Abstract
Limited data exist on the efficacy of low-load blood flow-restricted strength training (BFR), as compared directly to heavy-load strength training (HST). Here, we show that 12 wk of twice-a-week unilateral BFR [30% of one repetition maximum (1RM) to exhaustion] and HST (6-10RM) of knee extensors provide similar increases in 1RM knee extension and cross-sectional area of distal parts of musculus quadriceps femoris in nine untrained women (age 22 ± 1 yr). The two protocols resulted in similar acute increases in serum levels of human growth hormone. On the cellular level, 12 wk of BFR and HST resulted in similar shifts in muscle fiber composition in musculus vastus lateralis, evident as increased MyHC2A proportions and decreased MyHC2X proportions. They also resulted in similar changes of the expression of 29 genes involved in skeletal muscle function, measured both in a rested state following 12 wk of training and subsequent to singular training sessions. Training had no effect on myonuclei proportions. Of particular interest, 1) gross adaptations to BFR and HST were greater in individuals with higher proportions of type 2 fibers, 2) both BFR and HST resulted in approximately four-fold increases in the expression of the novel exercise-responsive gene Syndecan-4, and 3) BFR provided lesser hypertrophy than HST in the proximal half of musculus quadriceps femoris and also in CSApeak, potentially being a consequence of pressure from the tourniquet utilized to achieve blood flow restriction. In conclusion, BFR and HST of knee extensors resulted in similar adaptations in functional, physiological, and cell biological parameters in untrained women.
Keywords: low-load blood flow-restricted training, heavy-load strength training, muscle strength and mass, muscle fiber, gene expression
blood flow-restricted low-load strength training (BFR) provides comparable improvements in muscle strength and muscle mass to those seen after traditional heavy-load strength training (HST) (33, 34, 66). This similarity is evident despite utilization of protocols with training loads as low as 20% of one repetition maximum (1RM) (33), far below recommendations set for strength training, typically advocating loads >60–80% of 1RM to obtain sufficient mechanical stress to maximize muscular adaptations (52). It has, thus, been argued that muscular adaptations seen after BFR are dependent on cellular stressors, such as metabolic/oxidative stress (48, 73), hyperemia, and cell swelling (35, 48) rather than mechanical stressors. This could well involve signaling through alternative pathways (22), a notion that is supported by the unprecedented ability of BFR to activate myogenic satellite cells (46), its seemingly superior ability to stimulate type 1 fibers (8, 46), and its claimed ability to result in more pronounced changes in serum concentrations of human growth hormone (s-HGH) (40, 65). Contrasting this, others have speculated that BFR protocols exhibit type 2 fiber-dominant features (38) and display hormonal responses similar to HST (30).
It is surprising that a mere handful of studies have investigated gene expression responses to BFR. Such information would arguably provide hallmark information on cell-signaling events. Of the few studies that have looked into the subject, all have assessed a scarce number of genes (12, 32, 33, 39), with only one study having compared BFR to HST directly (33). Furthermore, no study has investigated effects of BFR on muscle fiber composition, which should be of particular interest given the hypothesized fiber-type specificity of BFR protocols, potentially leading to reduced stress of type 2 fiber (8), potentially skewing the expected transition of glycolytic 2X fibers into aerobic 2A fibers (1, 17, 63).
The aim of the present study was to compare functional and biological efficacies of typical BFR and HST leg extension protocols in previously untrained women using a within-subject design. The overall hypothesis was that BFR and HST would lead to similar increases in muscle strength and muscle cross-sectional area (CSA) but that they would result in different adaptations and responses on the cellular level. We investigated effects of 12 wk of twice-a-week training and acute responses to singular training sessions on outcome variables such as 1RM knee extension, CSA of distal and proximal halves of musculus quadriceps femoris (QUAD) and musculus vastus lateralis (VL), serum hormone levels, and muscle fiber composition and gene expression in VL. Gene expression analyses included 29 genes previously ascribed wide ranges of roles in muscle function or plasticity, with the overall aim being to compare BFR- and HST-induced responses using a correlation-based approach.
METHODS
Ethics statement.
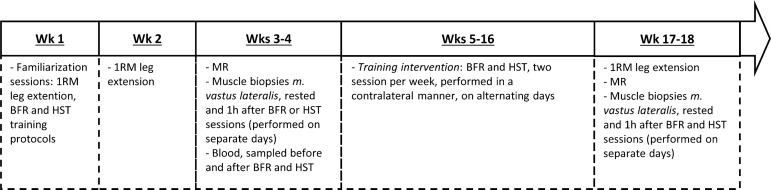
The Regional Committees for Medical and Health Research Ethics in Southeast Norway (2010/2679) approved the study, which was performed in accordance with the Declaration of Helsinki. All subjects signed a written informed consent prior to study participation. For an overview of the intervention protocol, see Fig. 1.
Fig. 1.
Overview of the strength-training intervention. BFR, blood flow-restricted strength training; HST, heavy-load strength training; 1RM, 1 repetition maximum; MR, magnetic resonance imaging.
Experimental design.
Fifteen healthy untrained female volunteers (23 ± 3 yr, 167 ± 8.0 cm, and 70 ± 20 kg) were recruited from a student population. Inclusion was limited to individuals who had performed less than one strength training session per week for the 6 mo leading up to the study. Three subjects did not complete the study for reasons unrelated to the study protocol. The training protocol consisted of 12 wk of two unilateral knee-extension exercises per leg per week (Technogym Silver Line, Gambettola, Italy). In a randomized manner, BFR was allocated to one leg, while HST was allocated to the other leg. The rationale behind choosing such a with-subject protocol, as opposed to a between-subject protocol, was to enable comparison of the two training modalities in a manner that was not limited by the large variation typically seen in training responses between human subjects (67). To lower the probability of contralateral effects of either of the two training protocols, i.e., to avoid training of one leg from affecting the other leg, BFR and HST were performed on different days in an alternating fashion; i.e., each subject performed training four days a week, two for each mode of training. This is in line with data presented by Madarame et al. (36), who found contralateral effects to BFR only in limbs (i.e., arms) that performed training on the same day. BFR and HST protocols utilized in the current study represent two quite typical and proven protocols for each type of exercise, which, indeed, was the practically relevant rationale for comparing them.
Prior to the start of the 12-wk training program, each subject was given supervised familiarization sessions to ensure proper lifting technique and testing procedures. For both BFR and HST protocols, the duration of each concentric and eccentric phase was set to 1 s, monitored using a metronome. Subsequent to the familiarization session (>48 h), 1RM in unilateral knee extension was determined, using the same knee-extension apparatus as was utilized during training. 1RM testing was repeated after 6 and 12 wk of training.
Throughout the intervention, each training session was preceded by 10 min of general warmup on a spinning cycle. Certified personnel supervised all training sessions. During BFR sessions, blood flow restriction was induced by a 18-cm-wide pressure cuff (Delfi Medical, Vancouver, Canada) connected to a tourniquet system (Welch Allyn, Skaneateles, NY). The cuff was wrapped around the proximal part of the thigh. For the initial 6 wk of the intervention, the cuff was inflated to 90 mmHg, whereupon the pressure was increased to 100 mmHg. The BFR protocol consisted of five sets of repetitions to failure at 30% of 1RM, calculated from 1RM tests performed at week 0 (wk0) and week 6, with 45-s rest between sets, and it was a modified version of the protocol presented in Wernbom et al. (72). This resulted in an average weekly training volume of 1,221 ± 320 reps × kg. Between 1RM tests, training progression occurred through increased numbers of repetitions (to exhaustion) at the constant workload of 30% of 1RM. As an example of the number of repetitions performed in each of the five sets in a given training session, in week 5, subjects typically performed the following ranges of repetitions: set 1, 35–45 reps; set 2, 20–30 reps; set 3, 10–20 reps; set 4, 8–15 reps; set 5, 6–12 reps. The cuff was kept in an inflated state throughout BFR sessions, including during periods of rest. For details about the conducted training, see Table 1.
Table 1.
Characteristics of blood-flow-restricted training and heavy-load strength training during the first (week 1), middle (week 6), and last week of training (week 12)
| BFR |
HST |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 6 | Week 12 |
Week 1 |
Week 6 |
Week 12 |
||||
| 3× 10 RM | 3× 6 RM | 3× 10 RM | 3× 6 RM | 3× 10 RM | 3× 6 RM | ||||
| Relative intensity, % 1RM | 30 ± 0 | 30 ± 0 | 30 ± 0 | 74 ± 9 | 85 ± 8 | 78 ± 6 | 88 ± 4 | 81 ± 5 | 92 ± 4 |
| Number of reps per session | 76 ± 19 | 103 ± 26 | 106 ± 25 | 30 ± 0 | 18 ± 0 | 30 ± 0 | 18 ± 0 | 30 ± 0 | 18 ± 0 |
| Sets per session | 5 | 5 | 5 | 3 | 3 | 3 | 3 | 3 | 3 |
| Training volume per session, kg | 934 ± 275 | 1215 ± 372* | 1324 ± 395* | 803 ± 194 | 553 ± 113 | 911 ± 164* | 617 ± 108* | 1004 ± 194* | 681 ± 136* |
Values are expressed as means ± SD.
BFR, blood flow-restricted training; HST, heavy-load strength training; RM, repetition maximum.
Significant changes in training volume from week 1 (P < 0.05).
The HST protocol consisted of three sets of 6-10RM, with 90-s rest between sets. Variation of training load was done on a session-to-session basis throughout the training period, with each subject performing the first HST session of each week with a 10RM load and the second session with a 6RM load, corresponding to 74–81% and 85–92% of 1RM, respectively, as referred to 1RM tests performed at weeks 0 and 6 (Table 1). This resulted in an average weekly training volume of 813 ± 138 reps × kg, which was significantly lower than the BFR training volume (1,221 ± 320 reps × kg, P < 0.05). Subjects continuously increased training loads to meet target RM loads, ensuring training progression. Assistance was allowed on the last repetition of each training set to ensure full ranges of motion and target training loads. For details about the conducted training, see Table 1.
Muscle biopsies and blood samples.
Biopsies were sampled from VL of both legs using the Bergström procedure, as previously described (16). Biopsy sampling was performed at the following time points: 1) in a rested state at wk0, 2) 1 h after completing BFR and 10RM HST sessions at wk0 (representing the two training sessions succeeding the familiarization session), 3) in a rested state at week 12 (wk12), and 4) 1 h after completing BFR and 10RM HST at wk12 (the two last training sessions). The first biopsy was sampled from an area situated ∼1/3 along the length of femur, as related to its distal end-point in the knee. Subsequent biopsies were sampled 2 cm proximally to the previous biopsy. Biopsy sampling was performed under local anesthesia (xylocain + adrenaline, 10 mg/ml + 5 μg/ml, AstraZeneca PLC, London, UK), and typically resulted in ∼200 mg of muscle tissue per subject per sampling day (2–3 × 50–150 mg). Biopsies were obtained from 9 of the 12 subjects, with the remaining three subjects choosing to refrain from such sampling. To reduce the total number of sampling events, rested-state biopsies at wk0 were collected from one leg only, allocated to either the right or the left leg in a randomized manner. Subjects were instructed to abstain from physical activity for the last 48 h leading up to biopsy-sampling events and were instructed to abstain from ingesting anything other than H2O for the last 2 h leading up to biopsy sampling/training. Adherence to these routines was ascertained through oral communication. For each particular subject, every biopsy-sampling event was performed at the same time of day. Biopsy material for immunohistochemistry were immersed immediately in 10% buffered formaldehyde solution (Chemi-Teknik AS, Oslo, Norway), wherein they were left to fixate for 3 or 4 days before further preparation. Biopsy material for RNA analyses were immersed immediately in RNAlater (Ambion, Life Technologies, Carlsbad, CA) and was treated according to the manufacturer's protocol before storage at −80°C until RNA extraction.
Blood samples for hormonal analyses were sampled at the following time points: 1) in a rested state at wk0 (immediately prior to BFR and HST sessions), 2) 1 min and 30 min after completing BFR or HST training at wk0, 3) in a rested state at wk12 (immediately prior to BFR or HST sessions), and 4) 1 min and 30 min after completing BFR or HST training at wk12. Unfortunately, in connection with BFR at wk0, we were unable to retrieve blood samples from two of the subjects. These subjects are, thus, not included in the wk0 data set. In resemblance with the protocol followed during biopsy sampling, subjects were instructed to abstain from physical activity for the 48 h leading up to blood-sampling events and to abstain from ingestion of anything other than water for the 2 h preceding sampling. Blood samples were drawn from an antecubital vein into serum-separating tubes and were incubated at room temperature for 30 min before centrifugation at 1,400 g for 15 min. Serum was stored at −80°C until analysis.
Magnetic resonance imaging.
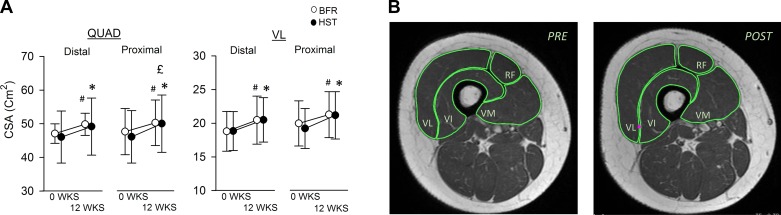
CSAs of QUAD and VL were assessed in all 12 subjects at wk0 and wk12 using magnetic resonance (MR) imaging (Philips Achieva 1.5 T, Philips Healthcare, Best, The Netherlands), according to manufacturer's protocol. Images were analyzed in a blinded fashion, using OsiriX version 5.6 (Pixmeo, Geneva, Switzerland). During subsequent analyses, QUAD and VL were divided into proximal and distal halves, which were treated separately. The boundaries between the two halves were defined by the two images showing the highest CSA. In QUAD, these two images were also utilized to calculate peak CSA (CSApeak). Prior to MR scanning, subjects were instructed to abstain from physical activity for the last 48 h leading up to scanning and were instructed to abstain from ingestion of food and drinks for the last 2 h. Analyses of MR images were performed in a blinded fashion. For representative MR images, see Fig. 4B.
Fig. 4.
A: effect of 12-wk BFR (○) and HST (●) on cross-sectional area (CSA) of distal and proximal parts of musculus quadriceps femoris (QUAD, left) and musculus vastus lateralis (VL, right) in previously untrained women (n = 12). Each individual performed the two protocols in a contralateral manner. The border between distal and proximal parts of muscles were defined using images showing the highest cross-sectional area. Data are expressed as means ± SD. #Significant effects of BFR on muscle (P < 0.05). *Significant effects of HST on muscle CSA (P < 0.05). £Significant differential effects of HST and BFR, measured as the difference in relative change of CSA from baseline (P < 0.05). B: representative MR image of the thigh from one subject taken before (PRE) and after (POST) 12 wk of BFR. The four muscle bellies of QUAD is highlighted in green: VL; VI, musculus vastus intermedius; VM, musculus vastus medialis; RF, musculus rectus femoris. This particular individual exhibited a 6.8% increase in the CSA of QUAD and a 9.4% increase in the CSA of VL.
Immunohistochemistry.
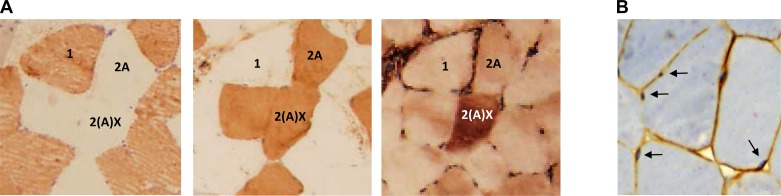
For determination of muscle fiber composition, formalin-fixed muscle biopsies were processed using an Shandon Excelsior ES (Thermo Scientific, Waltham, MA), before it was paraffin-embedded and sectioned (4 μm), whereupon transverse, serial sections were labeled for MyHC1 (A4.840), MyHC2A (EPR5280), and MyHC2X (6H1), as previously described (17). For images showing representative labeling, see Fig. 2A. A minimum of 200 fibers were assessed, as recommended by Blomstrand and Ekblom (3), performed using Photoshop CS5 Extended (Adobe, San Jose, CA), in a blinded fashion. Unfortunately, the specific protocol utilized for paraffin-embedment of tissues prohibited us from performing fiber type-specific analyses of CSA. In short, the embedment process caused something resembling dehydration of muscle fibers, leading to slight and somewhat unevenly distributed changes in fiber sizes. This did not affect muscle fiber integrity and thus had no effect on muscle fiber-type characterization. Moreover, because of a technical challenge with the 6H1 antibody, which might be related to the low stability of the antibody, as discussed in Ellefsen et al. (17), we lack complete data on MyHC composition from one subject, which was, hence, removed from the data set prior to analysis.
Fig. 2.
Representative immunohistochemical staining of myosin heavy chains (A) in serial sections of human skeletal muscle using antibodies toward MyHC1 (1; A4.840), MyHC2A (2A; EPR5280), and MyHC2X [2(A)X; 6H1] and dystrophin (B) with hematoxylin staining of nuclei. Arrows indicate myonuclei.
For the sake of the muscle fiber proportions calculated and presented in the current study, MyHC1/2A hybrid fibers were counted as 0.5 × MyHC1 and 0.5 × MyHC2A, i.e., as being half type 1 fiber and half type 2A fiber, as outlined in Ellefsen et al. (17); i.e., hybrid fibers are not presented as separate entities in our analyses. With regard to MyHC2A/2X hybrid fibers, a particular issue became evident during analyses, as all fibers that labeled positively using the 2X antibody also labeled positively using the 2A antibody, but not vice versa, resembling the observation recently made by Fry et al. (20). This either means that all 2X-positive fibers are 2X-2A hybrids, or that the 2A-antibody recognizes the 2X antigen in addition to recognizing the 2A antigen, as discussed in Ellefsen et al. (17). Importantly, the former claim cannot be disregarded, as a moderate to very large proportion of MyHC2X-positive fibers are known to coexpress MyHC2A (2, 31, 60, 61). Indeed, it has been proposed that only 1 in 1,000 MyHC2X-positive fibers express MyHC2X only (2). This being said, the second claim regarding a low specificity of 2A-antibodies has been argued before and seems to be a particular issue when working with human muscle biopsies (61). This was actually what led us to utilize the rather untested EPR5280 antibody in the first place, after first encountering the double-staining dilemma using the SC-71 antibody. For the sake of the present study, we have chosen to refer to all double-stained 2A-2X fibers as 2X fibers, in essence meaning that they are 2X-positive fibers, regardless of whether they represent hybrid fibers or not.
For determination of myonuclei numbers, we first stained the sarcolemma using a dystrophin-specific antibody (ab15277), as described above, followed by hematoxylin staining (Ventana Medical Systems, Oro Valley, AZ). During subsequent analyses, myonuclei were defined as nuclei that were located to the intracellular side of the dystrophin layer (Fig. 2B) and were counted on a per muscle fiber basis. On average, 101 ± 23 muscle fibers were analyzed per subject, being in the range of 52–140, and ∼1.8 myonuclei were counted per muscle fiber, which is somewhat lower than reported by Nielsen et al. (46) (2.3–3.4 myonuclei per muscle fiber). This slight discrepancy could be related to the utilization of thinner tissue sections in our study (4 μm vs. 8 μm), which would naturally contain fewer nuclei. Myonuclei quantification was performed in a blinded fashion. For a representative image of dystrophin and nuclei staining, see Fig. 2B.
Gene expression.
Gene-specific primers for reference genes and target genes were designed as previously described (16, 17), using Primer3 Plus (68). For each gene, a minimum of three primer pairs were designed (16), with exception of the MyHC genes, for which a minimum of five primer pairs were designed (17). To avoid genomic contamination from affecting gene expression analyses, primer pairs were either located to span exon-exon boundaries containing genomic introns more than ∼1,000 nucleotides or to include at least one primer positioned directly across an exon-exon boundary, whenever possible. HPLC-purified primers were ordered from Thermo Scientific. All primer pairs were tested using the below described quantitative RT-PCR protocol, employing a primer concentration of 100 nM and an annealing temperature of 60°C. The primer pair showing the lowest Ct value and at the same time showing distinct melting curves were chosen. Primers sequences are given in Table 2.
Table 2.
Sequences of quantitative RT-PCR primers utilized for analysis of mRNA expression
| Gene | Primers for qRT-PCR | qRT-PCR Characteristics | ||||
|---|---|---|---|---|---|---|
| PPIA | F | GGTTTATGTGTCAGGGTGGTG | R | TCCCCATAGATGGACTTGC | E = 2.00 ± 0.06 | Ct = 24.8 ± 1.5 |
| β2-m | F | GAGGCTATCCAGCGTACTCC | R | TCCATTCTCTGCTGGATGAC | E = 2.00 ± 0.04 | Ct = 22.6 ± 1.5 |
| RPL32 | F | TTAAGCGTAACTGGCGGAAAC | R | GGCCCTTGAATCTTCTACGAAC | E = 1.98 ± 0.13 | Ct = 21.1 ± 1.6 |
| PolR2A | F | GGAGATCTTCACGGTGCTG | R | AGCCATCAAAGGAGATGACG | E = 1.97 ± 0.05 | Ct = 29.2 ± 1.3 |
| B-actin | F | ACCCCGTGCTGCTGAC | R | AACATGATCTGGGTCATCTTCTC | E = 1.96 ± 0.05 | Ct = 25.5 ± 1.5 |
| MyHC1 | F | AGGAGCTCACCTACCAGACG | R | TGCAGCTTGTCTACCAGGTC | E = 1.93 ± 0.03 | Ct = 21.3 ± 1.5 |
| MyHC2A | F | AACATGAGAGGCGAGTGAAG | R | GTGTTGGATTGTTCCTCAGC | E = 1.83 ± 0.04 | Ct = 23.5 ± 1.5 |
| MyHC2X | F | TGGTGGACAAACTGCAAGC | R | TTGTTCCTCCGCTTCTTCAG | E = 1.79 ± 0.05 | Ct = 24.9 ± 1.7 |
| PGC1α s4 | F | TGTGCCATATCTTCCAGTGACC | R | TGCAGTTCCAGAGAGTTCCAC | E = 1.97 ± 0.07 | Ct = 24.8 ± 2.7 |
| Ankrd2 | F | AGAAGCTGCCCATGGACTTG | R | TTGGCCCTTCACCTTCTGC | E = 1.91 ± 0.04 | Ct = 25.5 ± 2.1 |
| FGF6 | F | TGGTGAGTCTCTTTGGAGTGAG | R | TTGTTGGGCAGGAGGGTTTC | E = 1.99 ± 0.05 | Ct = 27.4 ± 1.5 |
| IGF1Ea | F | ATGCCCAAGACCCAGAAGG | R | CATCCTGTAGTTCTTGTTTCCTG | E = 1.98 ± 0.10 | Ct = 28.7 ± 2.1 |
| β-catenin | F | TGGCAACCAAGAAAGCAAGC | R | ACAGATAGCACCTTCAGCACTC | E = 1.93 ± 0.09 | Ct = 26.3 ± 2.6 |
| Myostatin | F | CCATGCCTACAGAGTCTGATTTTC | R | AGAAGCAACATTTGGGTTTTCC | E = 1.95 ± 0.09 | Ct = 30.1 ± 2.9 |
| Follistatin | F | GTGCACTCCTAAAGGCAAGATG | R | CCGACAAGTCTTTTTACATCTGC | E = 1.83 ± 0.06 | Ct = 34.5 ± 1.7 |
| PAX7 | F | AGCTGGAGAAGGCCTTTGAG | R | TACTGAACCAGACCTGCACAC | E = 1.97 ± 0.05 | Ct = 31.5 ± 1.5 |
| MCAD | F | AGCGTCATCTACAGCATCCAG | R | AAACGCTCTTAGCCTGAAGC | E = 1.97 ± 0.05 | Ct = 30.1 ± 1.4 |
| ITGA7 | F | GGCTTTCCAGATATTGCAGTGG | R | TGCTCCCATGGTAGATGAAGAC | E = 2.00 ± 0.05 | Ct = 27.0 ± 1.4 |
| Jagged-1 | F | ACATGTGGCCATTTCTGCTG | R | CAATCAGCGAGCTGTTTCCATC | E = 1.97 ± 0.05 | Ct = 30.2 ± 1.2 |
| CAV-1 | F | CCTAAACACCTCAACGATGACG | R | TCGTCACAGTGAAGGTGGTG | E = 1.97 ± 0.04 | Ct = 26.0 ± 1.4 |
| MYOG | F | AGCGAATGCAGCTCTCACAG | R | AGATGATCCCCTGGGTTGG | E = 1.97 ± 0.05 | Ct = 28.4 ± 1.2 |
| MYOD | F | ACGGCATGATGGACTACAGC | R | TTGTAGTAGGCGCCTTCGTAG | E = 1.90 ± 0.05 | Ct = 31.8 ± 1.5 |
| MRF4 | F | ATAACGGCTAAGGAAGGAGGAG | R | AGAAAGGCATCGAAGGCTACTC | E = 1.99 ± 0.04 | Ct = 27.1 ± 0.9 |
| MYF5 | F | AACTGCTCTGATGGCATGC | R | AGTACTGCTCTTTCTGGACCAG | E = 1.96 ± 0.08 | Ct = 31.6 ± 1.6 |
| SYND4 | F | TGAAGTTGTCCATCCCTTGGTG | R | TCAACGGGTGAGATTCTCTTGG | E = 1.95 ± 0.13 | Ct = 26.7 ± 3.0 |
| MuRF1/TRIM63 | F | TGTGCAGACCATCATCACTCAG | R | AACTTCTGGCTCAGCTCTTCC | E = 1.97 ± 0.04 | Ct = 25.9 ± 1.4 |
| MuRF2/TRIM55 | F | TGCCCATCCTGTAGACATGAAG | R | GCTGGTCGGATTTCTTTTCTGG | E = 1.98 ± 0.05 | Ct = 27.4 ± 1.1 |
| REDD1 | F | TGGACAGCAGCAACAGTGG | R | TCACTGAGCAGCTCGAAGTC | E = 1.93 ± 0.10 | Ct = 28.9 ± 2.9 |
| REDD2 | F | TTGCTGGACTGTGGCTATCAC | R | CAAGGACCTTTGAGCAACCAAG | E = 1.96 ± 0.10 | Ct = 26.5 ± 1.8 |
| SRF | F | CAGTGCAGGCCATTCAAGTG | R | AGTTGTGGGCACGGATGAC | E = 1.95 ± 0.09 | Ct = 28.0 ± 2.7 |
| Arkadia/RNF111 | F | GGCTATGGATCAAGCATGGTTG | R | GCTTGATGATGAAGTGGCCTTG | E = 1.84 ± 0.04 | Ct = 33.3 ± 1.5 |
| Atrogin-1/FBXO32 | F | CGATGTTACCCAAGGAAAGAGC | R | TCAGTGCCCTTCCAGGAAAG | E = 1.98 ± 0.13 | Ct = 25.3 ± 3.3 |
| cSKI | F | CGACGTGAAGGAGAAATTCGAC | R | CGGCTTGTCCTTTTCGGAAG | E = 1.95 ± 0.09 | Ct = 29.7 ± 2.9 |
| SnoN | F | GGGGCTTTGAATCAGCTAAATGG | R | TCCTGATGGTGCATCTGTCTTG | E = 1.89 ± 0.09 | Ct = 27.9 ± 2.8 |
| Titin | F | TGAGTTCCAGCAGCTTTATGGG | R | TAGGCGGAATTCCTCTTATGCC | E = 1.93 ± 0.05 | Ct = 20.6 ± 1.7 |
| LMNA | F | GAACATCTACAGTGAGGAGCTG | R | TCTCAAACTCACGCTGCTTC | E = 1.86 ± 0.04 | Ct = 30.9 ± 1.1 |
Values are expressed as means ± SD. Priming efficiencies (E) and cycle threshold (Ct) values are given in the two rightmost columns as an average of all quantitative RT-PCR reactions.
F, forward primer; R, reverse primer; PPIA, peptidylprolyl isomerase A; β2-m, b2-microglobulin; RPL32, ribosomal protein L32; PolR2A, polymerase (RNA) II (DNA directed) polypeptide A; MyHC1, 2A, and 2X, myosin heavy chain 1, 2A and 2X; PGC1α s4, peroxisome proliferator-activated receptor gamma, coactivator 1 alpha, splice variant 4; Ankrd2, ankyrin repeat domain 2; FGF6, fibroblast growth factor 6; IGF1Ea, insulin-like growth factor 1, splice Ea; PAX7, paired box 7; MCAD, M-cadherin; ITGA7, integrin, α7; CAV1, caveolin 1; MYOG, myogenin/myogenic factor 4; MYOD, myogenic differentiation 1/myogenic factor 3; MRF4/MYF6, muscle regulatory factor 4/myogenic factor 6; MYF5, myogenic factor 5; SYND4, syndecan 4; MuRF1/TRIM63, muscle-specific RING finger protein 1/tripartite motif containing 63, E3 ubiquitin protein ligase; MuRF2/TRIM55, muscle-specific RING finger protein 2/tripartite motif containing 55; REDD1 and 2, protein regulated in development and DNA damage response 1 and 2; SRF, serum response factor; RNF111, ring finger protein 111; FBXO32, F-box protein 32; cSKI, V-Ski avian sarcoma viral oncogene homolog; SnoN, SKI-like oncogene; LMNA, lamin A/C.
