Abstract
Intrauterine growth restriction increases the risk of perinatal complications and predisposes the infant to diabetes and cardiovascular disease in later life. Mechanisms by which maternal nutrient restriction (MNR) reduces fetal growth are poorly understood. We hypothesized that MNR decreases placental amino acid (AA) transporter activity, leading to reduced transplacental transfer of AAs. Pregnant baboons were fed either a control (ad libitum, n = 7), or MNR diet (70% of control diet, n = 7) from gestational day (GD) 30. At GD 165 (0.9 gestation), placentas (n = 7 in each group) were collected, and microvillous plasma membrane vesicles (MVM) isolated. MVM system A and system L AA transport was determined in vitro using radiolabeled substrates and rapid filtration techniques. In vivo transplacental AA transport was assessed by infusing nine 13C- or 2H-labeled essential AA as a bolus into the maternal circulation (n = 5 control, n = 4 MNR) at cesarean section. A fetal vein-to-maternal artery mole percent excess ratio for each essential AA was calculated. Fetal and placental weights were significantly reduced in the MNR group compared with controls (P < 0.01). The activity of system A and system L was markedly reduced by 73 and 84%, respectively, in MVM isolated from baboon placentas at GD 165 following MNR (P < 0.01). In vivo, the fetal vein-to-maternal artery mole percent excess ratio was significantly reduced for leucine, isoleucine, methionine, phenylalanine, threonine, and tryptophan in MNR baboons (P < 0.05). This is the first study to investigate placental AA transport in a nonhuman primate model of MNR. We demonstrate that the downregulation of system A and system L activity in syncytiotrophoblast MVM in MNR leads to decreased transplacental AA transport and, consequently, reduced circulating fetal AA concentrations, a potential mechanism linking maternal undernutrition to reduced fetal growth.
Keywords: fetal growth restriction, nonhuman primate, maternal-fetal exchange
maternal undernutrition is a major public health concern world wide. