Abstract
Inwardly rectifying potassium channels play essential roles in renal physiology across phyla. Barium-sensitive K+ conductances are found on the basolateral membrane of a variety of insect Malpighian (renal) tubules, including Drosophila melanogaster. We found that barium decreases the lumen-positive transepithelial potential difference in isolated perfused Drosophila tubules and decreases fluid secretion and transepithelial K+ flux. In those insect species in which it has been studied, transcripts from multiple genes encoding inwardly rectifying K+ channels are expressed in the renal (Malpighian) tubule. In Drosophila melanogaster, this includes transcripts of the Irk1, Irk2, and Irk3 genes. The role of each of these gene products in renal tubule function is unknown. We found that simultaneous knockdown of Irk1 and Irk2 in the principal cell of the fly tubule decreases transepithelial K+ flux, with no additive effect of Irk3 knockdown, and decreases barium sensitivity of transepithelial K+ flux by ∼50%. Knockdown of any of the three inwardly rectifying K+ channels individually has no effect, nor does knocking down Irk3 simultaneously with Irk1 or Irk2. Irk1/Irk2 principal cell double-knockdown tubules remain sensitive to the kaliuretic effect of cAMP. Inhibition of the Na+/K+-ATPase with ouabain and Irk1/Irk2 double knockdown have additive effects on K+ flux, and 75% of transepithelial K+ transport is due to Irk1/Irk2 or ouabain-sensitive pathways. In conclusion, Irk1 and Irk2 play redundant roles in transepithelial ion transport in the Drosophila melanogaster renal tubule and are additive to Na+/K+-ATPase-dependent pathways.
Keywords: Malpighian tubule, epithelial ion transport, barium, Kir, Ir, Dir, dKirIII, ion-specific electrode, Ramsay assay
inwardly rectifying potassium channels play key roles in vertebrate and invertebrate renal physiology and epithelial ion transport (13, 27, 28, 31, 32, 36–38, 43, 44, 47). Mutations in KCNJ1/Kir1.1 (also known as renal outer medullary K+ channel, or ROMK) and KCNJ10/Kir4.1 result in human diseases characterized by salt-losing tubulopathies and electrolyte disturbances (1, 40, 42). Inwardly rectifying K+ channels also play important roles in insect iono- and osmoregulation. For example, in mosquitoes, pharmacological inhibition of inwardly rectifying K+ channels decreases renal tubule fluid secretion and alters both transepithelial ion fluxes in the tubule as well as whole-body ion clearances and urine excretion (28, 31, 32, 36, 38, 41). This results in decreased viability and flight capacity (31, 32, 38). As such, inwardly rectifying K+ channels are potential targets for insecticidal agents.
Drosophila melanogaster is an insect model organism that can lend insights into the biology of mammals as well as other insects, such as disease vector-carrying insects or insect pests. The Drosophila genome encodes three inwardly rectifying K+ channels, Irk1 (also called Ir, Dir, or DrKir1), Irk2 (also called DrKir2), and Irk3 (also called dKirIII or DrKir3) (7, 20, 23). Roles for Irk genes have been described in wing development (4) and cardiotropic viral infections (10). In addition, all three Irk genes are expressed in the principal cell of the Malpighian tubule, and pharmacological experiments using sulphonylureas have suggested a role for Irk channels in tubule function (11). Here, we used pharmacological and genetic approaches to examine the role of Irk channels on the physiological function of adult Drosophila melanogaster renal tubules. Our data are consistent with two of the Irk channels, Irk1 and Irk2, playing a role in transepithelial ion transport in the fly tubule.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals and reagents were from Sigma (St. Louis, MO) or Fisher (Pittsburgh, PA) unless otherwise specified.
Fly stocks and genetics.
The following Drosophila melanogaster strains were used: wBerlin (wild-type), obtained from Dr. Adrian Rothenfluh (University of Texas Southwestern Medical Center, Dallas, TX); w;c42-GAL4, expressing GAL4 in the principal cells of the main and lower segments, as well as bar-shaped cells in the initial and transitional segments (35), obtained from Dr. Julian Dow (University of Glasgow, Glasgow, UK) and outcrossed for five generations to wBerlin. w;UAS-Irk2RNAi, an RNA interference (RNAi) line targeting Irk2, and w;UAS-Irk1RNAi, targeting Irk1, were obtained from the Vienna Drosophila Resource Center (Vienna, Austria; lines 108140 and 28430) (6) and were outcrossed for five generations to wBerlin. yw;UAS-Irk1RNAi (y1v1; P{TRiP.JF01841}attP2) and yw;UAS-Irk3RNAi (y1v1; P{TRiP.JF02262}attP2) were obtained from the Bloomington Stock Center (Bloomington, IN) and outcrossed for five generations to ywBerlin.
Flies were reared at 28°C unless otherwise noted on cornmeal/yeast/molasses food prepared in a central kitchen at UT Southwestern. Female flies were collected within 48 h of eclosion and kept on standard food for 3–5 days before tubule dissection.
Quantitative RT-PCR.
Irk1, Irk2, and Irk3 mRNA levels were quantified in knockdown vs. control tubules. In each case, w;c42-GAL4 served as the control. Four sets of tubules, with 50 tubules/genotype in each set, were compared for each Irk gene. RNA was prepared from tubules dissected in Drosophila saline and transferred to 600 μl ZR RNA buffer from the Zymo Quick-RNA microprep kit (Zymo Research, Irvine, CA). Tubules were homogenized by 10–20 passes with a 27-G needle. RNA was isolated according to the Zymo protocol and eluted in 6 μl DNase/RNase-free H2O. RNA amounts were quantified using a NanoDrop 2000c (Thermo Scientific, Waltham, MA). Total RNA (150 ng) was used for reverse transcription using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexamer primers according to manufacturer's instructions. cDNA from 10 ng total RNA was used per reaction of qPCR. qPCR was performed using the CFX Connect Real-Time PCR detecting system (Bio-Rad, Hercules, CA) with the iTaq Universal Probes Supermix (Bio-Rad). The TaqMan primer/probe sets for Ir (Dm02143602_g1), Irk2 (Dm02143724_m1), Irk3 (Dm01796588_g1), and endogenous control RpL32 (Dm02151827_g1) were ordered from Invitrogen. The PCR cycle was 95°C × 3 min, 95°C × 10 s, 60°C × 30 s (repeated × 40). Results were analyzed with Bio-Rad CFX Manager.
Perfusion of isolated tubules and measurement of transepithelial potential difference.
Anterior Malpighian tubules were dissected free hand at room temperature from adult Drosophila females in Drosophila saline, consisting of the following (in mM): 117.5 NaCl, 20.0 KCl, 2.0 CaCl2, 8.5 MgCl2, 10.2 NaHCO3, 4.3 NaH2PO4, 15.0 HEPES, and 20.0 glucose, pH 7.0. Tubules were then transferred to a bath chamber of an inverted microscope. The tubule was cannulated with a concentric glass pipette. The lumen was perfused with Drosophila saline. Transepithelial potential difference was measured using the perfusion pipette as the bridge into the tubular lumen. The reference electrode was in the bathing solution that was also composed of Drosophila saline. The potential difference was measured using a Keithley Electrometer, model no. 6517B (Keithley, Cleveland, OH). After we obtained a stable transepithelial potential, the bathing solution was changed to Drosophila saline containing 2 mM BaCl2 and the potential recorded after the potential difference stabilized. The barium-containing bath was then exchanged with Drosophila saline, and the potential was recorded until a stable potential was again achieved.
Ramsay assay and ion-specific electrodes.
The Ramsay assay and ion-specific electrodes were used to measure fluid secretion and K+ concentration from isolated adult female Drosophila tubules as previously described (34, 46). Fluid secretion rates and K+ concentration were measured after 2 h of fluid secretion unless otherwise specified. Potassium ionophore I cocktail B was used for the K+ ionophore. The K+ flux of each tubule was calculated by multiplying K+ concentration by the secretion rate for each tubule. Unless otherwise indicated, the tubules were bathed in standard bathing medium consisting of a 1:1 mixture of Drosophila saline and Schneider's medium (Invitrogen). The composition of Schneider's medium is as follows (in mM): 3.330 glycine, 2.300 l-arginine, 3.010 l-aspartic acid, 0.496 l-cysteine, 0.417 l-cystine, 5.440 l-glutamic acid, 12.330 l-glutamine, 2.580 l-histidine, 1.150 l-isoleucine, 1.150 l-leucine, 9.020 l-lysine hydrochloride, 5.370 l-methionine, 0.909 l-phenylalanine, 14.780 l-proline, 2.380 l-serine, 2.940 l-threonine, 0.490 l-tryptophan, 2.760 l-tyrosine, 2.560 l-valine, 5.620 β-alanine, 5.410 CaCl2, 15.060 MgSO4, 21.330 KCl, 3.310 KH2PO4, 4.760 NaHCO3, 36.210 NaCl, 4.940 Na2HPO4, 1.370 α-ketoglutaric acid, 11.110 d-glucose, 0.862 fumaric acid, 0.746 malic acid, 0.847 succinic acid, 5.850 trehalose, and 2,000 mg/l yeastolate.
In experiments testing the effect of barium, large amounts of barium phosphate precipitation occurred when using standard bathing medium attributable to the higher concentrations of phosphate in this solution compared with Drosophila saline. Tubules were therefore incubated in amino acid-replete solution, which consists of Drosophila saline supplemented with amino acids and supports more fluid secretion in the Ramsay assay than Drosophila saline (19). Amino acid-replete solution consists of the following (in mM) (19): 117.50 NaCl, 20.00 KCl, 2.00 CaCl2, 8.50 Mg Cl2, 10.20 NaHCO3, 15.00 HEPES, 20.00 glucose, 4.30 NaH2PO4, 1.70 l-glycine, 7.00 l-proline, 6.16 l-glutamine, 0.95 l-histidine, 0.55 l-leucine, 4.50 l-lysine, and 1.30 l-valine, pH 7.0. Tubules were incubated for 1 h in the amino acid-replete solution. Fluid secretion and K+ activity were measured. BaCl2 (2 mM) was then added to the bathing solution. The tubules were incubated for an additional hour, and fluid secretion and K+ activity were determined.
In experiments to assess the effects of cAMP, 1 mM dibutyryl cAMP was added to standard bathing medium. In other experiments, 100 μM ouabain was added to standard bathing medium to inhibit the Na+/K+-ATPase. Tubules were bathed in the drug-containing standard bathing medium for the entirety of the experiment (2 h). Ouabain stocks were prepared fresh each day.
Statistics.
Paired t-test (2 comparisons) or one-way repeated-measures ANOVA (3 comparisons) were used for experiments in which the same tubule was analyzed over time under different conditions. In experiments with separate groups of tubules, unpaired t-test was used in experiments with two groups or one-way ANOVA in experiments with three or more groups. In those experiments in which there were three or more groups with more than one variable (for example, genotype and drug), two-way ANOVA was used. Significance level was set at P < 0.05. Post hoc comparisons were performed using Bonferroni's correction. All statistical analyses were performed using GraphPad Prism, version 5.0 or 6.0 (GraphPad Software, La Jolla, CA).
RESULTS
Urine generation by the main segment of the Drosophila renal tubule occurs through the secretion of KCl-rich fluid. In the principal cell, the apical vacuolar H+-ATPase generates a lumen-positive transepithelial potential difference that drives cation secretion, primarily K+ and to a lesser extent Na+, across the apical membrane by driving H+/cation exchange (5, 8, 19, 25, 33). Cl− secretion occurs in parallel through the transcellular flux of Cl− through the stellate cell (3, 25, 26). Previous studies have demonstrated an effect of barium, an inhibitor of inwardly rectifying K+ channels, on the basolateral membrane potential and K+ conductance in the Drosophila melanogaster Malpighian tubule (15, 25).
To determine whether barium also affects transepithelial ion flux, we measured transepithelial potential difference in isolated perfused tubules in the presence or absence of 2 mM peritubular barium. Barium reversibly decreased the lumen-positive transepithelial potential difference by 21 mV, from 30.5 ± 5.2 mV before barium to 9.5 ± 1.9 mV during barium treatment to 30.5 ± 4.0 mV after washout, means ± SE (Fig. 1A). Because the transepithelial potential difference drives transepithelial fluid secretion and cation flux (8, 25), we tested the effect of barium on transepithelial fluid secretion and K+ flux in the Ramsay assay, which measures fluid and K+ secretion by the main segment of the tubule (8). Barium decreased transepithelial K+ flux when added to the bathing solution in the Ramsay assay (Table 1 and Fig. 1B). These results suggest that basolateral barium-sensitive K+ channels play a role in transepithelial K+ secretion across the main segment of the tubule.
Fig. 1.
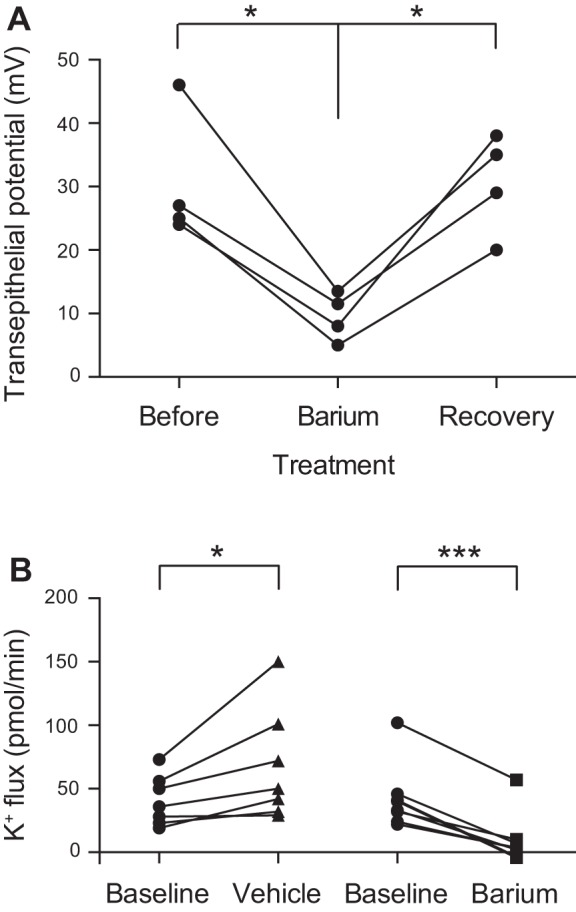
Barium applied to the basolateral membrane of the Drosophila melanogaster renal tubule decreases transepithelial potential difference and transepithelial K+ flux. A: transepithelial potential difference was measured in isolated perfused wild-type tubules (wBerlin, n = 4) at baseline, after the application of peritubular 2 mM barium, and after washout. Transepithelial potential difference was reversibly decreased after barium administration. The time to initial effect after changing the bath to the barium-containing bath was 4 ± 1 min. The time to maximal effect after changing the bath to the barium-containing bath was 11 ± 2 min. There was no signification difference between “before” and “recovery” transepithelial potentials. B: transepithelial K+ flux (pmol/min per tubule) was measured in wild-type (wBerlin) tubules using the Ramsay assay and ion-specific electrodes. K+ flux was measured for 1 h before drug addition (baseline) and in the same tubules for 1 h after the addition of 2 mM barium or vehicle (n = 7–8 tubules/condition). There was an increase in K+ flux after vehicle addition, whereas the addition of barium resulted in a decrease in K+ flux. All values shown in this and subsequent figures are means ± SE. *P < 0.05, ***P < 0.001.
Table 1.
