalation and exsanguination. The heart was removed and placed into ice-cold Krebs-Ringer solution (in mM: NaCl 121, KCl 5.9, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, glucose 8, aerated with 95% O2/5% CO2 for a pH of 7.4). The heart and lungs were weighed. The lungs were dried at 37°C and weighed again (dry lungs). The cardiac nerve plexus, located in the epicardium of the atrial walls, was dissected as described previously (35). Following dissection, tissue was superfused continuously (6–8 ml/min) with 35–37°C Krebs-Ringer.
Electrophysiological methods.
Intracellular voltage recordings from IC neurons were obtained with an Axoclamp 2B amplifier (Molecular Devices, Sunnyvale, CA) from cells impaled with 3 M KCl-filled glass micropipettes (40–80 MΩ). In the guinea pig model, the IC neurons, recorded with such sharp microelectrodes, are primarily cholinergic in nature (52). Data were collected, digitized, and analyzed using pCLAMP 10.2 (Molecular Devices). Individual neurons were used for data analysis if the resting membrane potential (RMP) was −40 mV or less and produced action potentials with an overshoot of at least 20 mV (31). Single action potentials were produced by brief depolarizing current injections (0.7–1.2 nA, 3 ms). Six individual traces were averaged and analyzed to determine afterhyperpolarization (AHP) amplitudes and durations. AHP durations were analyzed to determine the time needed to reach 50% of the amplitude from the peak of the AHP to the RMP. Neuronal input resistance was determined by injecting small hyperpolarizing current pulses of 0.1 and 0.2 nA using 500 ms pulse duration. Neuronal excitability was monitored by observing the number of action potentials generated in response to a series of long depolarizing current pulses (0.1–0.6 nA, 500 ms) before and after brief (1 s) application of NE 10−3 M (Sigma, St. Louis, MO), applied by local pressure ejection (4–6 psi; Picospritzer II; General Valve, Fairfield, NJ) through a small tip diameter (5–10 μm) glass micropipette, positioned 50–100 μm from the individual neuron. The cells were then washed (via the circulating Krebs solution) for several minutes until their response returned to control levels.
To activate synaptic inputs, an extracellular, bipolar, concentric electrode was placed on nerve-fiber bundles leading to the ganglion containing the neuron of interest (36, 39). Orthodromic responses to fiber-tract stimulation (0.1–10 V, 1 ms duration) were determined by the ability to generate an excitatory postsynaptic potential or by the presence of a time delay between the stimulus artifact and the neuronal response. Suprathreshold stimuli leading to action potentials were then given in 2 s trains at frequencies of 1, 2, 5, 10, and 20 Hz, and the number of action potentials produced by the neuron of interest at each stimulus frequency was determined.
Preparation of heart homogenate.
Concurrently, with the dissection of the cardiac neuronal plexus from the atria, the ventricles were washed briefly in PBS, and ventricular samples from each heart were grossly dissected into three tissue portions and clamped immediately with a set of tongs that was prechilled in liquid nitrogen. The portion (∼100 mg) that contained the infarct and some surrounding tissue were designated the central zone (CZ). Moving concentrically away from infarction, an ∼350-mg intermediate zone (IZ) and an ∼350-mg distal zone (DZ) were also obtained. They were then ground into a fine powder using a mortar and pestle under liquid nitrogen. The powdered heart samples were homogenized in radioimmunoprecipitation assay buffer composed of 50 mM Tris-HCl, pH 7.4 (Calbiochem, Darmstadt, Germany), 1% vol/vol Triton X-100 (Fisher Scientific, Pittsburgh, PA), 1% wt/vol sodium deoxycholate (Fisher Scientific), 0.1% wt/vol SDS (EMD Millipore, Billerica, MA), and 1 mM EDTA (Fisher Scientific), with 1:40 vol/vol protease inhibitor cocktail mix (Sigma). The homogenates were incubated on ice for 1 h and then centrifuged at 12,000 g at 4°C for 10 min. The supernatant was collected. Protein concentration for guinea pig heart homogenates was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL), according to the manufacturer's protocol.
SDS-PAGE and Western blot.
Phosphorylation status of GSK-3β and its substrate glycogen synthase (GS) was determined from each zone from the MI, VNS-MI, and control guinea pigs, essentially as described previously (73). Western blots were prepared from these gels and probed with antibodies specific for phosphorylated Ser641 of GS (p-GS), GS protein (GS), phosphorylated Ser9 of GSK-3β (p-GSK-3β), GSK-3β protein (GSK-3β), and proapoptotic Bcl-2-associated X (BAX) protein. Protein samples (60 μg/lane) were separated using SDS-PAGE in Pierce Tris-Hepes-SDS 4–20% precast polyacrylamide gels (Thermo Fisher Scientific). Proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Richmond, CA) at 75 V for 2 h. After transfer, Ponceau (Sigma) staining was used to ensure complete transfer and equal protein loading. Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) with 1% Tween 20 (TBS-T) for 1 h at room temperature. GS expression was probed with a rabbit primary MAb at 1:1,000 dilution in TBS-T. Other antibodies were used at the manufacturer's recommended dilutions. Membranes were incubated at 4°C overnight and washed for 5 min in TBS-T (5×) before incubation with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody. Protein bands were detected using the Pierce SuperSignal West Pico Chemiluminescence Substrate (Thermo Fisher Scientific) in the G:Box Chemiluminescence and Fluorescence Imaging System. Serial exposure times in increments of 10 s were recorded for up to 2 min. For densitometry analysis, lower exposure times were selected to ensure linearity, whereas higher exposures are shown in figures for visual clarity. In some of the figures, blots were cut and rearranged strictly for presentational purposes; however, within rows, images were from the same blot and treated identically. Unless otherwise noted, all antibodies were purchased from Cell Signaling Technology (Beverly, MA) and used according to the manufacturer's instructions. Band intensities were quantified using ImageJ software analysis.
