Abstract
The present study tested whether primary cilia on macula densa serve as a flow sensor to enhance nitric oxide synthase 1 (NOS1) activity and inhibit tubuloglomerular feedback (TGF). Isolated perfused macula densa was loaded with calcein red and 4,5-diaminofluorescein diacetate to monitor cell volume and nitric oxide (NO) generation. An increase in tubular flow rate from 0 to 40 nl/min enhanced NO production by 40.0 ± 1.2%. The flow-induced NO generation was blocked by an inhibitor of NOS1 but not by inhibition of the Na/K/2Cl cotransporter or the removal of electrolytes from the perfusate. NO generation increased from 174.8 ± 21 to 276.1 ± 24 units/min in cultured MMDD1 cells when shear stress was increased from 0.5 to 5.0 dynes/cm2. The shear stress-induced NO generation was abolished in MMDD1 cells in which the cilia were disrupted using a siRNA to ift88. Increasing the NaCl concentration of the tubular perfusate from 10 to 80 mM NaCl in the isolated perfused juxtaglomerular preparation reduced the diameter of the afferent arteriole by 3.8 ± 0.1 μm. This response was significantly blunted to 2.5 ± 0.2 μm when dextran was added to the perfusate to increase the viscosity and shear stress. Inhibition of NOS1 blocked the effect of dextran on TGF response. In vitro, the effects of raising perfusate viscosity with dextran on tubular hydraulic pressure were minimized by reducing the outflow resistance to avoid stretching of tubular cells. These results suggest that shear stress stimulates primary cilia on the macula densa to enhance NO generation and inhibit TGF responsiveness.
Keywords: macula densa, primary cilia, nitric oxide, tubuloglomerular feedback
the macula densa is a group of specialized epithelial cells located at the distal segment of the thick ascending limb (TAL) that serves as a sensor of luminal NaCl concentration. Increased NaCl delivery to the macula densa promotes the release of adenosine or ATP, which constricts the afferent arteriole (Af-Art) and decreases single nephron glomerular filtration rate (SNGFR) via a process known as tubuloglomerular feedback (TGF) (2, 37, 57). TGF is a negative feedback mechanism to prevent fluctuations in GFR and flow in the proximal tubule to deliver excess NaCl to the distal nephron and overwhelm its transport capacity.
Following acute volume expansion or the ingestion of a sodium load, increases in GFR facilitate the rapid elimination of salt load (29, 40). Baroreflex suppression of renal sympathetic tone and the renin-angiotensin system also contribute to the increase in sodium excretion by inhibiting proximal tubular reabsorption (6, 22). All of these factors result in a large increase in flow to the macula densa, which should trigger a TGF-mediated decrease in GFR that opposes the elimination of the sodium load. However, this decrease in GFR does not occur, partially because of inhibition of TGF responsiveness. Modulation of TGF response following volume expansion should be important in maintaining extracellular sodium and volume balance (42, 52, 53). Indeed, GFR typically increases by 20–30% in humans (4) and in dogs (10) during the postprandial period. Daily salt excretion peaks during the postprandial increase in GFR (21). In these situations, especially following ingestion of a salt load, reduced TGF responsiveness permits high distal NaCl delivery, which could facilitate the excretion of NaCl. The mechanisms for this TGF modulation have not been clarified.
Normal luminal flow rate in the distal tubule is about 2–7 nl/min, but it can be temporally reduced to 0 nl/min in salt and volume depletion, and it increases to 25–37 nl/min following acute volume expansion (11, 20, 43). In physiological conditions, increase of tubular flow to the macula densa activates TGF by increasing NaCl delivery (44). However, it also increases shear stress, stretch, and transmural pressure (54, 59); therefore, it possibly activates mechanosensors and other pathways that might modulate TGF responsiveness. The modulation of TGF responsiveness may depend, in part, on increased nitric oxide (NO) production (41, 48, 58), but the source and mechanism for the increase in NO are not known. Primary cilia extend from the surface of many eukaryotic cells (8, 36, 61). While the function of the primary cilia on most cells has remained elusive, they are known to serve as mechanosensors in the mammalian kidney and vascular endothelial cells (8, 36, 61). Primary cilia have been found on the macula densa cells of humans, rats, rabbits, and dogs (17, 33, 47, 50, 56). Little is known about their function (47). The present study examined the hypothesis that primary cilia on macula densa cells serve as a flow sensor to activate NOS1 activity and enhance NO generation, which inhibits TGF responsiveness.
METHODS
All procedures and experiments using rabbits were approved by the Institutional Animal Care and Use Committees at the University of South Florida College of Medicine and University of Mississippi Medical Center.
Isolation and microperfusion of the rabbit Af-art with attached macula densa.
Af-Art with attached macula densa was isolated and perfused, as we previously described (27, 49). Briefly, young male New Zealand White rabbits (1.5 to 2.0 kg) were anesthetized with pentobarbital sodium (40 mg/kg iv) and then injected with heparin (500 U iv). The kidneys were removed and sliced along the corticomedullary axis. Slices were placed in ice-cold MEM (Gibco, Grand Island, NY) containing 5% BSA and dissected under a stereomicroscope (SMZ 1500; Nikon). From each rabbit, a single superficial Af-Art and its intact glomerulus were microdissected together with adherent tubular segments consisting of portions of the TAL of the loop of Henle, macula densa, and the early distal tubule. Microdissection of each juxtaglomerular apparatus (JGA) was completed within 30 min at 8°C. The preparation was then transferred into a temperature-regulated perfusion chamber mounted on an inverted microscope (Eclipse TI; Nikon) equipped with Hoffmann modulation, digital charge-coupled device (CCD) camera (CoolSnap; Photometrics), xenon arc lamp (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10-3; Shutter Instruments). The perfusion bath was gradually warmed to 37°C and perfused with MEM at 1 ml/min.
Both the Af-Art and the end of either the distal tubule or TAL were cannulated with an array of glass pipettes. The Af-Art was perfused with MEM, and intraluminal pressure was maintained at 60 mmHg throughout the experiment. The tubule was perfused with a solution, which is composed of (in mM) 10 HEPES; 1.0 CaCO3, 0.5 K2HPO4, 4.0 KHCO3, 1.2 MgSO4, 5.5 glucose, 0.5 Na acetate, 0.5 Na lactate, l-arginine 0.5, and either 80 or 10 NaCl; pH was adjusted to 7.4. The tubule was perfused at 20 nl/min with a microperfusion pump, unless otherwise indicated. A 30-min equilibration period was allowed before taking any measurements.
In some experiments, the viscosity of the tubular perfusate was increased by adding a high molecular weight dextran (MW: 200,000; MP Biomedicals, Solon, OH) to a perfusate containing a high-concentration (80 mM) NaCl solution (28, 51). Viscosity was measured at 37°C with a glass capillary viscometer (Cannon Instrument, State College, PA). Osmolalities of the solutions without dextran were adjusted with mannitol to achieve the same osmolality as the solution with dextran.
In other experiments to determine the role of NOS1 in mediating the effects of shear stress on NO generation or TGF responses, we measured NO or TGF responses before and after the addition of a selective NOS1 inhibitor 7-nitroindazole (7-NI) (10−5 M) for 15 min to the tubular perfusate of isolated perfused JGA.
Measurement of cell volume and NO in isolated perfused macula densa.
Cell volume and NO production in macula densa cells were measured simultaneously using cell-permeant fluorescent volume indicator calcein red-AM and NO indicator 4, 5-diaminofluorescein diacetate (DAF-2 DA), as described previously with modifications (24, 26). Briefly, once the TAL was perfused, the macula densa was loaded with 10 μM DAF-2 DA from the lumen for 30 min and then washed with 10 mM NaCl for 10 min at 37°C. Next, the macula densa was loaded with 2 μM calcein-red-AM from the lumen for 10 min and washed with 10 mM NaCl for 10 min at 37°C. The luminal perfusate was then switched to 80 mM NaCl solution for 15 min and stopped for 5 min for measurement as basal. Fluorescent images of macula densa were recorded for 5 min in the absence of tubular flow to serve as the baseline control. Then, the images were collected again for 5 min when the tubule was perfused at 40 nl/min with an 80 mM NaCl solution. Emission of DAF-2 and calcein-red from macula densa were sampled at 0.2 Hz by exciting DAF-2 at 490 nm and calcein-red at 560 nm sequentially. Emissions were captured with a digital CCD camera through two band-pass filters centered at 530 nm and 610 nm, respectively. Collected images were displayed and analyzed with NIS-Elements imaging software (Nikon). Regions of interest (ROIs) were defined within the cytoplasm of macula densa cells. The mean intensity of each ROI from both spectral windows was measured for 5 min in each experimental period retrospectively. Relative changes in DAF-2 and calcein-red fluorescence intensity were calculated by percentage changes from basal fluorescence intensity. NO concentration was expressed as changes in relative DAF-2 intensity after correcting for the changes of cell volume (calcein-red emission) using the following equation: NO = [(F2 − F1)·100/F1]·V2; where F1 is DAF-2 intensity without flow and F2 is DAF-2 intensity with flow. V2 is the relative volume with flow measured with calcein-red.
Measurement of intracellular pH in isolated perfused macula densa.
Macula densa intracellular pH (pHi) was measured ratiometrically after the tubules were perfused with 5 μM 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)-AM for 15 min, as we described previously (23, 24). The luminal perfusate was switched to 80 mM NaCl solution for 15 min to wash out the loading solution and then stopped for 5 min for measurement of macula densa pHi under a no-flow condition. After that, the luminal perfusate was switched to 40 nl/min, and pHi was measured again. BCECF was excited alternatively at 440 nm and 490 nm, and emission was collected at 540 nm. The emission ratio was calibrated at the end of each experiment. pHi was monitored with the same protocol of changing tubular flows as described in the measurement of NO.
Identification of primary cilia on macula densa cells in the isolated perfused JGA and MMDD1 cells.
We detected primary cilia on macula densa with immunofluorescence in the isolated perfused rabbit JGA and on MMDD1 cells. Briefly, the JGA was mounted, and the TAL was cannulated and perfused for 30 min. Then the macula densa was fixed by adding 4% paraformaldehyde in PBS to both bath and lumen for 30 min and washed for 10 min by adding Tris-buffered saline Tween-20. The sample was permeabilized and blocked with 0.1% Triton X-100 and 3% BSA for 60 min. Cilia were immunolocalized by perfusing a primary antibody against acetylated tubulin for 60 min (Santa Cruz 6-11B-1; 1:800), and then an Alexa Fluor 488-conjugated goat anti-mouse IgG secondary antibody for 30 min (Invitrogen; 1:400). Cilia on the macula densa were visualized with fluorescence microscopy. The same protocol was used to immunolocalize the cilia on MMDD1 cells. Negative control was obtained by omitting the primary antibody.
Cell culture and NO measurement in MMDD1 cells.
Experiments were also performed using MMDD1 cells, a macula densa-like cell line (kindly provided by Dr. J. Schnermann, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) (5, 13, 60). Similar to the protocols that were described previously (12, 63, 64), MMDD1 cells at passages 20–25 were cultured on sterilized coverslips in DMEM nutrient mixture-Ham's F-12 (DMEM/F12, supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) and incubated in a humidified atmosphere of 95% room air and 5% CO2 at 37°C. After the cells reached 70–80% confluence, they were loaded with 10 μM DAF-2 DA for 30 min in MEM. Then, the coverslips were transferred to a glass chamber to be mounted on the stage of an inverted microscope, and temperature was maintained at 37°C. The chamber was perfused with MEM that was continuously exchanged at a rate of 1 ml/min. DAF-2 was excited at 490 nm, and emission was collected at 510–550 nm. ROIs were defined within the cytoplasm of the cells, and mean intensity of ROIs was sampled at 0.2 Hz with background correction. The rate of increase in fluorescence intensity was calculated (increase of units/min). After a 20-min equilibration period, NO was measured for 5 min at shear stress of 0.5 dynes/cm2. Then shear stress was increased to 5.0 dynes/cm2 for 10 min, and the change in the rate of rise of NO fluorescence was measured again for 5 min. Timed control experiments were performed using the same protocol without changing the shear stress.
Effect of shear stress on NO generation in MMDD1 cells.
A circular flow chamber system (Glyco Tech, Gaithersburg, MD) was used to quantitatively increase shear stress, and NO generation in MMDD1 cells was measured. MMDD1 cells were subcultured on coverslips for 24 h. The coverslips were sealed in the flow chamber, which was connected with a pump. Shear stress was adjusted by changing the flow rate in the chamber.
Preparation of small interfering RNA (siRNA).
Primary cilia function was disrupted by knocking down the expression of the ift88 gene with a siRNA. MMDD1 cells were transferred onto six-well plates (about 5 × 105 cells per well) and incubated for 24 h before transfection. A siRNA targeting ift88 (sequence: 5′-AAAG GUCG UCCU CAUC UGUU UCUG G-3′) or a scrambled control siRNA (Invitrogen) was transfected into cells using XtremeGene (Roche Molecular Systems, Alameda, CA), according to the manufacturer's instructions. The final concentration of siRNAs in the bath was 30 nM. The medium was changed 24 h after transfection, and the flow experiments were performed at 48 h. The effectiveness of the siRNA was confirmed by measuring the expression of ift88 mRNA using real-time PCR and by immunofluorescence imaging of primary cilia.
Real-time PCR measurement.