Effect of barium on secretion rate, [K+], and K+ flux
| Genotype | Secretion Rate, nl/min per tubule | [K+], mM | K+ flux, pmol/min per tubule | n |
|---|---|---|---|---|
| Effect of 2 mM barium | ||||
| wBerlin | ||||
| Baseline | 0.29 ± 0.048 | 139 ± 7.8 | 41 ± 7.5 | 7 |
| After vehicle | 0.37 ± 0.057 | 157 ± 8.3 | 68 ± 16.7 | 7 |
| Baseline | 0.31 ± 0.060 | 150 ± 21.9 | 43 ± 9.0 | 8 |
| After barium | 0.01 ± 0.010 | 156 ± 19.1 | 9 ± 7.0 | 8 |
| GAL4 control: w;c42-GAL4/+ | ||||
| Baseline | 0.21 ± 0.028 | 117 ± 6.4 | 25 ± 3.6 | 10 |
| After barium | 0.02 ± 0.028 | 116 ± 11.0 | 4 ± 1.6 | 10 |
| Irk1 knockdown: w/yw;UAS-Irk1RNAi/c42 | ||||
| Baseline | 0.23 ± 0.028 | 116 ± 10.3 | 28 ± 4.6 | 9 |
| After barium | 0.05 ± 0.029 | 121 ± 10.2 | 8 ± 3.5 | 9 |
| Irk2 knockdown: w;UAS-Irk2RNAi/+;c42-GAL4/+ | ||||
| Baseline | 0.24 ± 0.024 | 129 ± 11.0 | 32 ± 4.6 | 11 |
| After barium | 0.03 ± 0.010 | 131 ± 10.8 | 6 ± 1.7 | 11 |
| Irk1/Irk2 double knockdown: w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi | ||||
| Baseline | 0.18 ± 0.032 | 105 ± 10.6 | 20 ± 4.4 | 10 |
| After barium | 0.08 ± 0.022 | 123 ± 12.5 | 13 ± 4.1 | 10 |
All values shown are means ± SE. Note that K+ flux was calculated separately for each tubule analyzed, and therefore the value of mean secretion rate × mean [K+] may differ slightly from measured K+ flux, attributable to rounding.
Transcripts for all three Drosophila inwardly rectifying K+ channel genes are expressed in the Malpighian tubule principal cell (11). In addition, there are four annotated alternatively spliced products of Irk2, of which RA, RB, and RC are expressed in the tubule, with greatest expression of Irk2-RA (11). To test a role for each Irk gene, we used the GAL4-UAS system (2) and RNA interference (RNAi) to knock down each of the Irk genes, individually and in combination, in the principal cells of the tubule. c42-GAL4 was used to drive expression of hairpin RNAs in the tubule principal cells (35). The RNAi constructs used to knock down Irk2 target the four annotated Irk2 transcripts. Using quantitative RT-PCR, we demonstrated that each of the three Irk genes was efficiently knocked down (Figs. 2B, 3B, and 4B); the degree of transcript knockdown is likely underestimated because the genes are only being knocked down in a subset of the tubule cells examined. Despite the observed decreases in transcript abundance, knocking down Irk1, Irk2, or Irk3 individually did not result in a change in transepithelial K+ flux, as measured in the Ramsay assay (Table 2 and Figs. 2A, 3A, and 4A). Similarly, knocking down Irk1 and Irk3 in combination, or Irk2 and Irk3, also did not result in a change in K+ flux (Table 2 and Fig. 5). However, knocking down Irk1 and Irk2 simultaneously resulted in decreased transepithelial K+ flux (Table 2 and Fig. 6A), whereas Irk1/Irk2/Irk3 triple knockdown did not further decrease K+ flux (Table 2 and Fig. 6B). Together, these results suggest that Irk1 and Irk2 play redundant roles in transepithelial K+ flux, as a phenotype was only revealed when both genes were knocked down simultaneously, whereas no role for Irk3 was apparent in this process.
Fig. 2.
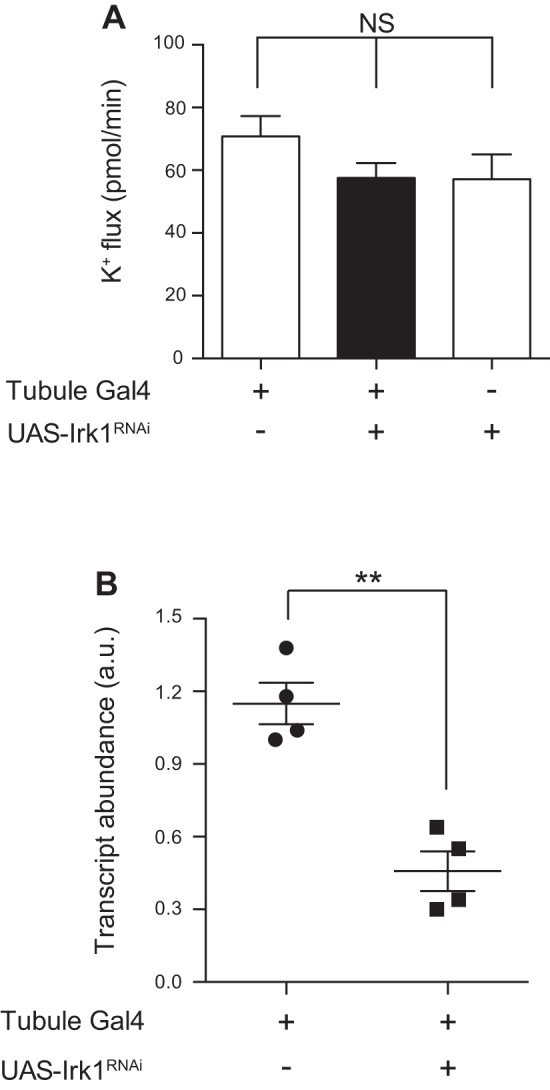
Knocking down Irk1 in the principal cell of the tubule decreases transcript levels but has no effect on K+ flux. The GAL4-UAS system, using the principal cell GAL4 driver c42-GAL4, was used to knock down Irk genes in the principal cells of the tubule. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+ and w/yw;UAS-Irk1RNAi/+) or from flies in which Irk1 was knocked down in the principal cells under the control of c42-GAL4 (w/yw;c42-GAL4/UAS-Irk1RNAi). K+ flux was unchanged in the Irk1 knockdown tubules; n = 13–14 tubules/genotype. P = 0.2537, 1-way ANOVA. B: Irk1 transcript levels were measured using quantitative RT-PCR from control tubules (w;c42-GAL4/+) or from tubules in which Irk1 was knocked down in the principal cells (w/yw;c42-GAL4/UAS-Irk1RNAi). Transcript levels were decreased in the knockdown tubules; n = 4 pairs of 50 tubules/genotype. **P < 0.01.
Fig. 3.
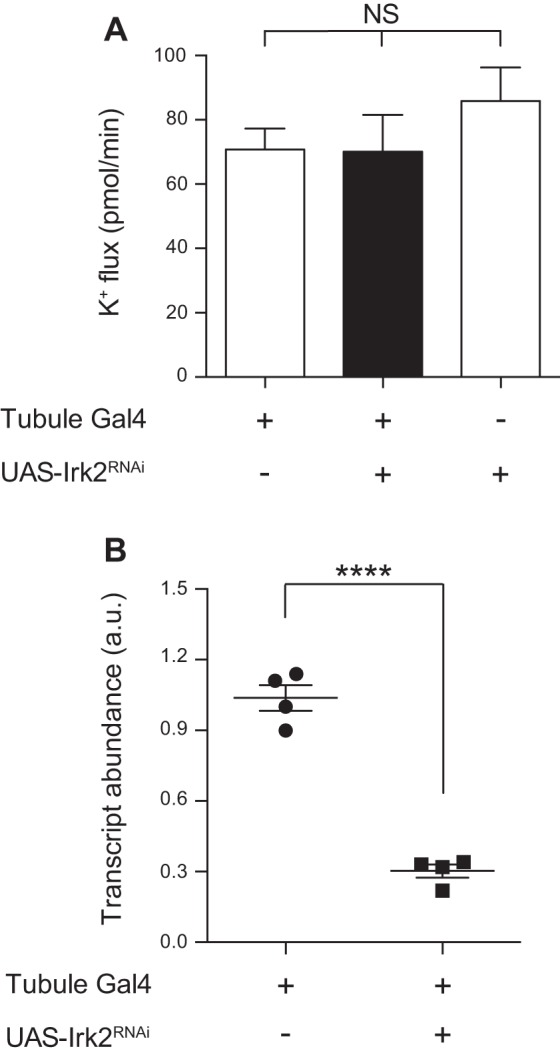
Knocking down Irk2 in the principal cell of the tubule decreases transcript levels but has no effect on K+ flux. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+ and w;UAS-Irk2RNAi/+) or from flies in which Irk2 was knocked down in the principal cells under the control of c42-GAL4 (w;c42-GAL4/UAS-Irk2RNAi). K+ flux was unchanged in the Irk2 knockdown tubules. This experiment was performed simultaneously with the experiment shown in Fig. 2; therefore, the value for w;c42-GAL4/+ is the same; n = 12–14 tubules/genotype. P = 0.4608, 1-way ANOVA. B: Irk2 transcript levels were measured using quantitative RT-PCR from control tubules (w;c42-GAL4/+) or from tubules in which Irk2 was knocked down in the principal cells (w;c42-GAL4/UAS-Irk2RNAi). Transcript levels were decreased in the knockdown tubules; n = 4 pairs of 50 tubules/genotype. ****P < 0.0001.