Statistical analysis.
Heart function using echocardiography was analyzed with a repeated-measures ANOVA to compare each animal with its baseline condition. The neuronal activity determined by intracellular current injections (see Figs. 1 and 2) was not normally distributed when analyzed using a Shapiro-Wilk test. A nonparametric Friedman test at the ordinal level and post hoc Wilcoxon signed-rank tests with a Bonferroni correction were done to determine differences among study groups. The data related to tissue weights (see Table 2), neuronal transmembrane properties (see Table 3), synaptic properties (see Fig. 3B), and myocyte function (see Figs. 4 and 5) were continuous and normally distributed by using a Shapiro-Wilk test. These data were analyzed using a simple or a mixed-model ANOVA, followed by a Newman-Keuls post hoc analysis. Results with P < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS software.
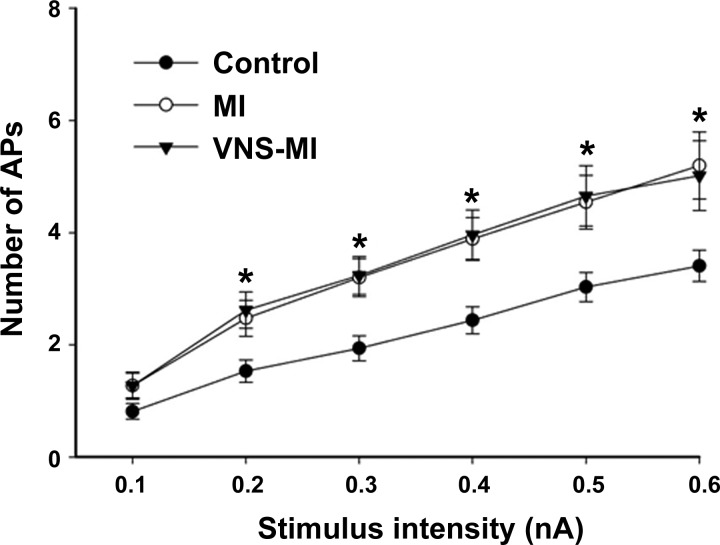
Fig. 1.
Evoked action potential (AP) frequencies with increasing intracellular stimulus intensities (0.1–0.6 nA, 500 ms) were determined by intracellular voltage recordings from intrinsic cardiac (IC) neurons in control preparations, in preparations at 90 days post-myocardial infarction (MI), and in preparations at 90 days post-MI that included 80 days of autonomic regulation therapy [vagus nerve stimulation (VNS)-MI], starting 10 days post-MI induction. A nonparametric Friedman test was used to evaluate difference among groups, followed by Wilcoxon signed-rank post hoc tests using a Bonferroni correction. Points represent the means ± SE from ∼60 cells for each condition. *P < 0.05, control vs. MI and VNS-MI.
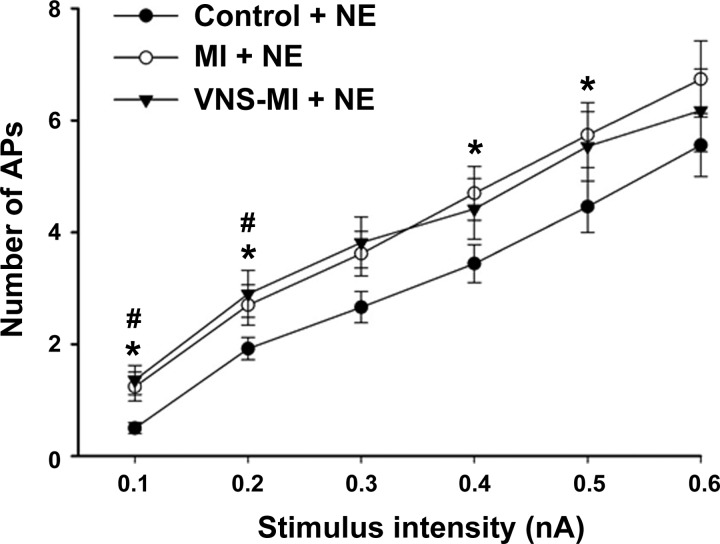
Fig. 2.
Evoked action potential frequencies in response to increasing intracellular stimulus intensities were evaluated, concurrent with brief (1 s), local exposure to exogenous norepinephrine (NE) in IC soma derived from control animals and animals following MI, with and without chronic VNS. A nonparametric Friedman test was used to see differences among groups, followed by Wilcoxon signed-rank post hoc tests using a Bonferroni correction. Points represent the means ± SE from ∼60 cells for each condition. *P < 0.05, control vs. MI; #P < 0.05, control vs. VNS-MI.
Table 2.
Analysis of tissue weights in controls, MI, and VNS-MI
| Controls, n = 8 | MI, n = 7 | VNS-MI, n = 7 | |
|---|---|---|---|
| Age at termination, wk | 28.2 ± 1.1 | 30.5 ± 0.5 | 29.2 ± 1.9 |
| Postoperative recovery, wk | 12.8 ± 0.2 | 12.6 ± 0.3 | |
| Body weight (wt), g | 1011 ± 56 | 1106 ± 52 | 1001 ± 94 |
| Heart weight, % of body wt | 0.65 ± 0.08 | 0.61 ± 0.23 | 0.66 ± 0.06 |
| Wet lung weight, % of body wt | 0.51 ± 0.05 | 0.48 ± 0.15 | 0.50 ± 0.05 |
| Dry lung weight, % of body wt | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.10 ± 0.01 |
Values are means ± SD. A Shapiro-Wilk test showed normality, and no significant effect (P < 0.05) was found among groups using an ANOVA.
Table 3.
Properties of intracardiac neurons of controls, MI, and VNS-MI
| Property | Controls, n = 64 | MI, n = 55 | VNS-MI, n = 55 |
|---|---|---|---|
| Resting membrane potential, mV | −49.6 ± 0.8 | −44.4 ± 0.6* | −49.5 ± 1.0 |
| AHP amplitude, mV | 15.7 ± 0.6 | 16.4 ± 0.5 | 16.5 ± 0.5 |
| AHP half-decay time, ms | 119.5 ± 6.2 | 105.9 ± 7.7 | 106.0 ± 8.2 |
Values expressed as means ± SE, with number of neurons shown. A Shapiro-Wilk test showed normality, and a significant effect (
P < 0.05) with ANOVA is shown for resting membrane potential and input resistance, MI vs. controls and VNS-MI. AHP, afterhyperpolarization.
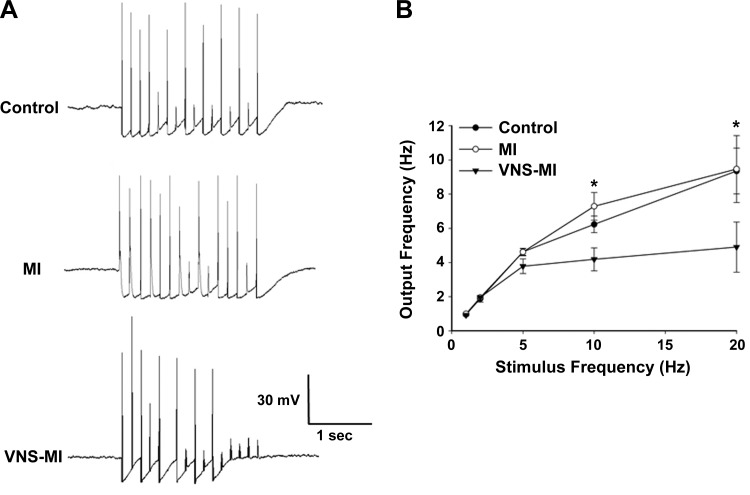
Fig. 3.
Chronic VNS reduces synaptic efficacy of IC neurons. Nerve fibers synapsing with the IC neurons were stimulated via an extracellular concentric electrode (0.1–10 V, 2 ms) for 2 s at frequencies of 1, 2, 5, 10, and 20 Hz. A: representative examples of recordings derived from control, MI, and VNS-MI preparations when nerves were stimulated at 10 Hz. B: average data derived from ∼20 cells for each condition. An ANOVA analysis indicated significant differences among treatments and was followed by Newman-Keuls post hoc analysis. Points are the means ± SE. *P < 0.05, control and MI vs. VNS-MI neurons.
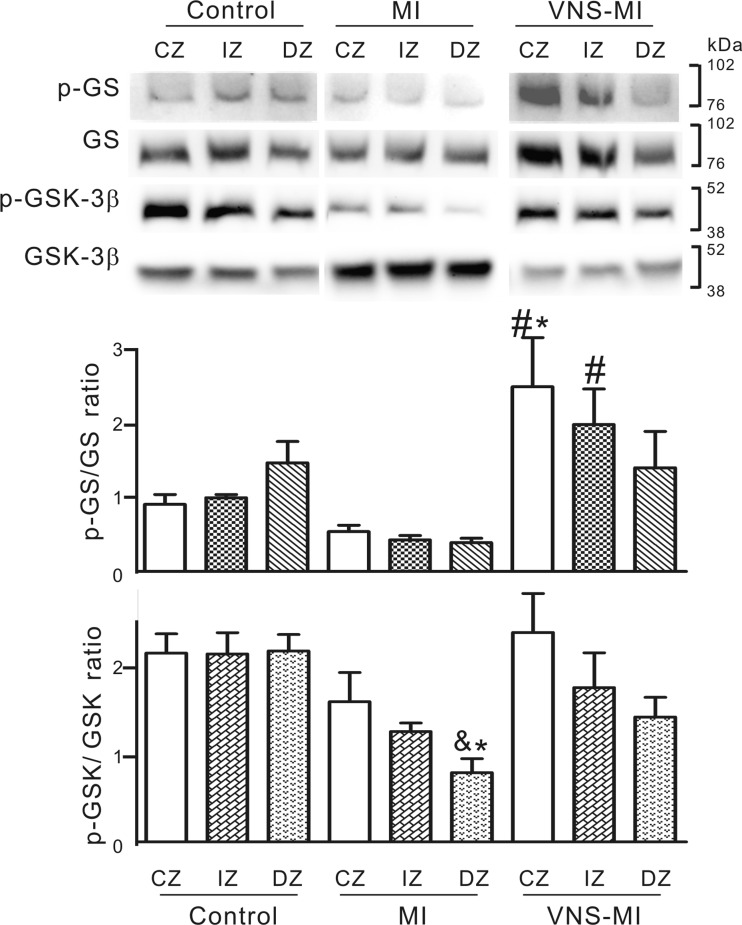
Fig. 4.
Phosphorylation status of GSK-3β and its substrate glycogen synthase (GS) in heart tissue derived from the MI (n = 5), VNS-MI (n = 4), and control (n = 3) animals. Shown are representative Western blots probed with antibodies specific for phosphorylated Ser641 of GS (p-GS), GS protein (GS), phosphorylated Ser9 of GSK-3β (p-GSK-3β), and GSK-3β protein (GSK-3β). Densitometry analysis of protein band intensity was performed for all Westerns. The graphs show the ratio of the p-GS/GS and p-GSK-3β/GSK-3β, where the protein bands were expressed in arbitrary densitometric units. ANOVA analysis indicating differences among the treatments was followed by Newman-Keuls post hoc analysis. *P < 0.05 vs. control central zone (CZ), intermediate zone (IZ), and distal zone (DZ); #P < 0.05 vs. MI-CZ, MI-IZ, and MI-DZ; and &P < 0.05 vs. VNS-MI-CZ.
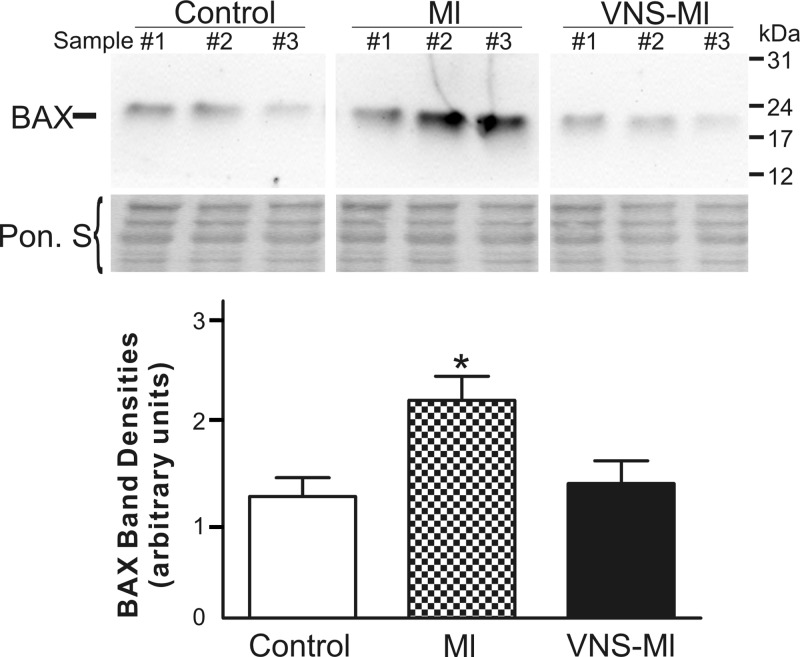
Fig. 5.
The elevation of proapoptotic Bcl-2-associated X (BAX) in MI hearts is mitigated by VNS. A representative Western blot probed with antibodies specific for BAX protein is shown (30 μg total protein/lane). The experiment was repeated 4 times with all hearts. The graph shows the densitometry analysis of protein band intensity, which was performed for all Westerns for control (n = 3), MI (n = 5), and VNS-MI (n = 4). The blot stained with Ponceau S (Pon. S) is shown as protein-loading control. ANOVA analysis indicated significant differences among the treatments and was followed by Newman-Keuls post hoc analysis. *P < 0.05 MI vs. control and VNS-MI hearts.
RESULTS
Echocardiography was performed on each animal before MI (baseline) and compared with the data acquired at day of termination (cf. 90 days post-MI). At 90 days post-MI, LVESV was increased significantly by 30%, whereas LVEF was reduced by 6.5%. VNS prevented these MI-induced changes in LVESV and LVEF (Table 1). Following echocardiographic recordings, IC ganglia, heart, and lungs were harvested. There was no significant difference in heart weight or lung weight (wet and dry) as percentage to body weight among all treated groups (Table 2).
Table 1.
Echocardiogram measurement to determine heart function
| Baseline | 90 Days Post-MI | Baseline | 90 Days Post-MI | |
|---|---|---|---|---|
| LVESV, ml | LVESV, ml | LVEF, % | LVEF, % | |
| MI, n = 7 | 0.44 ± 0.07 | 0.57 ± 0.12*† | 80.3 ± 1.7 | 74.9 ± 2.7*† |
| VNS-MI, n = 7 | 0.43 ± 0.11 | 0.41 ± 0.13 | 79 ± 4.4 | 81.4 ± 4.4 |
Values expressed as means ± SE, with number of animals shown. Significant effect (
P < 0.05) compared with their baseline level using repeated-measures ANOVA and significant effect (
P < 0.05) compared with vagus nerve stimulation (VNS)-myocardial infarction (MI). LVESV, left ventricular end systolic volume; LVEF, LV ejection fraction.
IC neuronal properties.
The membrane properties of IC neurons derived from MI (n = 7; 55 IC neurons), VNS-MI (n = 7; 55 IC neurons), and time-matched control guinea pigs (n = 8; 64 neurons) are summarized in Table 3. A significant depolarization (∼5 mV) of the RMP in the MI group was identified compared with controls. VNS was effective in restoring these RMPs to control values. Neuronal input resistance increased significantly in the MI group. VNS mitigated this MI-induced increase in input resistance. There were no significant differences in the amplitude or duration of the AHP half-decay times among IC neurons derived from all three groups.
Soma excitability was assessed by measuring the number of evoked action potentials in response to intracellular depolarizing current steps. Neuronal excitability increased in MI and VNS-MI groups compared with controls (Fig. 1). Previous studies demonstrated that chronic MI produces an increase in IC neuron sensitivity to NE, as seen in an increase in the number of evoked action potentials in response to depolarizing currents with NE application (38). In the current study, IC neuronal sensitivity to NE was increased in the MI group at lower (0.1 and 0.2 nA) and higher (0.4 and 0.5 nA) intensities compared with controls. Neural sensitivity also increased in the VNS-MI group at lower intensities (0.1 and 0.2 nA) compared with controls (Fig. 2).
Synaptic efficacy.