RNA was extracted from MMDD1 cells using TRIzol reagent (Life Technologies, Carlsbad, CA), and then mRNA was prepared using Pure-Link RNA mini kit (Life Technologies). All RNA preparations were treated with DNase 1 (Life Technologies). After quantification of the RNA concentrations, the samples were reverse-transcribed with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR analysis was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and CFX96 real-time detection system (Bio-Rad). The real-time PCR reactions contained 1 μl of the RT reaction and 0.1 μM of the forward and reverse primers in 25-μl volume. The cycling conditions were 1 cycle at 95°C for 3 min, followed by 40 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The relative expression of the target genes were compared using the delta CT method. Following were the primers used to amplify the target genes: ift88 forward (F) 5′-AGGC ACCA TCCA TCAC CTT-3′ and reverse (R) 5′-TGGA AAAG TGCT TGCC TTGC-3′; NOS1: F 5′-ATGA AGTG ACCA ACCG CCTT-3′ and R 5′-CGTG TGTG TCCC CGTT TAGT-3′. β-actin was used as a housekeeping gene.
Statistical analysis.
Mean values ± SE are presented. The significance of differences in mean values between and within groups were determined by ANOVA for repeated measures and a post hoc Fisher LSD test or a paired t-test where appropriate. A P < 0.05 was considered to be statistically significant.
RESULTS
Tubular flow enhances NO generation by the macula densa.
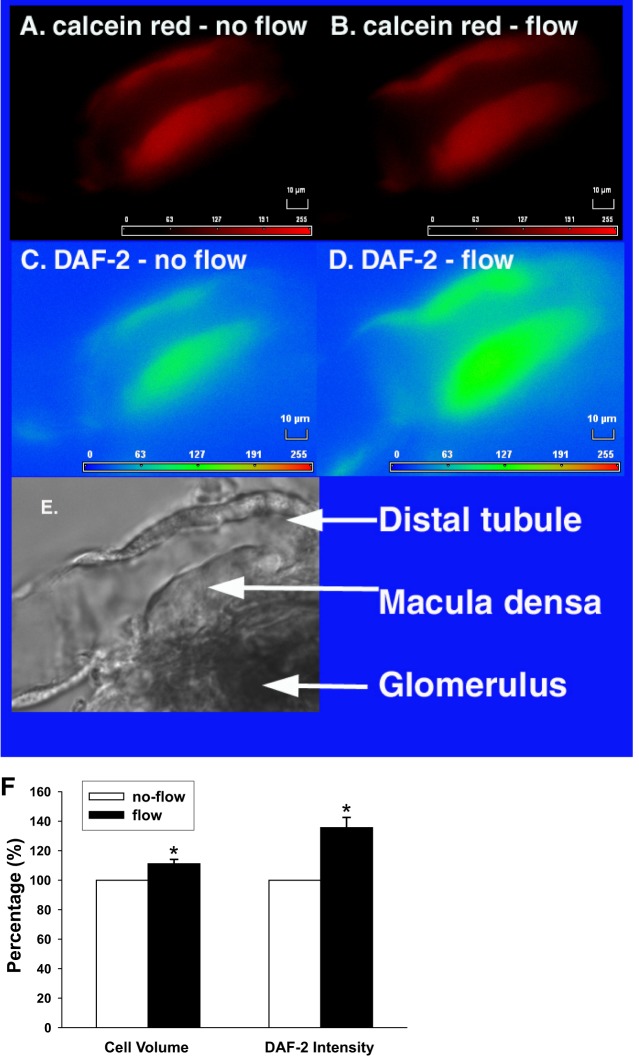
We first examined whether tubular flow enhances NO generation in the macula densa. To correct the volume effect on 4,5-diaminofluorescein (DAF-2) emission, calcein red was used as a cell volume marker. When tubular flow was increased from 0 to 40 nl/min, the calcein red intensity decreased from 428.1 ± 4.2 to 380.0 ± 4.1 arbitrary units (AU), which was an 11.3 ± 0.3% increase in cell volume. In contrast, the DAF-2 signal increased from 124.2 ± 0.8 to 168.7 ± 1.3 AU at the same time, which indicated a 35.9 ± 0.7% increase (Fig. 1). After correcting for the change in cell volume, NO generation increased by 40.0 ± 1.2% (P < 0.05 vs. no flow; n = 7; Fig. 2), indicating tubular flow enhanced NO generation.
Fig. 1.
Effect of tubular flow on cell volume and nitric oxide (NO) generation by the macula densa. The macula densa was loaded with both calcein red AM and 4, 5-diaminofluorescein diacetate (DAF-2 DA). When tubular flow was increased from 0 to 40 nl/min, cell volume increased by 11.3 ± 0.3%, indicated by the decreases of calcein red intensity (A and B). DAF-2 intensity increased by 35.9 ± 0.7% (C and D). E: image of light microscopy showing anatomy structure of the same perfused JGA. F: average data (*P < 0.05 vs. no flow; n = 7).
Fig. 2.
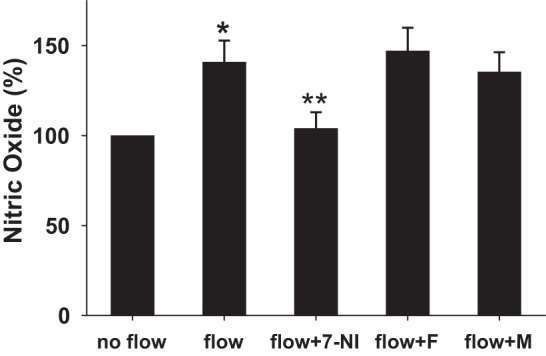
Roles of NOS1 and NKCC2 on flow-induced NO production. The flow-induced NO generation by the macula densa increased by 40.0 ± 1.2% (*P < 0.05 vs. no flow; n = 7). A selective NOS1 inhibitor 7-NI (10−5 M) inhibited flow-induced NO generation (**P < 0.01 vs. flow; n = 5). A NKCC2 inhibitor furosemide (F, 10−4 M) had no effect on flow-induced NO generation (n = 6). When the electrolytes in tubular perfusate were replaced with mannitol (M), there was no significant difference between the tubular flow-induced NO and the control (n = 4).
Flow-induced NO production is dependent on NOS1 but not NKCC2.
7-NI was used to determine whether flow-induced NO production is mediated by NOS1 in the macula densa. 7-NI (10−5 M) was added to the tubular perfusate for 15 min. Then tubular flow was switched to 0 nl/min for 5 min, and the baseline NO signal was determined. When tubular flow was increased to 40 nl/min, NO generation only increased by 4.3 ± 9% (n = 5; Fig. 2) after the tubule was treated with 7-NI.
Previously, we and others found that increases in NaCl concentration of the perfusate could enhance NO generation in the macula densa (19, 26) by increasing intracellular pH (23). To determine whether the present flow-induced increase in NO production requires NKCC2 activity, furosemide (10−4 M) was added to the perfusate, as described above. When tubular flow rate was increased from 0 to 40 nl/min, furosemide had no effect on the flow-induced NO generation, which increased by 47.4 ± 13% (P < 0.05 vs. no flow; n = 6; Fig. 2).