Total RNA was extracted from muscle biopsies using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA), as previously described (14). Care was taken to remove all remnants of RNAlater from biopsies. Two-hundred-and-fifty-picogram external reference gene (mw2060) was added per milligram muscle tissue prior to homogenization to allow validation of internal reference gene expression on a per milligram tissue basis, as developed in Ellefsen et al. (14). RNA quantities were obtained using Nanodrop (Thermo Scientific, Waltham, MA), whereupon reverse transcription was performed on 500 μg of total RNA using Superscript III reverse transcriptase (Invitrogen), primed with both random hexamers (Ambion, Life Technologies) and oligo d(T) (Ambion, Life Technologies), according to the manufacturer's protocol. For each sample, duplicate cDNA syntheses were performed.
Quantitative real-time PCR (qRT-PCR) was performed on 1/30 dilutions of cDNA using either PerfeCTa SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD) or SYBR Select Master Mix (Invitrogen) and the 7500 Fast Real-Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA). For PerfeCTa-based reactions, cycling consisted of an initial denaturation step at 94°C for 30 s, followed by 39 repeats of 94°C for 3 s and 60°C for 30 s. For SYBR Select Master Mix-based reaction, cycling consisted of initial UDG activation at 50°C for 2 min, followed by denaturation at 95°C for 2 min and 39 repeats of 94°C for 3 s and 60°C for 30 s. For each cDNA synthesis, one qRT-PCR reaction was performed for each gene, meaning that two qRT-PCR reactions were performed per muscle biopsy per gene. For each qRT-PCR reaction, cycle threshold (Ct) was calculated using the 7500 fast real-time PCR System software in an automated manner and priming efficiencies (E) were calculated using the LinRegPCR software (51, 55). For final calculations of target gene expression, average priming efficiencies were utilized and calculated separately for each primer pair. Average E and Ct values are given for each primer pair in Table 2. qRT-PCR analyses were performed in a blinded fashion.
Calculation of target gene expression was either performed using gene-family profiling (GeneFam; for MyHC1, 2A and 2X) (15, 17) or GeNorm (for the remainder of target genes) (69). GeNorm-based analyses were based on geometric evaluation of the expression of five frequently utilized reference genes: peptidylprolyl isomerase A (PPIA, cyclophilin A), β2-microglobulin (β2m), ribosomal protein L32 (RPL32), β-actin (β-a), and polymerase (RNA) II (DNA directed) polypeptide A (PolR2A). PPIA and RPL32 were evaluated to be the two most stable references genes, with M values below the 0.7 limit set by Vandesompele et al. (69) and were, therefore, utilized for final calculations of normalization factors. The stability of these genes were confirmed on a per-milligram tissue basis using mw2060 (data not shown), as was also done in Ellefsen et al. (16).
Hormone assessment.
Serum concentrations of s-HGH, cortisol (s-COR), sex hormone-binding globulin (s-SHBG), and androstendione (s-AND) were measured on an Immulite 2000 analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany), using kits from the Immulite Immunoassay Systems menu (Siemens Healthcare Diagnostics), performed according to manufacturer's protocols. Reference intervals were as follows: s-HGH (<24 mIU/l), s-COR (138–690 nmol/l, morning value), s-SHBG (18–144 nmol/l), and s-AND (1.0–11.5 nmol/l). Coefficients of variation for the analyses were s-HGH 6.5%, s-COR 6.8%, s-SHBG 4.0%, and s-AND 8.5%. Hormone analyses were performed in a blinded fashion.
Statistics.
Student's paired t-tests were performed to evaluate within-group effects of BFR and HST on 1RM, MyHC composition (immunohistochemistry and GeneFam-based), myonuclei per muscle fiber, and GeNorm-normalized mRNA expression. To adjust for multiple comparisons, Bonferroni corrections were performed for analyses of mRNA expression (α/3). Two-way ANOVA with Holm-Sidak post hoc tests were performed to evaluate effects of BFR and HST on CSA (of VL and QUAD) and serum hormone concentrations. Student's paired t-tests were performed to evaluate between-group effects of BFR and HST on all parameters. In these analyses, Bonferroni corrections were performed for CSA (α/2), serum hormone concentrations (α/3), and mRNA expression (α/3). Pearson correlation was utilized for correlation analyses. Correlation coefficients (r) were interpreted according to Hopkins et al. (25): r < 0.1 = trivial, 0.1–0.3 = small, 0.3–0.5 = moderate, 0.5–0.7 = large, 0.7–0.9 = very large, 0.9 = nearly perfect, and 1.0 = perfect.
For Pearson correlation analyses of MyHC composition and training-induced changes in 1RM and CSA, a special measure of MyHC was developed, wherein immunohistochemistry- and GeneFam-based data were combined, using the ranking principle presented in Vegge et al. (70) and Ellefsen et al. (16). First, individual proportions of MyHCs (1, 2A, or 2X) were determined using each of the two methods. Data in each of the two data sets were then ranked separately based on MyHC1, 2A, and 2X proportions. To ensure that the two data sets had compatible numerical values prior to calculation of mean values, values from individuals with lesser proportions of MyHC1, 2A, or 2X were divided by values from individuals with higher proportions of each MyHC variant (i.e., data were adjusted to the same scale, with 1.0 as the highest value). In effect, this set the individual with the highest MyHC-content to a MyHC quantity of 1.0, while all others were set to values between 0 and 1. Finally, we calculated mean values of MyHC proportions, using both immunohistochemistry- and GeneFam-data. In effect, this provided us with a combined measure of individually ranked MyHC proportions. Prior to statistical testing, these data were arcsine-transformed, as previously described. It is worth stressing that the outcome of these correlation analyses were not dependent on utilization of our developed combined measure of MyHC, meaning that similarly strong correlations were found using immunohistochemistry- and GeneFam-based data alone. However, in our hands the combined MyHC measure typically provides more consistent data, perhaps by taking into account volumetric variation in MyHC proportions (i.e., through the mRNA-based approach) or by eradicating some of the issues relating to the presence of hybrid fibers, which is a particular issue when quantifying 2X fibers (17).
To ensure normal distribution, data on serum hormone concentrations and GeNorm-normalized mRNA expression (10,000× factorized) were log2-transformed prior to statistical testing. Similarly, data on MyHC composition were square-root arcsine-transformed, which represents the recommended mode for transformation of proportional data between −1 and 1 (13). In general, data are presented as mean, mean ± SD, or individual values. MyHC data are presented as means ± 95% confidence intervals (CI). P < 0.05 was considered significant. Statistical calculations were performed using Excel (Microsoft, Redmond, WA), SigmaPlot 12.5 (Systat Software, San Jose, CA), or R (R Core Team, 50).
RESULTS
Effects of 12 wk of BFR and HST on 1RM and muscle CSA.
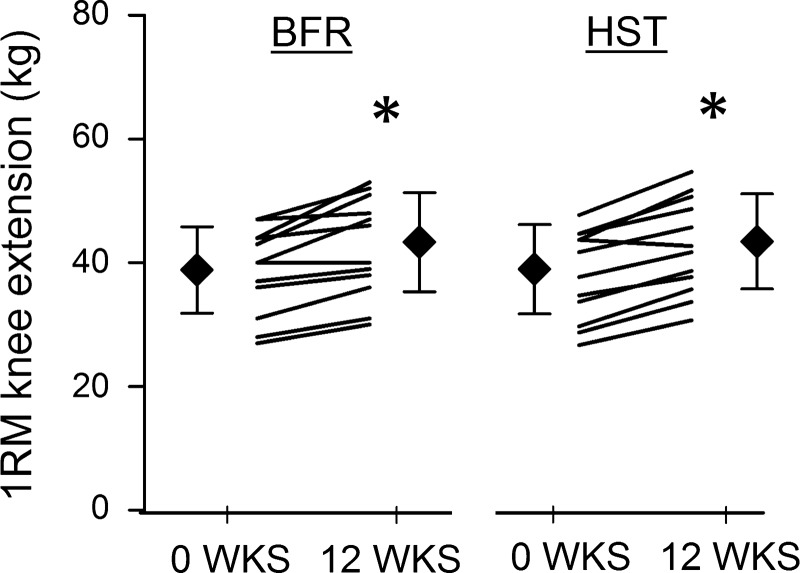
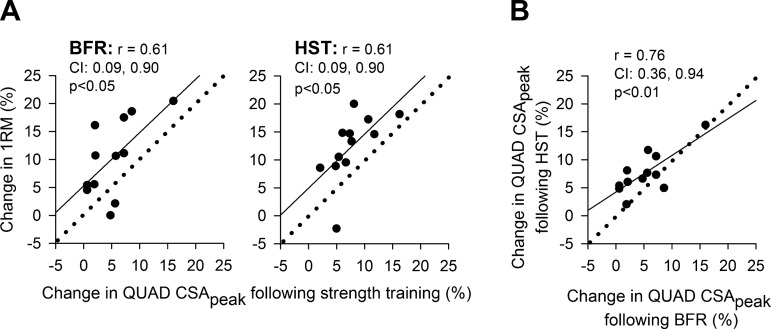
Twelve weeks of BFR resulted in 10 ± 7% increase in unilateral 1RM knee extension performance in previously untrained women (P < 0.05; Fig. 3). This was similar to the efficacy of HST, performed by the contralateral leg, which resulted in a 12 ± 6% increase in 1RM (P < 0.05; Fig. 3). In both legs, increases in 1RM were accompanied by increases in CSA of both distal and proximal halves of QUAD and VL (P < 0.05; Fig. 4A). In QUAD, increases in distal CSA was similar between BFR and HST legs (6 ± 4% vs. 7 ± 8%, respectively; Fig. 4A), whereas increases in proximal CSA was more pronounced in the HST leg (6 ± 4% vs. 9 ± 4%, P < 0.05; Fig. 4A). In VL, increases in distal and proximal CSA were similar between training protocols (8 ± 6% vs. 7 ± 5% and 7 ± 5% vs. 10 ± 5%, respectively; Fig. 4A). In QUAD, CSApeak was increased in both BFR and HST legs (5 ± 4% and 8 ± 4%, respectively; P < 0.05), with a statistical difference being detected between legs (P < 0.05), favoring HST. Changes in CSApeak of QUAD correlated largely with changes in 1RM in both BFR and HST legs (r = 0.61 and r = 0.61, P < 0.05; Fig. 5A). In addition, changes in CSApeak of QUAD in BFR and HST legs correlated very largely with each other (r = 0.76, P < 0.05; Fig. 5B).
Fig. 3.
The effect of 12-wk blood flow-restricted strength training (BFR, left) and heavy-load strength training (HST, right) on 1RM knee extension in previously untrained women (n = 12). Each individual performed the two protocols in a contralateral manner. Data are expressed as means ± SD (⧫ with error bars) and individual values (black lines). *Significant effects of HST and BFR on 1RM performance (P < 0.05). No difference was found between HST and BFR.
Fig. 5.
A: correlation between changes in peak cross-sectional area (CSApeak) of musculus quadriceps femoris (QUAD) and 1RM knee extension performance following 12 wk of BFR and HST in previously untrained women (n = 12). CSApeak was defined by the part of QUAD showing the largest CSA in postintervention images, assessed using magnetic resonance imaging. Data are individual values. Straight lines illustrate trend lines, while dotted lines indicate a perfect 1:1 slope. CI denotes the 95% confidence interval of Pearson r. B: correlation between changes in CSA in QUAD following 12 wk of HST and BFR.
Effects of 12 wk of BFR and HST on MyHC composition and myonuclei number.
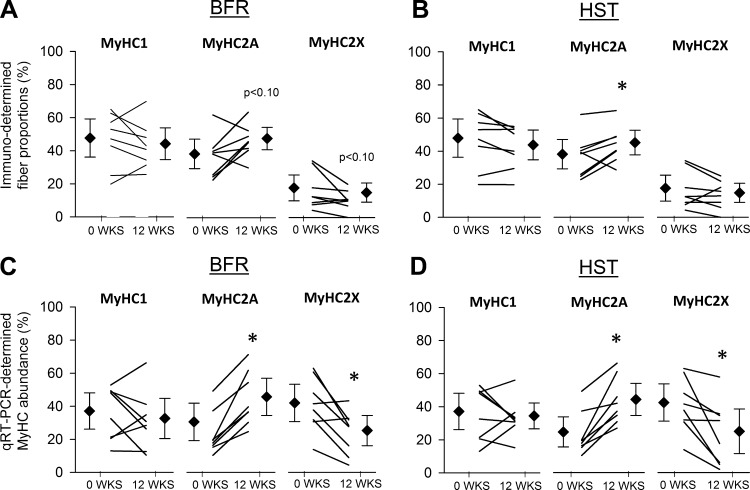
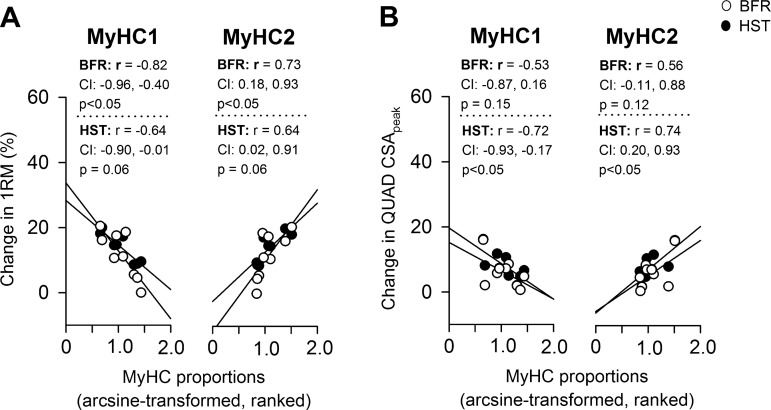
In muscle biopsies from VL, BFR and HST led to similar changes in MyHC composition. Whereas neither of the training protocols resulted in altered MyHC1 proportions, measured using either immunohistochemistry or GeneFam (Fig. 6), they generally resulted in increased levels of MyHC2A and decreased levels of MyHC2X (Fig. 6), with no differences being found between protocols. MyHC1/2A hybrid fibers were scarcely abundant and amounted to 1.0 ± 0.1% at wk0 and 0.9 ± 0.1% at wk12 (in both BFR- and HST-trained legs), with no statistically significant changes being found. Proportions of MyHC1 and MyHC2 at wk0 correlated largely to very largely with changes in 1RM knee extension (Fig. 7A) and CSA of QUAD, respectively (Fig. 7B). Whereas MyHC1 generally showed a large to very large negative correlation with training adaptations (Fig. 7), MyHC2 showed a large to very large positive correlation (Fig. 7). Neither BFR nor HST had effects on myonuclei numbers per muscle fiber (Fig. 8).
Fig. 6.
The effect of 12 wk of BFR (left) and HST (right) on immunohistochemistry (Immuno)-determined muscle fiber proportions (A and B) and qRT-PCR-determined myosin heavy chain (MyHC) composition (C and D) in musculus vastus lateralis of previously untrained women (n = 8). Data appear as proportions of overall My.HC expression. Data are back-transformed means (◇) with 95% confidence intervals and back-transformed individual values (black lines). *Significant effects of BFR and HST on MyHC proportions (P < 0.05). No difference was found between HST and BFR.
Fig. 7.
Correlation between baseline MyHC1 and MyHC2 proportions in musculus vastus lateralis and changes in 1RM performance (A) and CSApeak (B) in musculus quadriceps femoris (QUAD) following 12 wk of BFR (○) and HST (●) in previously untrained women (n = 9). Values are individual. MyHC proportions are presented as combined IMMUNO- and qPCR-data, calculated as described in methods. CI denotes the 95% confidence interval of Pearson r. Straight lines illustrate trend lines.
Fig. 8.
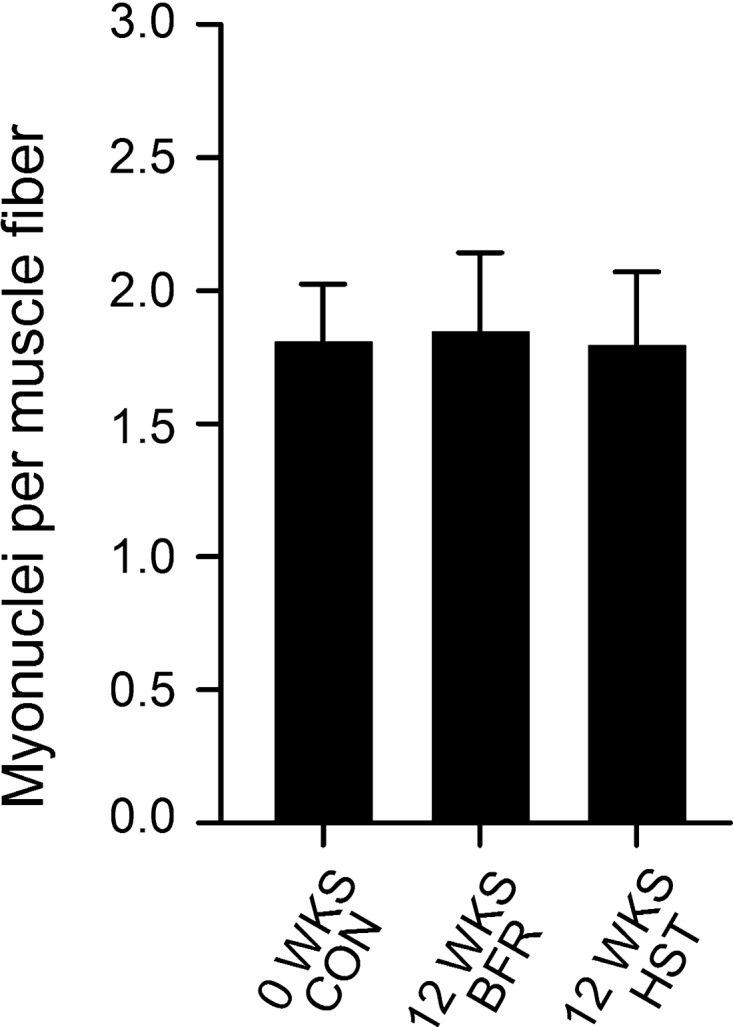
The effect of 12 wk of BFR and HST on myonuclei numbers, measured per muscle fiber, in musculus vastus lateralis of previously untrained women (n = 7). Data are expressed as means ± SD. No effect was found of HST or BFR. No difference was found between HST and BFR.
Effects of BFR and HST on serum hormone levels.
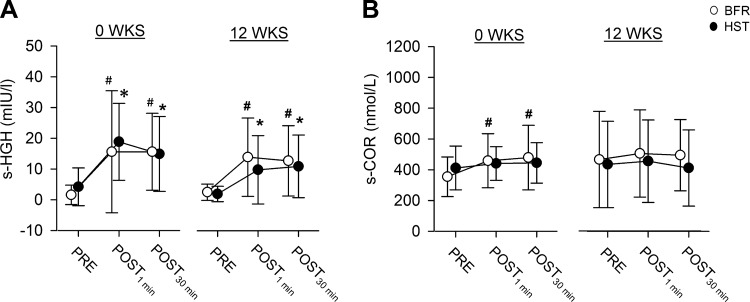
At wk0 and wk12, acute sessions of BFR and HST led to equally substantial elevations of s-HGH, both 1 min and 30 min after training (P < 0.05, Fig. 9A). In addition, at wk0, BFR led to slight elevations of s-COR at both 1 min and 30 min (P < 0.05, Fig. 9B), while at wk12, it led to slight elevation of s-SHBG at 1 min (P < 0.05, Table 3). Training had no effect on s-AND (Table 3). Overall, acute hormonal responses to exercise did not differ between training protocols. Notably, we also tried to quantify serum testosterone levels. Unfortunately, these data had to be excluded from subsequent analyses because only three individuals showed serum concentrations above the technical detection limit, apparently without showing signs of training-induced alterations in abundances.
Fig. 9.