Fig. 4.
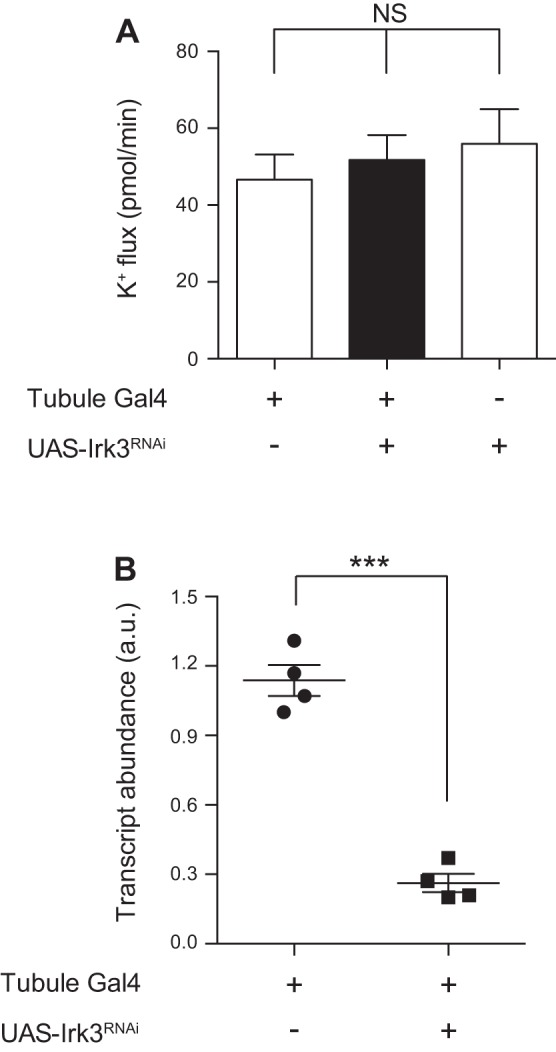
Knocking down Irk3 in the principal cell of the tubule decreases transcript levels but has no effect on K+ flux. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+ and w/yw;UAS-Irk3RNAi/+) or from flies in which Irk3 was knocked down in the principal cells under the control of c42-GAL4 (w/yw;c42-GAL4/UAS-Irk3RNAi). K+ flux was unchanged in the Irk3 knockdown tubules; n = 14–15 tubules/genotype. P = 0.6862, 1-way ANOVA. B: Irk3 transcript levels were measured using quantitative RT-PCR from control tubules (w;c42-GAL4/+) or from tubules in which Irk3 was knocked down in the principal cells (w/yw;c42-GAL4/UAS-Irk3RNAi). Transcript levels were decreased in the knockdown tubules; n = 4 pairs of 50 tubules/genotype. ***P < 0.001.
Table 2.
Secretion rate, [K+], and K+ flux
| Genotype | Secretion Rate, nl/min per tubule | [K+], mM | K+ Flux, pmol/min per tubule | n |
|---|---|---|---|---|
| Irk1 and Irk2 knockdown | ||||
| w;c42-GAL4/+ | 0.47 ± 0.032 | 150 ± 9.1 | 71 ± 6.5 | 13 |
| w/yw;c42-GAL4/UAS-Irk1RNAi | 0.37 ± 0.027 | 158 ± 5.9 | 58 ± 4.8 | 14 |
| w/yw;UAS-Irk1RNAi/+ | 0.38 ± 0.040 | 148 ± 11.3 | 57 ± 7.9 | 13 |
| w;UAS-Irk2RNAi/+;c42-GAL4/+ | 0.47 ± 0.070 | 147 ± 5.0 | 70 ± 11.4 | 14 |
| w;UAS-Irk2RNAi/+ | 0.59 ± 0.090 | 150 ± 8.1 | 86 ± 10.5 | 12 |
| Irk3 knockdown | ||||
| w;c42-GAL4/+ | 0.31 ± 0.035 | 147 ± 8.7 | 47 ± 6.5 | 14 |
| w/yw;c42-GAL4/UAS-Irk3RNAi | 0.32 ± 0.035 | 157 ± 8.0 | 52 ± 6.5 | 15 |
| w/yw;UAS-Irk3RNAi/+ | 0.33 ± 0.052 | 166 ± 6.6 | 56 ± 9.1 | 15 |
| Irk1/Irk3 double knockdown | ||||
| w;UAS-Irk1RNAi/+;UAS-Irk3RNAi/+ | 0.40 ± 0.042 | 164 ± 8.9 | 66 ± 7.0 | 13 |
| w;UAS-Irk1RNAi/+;c42-GAL4/UAS-Irk3RNAi | 0.34 ± 0.047 | 150 ± 12.0 | 52 ± 9.1 | 10 |
| Irk2/Irk3 double knockdown | ||||
| w;c42-GAL4/+ | 0.36 ± 0.052 | 145 ± 10.3 | 54 ± 9.3 | 10 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk3RNAi | 0.48 ± 0.053 | 144 ± 8.8 | 70 ± 8.7 | 7 |
| w;UAS-Irk2RNAi/+;UAS-Irk3RNAi/+ | 0.41 ± 0.046 | 160 ± 12.9 | 63 ± 6.3 | 8 |
| Irk1/Irk2 double knockdown | ||||
| w;c42-GAL4/+ | 0.45 ± 0.024 | 154 ± 6.7 | 70 ± 4.0 | 42 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi | 0.37 ± 0.018 | 136 ± 5.6 | 53 ± 4.7 | 41 |
| w;UAS-Irk2RNAi/+;UAS-Irk1RNAi/+ | 0.49 ± 0.030 | 158 ± 3.9 | 77 ± 5.0 | 41 |
| Irk1/Irk2/Irk3 triple knockdown | ||||
| w;UAS-Irk2RNAi/+;UAS-Irk1RNAi/+ | 0.64 ± 0.051 | 166 ± 6.8 | 109 ± 9.7 | 22 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi | 0.38 ± 0.049 | 135 ± 14.7 | 60 ± 11.5 | 21 |
| w;UAS-Irk2RNAi/+;c42-GAL4 UAS-Irk3RNAi/UAS-Irk1RNAi | 0.47 ± 0.058 | 147 ± 11.2 | 77 ± 12.0 | 20 |
| Effect of cAMP | ||||
| w;c42-GAL4/+, vehicle | 0.33 ± 0.051 | 98 ± 6.5 | 35 ± 6.2 | 19 |
| w;c42-GAL4/+, cAMP | 0.55 ± 0.067 | 110 ± 6.5 | 64 ± 9.6 | 19 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi, vehicle | 0.16 ± 0.037 | 62 ± 6.2 | 11 ± 3.1 | 18 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi, cAMP | 0.38 ± 0.070 | 84 ± 6.7 | 37 ± 8.6 | 19 |
| Effect of ouabain | ||||
| w;c42-GAL4/+, vehicle | 0.37 ± 0.050 | 140 ± 8.9 | 56 ± 8.2 | 16 |
| w;c42-GAL4/+, ouabain | 0.27 ± 0.050 | 81 ± 7.0 | 25 ± 6.1 | 18 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi, vehicle | 0.29 ± 0.051 | 132 ± 12.8 | 43 ± 9.0 | 12 |
| w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi, ouabain | 0.16 ± 0.021 | 94 ± 6.6 | 15 ± 2.6 | 13 |
All values shown are means ± SE. Note that K+ flux was calculated separately for each tubule analyzed, and therefore the value of mean secretion rate × mean [K+] may differ slightly from measured K+ flux, attributable to rounding.