To test whether the increase of tubular flow and not NaCl concentration reaching the macula densa per se is sufficient to induce NO production, all electrolytes in the luminal perfusate were removed and replaced with mannitol to maintain the osmolality of the perfusate. When tubular flow rate was increased from 0 to 40 nl/min using a Na+-free perfusate, NO generation still increased by 35.3 ± 11% (P < 0.05 vs. no flow; n = 4; Fig. 2).
Tubular flow does not affect intracellular pHi of the macula densa.
To test whether flow-induced NO production is associated with intracellular alkalization, macula densa pHi was measured using BCECF. When tubular flow rate was increased from 0 to 40 nl/min, there was no significant change in the pHi in the macula densa (n = 5; Fig. 3). These data indicate that the flow-induced NO generation is involved in a different signal pathway from that which initiates a TGF response and independent of NKCC2 cotransporter and intracellular pH.
Fig. 3.
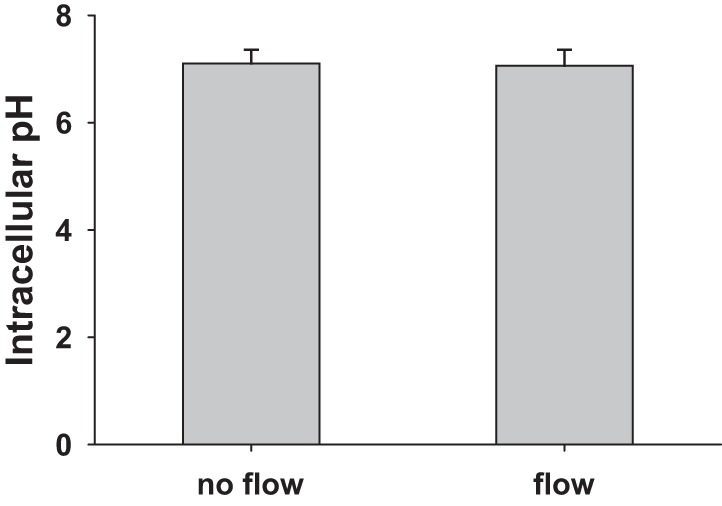
Effect of tubular flow on intracellular pH (pHi) of the macula densa. BCECF-AM was used for measuring the changes of macula densa pHi under different tubular flow conditions. Without tubular flow, pHi of macula densa cells was 7.10 ± 0.26. After tubular flow was increased to 40 nl/min, the pHi had no significant change (7.07 ± 0.24) (n = 5).
Shear stress enhances NO generation in MMDD1 cells mediated by primary cilia.
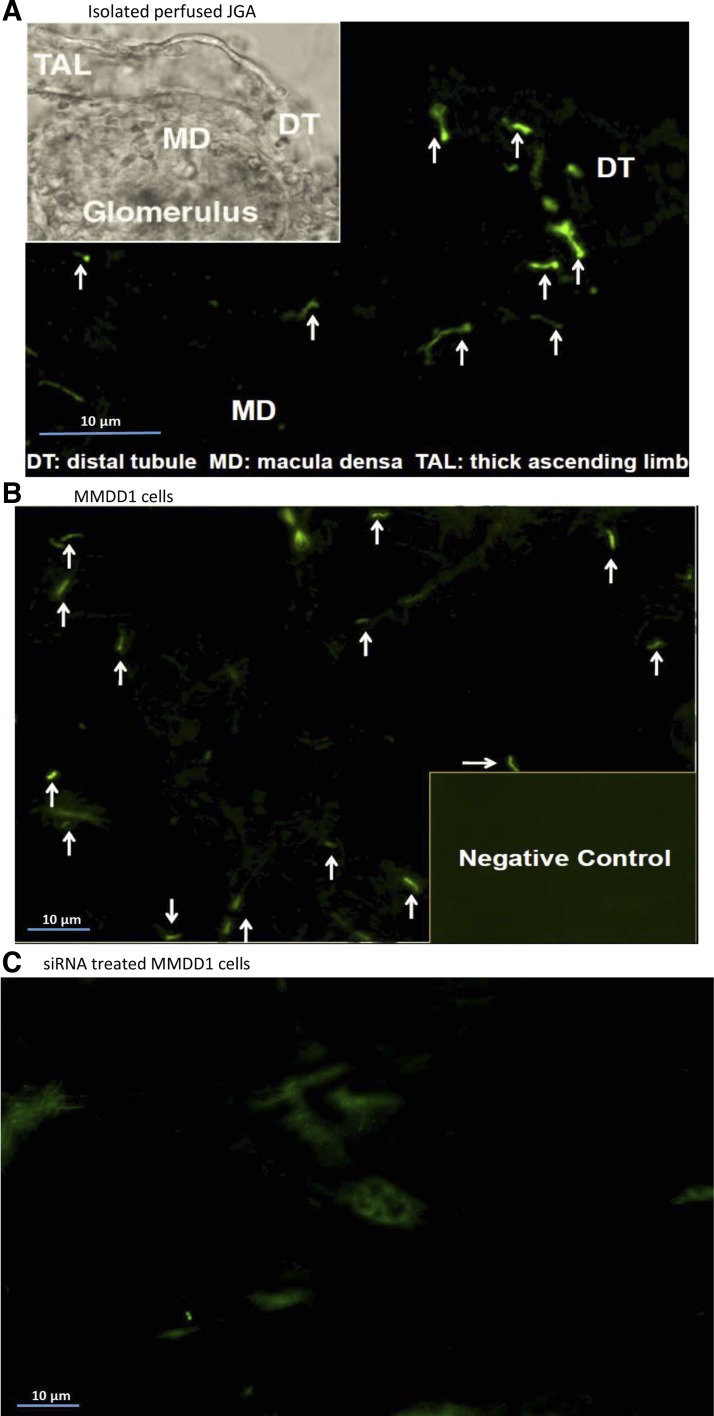
Primary cilia were clearly visible on isolated perfused rabbit macula densa cells using immunofluorescence microscopy, as shown in Fig. 4A and on cultured MMDD1 cells, as shown in Fig. 4B. To test whether primary cilia are required for the shear stress-mediated NO generation, siRNA against ift88 was used to disrupt formation of the primary cilia in MMDD1 cells. As shown in Fig. 4C, most of the primary cilia were deleted from MMDD1 cells after treatment with a siRNA for 24 h. The efficiency of silencing of the ift88 gene was determined by measuring the expression of ift88 mRNA with real-time PCR. The expression of ift88 mRNA fell by 93.7 ± 4.5% in cells treated with siRNA vs. the levels seen in control cells treated with scrambled siRNA (n = 8; Fig. 5).
Fig. 4.
Primary cilia are present on rabbit macula densa and cultured MMDD1 cells. By using immunofluorescence microscopy, primary cilia are clearly visualized on rabbit macula densa cells in isolated perfused JGA (A) and cultured MMDD1 cells (B). Most of the primary cilia were deleted from MMDD1 cells by treating with a siRNA against ift88 for 24 h (C).