Synaptic efficacy was evaluated among the three groups by measuring neuronal responses to stimulating axon bundles leading to the ganglion containing the neuron of interest (2 s suprathreshold train at 1, 2, 5, 10, and 20 Hz). Whereas the responses between MI and controls did not differ, the number of action potentials so produced was reduced significantly in the VNS-MI compared with MI and control animals (Fig. 3). The maximum neuronal output firing frequency was ∼8 Hz in control and MI animals; it was reduced to ∼4 Hz with chronic VNS.
Myocyte function.
Three ventricular tissue samples were removed at time of termination, including from the infarct or CZ, the IZ, and a DZ. The ratio of the p-GS (inactive) to the active form of GS (GS) was determined by Western blot analysis in all three ventricular regions in control, MI, and VNS-MI heart samples. The VNS-MI heart tissue showed a significantly elevated p-GS/GS ratio compared with MI hearts in both the CZ and IZ (Fig. 4). In VNS-MI animals, the p-GS/GS ratio in the CZ was significantly higher compared with control animals (Fig. 4). Besides its role in glycogen metabolism, GS is of interest as a downstream substrate of the key stress signaling kinase, GSK-3β, which is also regulated by phosphorylation. p-GSK-3β is inactive as a kinase but is considered the cardioprotective form of the protein (67). The ratio of p-GSK-3β/GSK-3 β decreased in the DZ of MI hearts compared with controls; this level was re-established in the CZ of the VNS-MI animals (Fig. 4).
To evaluate the potential of VNS to exert cardioprotection via modulation of the apoptotic pathway in cardiac myocytes, we evaluated the expression of several members of the mitochondrial apoptotic machinery (Bcl-2, Bcl-xl, and BAX) (56). No significant differences in the levels of Bcl-2 or Bcl-x1 were identified (data not shown). However, the level of the proapoptotic protein BAX was elevated twofold in MI hearts. VNS mitigated the increase in BAX level, such that no difference was evident between control and VNS-MI group data (Fig. 5).
DISCUSSION
MI remodels both the cardiac nervous system and the cardiac tissue that it modulates. These changes are dynamic, persisting for several weeks after the initial insult (36, 38). Associated with the loss of tissue (∼8%) post-MI in the guinea pig model (38), LVESV increased (30%), and LVEF fell (6.5%). Whereas sufficient to alter contractile function, the level of cardiac damage induced by this MI was not sufficient for progression into congestive heart failure. At the cellular level, IC neurons post-MI displayed depolarization of their RMPs, increases in input resistance, and increased excitability. Since these IC neurons were not in the ischemic zone (i.e., their blood supply was uninterrupted), these IC neuronal changes presumably were a consequence of infarct-induced changes in afferent neuronal feedback to the ICNS (6, 36, 68). Presumably, they also reflect an MI reflex-induced decrease in parasympathetic efferent preganglionic neuronal drive (6, 11, 47).
The increase in the parasympathetic efferent neuronal drive to the ICNS by application of chronic VNS restored the IC neuronal RMPs and input resistances toward control values (Table 3). Furthermore, VNS also reduced synaptic efficacy by 50% for network interactions within the ICNS. The changes that VNS induced within the ICNS occurred in conjunction with the following: 1) preservation of LV function (as evidenced by echocardiographic data), 2) improvement in cardiomyocyte metabolic capacity, and 3) a reduced potential for ventricular myocyte apoptosis.
Regional LV MI produces an eccentric insult to the heart, which would change afferent neuronal signaling derived from the region where sensory neurites are located, namely, ischemic vs. normal zones. Such altered afferent input is transduced to somata in IC, intrathoracic, and central components of the cardiac neuroaxis (22, 27, 68). Such alteration in afferent inputs has the potential to induce reactive changes in neuronal processing throughout the cardiac neuroaxis (2, 42, 68). With respect to IC neurons, such remodeling is most dynamic at 7 days post-MI, becoming stabilized by 14 days post-MI (1, 25, 36). In the current study, VNS was initiated during the peak of this remodeling process (cf. 7 days post-MI). As such, this strategy was effective in mitigating not only adverse cardiac functional changes but also targeted MI-induced remodeling of the ICNS.
IC neuronal properties.
In this study, increased IC neuronal excitability post-MI presumably was due to the following: 1) enhancement in neuronal input resistance, which as a consequence, necessitated lower current density to modify the RMP, and 2) depolarization of the IC neuronal RMP by ∼5 mV, thereby decreasing the absolute voltage change needed to induce action potentials. As a consequence, increasing numbers of action potentials were evoked with progressive increases in input stimulus intensities, an effect enhanced by the IC neuronal changes evoked post-MI (36). The most dramatic effects identified among groups were related to neuronal, following frequencies elicited when stimulating axons innervating IC ganglia containing somata of interest. IC neurons derived from both control and MI groups showed no differences in neuronal output frequencies (Fig. 3). Likewise, whereas MI by itself increases IC soma sensitivity to NE, VNS therapy did not alter it. In contrast, the VNS-MI group showed a dramatic reduction in the efficacy of synaptic transmission within the ICNS, as evidenced by the decrease in activation at equivalent stimulation frequencies.
Previous studies have demonstrated that IC neuronal synaptic efficacy increases in animals subjected to chronic pressure overload (34) or animals, 7 days post-MI (36). In both cases, neurons were able to follow input frequencies up to ∼25 Hz with very good efficacy. This abnormal, high frequency of synaptic transmission in the IC network presumably is, in part, an adaptive response to altered afferent inputs derived from the stressed myocardium (8, 28, 68), with a corresponding decrease in central neuronal drive from medullary parasympathetic efferent preganglionic neurons (11, 13, 22, 75). With the use of chronic VNS to restore biomimetic levels of parasympathetic efferent neuronal drive to the ICNS, synaptic efficacy was reduced by 50%. VNS also activates afferent fibers, with the potential to alter central efferent drive (12, 14). However, it should be recognized that the ICNS will process both exogenously and endogenously derived inputs and thereby functions as a primary target for VNS (14, 60). It is postulated that VNS, by reducing IC synaptic efficacy, will blunt network hyperexcitability within the ICNS occurring secondary to ischemia-induced afferent feedback (46, 47). This would be analogous to placing a governor on an internal combustible engine. These neural influences are likely mediated via descending projections to local circuit neurons contained within the ICNS (10, 30).