Serum concentrations of human growth hormone (sHGH) (A) and cortisol (sCOR) (B) measured before (PRE), directly after (POST1min), and 30 min after (POST30 min) bouts of unilateral knee extension exercise of BFR (○) and HST (●) in previously untrained women (n = 7 at 0 WKS and n = 9 at 12 WKS). Each individual performed each of the two training protocols in a contralateral manner, with acute HST and BFR sessions being performed both before (week 0) and after (week 12) 12 wk of twice weekly HST and BFR, in each case on different days, separated by 4–6 days. mIU/l, milli-international units per liter. Data are expressed as means ± SD. #Significant changes caused by BFR (P < 0.05). *Significant changes caused by HST (P < 0.05). No difference was found between HST and BFR.
Table 3.
Acute effects of bouts of BFR and HST on serum concentrations of sex hormone binding globulin and androstenedione in previously untrained women (n = 9), measured 1 min and 30 min after training sessions
| BFR |
HST |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Week 0 |
Week 12 |
Week 0 |
Week 12 |
|||||||||
| PRE | POST 1 min | POST 30 min | PRE | POST 1 min | POST 30 min | PRE | POST 1 min | POST 30 min | PRE | POST 1 min | POST 30 min | |
| s-SHBG, nmol/l | 117 ± 107 | 117 ± 96 | 112 ± 90 | 110 ± 90 | 119 ± 96* | 112 ± 99 | 111 ± 78 | 106 ± 71 | 107 ± 77 | 108 ± 87 | 113 ± 93 | 107 ± 94 |
| s-AND, nmol/l | 10.7 ± 4.8 | 11.5 ± 7.3 | 12.2 ± 8.5 | 10.4 ± 4.8 | 11.2 ± 7.0 | 11.0 ± 5.7 | 13.4 ± 7.8 | 14.2 ± 9.6 | 12.6 ± 9.7 | 11.1 ± 6.4 | 12.7 ± 5.7 | 11.2 ± 7.9 |
Values are expressed as means ± SD. Each individual performed each of the two training protocols in a contralateral manner, with singular sessions of HST and BFR being performed both before (week 0; n = 7) and after (week 12; n = 9) 12 weeks of HST and BFR, in each case on different days, separated by 4–6 days.
s-SHBG, sex hormone binding globulin; s-AND, sex hormone binding androstenedione.
Signficant changes from baseline caused by BFR or HST (P < 0.05). No difference was found between HST and BFR.
Effects of BFR and HST on gene expression.
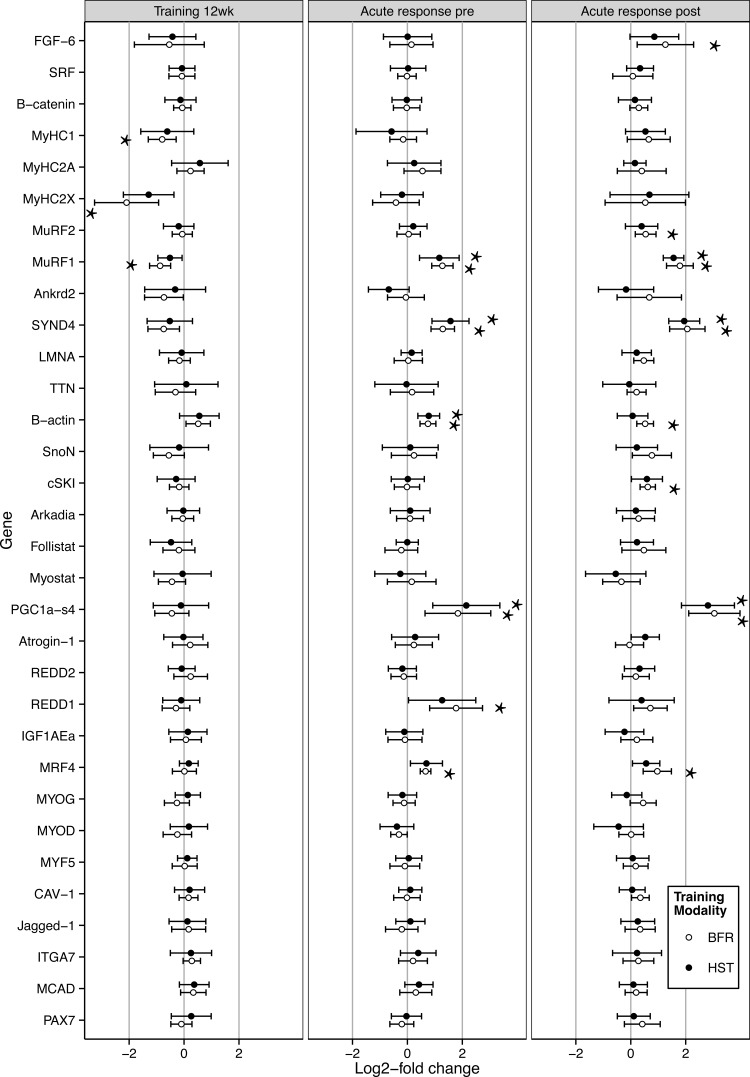
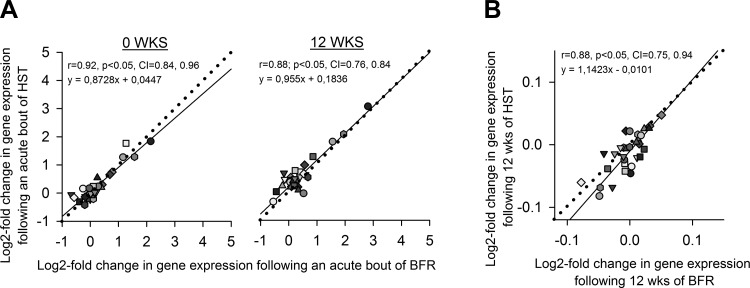
In VL biopsies, BFR and HST had similar effects on expression of the 29 genes involved in muscle function and plasticity investigated (Fig. 10), measured both acutely after training sessions at wk0 and wk12 and in the rested state at wk12. While 12 wk of training had no effect on markers for satellite cells (Fig. 10), training sessions led to marked acute increases in mRNA levels of well-known responder genes to strength training such as PGC1αs4 and MuRF1 (42, 54) at both wk0 and wk12 (P < 0.05, Fig. 10). Of particular interest, acute sessions of BFR and HST led to 1.6–4.2-fold increases in SYND4 expression (P < 0.05, Fig. 10), a gene whose responses to training has not previously been investigated. There were no differences in gene expression responses between the two protocols for any gene at any time point (Fig. 10). This similarity was particularly evident from the very large to nearly perfect correlation between gene expression responses, measured both acutely at wk0 and wk12 and in the rested state after 12 wk of training (Fig. 11). The relative sizes of responses to BFR and HST displayed an approximate 1:1 relationship, as evident from the trend lines of Fig. 11, exhibiting slopes of 0.87, 0.96, and 1.14.
Fig. 10.
Log2-fold changes in expression of 29 genes involved in muscle function and plasticity in musculus vastus lateralis of previously untrained women (n = 9) following 12 wk of BFR (○) and HST (●) (left), and 1 h after acute bouts of BFR and HST at 0 wk (Pre, middle), and 12 wk (Post, right). Each individual performed each training protocol in a contralateral manner. Values are expressed as means ± 95% CI. *Significant changes in expression caused by BFR or HST (P < 0.05). No differences were found between HST and BFR.
Fig. 11.
Correlation between log2-fold changes in gene expression in musculus vastus lateralis following acute bouts of BFR and HST (A), or 12 wk of HST and BFR (assessed as rested-state expression) in previously untrained women (n = 9) (B). Each individual performed each of the two training protocols in a contralateral manner, with sessions of HST and BFR being performed both before (week 0) and after (week 12) the strength training intervention, in each case on different days, separated by 4–6 days. One particular symbol represents the average GeNorm-normalized log2-fold change in expression of one particular gene (n = 32 genes). CI denotes the 95% confidence interval of Pearson r. Straight lines illustrate trend lines, while dotted lines indicate the perfect 1:1 slope. For detailed information on the expression of singular genes, see Fig. 10.
DISCUSSION
To our knowledge, this is the first study to show that BFR and HST result in similar alterations in muscle fiber composition and gene expression in humans, and the first to compare general responses to these two training modes in women using a within-subject design. The similarities in muscle fiber transitions were evident as increases in MyHC2A proportions and decreases in MyHC2X proportions following 12 wk of training, measured at both the cellular (i.e., protein) and the mRNA level. In addition, training had no effect on MyHC1 proportions. These observations fit nicely with previously observed effects of HST on muscle fiber composition (1, 17, 63). In contrast to our hypothesis, the two protocols had similar effects on expression of 29 genes involved in muscle function and plasticity, with no difference being evident between protocols for any gene at any time point. This similarity is underlined by the ∼1:1 relationship in gene expression responses to BFR and HST, measured both acutely after training sessions at wk0 and wk12 and as rested-state expression following 12 wk of training. Of particular interest, SYND-4 expression increased acutely after sessions of both protocols, providing the first indication of a role of this gene in cellular adaptations to training. Accompanying these similarities, BFR and HST resulted in similar changes in parameters such as 1RM, CSA of distal QUAD (but not proximal QUAD and CSApeak), and acute elevations of s-HGH, all of which support previous findings (30, 33, 34, 66), though discrepancies exist, particularly for s-HGH responses, for which BFR has been previously suggested to be particularly potent (48).
Strength training and muscle fiber composition.
The fact that BFR and HST were associated with similar 2X→2A shifts in muscle fiber composition in VL is of interest both in a performance and in a health perspective. This particular adaptation suggests improvements in muscle functionality and is especially beneficial for individuals with elevated MyHC2X levels, such as is observed in many patient groups (37, 43, 45, 64) and as a consequence of aging (9), for whom low-load strength training such as BFR may represent particularly adequate modes of training.