Fig. 5.
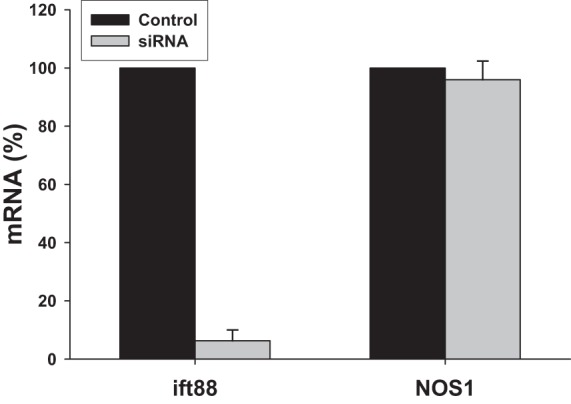
Effects of siRNA on mRNA levels of ift88 and NOS1. Gene expression levels were measured with real-time PCR for ift88 to determine the silencing efficiency of the siRNA (n = 8); and for NOS1 to determine whether siRNA or deletion of cilia affects NOS1 expression (n = 5).
The influence of shear stress on NO generation in MMDD1 cells was investigated by culturing MMDD1 cells in a circular chamber. When the shear stress increased from 0.5 to 5.0 dynes/cm2, NO generation increased from 174.8 ± 21 to 276.1 ± 24 units/min, which increased by 57.9 ± 6.4% (P < 0.01 vs. 0.5 dynes/cm2; n = 17; Fig. 6). NO generation was significantly inhibited and only increased from 48.6 ± 17 to 62.7 ± 15 units/min in cells treated with an ift88 siRNA (P < 0.01 vs. control; Fig. 6).
Fig. 6.
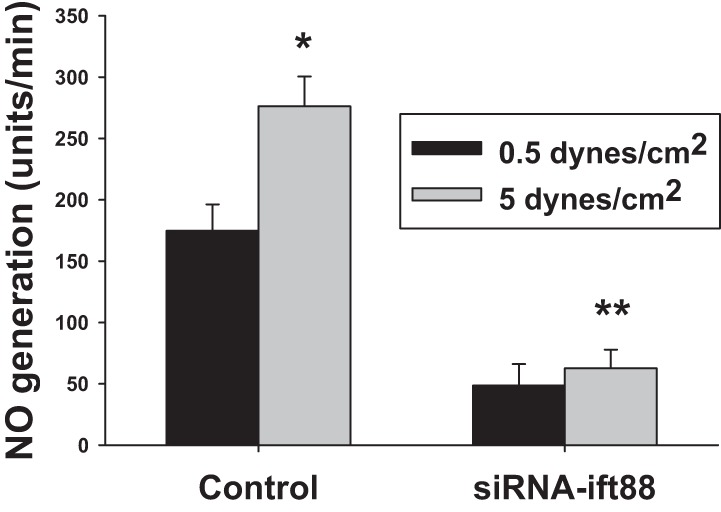
Effects of shear stress and primary cilia on NO generation in MMDD1 cells. The MMDD1 cells were subcultured in a circular chamber. When the shear stress increased from 0.5 to 5.0 dynes/cm2, NO generation increased by 57.9 ± 6.4%, from 174.8 ± 21 to 276.1 ± 24 units/min. (*P < 0.01 vs. 0.5 dynes/cm2; n = 17). In MMDD1 cells treated with a siRNA against ift88 to remove the primary cilia, the shear stress-induced NO generation was inhibited (**P < 0.01 vs. scrambled-siRNA-treated cells).
To determine whether the siRNA or deletion of cilia has any effect on NOS1 expression, we measured NOS1 mRNA expression with real-time PCR and compared its expression with cells treated with scrambled siRNA. Treatment with the siRNA had no significant effect on the expression of NOS1 mRNA in the MMDD1 cells (n = 5; Fig. 5).
Increase of shear stress by elevating viscosity blunts TGF response.
To test the effect of shear stress on TGF responsiveness, we increased viscosity by adding dextran to the tubular perfusate while maintaining a constant tubular perfusion at 40 nl/min. Shear stress was calculated according to the equation for laminar flow of Newtonian fluid: (28, 51) τ = 6μQ/πr2L, where τ is shear stress, μ is fluid viscosity, Q is flow rate, r is the radius of the tube, and L is tube length. We increased shear stress of tubular perfusate from 0.78 ± 0.06 to 1.55 ± 0.09 millipascal-second (mPa·s) by adding dextran. The TGF response was determined by measuring the vasoconstriction of Af-Art in response to an increase in the NaCl concentration of the tubular perfusate from 10 to 80 mM in the presence or absence of dextran.
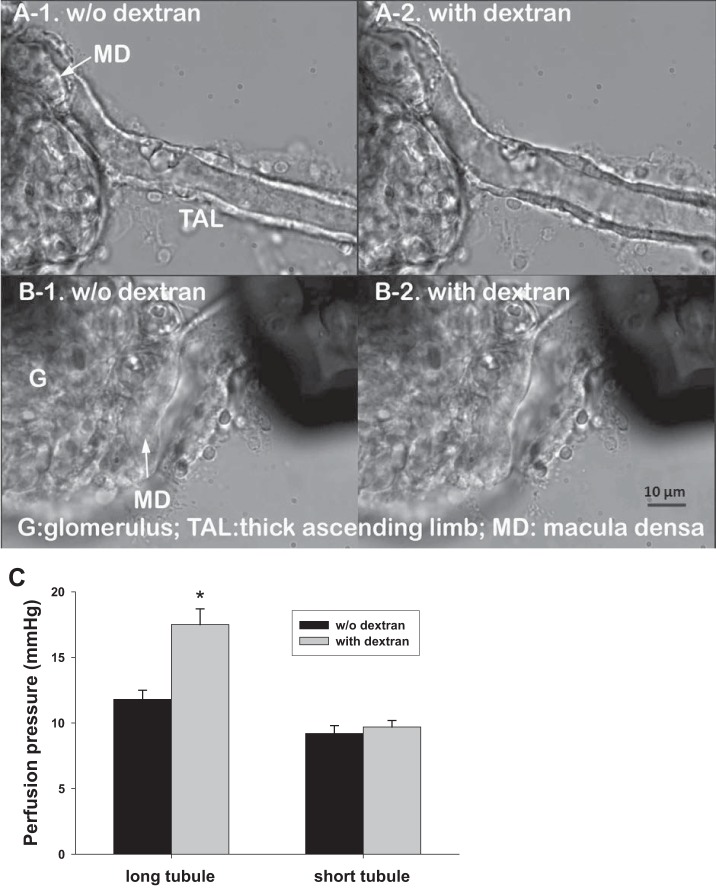
Increased perfusate viscosity could increase tubular hydraulic pressure. However, the effect of viscosity on hydraulic pressure was minimized by reducing outflow resistance using a short length of TAL beyond the macula densa. To demonstrate this, tubular hydraulic pressure was measured and compared between long (about 90 μm) and short (about 10 μm) TALs of the JGAs perfused with and without dextran at 40 nl/min. The tubular hydraulic pressure was 11.8 ± 0.7 mmHg when the JGA with long TALs were perfused without dextran, and it increased to 17.5 ± 1.2 mmHg after adding dextran to the perfusate (P < 0.01 vs. without dextran; n = 5). As shown in Fig. 6A when the long TAL was perfused with dextran, the tubule was obviously dilated compared with perfusion without dextran. In contrast, the tubular hydraulic pressure was 9.2 ± 0.6 and 9.7 ± 0.5 mmHg when the JGA was perfused with a short TAL segment without and with dextran (n = 5; Fig. 7C). Moreover, there was no obvious dilation to the tubule when short TAL was perfused with dextran (Fig. 7B).