Fig. 5.
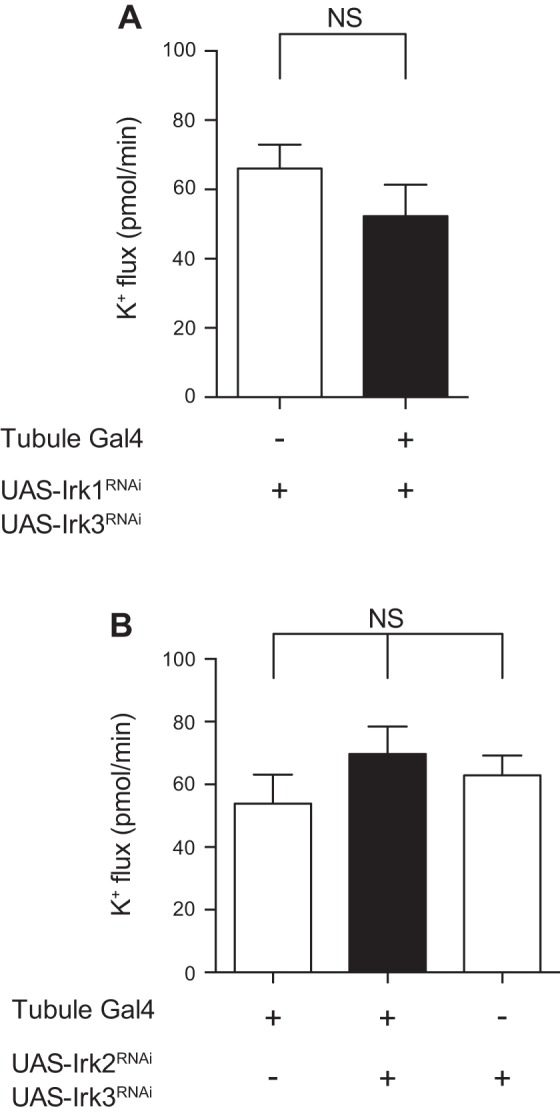
Knocking down Irk1 and Irk3, or Irk2 and Irk3, in the principal cell of the tubule has no effect on K+ flux. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;UAS-Irk1RNAi/+;UAS-Irk3RNAi/+) or from flies in which Irk1 and Irk3 were knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk1RNAi/+;c42-GAL4/UAS-Irk3RNAi). K+ flux was unchanged in the Irk1/Irk3 double-knockdown tubules; n = 10–13 tubules/genotype. P = 0.2371, unpaired t-test. B: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+ and w;UAS-Irk2RNAi/+;UAS-Irk3RNAi/+) or from flies in which Irk2 and Irk3 were knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk3RNAi). K+ flux was unchanged in the Irk1/Irk3 double-knockdown tubules; n = 7–10 tubules/genotype. P = 0.6862, 1-way ANOVA.
Fig. 6.
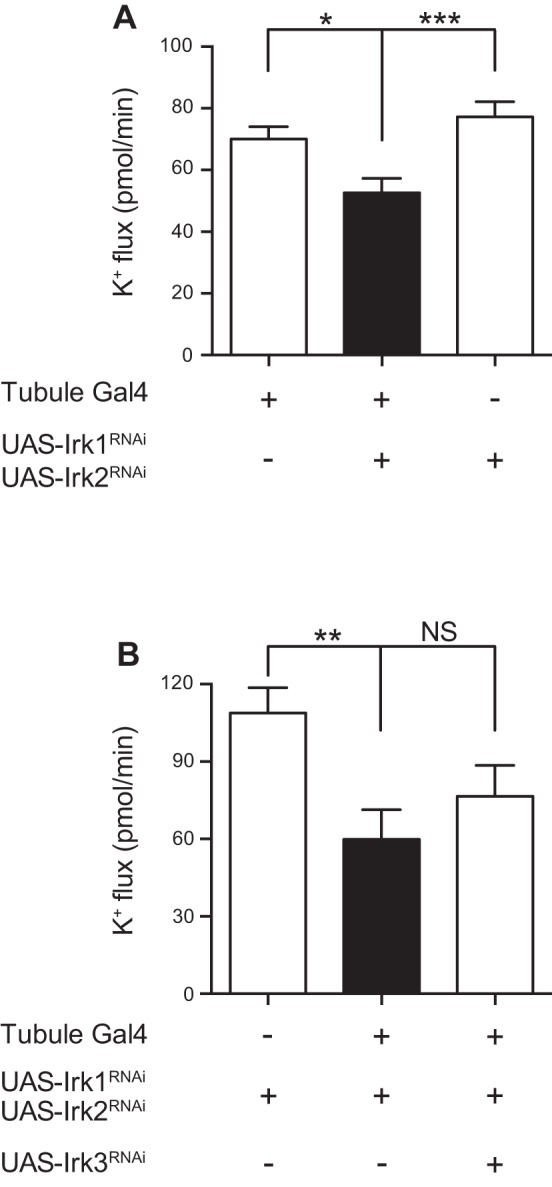
Knockdown of Irk1 and Irk2 together reveals a K+ secretory defect, with no additional effect of Irk3 knockdown. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+ and w;UAS-Irk2RNAi/+;UAS-Irk1RNAi/+) or from flies in which Irk1 and Irk2 were simultaneously knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi). K+ flux was decreased in the Irk1/Irk2 double-knockdown tubules. Flies in this experiment were reared at room temperature, ∼22–23°C; n = 41–42 tubules/genotype. *P < 0.05; ***P < 0.001. B: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;UAS-Irk2RNAi/+;UAS-Irk1RNAi/+) or from flies in which Irk1 and Irk2 were simultaneously knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi) or from flies in which Irk1, Irk2, and Irk3 were simultaneously knocked down (w;UAS-Irk2RNAi/+;c42-GAL4 UAS-Irk3RNAi/UAS-Irk1RNAi). K+ flux was decreased in the Irk1/Irk2 double-knockdown tubules but was not further decreased in the Irk1/Irk2/Irk3 triple-knockdown tubules. Flies in this experiment were reared at room temperature, ∼22–23°C; n = 20–22 tubules/genotype. **P < 0.01.
Having determined that Irk1 and Irk2 appear to function redundantly in transepithelial K+ flux, we next wanted to determine how much of the barium-sensitive component of K+ flux seen in wild-type tubules (Fig. 1) can be explained by Irk1 and Irk2. We therefore measured K+ flux before and after addition of 2 mM barium in control tubules and in tubules in which Irk1, Irk2, or both Irk1 and Irk2 were knocked down in the principal cell under the control of the c42-GAL4 driver (Table 1 and Fig. 7). Similar to the results shown in Fig. 1, ∼80% of K+ flux was abolished by the addition of barium in the control tubules, as well as in tubules in which Irk1 and Irk2 were individually knocked down. In contrast, only 44% of the K+ flux in the Irk1/Irk2 double-knockdown tubules was barium sensitive. This suggests that approximately half of the barium-sensitive K+ flux in control tubules is mediated by Irk1 and Irk2. These data are also consistent with the idea that either Irk1 or Irk2 compensates for each other when either channel is knocked down individually, or that other barium-sensitive channels are upregulated.
Fig. 7.