The second major neural influence derived from chronic VNS therapy is its antiadrenergic influences. In large animal studies, data demonstrate that peripheral sympathetic-parasympathetic efferent interactions occur within IC ganglia (54, 58) and at the efferent neural-myocyte interface [both presynaptic (49) and postsynaptic (48)]. Both preclinical (70, 71) and clinical (20, 57) studies indicate that the benefits of VNS can be made manifest at stimulation levels that exert minimal effects on resting heart rate. Since the cervical vagus is a mixed nerve, 80% of which is afferent fibers (12, 72), it is the interaction among multiple levels of the cardiac neural hierarchy that ultimately determines functional effects on target organs (4, 7, 12, 53).
Cardiomyocyte function.
Examination of the metabolic enzyme markers in ventricular myocytes demonstrated a clear trend of increasing the p-GS/GS ratio in normally perfused tissue located more distal to the arterial ligation in VNS-MI hearts. Increases in the p-GS/GS ratio represent an increase in glycogen use rather than storage via inactivation of glycogen synthetic enzymes. These results indicate that VNS supports glycogen mobilization and glucose use in recovering and/or compensating cardiac tissues. Whereas additional work is needed to ascertain the precise nature of the effects of VNS on such glucose metabolism and whether the increased p-GS/GS ratio indeed represents an upregulation of glycogenolysis, these data suggest that VNS exerts a profound regulatory influence upon glycogen metabolism in the post-MI heart.
Glucose provides more ATP per mole of O2 than free fatty acids (FFA). Shifting metabolism from FFA oxidation to glucose oxidation has been suggested as a therapeutic approach in heart failure (65, 66). Whereas cardiac tissue has a relatively small pool of glycogen, its turnover rates are high—reported to account for ≤40% of glucose-derived ATP production (40). As mobilization of glycogen stores represents a classic response to ischemia, any increase in phosphorylation of GS post-MI with VNS suggests less glycogen synthesis. This, in turn, implies more glucose use. Since the border and CZs have less O2 availability, glucose therein would be available for anaerobic metabolism.
Multiple stress pathways converge upon GSK-3β, such that it has emerged recently as a pivotal cardioprotective molecule. p-GSK-3β inhibits its kinase activity and increases its cardioprotective potential through poorly understood effects at the mitochondrial level (67). The current study did not show any decrease in p-GSK-3 in tissue samples with increased p-GS/GS ratios. This finding indicates that increased GSK-3β activity is not responsible for the higher p-GS/GS ratio that we observe in these samples. Since a multitude of other kinases and phosphatases exerts regulatory influence upon GS, any disconnect between the apparent activity of p-GSK-3β and p-GS status is not entirely unexpected. Regardless of the influence of GSK-3β on GS under these particular circumstances, the findings of an increased p-GSK-3β/GSK-3β ratio, as well as the normalization of the mitochondrial proapototic protein BAX in VNS-treated hearts, potentially represent important findings pertinent to cardioprotection. These cellular data are especially relevant when considered in light of evidence of improved function of VNS-MI hearts in both small (Table 1) and large animal models of ischemic heart disease (51, 62, 71).
Study limitations.
Whereas we were able to demonstrate functional manifestations of VNS-mediated cardioprotection, no histopathologic results from the ventricles were performed in parallel to confirm such molecular findings—the former being a more sensitive index of change. Correspondingly, in the past, we have identified no histological changes in the ICNS unless there is direct obstruction of the arterial supply of neurons so targeted (6, 42). As this was not the case in this study, we did not attempt to detect histologically changes to neuronal somata that had normal blood supplies. As for VNS, we evaluated only one frequency (20 Hz) at a stimulus intensity where afferent- and efferent-evoked responses balanced each other, such that little or no heart rate changes were evoked (45). As such, VNS was able to engage the cardiac nervous system while minimizing the potential for rebound effects during the off phase that accompanies the intermittent VNS protocol. Future studies should evaluate other VNS stimulus paradigms (duty cycle, frequency, intensity, and pulse width). Finally, we did not characterize the phenotype of the IC neurons being recorded (41) nor could we distinguish between postganglionic soma and interneurons contained within the IC nervous system (7) with the techniques used. At least in the guinea pig model, the IC neurons recorded with sharp electrodes have been shown to be primarily cholinergic (52). Future studies should consider the possible contribution of cardiac disease-induced changes in IC neural phenotype (29) and network interactions contributing to autonomic dysautonomia associated with ischemic and nonischemic heart disease (26, 29, 61).
Significance and perspectives.
VNS is an emerging neuromodulation therapy that is currently being evaluated for treating cardiac arrhythmias (13, 74) and heart failure (21, 57, 59). In animal models, the deleterious consequences of MI on cardiac structure and function have been shown to be attenuated by chronic VNS, such that survival improves (51, 71). VNS protects cardiomyocytes against apoptosis (43, 44), mitigates mitochondrial dysfunction (62), and reduces the inflammatory responses (15, 69). At the level of the ICNS, VNS does not alter MI-induced increases in neuronal excitability; VNS did reduce ICNS synaptic efficacy; and VNS can exert antiadrenergic effects within peripheral autonomic ganglia (54, 58) and at their end-terminus (49). Together, these effects of VNS would moderate overall network processing within the ICNS in transducing myocardial ischemia to shift the autonomic balance at the neural-myocyte interface away from pathological levels of adrenergic hyperactivity (7, 45). VNS, both directly and indirectly (via the nervous hierarchy), improved cardiac myocyte metabolic function, while reducing cardiac myocyte apoptotic state.