Fig. 7.
Effects of tubular length and perfusate viscosity on perfusion pressure. When long thick ascending limbs (TALs; > 90 μm) were perfused, increases of viscosity by adding dextran in tubular perfusate dilated the tubules (A-1, A-2) and increased hydraulic pressure from 11.8 ± 0.7 to 17.5 ± 1.2 mmHg (*P < 0.01 vs. without dextran; n = 5; C). When short TALs (<10 μm) were perfused, adding dextran did not obviously dilate the tubules (B-1, B-2) nor changed the hydraulic pressure (n = 5; C).
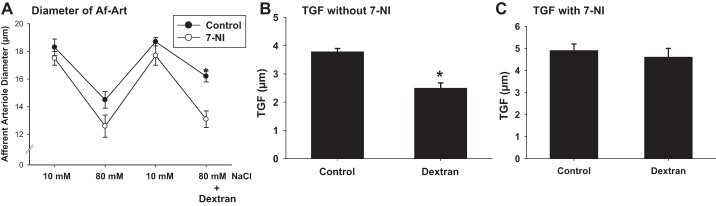
We then compared the effects of increasing shear stress with dextran on TGF response in isolated and double-perfused JGAs. When the NaCl concentration of the perfusate was increased from 10 to 80 mM, the diameter of the Af-Art was reduced from 18.3 ± 0.5 to 14.5 ± 0.6 μm. Thus, the TGF response was 3.8 ± 0.1 μm. After the addition of dextran to raise the viscosity of the tubular perfusate, the Af-Art constricted from 18.7 ± 0.3 to 16.2 ± 0.4 μm, and the TGF-mediated response was reduced to 2.5 ± 0.2 μm (P < 0.05 vs. without dextran; n = 7; Fig. 8, A and B).
Fig. 8.
Effect of increasing perfusate viscosity on TGF in the isolated perfused JGA. The control TGF response without dextran was 3.8 ± 0.1 μm. The Af-Art constricted from 18.3 ± 0.5 to 14.5 ± 0.6 μm when the concentration of NaCl in the tubular perfusate was increased from 10 to 80 mM. In the presence of dextran TGF response reduced to 2.5 ± 0.2 μm. The Af-Art constricted from 18.7 ± 0.3 to 16.2 ± 0.4 μm after tubular solution was switched again in the presence of dextran (*P < 0.05 vs. without dextran; n = 6). NOS1 inhibitor 7-NI enhanced TGF response and blocked dextran-induced TGF inhibition (n = 4). A: diameter of the Af-Art. B: mean TGF-mediated vasoconstriction without 7-NI (*P < 0.05). C: data in the presence of 7-NI.
To determine whether the shear-stress-induced TGF inhibition is mediated by NOS1 in the macula densa, we repeated the experiment in the presence of a selective NOS1 inhibitor 7-NI (10−5 M). When the NaCl concentration of the perfusate was increased from 10 to 80 mM, the diameter of the Af-Art was reduced from 17.5 ± 0.5 to 12.6 ± 0.8 μm, the TGF response was 4.9 ± 0.3 μm. In the presence of 7-NI, the addition of dextran to the tubular perfusate reduced the diameter of the Af-Art from 17.7 ± 0.7 to 13.1 ± 0.6 μm, and the TGF-mediated response was 4.6 ± 0.4 μm (n = 4; Fig. 8, A and C).
DISCUSSION
The present study examined whether modulation of tubular flow rate through increases in shear stress can affect the production of NO in the macula densa and blunt TGF responsiveness mediated by the primary cilia. We first examined whether tubular flow alters NO generation by the macula densa. DAF-2 is a nonratiometric fluorescent reporter of NO; consequently, monitoring precise changes in cell volume while increasing tubular flow is critical for accurate measurements of NO concentration (18, 31, 32). We measured NO and cell volume simultaneously by using both DAF-2 and calcein-red, which was a modification from our previous methods (25, 26). Changes in calcein-red emission were used as an index of changes of cell volume, which was used to calculate NO production by correcting the intensity of DAF-2 due to the volume effect. We found that tubular flow enhanced NO generation in macula densa and that selective NOS1 inhibitor 7-NI blocked the flow-induced NO. However, inhibition of NKCC2 with furosemide did not block the flow-induced NO generation. These observations suggested that the signaling pathway for the flow-induced NOS1 activation is different from that of TGF response, as we reported previously (23, 26). To confirm that flow-induced NO generation does not depend on ion transporter activities, we replaced all electrolytes of the tubular perfusate with mannitol. Removal of electrolytes from tubular perfusate had no effect on flow-induced NO generation in the macula densa, indicating that NOS1 was stimulated by tubular flow and was independent of ion transporter activities.
In previous studies, we and others reported that an increase of tubular NaCl concentration enhanced NOS1 activity and NO production by the macula densa (19, 26). We further found that the increase in tubular NaCl concentration enhanced Na/H exchange, which raised macula densa pHi and contributed to the activation of NOS1 (23). In the present study, we changed the tubular flow rate while the tubular NaCl concentration (80 mM) was fixed at a level to maximally stimulate TGF. Under these conditions, we did not expect to alter Na/H exchange. To confirm this, we measured pHi in the macula densa. We found that an increase of tubular flow per se had no effect on macula densa pHi, which further supported that the flow-induced NOS1 activation is involved in a separate signal pathway from the NaCl-induced NO generation by the macula densa.
The macula densa cells were exposed to at least three types of mechanical signals when luminal perfusion was switched from stationary luminal fluid (0 nl/min) to 40 nl/min. They are shear stress due to the flow, circumferential stress, and axial stress due to pressure gradient. To test whether an increase in shear stress per se is sufficient to increase NO generation in macula densa, we monitored the shear stress dependence of NO production in MMDD1 cells, a macula densa-like cell line, with or without intact cilia. We found that increases of shear stress enhanced NO generation in cultured MMDD1 cells. Primary cilia on the macula densa have been suggested as flow sensors in mice (47). To test whether the cilia on the macula densa are required to mediate shear stress-induced NO production, a siRNA against ift88 was used to remove cilia in MMDD1 cells. Ift88 is a product of the Tg737 gene, which is required for assembly and function of primary cilia (62). After siRNA treatment, shear stress-induced NO generation was significantly inhibited. These data indicate that shear stress-induced NO generation in MMDD1 cells requires intact cilia. Our findings are in agreement with previous reports that primary cilia serve as flow sensors in other tissues (8, 36, 61). We noticed that the rate of NO generation was significantly reduced, but not abolished. It seems like this NO reflects the basal activity of NOS1 as a constitutively expressed and active enzyme. Because the siRNA had no effect on NOS1 expression, we do not think this was due to an off target effect of the siRNA.