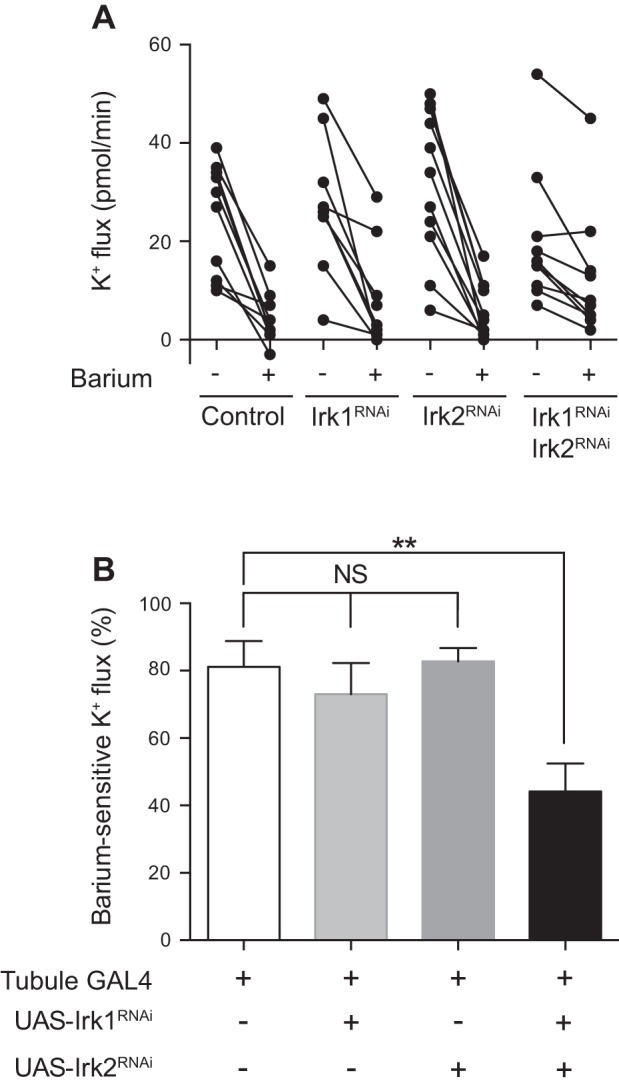
The barium-sensitive component of K+ flux is decreased in Irk1/Irk2 double-knockdown tubules. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+), flies from which Irk1 or Irk2 were individually knocked down in the principal cells under the control of c42-GAL4 (w/yw;UAS-Irk1RNAi/c42 and w; UAS-Irk2RNAi/+;c42-GAL4/+), or flies in which Irk1 and Irk2 were simultaneously knocked down in the principal cells (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi). K+ flux was measured for 1 h before drug addition (baseline) and in the same tubules for 1 h after the addition of 2 mM barium (n = 9–11 tubules/genotype). B: barium-sensitive K+ flux was calculated from the data shown in A. Barium-sensitive K+ flux was similar in control tubules and the Irk1 and Irk2 knockdown tubules but decreased in the Irk1/Irk2 double-knockdown tubules. **P < 0.01.
Previous studies have demonstrated an increase in fluid secretion (8) and K+ flux (19) after stimulation of the Drosophila tubule with cAMP. We previously observed that tubules carrying a homozygous null mutation in the fly sodium-potassium-2-chloride cotransporter (NKCC) remain sensitive to the stimulatory effect of cAMP (34). To determine whether the inwardly rectifying K+ channels play a role in the cAMP response, we stimulated control tubules or Irk1/Irk2 principal cell double-knockdown tubules with cAMP. Increased K+ flux was seen with cAMP treatment in both genotypes (Table 2 and Fig. 8A). Whether this is due to stimulation of other transport pathways in the principal cells or the stimulation of separate pathways in the stellate cell, which are also cAMP responsive (9), is presently unknown.
Fig. 8.
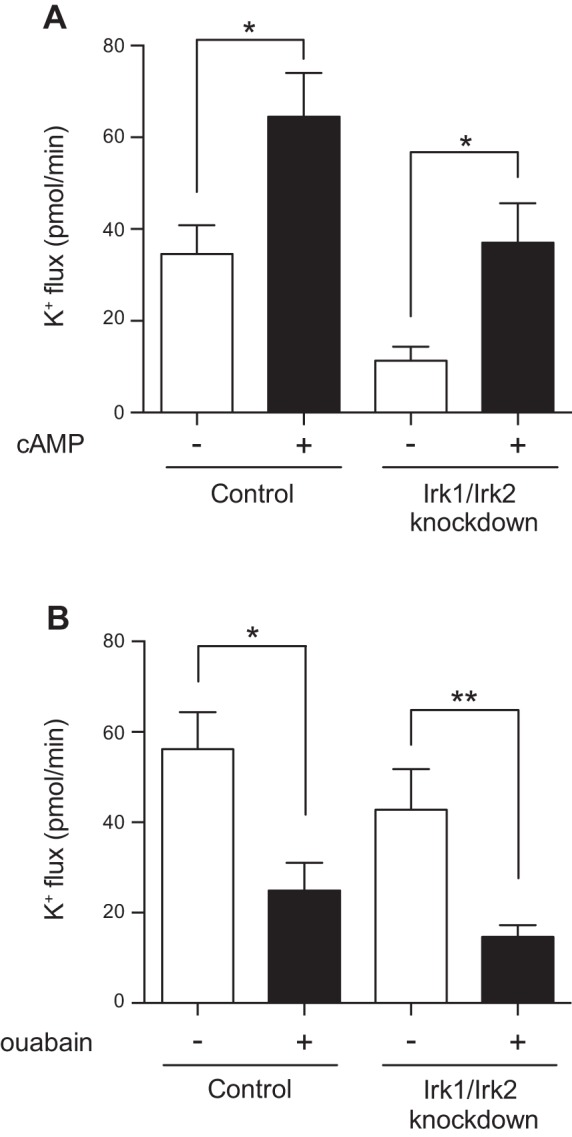
Tubule response to cAMP and ouabain is unchanged in Irk1/Irk2 double-knockdown tubules. A: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+) or from flies in which Irk1 and Irk2 were simultaneously knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi), in the presence of 1 mM cAMP or vehicle control. cAMP stimulated K+ flux in both genotypes; n = 18–19 tubules/group. *P < 0.05. B: transepithelial K+ flux (pmol/min per tubule) was measured in tubules from control flies (w;c42-GAL4/+) or from flies in which Irk1 and Irk2 were simultaneously knocked down in the principal cells under the control of c42-GAL4 (w;UAS-Irk2RNAi/+;c42-GAL4/UAS-Irk1RNAi), in the presence of 100 μM ouabain, an Na+/K+-ATPase inhibitor, or vehicle control. K+ flux was decreased by ouabain treatment in both genotypes. Flies in this experiment were reared at room temperature, ∼22–23°C; n = 12–18 tubules/group. *P < 0.05; **P < 0.01.
We previously demonstrated that ouabain, an inhibitor of the Na+/K+-ATPase, decreases K+ flux in control tubules (34). No effect of ouabain was seen in NKCC-null mutant tubules (34), indicating that the primary role of the Na+/K+-ATPase in transepithelial K+ flux is to generate a favorable gradient for NKCC activity. A possible role of inwardly rectifying K+ channels is to recycle the K+ entering through the Na+/K+-ATPase. If this is the case for Irk1 and Irk2, addition of ouabain to the Irk1/Irk2 double-knockdown tubules would not be expected to further decrease K+ flux. Instead, a decrease in K+ flux was observed in both control and Irk1/Irk2 double-knockdown tubules (Table 2 and Fig. 8B). This suggests that inwardly rectifying K+ channels have roles independent of the Na+/K+-ATPase. Additionally, in this experiment, ∼75% of transepithelial K+ flux could be attributed to the combination of the Na+/K+-ATPase, which as described above drives the activity of the NKCC, and the inwardly rectifying K+ channels Irk1 and Irk2.