VNS is currently in multiple clinical trials for reduced ejection-fraction heart failure (14, 19). These include the Increase of Vagal Tone in CHF (INOVATE-HF), Neural Cardiac Therapy for Heart Failure (NECTAR-HF), and Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure (ANTHEM-HF). Initial results of these trials have been positive for INOVATE-HF and ANTHEM-HF, with neutral effects for NECTAR-HF (20, 21, 57). One of the key differences among these trials is the choice of stimulation parameters (current, frequency, pulse width, and duty cycle) and especially, the different levels of stimulus intensity. The understanding mechanistically of what is being stimulated within the autonomic nervous system by any bioelectric therapy and how the neural network-heart interface reacts to such stimuli is essential for optimizing stimulation parameters and for the future development of effective autonomic regulation therapies (14). As demonstrated here, targeted VNS exerts multiple effects on the cardiac nervous system and the cardiac tissues it regulates and ultimately, preserves contractile function and as such, cardiac output.
GRANTS
Support for this work was provided by the National Heart, Lung, and Blood Institute (Grants HL71830 to J. L. Ardell and HL09889 to J. C. Hardwick and E. M. Southerland) and by Cyberonics (to J. L. Ardell).
DISCLOSURES
J. L. Ardell and J. A. Armour serve as scientific advisors to Cyberonics. B. H. KenKnight is an employee of Cyberonics.
AUTHOR CONTRIBUTIONS
Author contributions: E.B., G.L.W., B.H.K., and J.L.A. conception and design of research; E.B., E.M.S., J.C.H., G.L.W., S.R., and Y.L. performed experiments; E.B., E.M.S., J.C.H., G.L.W., S.R., Y.L., and J.L.A. analyzed data; E.B., E.M.S., J.C.H., G.L.W., B.H.K., J.A.A., and J.L.A. interpreted results of experiments; E.B., G.L.W., S.R., and Y.L. prepared figures; E.B. and G.L.W. drafted manuscript; E.B., J.C.H., G.L.W., B.H.K., J.A.A., and J.L.A. edited and revised manuscript; E.B., E.M.S., J.C.H., G.L.W., Y.L., B.H.K., J.A.A., and J.L.A. approved final version of manuscript.
REFERENCES
- 1.Ahonen A, Harkonen M, Juntunen J, Kormano M, Penttila A. Effects of myocardial infarction on adrenergic nerves of the rat heart muscle, a histochemical study. Acta Physiol Scand 93: 336–344, 1975. [DOI] [PubMed] [Google Scholar]
- 2.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305: H1031–H1040, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoni ML, Boden H, Hoogslag GE, Ewe SH, Auger D, Holman ER, van der Wall EE, Schalij MJ, Bax JJ, Delgado V. Prevalence of dyssynchrony and relation with long-term outcome in patients after acute myocardial infarction. Am J Cardiol 108: 1689–1696, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Ardell JL. Intrathoracic neuronal regulation of cardiac function In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford University Press, 2004, p. 118–152. [Google Scholar]
- 5.Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res 41: 41–54, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 79–117. [Google Scholar]
- 9.Armour JA, Linderoth B, Arora RC, DeJongste MJ, Ardell JL, Kingma JG Jr, Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischaemic hearts. Auton Neurosci 95: 71–79, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther 111: 808–835, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 25: 208–221, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Brack KE, Winter J, Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation—tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev 18: 389–408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley U, Shivkumar K, Ardell JL. Autonomic regulation therapy in heart failure. Curr Heart Fail Rep 12: 284–293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvillo L, Vanoli E, Andreoli E, Besana A, Omodeo E, Gnecchi M, Zerbi P, Vago G, Busca G, Schwartz PJ. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol 58: 500–507, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Cardinal R, Ardell JL, Linderoth B, Vermeulen M, Foreman RD, Armour JA. Spinal cord activation differentially modulates ischaemic electrical responses to different stressors in canine ventricles. Auton Neurosci 111: 37–47, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 95: 957–970, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dawson TA, Li D, Woodward T, Barber Z, Wang L, Paterson DJ. Cardiac cholinergic NO-cGMP signaling following acute myocardial infarction and nNOS gene transfer. Am J Physiol Heart Circ Physiol 295: H990–H998, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ferrari GM. Vagal stimulation in heart failure. J Cardiovasc Transl Res 7: 310–320, 2014. [DOI] [PubMed] [Google Scholar]
- 20.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ; CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32: 847–855, 2011. [DOI] [PubMed] [Google Scholar]
- 21.De Ferrari GM, Tuinenburg AE, Ruble S, Brugada J, Klein H, Butter C, Wright DJ, Schubert B, Solomon S, Meyer S, Stein K, Ramuzat A, Zannad F. Rationale and study design of the NEuroCardiac TherApy foR Heart Failure Study: NECTAR-HF. Eur J Heart Fail 16: 692–699, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Ferrari GM, Vanoli E, Schwartz PJ. Cardiac vagal activity, myocardial ischemia and sudden death. In: Cardiac Electrophysiology: From Cell to Bedside (2nd ed), edited by Zipes DP and Jalifa J. Philadelphia, PA: WB Sanders, 1995, p. 422–434. [Google Scholar]
- 23.Dell'Italia LJ. Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circ Res 109: 437–452, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res 5: 837–847, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48: 504–511, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 61: 143–167, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Fu LW, Longhurst JC. Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol 194: 185–225, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA, Ardell JL. Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302: R357–R364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girasole AE, Palmer CP, Corrado SL, Marie Southerland E, Ardell JL, Hardwick JC. Angiotensin II potentiates adrenergic and muscarinic modulation of guinea pig intracardiac neurons. Am J Physiol Regul Integr Comp Physiol 301: R1391–R1399, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez GE, Seropian IM, Krieger ML, Palleiro J, Lopez Verrilli MA, Gironacci MM, Cavallero S, Wilensky L, Tomasi VH, Gelpi RJ, Morales C. Effect of early versus late AT(1) receptor blockade with losartan on postmyocardial infarction ventricular remodeling in rabbits. Am J Physiol Heart Circ Physiol 297: H375–H386, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A, Dell'Italia LJ. Beta1-Adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol 291: H147–H151, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Hardwick JC, Baran CN, Southerland EM, Ardell JL. Remodeling of the guinea pig intrinsic cardiac plexus with chronic pressure overload. Am J Physiol Regul Integr Comp Physiol 297: R859–R866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 166–174, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 181: 4–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardwick JC, Ryan SE, Powers EN, Southerland EM, Ardell JL. Angiotensin receptors alter myocardial infarction-induced remodeling of the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 309: R179–R188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardwick JC, Southerland EM, Ardell JL. Chronic myocardial infarction induces phenotypic and functional remodeling in the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 295: R1926–R1933, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardwick JC, Southerland EM, Girasole AE, Ryan SE, Negrotto S, Ardell JL. Remodeling of intrinsic cardiac neurons: effects of beta-adrenergic receptor blockade in guinea pig models of chronic heart disease. Am J Physiol Regul Integr Comp Physiol 303: R950–R958, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation 93: 1549–1555, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res 62: 112–121, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins DA, Macdonald SE, Murphy DA, Armour JA. Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec 259: 424–436, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett 579: 2111–2118, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg 137: 223–231, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Kember G, Ardell JL, Armour JA, Zamir M. Vagal nerve stimulation therapy: what is being stimulated? PLoS One 9: e114498, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kember G, Armour JA, Zamir M. Dynamic neural networking as a basis for plasticity in the control of heart rate. J Theor Biol 317: 39–46, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Kember G, Armour JA, Zamir M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45: 638–644, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel lecture). Angew Chem Int Ed Engl 52: 6366–6378, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res 29: 437–445, 1971. [DOI] [PubMed] [Google Scholar]
- 50.Levy MN, Ng M, Martin P, Zieske H, Rogoff T. Sympathetic and parasympathetic interactions upon the left ventricle of the dog. Circ Res 19: 5–10, 1966. [Google Scholar]
- 51.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Mawe GM, Talmage EK, Lee KP, Parsons RL. Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res 285: 281–286, 1996. [DOI] [PubMed] [Google Scholar]
- 53.McAllen RM, Salo LM, Paton JF, Pickering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol 589: 5801–5818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGuirt AS, Schmacht DC, Ardell JL. Autonomic interactions for control of atrial rate are maintained after SA nodal parasympathectomy. Am J Physiol Heart Circ Physiol 272: H2525–H2533, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Mollema SA, Liem SS, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Gorcsan J 3rd, Bax JJ. Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol 50: 1532–1540, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res 94: 7–16, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF Trial. J Card Fail 20: 808–816, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285: R1066–R1075, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz PJ. Vagal stimulation for the treatment of heart failure: a translational success story. Heart 98: 1687–1689, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Shen MJ, Zipes DP. Interventional and device-based autonomic modulation in heart failure. Heart Fail Clin 11: 337–348, 2015. [DOI] [PubMed] [Google Scholar]
- 61.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114: 1004–1021, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH, Chattipakorn N. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm 10: 1700–1707, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Southerland EM, Gibbons DD, Smith SB, Sipe A, Williams CA, Beaumont E, Armour JA, Foreman RD, Ardell JL. Activated cranial cervical cord neurons affect left ventricular infarct size and the potential for sudden cardiac death. Auton Neurosci 169: 34–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, Subramanian V, Singh M, Singh K, Ardell JL. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 292: H311–H317, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Stanley WC, Sabbah HN. Metabolic therapy for ischemic heart disease: the rationale for inhibition of fatty acid oxidation. Heart Fail Rev 10: 275–279, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res 90: 377–379, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q, Cheng Y, Xue FS, Yuan YJ, Xiong J, Li RP, Liao X, Liu JH. Postconditioning with vagal stimulation attenuates local and systemic inflammatory responses to myocardial ischemia reperfusion injury in rats. Inflamm Res 61: 1273–1282, 2012. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Yu L, Chen M, Wang S, Jiang H. Transcutaneous electrical stimulation of auricular branch of vagus nerve: a noninvasive therapeutic approach for post-ischemic heart failure. Int J Cardiol 177: 676–677, 2014. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Yu L, Wang S, Huang B, Liao K, Saren G, Tan T, Jiang H. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail 7: 1014–1021, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia 31, Suppl 2: S7–S19, 1990. [DOI] [PubMed] [Google Scholar]
- 73.Wu J, Chen P, Li Y, Ardell C, Der T, Shohet R, Chen M, Wright GL. HIF-1alpha in heart: protective mechanisms. Am J Physiol Heart Circ Physiol 305: H821–H828, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Mazgalev TN. Arrhythmias and vagus nerve stimulation. Heart Fail Rev 16: 147–161, 2011. [DOI] [PubMed] [Google Scholar]
- 75.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]