When the shear stress was increased in these cells, we observed an increase in NO generation, even though significantly less than control cells. The possible reason for the remaining response is that the cilia were not totally deleted, as noted in Fig. 4C. But we cannot exclude that other mechanisms in addition to the primary cilia may contribute to the increase in NO generation with flow, including cell deformation, which has been shown to activate several cell signaling pathways (7, 9). Moreover, it should be noted that MMDD1 cells may not behave the same as native macula densa cells (13). So the results obtained from MMDD1 cells need to be further confirmed in isolated perfused JGAs, if possible.
When electrolytes, including Ca2+ were removed from tubular perfusate, increases in flow still enhanced NO generation by the macula densa. It seems that the signal pathway is independent of influx of Ca2+. However, the calcium concentration in the bath was still 1 mM, which leaves a possibility of Ca2+ influx from basolateral membrane of the macula densa cells. In addition, we did not add calcium chelator in the “calcium-free” solution, in which the calcium concentration could reach micromolar concentrations (16, 30), which is still higher than intracellular calcium concentrations at nanomolar concentrations. Therefore, the possible mechanism of cilia-mediated NOS1 activation likely involves signal transductions that increases intracellular calcium concentrations mediated by either membrane calcium channels, such as polycystin cation channels, or calcium-induced intracellular calcium release.
Since NO is a well-established negative modulator of TGF, our findings suggest that flow-mediated increases in shear stress might attenuate TGF responsiveness. There has been a long-standing debate as to whether tubular flow rate has a direct effect on TGF responsiveness independent of changes in sodium concentration of the tubular perfusate. An increase in tubular flow typically initiates a TGF response by increasing luminal NaCl concentration to the macula densa and enhancing NKCC2-dependent transport. When NKCC2 was blocked by adding furosemide to the tubular perfusate, flow-induced constriction of the Af-Art was totally abolished (1, 3, 14, 45, 46, 58). In such case, even though tubular flow enhances NO generation, the modulatory effect of NO on TGF cannot be observed because the TGF response is eliminated by furosemide.
In the present study, we took advantage of perfused JGA preparation, in which it is possible to directly measure NO production in macula densa, while the luminal shear stress is increased. We increased viscosity by adding dextran to the tubular perfusate to increase shear stress. It is known that increased perfusate viscosity by adding dextran will increase tubular hydraulic pressure and, thus, generate a pressure gradient across the macula densa. We minimized the increase in tubular hydraulic pressure by shortening the perfused distal tubule and TAL to less than 10 μm, which was confirmed by directly measuring tubular hydraulic pressure. Intratubular hydraulic pressure was measured by the Landis technique, as in measuring perfusion pressure of the Af-Art (27, 38, 39, 49), Using this approach, we could maintain a constant hydraulic pressure and minimize the changes in pressure gradient across the macula densa, while increasing shear stress at the macula densa. By using this setup, we were able to demonstrate that the increase of shear stress impairs TGF-mediated vasoconstriction of Af-Art. It is known that NO production from TAL is also flow-dependent (15, 35), and the NO produced from TAL could modulate TGF response (55). However, this is unlikely in the present study because the length of TAL in perfused JGA is very short. In addition, NOS1 inhibition with 7-NI totally blocked the increase in NO in the macula densa, which indicates that NO generated from endothelial NOS in the TAL does not contribute to the NO in the macula densa at least in our isolated perfused JGA preparation.
Our findings are not consistent with those of Sipos et al. (47), who previously reported that direct stimulation of cilia on the macula densa cells activate, rather than inhibit, TGF using sophisticated and technically demanding methods. However, these studies were very different in several aspects. In the paper by Sipos et al., they removed TAL and exposed the apical side of the macula densa to the bath directly in some protocols. The primary cilia were directly stimulated by high-velocity flow from a pipette and mechanical bending with a pipette, which may intensely stimulate the cilia and macula densa cells and induce the release of adenosine or ATP that constrict the Af-Art. Beyond this, the main difference between the two studies was that 1 mM l-arginine was included in our tubular perfusate, whereas it was absent in the studies of Sipos et al. This is an important difference in the experimental approach in that previous studies by Ortiz et al. (34) indicated that l-arginine in the tubular perfusate is critical for the generation of NO in TAL, and we assume the same is true about the macula densa. Blockade of NO augments TGF responsiveness and eliminates the modulatory effect of NO on the TGF response. Thus, the macula densa preparations studied by Sipos et al. were likely NO-deficient. However, it could be interesting to further investigate the mechanisms for cilia-mediated TGF regulation in conditions with and without NO generation by the macula densa in future studies.
Perspectives and Significance
In conclusion, we found that an increase in tubular flow blunts TGF-mediated vasoconstriction of the Af-Art, which is partially mediated by shear stress, and enhances NO production in the macula densa. We provided evidence that flow can activate primary cilia on the macula densa through an increase of shear stress, which enhances NOS1 activity and NO generation in the macula densa. This mechanism of TGF inhibition may be important for the elevations in GFR following a salt loading to facilitate sodium and water excretion. As the excess sodium load is excreted, flow rate to the macula densa returns to normal, and this flow-mediated modulation mechanism would be withdrawn, which allows restoration of TGF responsiveness to control GFR to avoid excessive sodium and water loss.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.W., S.W., and R.J.R. conception and design of research; L.W., C.S., H.L., and S.W. performed experiments; L.W., C.S., H.L., S.W., J.W., J.Z., and R.L. analyzed data; L.W., C.S., X.C., Y.L., and R.L. interpreted results of experiments; L.W. and C.S. prepared figures; L.W., C.S., K.-P.Y., and R.L. drafted manuscript; L.W., X.C., R.J.R., L.A.J., Y.L., J.W., K.-P.Y., and R.L. edited and revised manuscript; L.W., C.S., X.C., R.J.R., L.A.J., Y.L., J.W., J.Z., K.-P.Y., and R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants DK-099276 (to R. Liu), DK-098582 (to R. Liu), HL-36279, and DK-104184 (to R. J. Roman) and core facilities of HL-051971 and GM-104357.