DISCUSSION
Inwardly rectifying K+ channels play important roles in invertebrate and vertebrate renal physiology. In insects, basolateral membrane barium-sensitive K+ conductances have been demonstrated in a variety of insect renal tubules. Examples include the yellow fever mosquito Aedes aegypti (22, 41), the forest ant Formica polyctena (17), the Chagas vector Rhodnius prolixus (12), the agricultural pest Locusta migratoria (14), and the mealworm Tenebrio molitor (45). In addition, transcripts for inwardly rectifying K+ channels are expressed in the Malpighian tubules of Aedes aegypti, the vector for yellow fever, dengue, and chikungunya (29); Anopheles gambiae, the malaria vector (30); and the bed bug Cimex lectularius (21). Drugs targeting renal tubule inwardly rectifying K+ channels are currently being developed as mosquitocidal insecticides in Aedes aegypti (31, 32, 38). These channels may represent targets for the control of other insect pests as well, although killing of benign or beneficial insects will need to be avoided. Our study extends the understanding of the role of these channels in insect renal tubule function.
Barium has been extensively used to probe the function of inwardly rectifying K+ channels in insects. Here, we replicated previous findings that barium decreases fluid secretion in the fly renal tubule (25) and showed that it decreases transepithelial K+ flux. We also observed a decrease (lumen less positive) in the transepithelial potential difference of 21 mV. This is consistent with prior reports of hyperpolarization of the basolateral membrane potential of similar magnitude (15, 25), likely explaining the effect on the transepithelial potential difference. Both Irk1 and Irk2 are sensitive to barium when expressed in heterologous cells (7), and barium blocks the basolateral K+ conductance of the Drosophila tubule (15). Barium may also have additional effects on other, undefined ion channels on the basolateral membrane of the fly tubule (15). Indeed, Irk1 and Irk2 appear to account for only half of the barium sensitivity we observed for transepithelial K+ flux. Genetic studies, in which specific channels can be manipulated, therefore provide complementary information to pharmacological studies. This is particularly important given the number of genes encoding inwardly rectifying K+ channels. The genomes of the mosquito species Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus encode Irk1 and Irk3 homologs, whereas the Irk2/Kir2 gene has undergone a duplication event, resulting in Kir2A and Kir2B genes. Additional duplication events have resulted in Kir2A and Kir2A′ genes in Anopheles and Culex, and Kir2B and Kir2B′ genes in Aedes. In Culex and Anopheles, the Kir3 gene has also been duplicated to result in Kir3A and Kir3B genes (23, 29, 30).
Here, we observed that Irk1 and Irk2, but not Irk3, are important for transepithelial fluid secretion and K+ flux. Interestingly, no functional channel activity was observed with attempts at expression of Irk3 in Xenopus oocytes or Drosophila S2 cultured cells, whereas both Irk1 and Irk2 possessed inwardly rectifying K+ channel activity (7). Similarly, no channel activity was observed in Xenopus oocytes expressing the Aedes aegypti Irk3 homolog, AeKir3, although it is highly expressed in the mosquito tubule (29). In addition, recent immunolocalization data indicates that AeKir3 is expressed in intracellular punctae in the mosquito tubule (28). In bed bugs, the Irk3 homolog ClKir3 transcript is expressed at very high levels in the Malpighian tubules, yet whole-organism silencing using RNA interference had no effect on bed bug viability (21). The functional role of Irk3 and its homologs in other insects in the renal tubule is therefore unclear.
In contrast, compounds that inhibit AeKir1 and AeKir2B have inhibitory effects on whole-mosquito urine excretion (31, 32, 38) and on transepithelial fluid secretion and K+ flux in the Ramsay assay (28, 36). This is broadly consistent with our results that both Irk1 and Irk2 play roles in the fly tubule although there are interesting differences; in Aedes, AeKir1 is located in the stellate cell, whereas AeKir2B is in the principal cell, and inhibition of both AeKir1 and AeKir2 has additive effects on K+ flux. However, this may also reflect the effects of acutely inhibiting the channels pharmacologically, as opposed to the longer-term genetic knockdown used in this study. Additional open questions remain. For example, why is expression of AeKir2B enriched in the mosquito tubule, rather than the fly Irk2 homolog, AeKir2A? Does AeKir2B play a specific role in fluid secretion and ion flux after a blood meal, a situation not faced by Drosophila? Do specific splice isoforms of Irk2/Kir2A, demonstrated in Drosophila, Aedes aegypti, and Anopheles gambiae (11, 37), have specific functional roles in the tubule? Given the ease of genetic manipulation and transgenesis in Drosophila, the fly renal tubule could serve as a platform to explore, not only the role of the Drosophila channels, but potentially also the physiological roles and/or the drug sensitivities of various inwardly rectifying K+ channels of other insects, aiding in the development of pharmacological agents to control insect disease vectors or insect pests.
Irk1 and Irk2 both have K+ channel activity as homomeric channels when heterologously expressed (7). It is possible that they could also function as heteromeric channels, as is the case for some other inwardly rectifying K+ channels (13). However, the fact that Irk1 and Irk2 must simultaneously be knocked down to see a change in transepithelial flux suggests that, even if the two channels do have heteromeric interactions, these are not absolutely required for their function in the tubule.
What roles are Irk1 and Irk2 playing in transepithelial ion flux? One possibility is that Irk1 and Irk2 constitute all or part of the basolateral K+ conductance and are important for maintaining the basolateral membrane potential. Could they also serve as a conductive pathway for K+ entry from the hemolymph into the principal cell? In an analysis by Ianowski and O'Donnell, EK (−52 mV) was close to the basolateral membrane potential (−43 mV), with a net outward driving force for K+ movement from cell to bath (15). Because EK and the basolateral membrane potential were close to one another, relatively modest changes in conditions could affect the direction of the driving force. Indeed, the bathing solution for our Ramsay assay studies of genetically modified tubules differed from those used by Ianowski and O'Donnell (15). In the Formica tubule, EK was also close to the basolateral membrane potential, and, depending on the measurement technique as well as the bath K+ concentration, driving forces were observed that were either inward, outward, or zero (18). A subsequent study proposed that, at high hemolymph K+ concentrations, K+ uptake occurs through basolateral K+ channels (17). Similarly, studies in the deoxycorticosterone-treated rabbit cortical collecting duct demonstrated an inward (bath to cell) driving force for potassium across the basolateral membrane (16, 39). In this preparation, transepithelial K+ flux from bath to lumen increased when bath K+ concentration increased, and this increase was attenuated by the basolateral application of barium, indicating that basolateral K+ channels allow K+ uptake into the cortical collecting duct principal cell (24). Similarly, application of an AeKir1/AeKir2B inhibitor to the basolateral membrane of the Aedes aegypti tubule depolarized the basolateral membrane potential and decreased input conductance under high bath K+ (34 mM) conditions (28).
We found in a previous study that the Na+/K+-ATPase generates the driving force for NKCC activity in the fly renal tubule (34). Another potential role for K+ channels could be to recycle the K+ entering through the Na+/K+-ATPase. However, we observed additive effects of the Na+/K+-ATPase inhibitor ouabain and knockdown of Irk1 and Irk2, indicating that Irk1 and Irk2 have functions beyond recycling the K+ entering through the Na+/K+-ATPase. Indeed, about 75% of transepithelial K+ flux was mediated by Irk1, Irk2, and ouabain-sensitive pathways, which could include the Na+/K+-ATPase itself as well as secondary active K+ uptake by the NKCC.
Our genetic and pharmacological results are most consistent with a role for the inwardly rectifying K+ channels, Irk1 and Irk2, on the basolateral membrane of the Drosophila melanogaster main segment principal cell. Possible roles of Irk1 and Irk2 are the maintenance of the basolateral membrane potential or to allow the movement of K+ from the hemolymph into the principal cell during transepithelial K+ flux. Because flies eat a K+-rich diet, the existence of multiple mechanisms to allow principal cell K+ uptake, Irk1 and Irk2 as well as the Na+/K+-ATPase and NKCC, builds redundancy into the system for renal K+ excretion.