The 2X→2A shift suggests that the two protocols led to similar activation of 2X fibers. According to the size principle of motor unit recruitment (23), these fibers are the last to be recruited during strenuous exercise, and their activation typically triggers differentiation into aerobic 2A phenotypes (1, 17, 63). Fitting with such effective activation of type 2 fibers, BFR and HST affected 1RM and muscle CSA in a manner that was positively associated with increasing proportions of type 2 fibers, which fits generally accepted response patterns to traditional HST (26). While this similarity between BFR and HST may not be entirely surprising, as type 2 fiber-specific responses to BFR have been previously suggested (38), it can be viewed as a contradiction of recent indications of superior effects of BFR on type 1 fibers, as suggested by Nielsen et al. (46) and Cumming et al. (8). Unfortunately, we cannot conclude on this subject, as our paraffin-embedment protocol resulted in dehydration of muscle fibers, with slight and somewhat unevenly distributed changes in fiber sizes, precluding assessment of muscle fiber CSA. Importantly, however, muscle fibers remained intact, leaving other types of immunohistochemical analyses, such as fiber typing and myonuclei quantification, fully feasible.
Strength training, CSA, and myonuclei.
The observed increases in CSA of QUAD accumulated at rates averaging 0.07% and 0.09% per day in BFR and HST legs, respectively. This is slightly below the training progression generally seen in QUAD in response to periods of two to three weekly strength-training sessions, averaging 0.11% per day (74). Still, the efficiency is relatively high, given the low volume of both training protocols, with each subject performing one single exercise only and doing so in a one-limb-at-a-time manner, reducing the impact of factors such as anabolic hormones and mechanical stress on muscle biology compared with protocols that are more elaborate. The potency of each of the two training protocols were also evident from the marked effects of training on expression of genes such as PGC1αs4 and MuRF1.
Somewhat surprisingly, whereas BFR and HST resulted in similar increases in CSA of distal QUAD, HST resulted in superior increases in CSA of proximal QUAD and CSApeak. Interestingly, the proximal part of QUAD coincides with the location of the tourniquet during BFR, suggesting that the tourniquet itself may have had a negative impact on training adaptations. Indeed, studies in rabbits have shown that prolonged placing of an inflated tourniquet (125 mmHg) around the thigh per se results in decreased muscle strength (44) and potentially also decreased muscle fiber CSA (18). In humans, a similar finding was made by Daniels et al. (10), wherein placing a tourniquet around the thigh during anterior cruciate ligament surgery resulted in impaired postoperative recovery of QUAD strength and girth. The hypothesis that the inflated tourniquet itself exerts impairing effects on hypertrophic events during BFR is appealing, perhaps acting to impair hypertrophic events or even induce atrophic events. Indications of such a phenomenon was also found by Kazin and Strazar (27), who noted a tendency toward suppressed increases in CSA in QUAD following BFR at the location under the tourniquet. This impairment has several potential explanations: 1) it may be a direct result of the sheer pressure exerted by the inflated tourniquet itself, 2) it may result from the accompanying extraordinary metabolic/ischemic stress (resulting in “too much” stress), or 3) it may result from altered mechanics of the exercise. Further research is needed to explore this phenomenon and also to elucidate why the impairment was more pronounced in some muscle bellies than in others, being absent or at least less pronounced in VL.
It was also somewhat surprising that the increased CSA of QUAD/VL was not accompanied by increased myonuclei numbers per muscle fiber. Such myonuclei accretion is regarded as an important adaptation to strength training (29, 47), providing the cellular framework for increases in muscle fiber mass. However, there are also studies supporting our finding, and it seems that myonuclei accretion does not always occur after strength training (24, 47, 53). Instead, the increased muscle mass may be supported by increased sizes of myonuclei domains. This would, in essence, mean that strength training leads to better exploitation of available cellular resources, i.e., releasing the full potential of the myonuclei. The lack of increased myonuclei numbers after 12 wk of BFR or HST are supported by the lack of changes in the mRNA expression of satellite cell markers (both acutely and after 12 wk of training). This suggests that the absence of pronounced satellite cell activation, which, in turn, would blunt nuclei accretion, and is highly contradictive to the general finding after both BFR (46, 72) and HST (28). Obviously, this is a controversial interpretation and may well be an artifact of our assessment of satellite cells at the level of mRNA rather than at the level of proteins/cells. It is currently difficult to discern the feasibility of such an mRNA approach, although it has been suggested to be a viable alternative (21). Satellite cell activation needs to be further addressed using immunohistochemistry.
Strength training and gene expression.
Importantly, the lack of gene expression responses for many of the other genes investigated, such as the myogenic factors (MRFs), do not provide evidence for inadequacies of training protocols. The cyclic nature of gene expression regulation means that training-induced alterations in gene expression are only visible for a limited window of time. This is well exemplified by the up-regulation and down-regulation of MRFs (e.g., 49), representing a biphasic mode of regulation that was certainly also the case in the present study, although being left impossible to detect using the current one-biopsy-only posttraining sampling protocol. Such issues underline the vulnerability of gene expression studies to factors such as study design and individual variation in gene expression responses. Indeed, existing studies of acute gene expression responses to BFR serve as good examples of how such pitfalls complicate study conclusions. In two of the studies, opposite conclusions were reached on the effect of training on MuRF1 expression (12, 39). The underlying explanation behind these discrepancies may well have been related to differences in biopsy sampling timing, being performed at 3 h posttraining in Drummond et al. (12) and at 8 h in Manini et al. (39). Notably, the two studies also incorporated different nutritional strategies, which may also have affected gene expression. Overall, the suitability of the gene expression data presented in the current study is restricted to comparing responses between BFR and HST within the current protocol.
The similarities between gene expression responses to BFR and HST in VL, suggests similar activation of molecular pathways by the two modes of training, leaving behind similar transcriptional fingerprints. Indeed, this is supported by protein data from other studies, supporting roles for kinases such as mTORC1 (11, 22) and MAPK (19, 75) in responses to both training modalities. Interestingly, the close coherence between responses to BFR and HST should make the two training modes similarly vulnerable to confounding factors related to genetic, epigenetic, or nutritional traits, which are known to result in widespread interindividual variation in training responses (67). Indeed, in the present study, such a mutual reliance on genotypic and phenotypic traits was evident through the close relationship between individual training adaptations to BFR and HST, exemplified by the very large correlation between changes in CSApeak of QUAD, with between-subject variation being as high as 0% to 17%.
Of the 29 genes investigated in the present study, two observations deserve particular attention. First, this is the first study to show that SYND4 is an exercise-responsive gene, displaying robust increases in expression in response to both BFR and HST in human skeletal muscle. From previous studies, expression of SYND4 is known to be restricted to satellite cells (6), where it plays essential roles in controlling muscle regeneration (7). It displays increased expression following induced muscle damage (5), likely acting to facilitate signaling through fibroblast growth factors/hepatocyte growth factors (7). Mice lacking functional SYND4 proteins exhibit severely impaired capabilities of skeletal muscle regeneration (7), and SYND4 seems important for directional migration of satellite cells in skeletal muscle (59). The increased SYND4 expression in response to BFR and HST was, thus, likely to be restricted to satellite cells, perhaps increasing their sensitivity to hypertrophic agents and improving their mobility, setting the stage for proliferation and formation of novel myonuclei.
Second, the training-induced 2X→2A shift in muscle fiber proportions was evident using both mRNA-based GeneFam and immunohistochemistry. In contrast, GeNorm-normalization of mRNA data were not able to disclose this hallmark trait of strength training. This supports the applicability of GeneFam for such analyses. Indeed, large to very large correlations were found between GeneFam-based and immunohistochemistry-based fiber type proportions (r = 0.54 to 0.74, data not shown), fitting well with findings in our recent proof-of-principle publication (17).
The training intervention.
While BFR and HST protocols resulted in similar changes in functional and biological parameters in the present study, we cannot rule out the possibility that either of the protocols exerted contralateral effects; i.e., that training of one leg led to adaptations in the opposite leg through neurological or hormonal pathways (4). However, efforts were made to counteract such treatment interactions by including two features into the study flow: 1) subjects were set to perform familiarization sessions to both BFR and HST prior to testing and intervention upstart, and 2) subjects were not allowed to perform BFR and HST on the same day. The latter ensured complete washout of hormonal responses prior to the next session, which could have affected training outcomes (56). Indeed, in a study by Madarame et al. (36), supplementing light resistance training of one arm with BFR training of the legs markedly increased gains in arm muscle size. This effect was not evident in the untrained arm, suggesting that the interaction between the systemic responses elicited by BFR and light training of the arm required training of the arm to take place within the same training session.
Still, we cannot dismiss the possibility of treatment interactions. For example, previous studies have demonstrated that unilateral strength training protocols result in contralateral increases in muscle strength that may be related to neuronal adaptations (4). In the present data set, such neurological adaptations do not seem to have played a major role in the observed ergogenic effects, as there was a close correlation between increases in 1RM performance and increases in muscle CSApeak in both BFR and HST legs, suggesting a dominant role for increases in muscle mass in the strength gain.
Notably, in the current study, the BFR protocol was associated with higher training volumes than the HST protocol, leaving open the possibility of overestimation of the BFR efficacy (measured per training volume). However, in our view this seems unlikely, as high and low volumes of BFR were recently shown to result in similar increases in muscle strength and mass (41). In essence, this means that lowering the BFR training volume to match the HST volume would not have affected relative efficacies. Importantly, our rationale behind the choice of protocols was not based on performing equal amounts of work with both thighs, but on comparing two quite typical and proven protocols for each type of exercise (46, 73, 74).
Conclusion.
In the present study, we utilized a within-subject protocol to show that BFR and HST of knee extensors resulted in similar functional and biological adaptations in previously untrained women. Twelve weeks of BFR and HST was associated with similar increases in 1RM performance, similar increases in CSA of distal QUAD, similar muscle fiber transitions in the 2X→2A direction, and similar changes in gene expression. In contrast to our hypothesis, singular sessions of BFR and HST were associated with similar hormonal responses and similar changes in gene expression, with finding of no changes in myonuclei per muscle cell. Of particular interest, 1) responses to BFR and HST were greater in individuals with higher proportions of type 2 fibers, 2) SYND4 seems to be an exercise-inducible gene in skeletal muscle, and 3) muscle hypertrophy in proximal QUAD was impaired in the BFR leg, potentially being a passive effect of the presence of an inflated tourniquet during training.