REFERENCES
- 1.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol 65: 32.1–32.20, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Briggs JP, Schnermann J. Macula densa control of renin secretion and glomerular vascular tone: evidence for common cellular mechanisms. Renal Physiol (Basel) 9: 193–203, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Burke TJ, Duchin KL. Glomerular filtration during furosemide diuresis in the dog. Kidney Int 16: 672–680, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Centonza L, Castoldi G, Chianca R, Busca G, Golin R, Zanchetti A, Stella A. Short-term analysis of the relationship between blood pressure and urinary sodium excretion in normotensive subjects. Clin Sci (Lond) 98: 495–500, 2000. [PubMed] [Google Scholar]
- 5.Cook N, Fraser SA, Katerelos M, Katsis F, Gleich K, Mount PF, Steinberg GR, Levidiotis V, Kemp BE, Power DA. Low-salt concentrations activate AMP-activated protein kinase in mouse macula densa cells. Am J Physiol Renal Physiol 296: F801–F809, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Cowley AW Jr., Guyton AC. Baroreceptor reflex effects on transient and steady-state hemodynamics of salt-loading hypertension in dogs. Circ Res 36: 536–546, 1975. [DOI] [PubMed] [Google Scholar]
- 7.Dahl KN, Kalinowski A, Pekkan K. Mechanobiology and the microcirculation: cellular, nuclear and fluid mechanics. Microcirculation 17: 179–191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 289: F1159–F1169, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JO, Howell DS. Mechanisms of fluid and electrolyte retention in experimental preparations in dogs. II. With thoracic inferior vena cava constriction. Circ Res 1: 171–178, 1953. [DOI] [PubMed] [Google Scholar]
- 11.Ellison DH, Velazquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 83: 113–126, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H, Podymow T, Zimpelmann J, Burns KD. NO inhibits Na+-K+-2Cl-cotransport via a cytochrome P-450-dependent pathway in renal epithelial cells (MMDD1). Am J Physiol Renal Physiol 284: F1235–F1244, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Holstein-Rathlou NH. A closed-loop analysis of the tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 261: F880–F889, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Hong NJ, Silva GB, Garvin JL. PKC-α mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F891, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard JI, Jones SF, Landau EM. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol 194: 355–380, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol Renal Fluid Electrolyte Physiol 248: F890–F894, 1985. [DOI] [PubMed] [Google Scholar]
- 18.Kojima H, Sakurai K, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem Pharm Bull 46: 373–375, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Kovács G, Komlósi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase: its role and regulation in macula densa cells. J Am Soc Nephrol 14: 2475–2483, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kunau RT Jr, Webb HL, Borman SC. Characteristics of the relationship between the flow rate of tubular fluid and potassium transport in the distal tubule of the rat. J Clin Invest 54: 1488–1495, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladd M, Raisz LG. Response of the normal dog to dietary sodium chloride. Am J Physiol 159: 149–152, 1949. [DOI] [PubMed] [Google Scholar]
- 22.LaGrange LP, Toney GM, Bishop VS. Chronic angiotensin II infusion attenuates the renal sympathoinhibitory response to acute volume expansion. Am J Physiol Regul Integr Comp Physiol 284: R1098–R1107, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Carretero OA, Ren Y, Wang H, Garvin JL. Intracellular pH regulates superoxide production by the macula densa. Am J Physiol Renal Physiol 295: F851–F856, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Persson AE. Simultaneous changes of cell volume and cytosolic calcium concentration in macula densa cells caused by alterations of luminal NaCl concentration. J Physiol 563: 895–901, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13: 2688–2696, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Proc Natl Acad Sci USA 101: 10,060–10,065, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 59: 643–650, 1979. [DOI] [PubMed] [Google Scholar]
- 30.Miledi R, Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol 212: 245–257, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagano T. Practical methods for detection of nitric oxide. Luminescence 14: 283–290, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett 427: 263–266, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Norgaard T. The ultrastructure of the macula densa during altered sodium intake. A morphometric study of the macula densa in the rabbit nephron. Acta Pathol Microbiol Immunol Scand [A] 90: 67–73, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO3− transport by rat thick ascending limb. Kidney Int 58: 2069–2074, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol 67: 515–529, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Ren Y, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 58: 2053–2060, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ren Y, Garvin JL, Liu R, Carretero OA. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int 71: 861–866, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Roman RJ, Cowley AW Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats: cortical and medullary hemodynamics. Hypertension 12: 168–176, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol Regul Integr Comp Physiol 274: R263–R279, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Schnermann J, Briggs JP. Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 259: F565–F572, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA 95: 9660–9664, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnermann J, Ploth DW, Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflügers Arch 362: 229–240, 1976. [DOI] [PubMed] [Google Scholar]
- 45.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int 54 (Suppl 67): S40–S45, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Schnermann J, Valtin H, Thurau K, Nagel W, Horster M, Fischbach H, Wahl M, Liebau G. Micropuncture studies on the influence of antidiuretic hormone on tubular fluid reabsorption in rats with hereditary diabetes insipidus. Pflügers Arch 306: 103–118, 1969. [DOI] [PubMed] [Google Scholar]
- 47.Sipos A, Vargas S, Peti-Peterdi J. Direct demonstration of tubular fluid flow sensing by macula densa cells. Am J Physiol Renal Physiol 299: F1087–F1093, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science 237: 1618–1620, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Song J, Lu Y, Lai EY, Wei J, Wang L, Chandrashekar K, Wang S, Shen C, Juncos LA, Liu R. Oxidative status in the macula densa modulates tubuloglomerular feedback responsiveness in angiotensin II-induced hypertension. Acta Physiol (Oxf) 212: 1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sottiurai V, Malvin RL. The demonstration of cilia in canine macula densa cells. Am J Anat 135: 281–286, 1972. [DOI] [PubMed] [Google Scholar]
- 51.Sutera SP, Skalak R. The history of Poiseuille's Law. Ann Rev Fluid Mech 25: 1–20, 1993. [Google Scholar]
- 52.Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461–F468, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Thurau K, Schnermann J. The Na concentration of the macula densa cells as a factor regulating glomerular filtration rate (micropuncture studies). 1965 J Am Soc Nephrol 9: 925–934, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Traub O, Berk BC. Laminar shear stress. Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Carretero OA, Garvin JL. Nitric oxide produced by THAL nitric oxide synthase inhibits TGF. Hypertension 39: 662–666, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Webber WA, Lee J. Fine structure of mammalian renal cilia. Anat Rec 182: 339–343, 1975. [DOI] [PubMed] [Google Scholar]
- 57.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HHW. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11,993–11,997, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu CC, Chao YC, Chen CN, Chien S, Chen YC, Chien CC, Chiu JJ, Linju YB. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech 41: 813–821, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 275: 37,922–37,929, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 18: 1381–1388, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R. Isoforms and functions of NADPH oxidase at the macula densa. Hypertension 53: 556–563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X, Manning RD Jr, Lu D, Gomez-Sanchez CE, Fu Y, Juncos LA, Liu R. Aldosterone stimulates superoxide production in the macula densa cells. Am J Physiol Renal Physiol 301: F529–F535, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]