GRANTS
This work was supported by NIDDK DK091316 (to A. Rodan) and DK079328 (UTSW O'Brien Center Pilot and Feasibility Award to A. Rodan); DK007257 (institutional T32), DK59530 (to C.-L. Huang), DK41612, and DK078596 (to M. Baum); and the American Society of Nephrology Gottschalk Award (to A. Rodan).
DISCLOSURES
A. Rodan has received a speaker's fee from Eli Lilly that was not related to this work. The remaining authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Y.W., M.B., and A.R.R. performed experiments; Y.W., M.B., and A.R.R. analyzed data; Y.W. prepared figures; Y.W., M.B., C.-L.H., and A.R.R. edited and revised manuscript; Y.W., M.B., C.-L.H., and A.R.R. approved final version of manuscript; M.B., C.-L.H., and A.R.R. conception and design of research; M.B. and A.R.R. interpreted results of experiments; A.R.R. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Julian Dow and Adrian Rothenfluh for generous gifts of fly strains. Fly strains were also obtained from the Vienna Drosophila Resource Center and the Bloomington Stock Center.
REFERENCES
- 1.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JA. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci USA 111: 14301–14306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahal GR, Rawson J, Gassaway B, Kwok B, Tong Y, Ptacek LJ, Bates E. An inwardly rectifying K+ channel is required for patterning. Development 139: 3653–3664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JA. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci 121: 2612–2619, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Doring F, Wischmeyer E, Kuhnlein RP, Jackle H, Karschin A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors Kir channel function. J Biol Chem 277: 25554–25561, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Efetova M, Petereit L, Rosiewicz K, Overend G, Haussig F, Hovemann BT, Cabrero P, Dow JA, Schwarzel M. Separate roles of PKA and EPAC in renal function unraveled by the optogenetic control of cAMP levels in vivo. J Cell Sci 126: 778–788, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eleftherianos I, Won S, Chtarbanova S, Squiban B, Ocorr K, Bodmer R, Beutler B, Hoffmann JA, Imler JL. ATP-sensitive potassium channel (K(ATP))-dependent regulation of cardiotropic viral infections. Proc Natl Acad Sci USA 108: 12024–12029, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol 208: 3771–3783, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Haley C, Donnell M. K+ reabsorption by the lower Malpighian tubule of Rhodnius prolixus: inhibition by Ba2+ and blockers of H+/K+-ATPases. J Exp Biol 200: 139–147, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hyde D, Baldrick P, Marshall SL, Anstee JH. Rubidium reduces potassium permeability and fluid secretion in Malpighian tubules of Locusta migratoria, L. J Insect Physiol 47: 629–637, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Ianowski JP, O'Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl- cotransport and Cl− conductance. J Exp Biol 207: 2599–2609, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Koeppen B, Giebisch G. Cellular electrophysiology of potassium transport in the mammalian cortical collecting tubule. Pflugers Arch 405, Suppl 1: S143–S146, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Leyssens A, Dijkstra S, Van Kerkhove E, Steels P. Mechanisms of K+ uptake across the basal membrane of malpighian tubules of Formica polyctena: the effect of ions and inhibitors. J Exp Biol 195: 123–145, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Leyssens A, Van Kerkhove E, Zhang SL, Weltens R, Steels P. Measurement of intracellular and luminal K+ concentrations in a Malpighian tubule (Formica). Estimate of basal and luminal electrochemical K+ gradients. J Insect Physiol 39: 945–948, 1993. [Google Scholar]
- 19.Linton SM, O'Donnell MJ. Contributions of K+:Cl− cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 202: 1561–1570, 1999. [DOI] [PubMed] [Google Scholar]
- 20.MacLean SJ, Andrews BC, Verheyen EM. Characterization of Dir: a putative potassium inward rectifying channel in Drosophila. Mech Dev 116: 193–197, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Mamidala P, Mittapelly P, Jones SC, Piermarini PM, Mittapalli O. Molecular characterization of genes encoding inward rectifier potassium (Kir) channels in the bed bug (Cimex lectularius). Comp Biochem Physiol B Biochem Mol Biol 164: 275–279, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Masia R, Aneshansley D, Nagel W, Nachman RJ, Beyenbach KW. Voltage clamping single cells in intact malpighian tubules of mosquitoes. Am J Physiol Renal Physiol 279: F747–F754, 2000. [DOI] [PubMed] [Google Scholar]
- 23.McCormack TJ. Comparison of K+-channel genes within the genomes of Anopheles gambiae and Drosophila melanogaster. Genome Biol 4: R58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muto S, Giebisch G, Sansom S. An acute increase of peritubular K stimulates K transport through cell pathways of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 255: F108–F114, 1988. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J Exp Biol 199: 1163–1175, 1996. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Paulais M, Lourdel S, Teulon J. Properties of an inwardly rectifying K+ channel in the basolateral membrane of mouse TAL. Am J Physiol Renal Physiol 282: F866–F876, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Piermarini PM, Dunemann SM, Rouhier MF, Calkins TL, Raphemot R, Denton JS, Hine RM, Beyenbach KW. Localization and role of inward rectifier K channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol. doi: 10.1016/j.ibmb.2015.06.006. [DOI] [PubMed]
- 29.Piermarini PM, Rouhier MF, Schepel M, Kosse C, Beyenbach KW. Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti. Insect Biochem Mol Biol 43: 75–90, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raphemot R, Estevez-Lao TY, Rouhier MF, Piermarini PM, Denton JS, Hillyer JF. Molecular and functional characterization of Anopheles gambiae inward rectifier potassium (Kir1) channels: a novel role in egg production. Insect Biochem Mol Biol 51: 10–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raphemot R, Rouhier MF, Hopkins CR, Gogliotti RD, Lovell KM, Hine RM, Ghosalkar D, Longo A, Beyenbach KW, Denton JS, Piermarini PM. Eliciting renal failure in mosquitoes with a small-molecule inhibitor of inward-rectifying potassium channels. PLoS One 8: e64905, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphemot R, Rouhier MF, Swale DR, Days E, Weaver CD, Lovell KM, Konkel LC, Engers DW, Bollinger SF, Hopkins C, Piermarini PM, Denton JS. Discovery and characterization of a potent and selective inhibitor of Aedes aegypti inward rectifier potassium channels. PLoS One 9: e110772, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rheault MR, O'Donnell MJ. Analysis of epithelial K+ transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. J Exp Biol 204: 2289–2299, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Rodan AR, Baum M, Huang CL. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol 303: C883–C894, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosay P, Davies SA, Yu Y, Sozen MA, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Rouhier MF, Hine RM, Park ST, Raphemot R, Denton J, Piermarini PM, Beyenbach KW. Excretion of NaCl and KCl loads in mosquitoes. 2. Effects of the small molecule Kir channel modulator VU573 and its inactive analog VU342. Am J Physiol Regul Integr Comp Physiol 307: R850–R861, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouhier MF, Piermarini PM. Identification of life-stage and tissue-specific splice variants of an inward rectifying potassium (Kir) channel in the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol 48: 91–99, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Rouhier MF, Raphemot R, Denton JS, Piermarini PM. Pharmacological validation of an inward-rectifier potassium (Kir) channel as an insecticide target in the yellow fever mosquito Aedes aegypti. PLoS One 9: e100700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sansom SC, Agulian S, Muto S, Illig V, Giebisch G. K activity of CCD principal cells from normal and DOCA-treated rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 256: F136–F142, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott BN, Yu MJ, Lee LW, Beyenbach KW. Mechanisms of K+ transport across basolateral membranes of principal cells in Malpighian tubules of the yellow fever mosquito, Aedes aegypti. J Exp Biol 207: 1655–1663, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol 33: 215–228, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol 297: F849–F863, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiehart UI, Nicolson SW, and Van Kerkhove E. K+ transport in Malpighian tubules of Tenebrio molitor L: a study of electrochemical gradients and basal K+ uptake mechanisms. J Exp Biol 206: 949–957, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Schellinger JN, Huang CL, Rodan AR. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the NCC69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem 289: 26131–26142, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