Perspectives and Significance
The present intervention involved two sessions of BFR a week, representing a practical approach to BFR that can be implemented into any training and rehabilitation program. This may be of particular interest to individuals with limited tolerance to training with high loads, such as those suffering from rheumatoid diseases or similar. In the future, it will, thus, be important to explore the efficacy of BFR in other demographic groups, with particular focus on patient groups. This being said, it will also be interesting to explore the effects of BFR in athletes, e.g., investigating its effect on endurance performance, wherein HST has come to play an important role (57, 58, 71).
GRANTS
This study was supported by grant 150208 from the Innlandet Hospital Trust, Norway.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.E., T.A.S., E.Z., J.E.W., M.H., R.R., T.R., and B.R.R. conception and design of research; S.E., D.H., E.Z., J.E.W., I.R., H.N., G.V., M.H., M.W., K.T.C., R.R., T.R., and B.R.R. performed experiments; S.E., D.H., E.Z., J.E.W., I.R., H.N., G.V., M.H., M.W., K.T.C., T.R., and B.R.R. analyzed data; S.E., D.H., T.A.S., E.Z., J.E.W., I.R., H.N., G.V., M.W., K.T.C., T.R., and B.R.R. interpreted results of experiments; S.E., D.H., T.R., and B.R.R. prepared figures; S.E. drafted manuscript; S.E., D.H., T.A.S., E.Z., J.E.W., I.R., H.N., G.V., M.H., M.W., K.T.C., R.R., T.R., and B.R.R. edited and revised manuscript; S.E., D.H., T.A.S., E.Z., J.E.W., I.R., H.N., G.V., M.H., M.W., K.T.C., R.R., T.R., and B.R.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the students Mads Hansen and Sindre Habberstad for assistance during intervention follow-up and data sampling.
S. Ellefsen, D. Hammarström, T. A. Strand, E. Zacharoff, J. E. Whist, I. Rauk, H. Nygaard, G. Vegge, M. Hanestadhaugen, and B. R. Rønnestad are members of The Lillehammer Research Center for Medicine and Exercise Physiology.
REFERENCES
- 1.Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol 74: 911–915, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand 151: 135–142, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Blomstrand E, Ekblom B. The needle biopsy technique for fibre type determination in human skeletal muscle-a methodological study. Acta Physiol Scand 116: 437–442, 1982. [DOI] [PubMed] [Google Scholar]
- 4.Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol 101: 1514–1522, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci 117: 73–84, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cornelison DDW, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and Syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 239: 79–94, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cornelison DDW, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev 18: 2231–2236, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cumming KT, Paulsen G, Wernbom M, Ugelstad I, Raastad T. Acute response and subcellular movement of HSP27, αB-crystallin and HSP70 in human skeletal muscle after blood-flow-restricted low-load resistance exercise. Acta Physiol 211: 634–646, 2014. [DOI] [PubMed] [Google Scholar]
- 9.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel DM, Lumkong G, Stone ML, Pedowitz RA. Effects of tourniquet use in anterior cruciate ligament reconstruction. J Arthro Rel Surg 11: 307–311, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MJ, Fujita S, Takash A, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dytham C. Choosing and Using Statistics: A Biologist's Guide. New York: Wiley, 2011. [Google Scholar]
- 14.Ellefsen S, Stenslokken KO, Sandvik GK, Kristensen TA, Nilsson GE. Improved normalization of real time RT-PCR data using an external RNA control. Anal Biochem 376: 83–93, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Ellefsen S, Stensløkken KO. Gene-family profiling: a normalization-free real-time RT-PCR approach with increased physiological resolution. Physiol Genomics 42: 1–4, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Ellefsen S, Vikmoen O, Slettaløkken G, Whist JE, Nygaard H, Rauk I, Vegge G, Strand TA, Hollan I, Raastad T, Rønnestad BR. Irisin and FNDC5—effects of 12 wks strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur J Appl Physiol 114: 1875–1888, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Ellefsen S, Vikmoen O, Zacharoff E, Rauk I, Slettaløkken G, Strand TA, Whist JE, Hanestadhaugen M, Vegge G, Fagernes CE, Nygaard H, Hollan I, Rønnestad BR. Reliable determination of training-induced alterations in muscle fibre composition in human skeletal muscle using qPCR. Scand J Med Sci Sports 24: e332–e342, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Fridén J, Pedowitz RA, Thornell LE. Sensitivity of different types of fibres in rabbit skeletal muscle to pneumatic compression by tourniquet and to ischaemia. Scand J Plast Reconstr Hand Surg 28: 87–94, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 108: 1199–1209, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4: e5205, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306: E1198–E1204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965. [DOI] [PubMed] [Google Scholar]
- 24.Hikida RS, Staron RS, Hagerman FC, Walsh S, Kaiser E, Shell S, Hervey S. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol Ser A Biol Sci Med Sci 55: B347–B354, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41: 3–13, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Häkkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Häkkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53: B415–B423, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports 21: e231–e241, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113: 99–103, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kim E, Gregg LD, Kim D, Sherk VD, Bemben MG, Bemben DA. Hormone responses to an acute bout of low intensity blood flow-restricted resistance exercise in college-aged females. J Sports Sci Med 13: 91–96, 2014. [PMC free article] [PubMed] [Google Scholar]
- 31.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44: 2077–2083, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M, Aihara AY, Fernandes AdRC, Tricoli V. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44: 406–412, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Loenneke J, Wilson J, Marín P, Zourdos M, Bemben M. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112: 1849–1859, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 78: 151–154. [DOI] [PubMed] [Google Scholar]
- 36.Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc 40: 258–263, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Man WDC, Kemp P, Moxham J, Polkey MI. Skeletal muscle dysfunction in COPD: clinical and laboratory observations. Clin Sci 117: 251–264, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev 37: 78–85, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol 201: 255–263, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manini TM, Yarrow JF, Buford TW, Clark BC, Conover CF, Borst SE. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res 22: 167–172, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín-Hernández J, Marín PJ, Menéndez H, Ferrero C, Loenneke JP, Herrero AJ. Muscular adaptations after two different volumes of blood flow-restricted training. Scand J Med Sci Sports 23: e114–e120, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294: E43–E51, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with Type 2 diabetes. Diabetes 56: 1592–1599, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Mohler LR, Pedowitz RA, Lopez MA, Gershuni DH. Effects of tourniquet compression on neuromuscular function. Clin Orthop Relat Res 359: 213–220, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Molsted S, Eidemak I, Sorensen HT, Kristensen JH, Harrison A, Andersen JL. Myosin heavy-chain isoform distribution, fibre-type composition and fibre size in skeletal muscle of patients on haemodialysis. Scand J Urol Nephrol 41: 539–545, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen JL, Aagaard P, Bech RD, Nygaard T, Hvid LG, Wernbom M, Suetta C, Frandsen U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 590: 4351–4361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrella JK, Kim Js Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Pope ZK, Willardson JM, Schoenfeld BJ. Exercise and blood flow restriction. J Strength Cond Res 27: 2914–2926, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol 95: 1038–1044, 2003. [DOI] [PubMed] [Google Scholar]
- 50.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2014 (http://www.R-project.org/).
- 51.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Ratamess NA, Alvar BA, Evetoch TE, Housh TJ, Ben Kibler W, Kraemer WJ, Triplett NT. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol Ser A Biol Sci Med Sci 56: B240–B247, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Ruas Jorge L, White James P, Rao Rajesh R, Kleiner S, Brannan Kevin T, Harrison Brooke C, Greene Nicholas P, Wu J, Estall Jennifer L, Irving Brian A, Lanza Ian R, Rasbach Kyle A, Okutsu M, Nair KS, Yan Z, Leinwand Leslie A, Spiegelman BM. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151: 1319–1331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: 12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rønnestad B, Nygaard H, Raastad T. Physiological elevation of endogenous hormones results in superior strength training adaptation. Eur J Appl Physiol 111: 2249–2259, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Rønnestad BR, Hansen EA, Raastad T. Strength training improves 5-min all-out performance following 185 min of cycling. Scand J Med Sci Sports 21: 250–259, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Rønnestad BR, Hansen J, Hollan I, Ellefsen S. Strength training improves performance and pedaling characteristics in elite cyclists. Scand J Med Sci Sports 25: e89–e98, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Shin J, McFarland D, Velleman S. Migration of turkey muscle satellite cells is enhanced by the syndecan-4 cytoplasmic domain through the activation of RhoA. Mol Cell Biochem 375: 115–130, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Smerdu V, Eržen I. Dynamic nature of fibre-type specific expression of myosin heavy chain transcripts in 14 different human skeletal muscles. J Muscle Res Cell Motil 22: 647–655, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Smerdu V, Soukup T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52: 179–190, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76: 1247–1255, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990. [DOI] [PubMed] [Google Scholar]
- 65.Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 88: 61–65, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88: 2097–2106, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol 110: 846–853, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–W74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vegge G, Rønnestad B, Ellefsen S. Improved cycling performance with ingestion of hydrolyzed marine protein depends on performance level. J Int Soc Sports Nutr 9: 14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vikmoen O, Ellefsen S, Trøen Ø, Hollan I, Hanestadhaugen M, Raastad T, Rønnestad BR. Strength training improves cycling performance, fractional utilization of V̇o2 max and cycling economy in female cyclists. Scan J Med Sci Sports In press. [DOI] [PubMed] [Google Scholar]
- 72.Wernbom M, Apro W, Paulsen G, Nilsen TS, Blomstrand E, Raastad T. Acute low-load resistance exercise with and without blood flow restriction increased protein signalling and number of satellite cells in human skeletal muscle. Eur J Appl Physiol 113: 2953–2965, 2013. [DOI] [PubMed] [Google Scholar]
- 73.Wernbom M, Augustsson J, Raastad T. Ischemic strength training: a low-load alternative to heavy resistance exercise? Scand J Med Sci Sports 18: 401–416, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 37: 225–264, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547: 977–987, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]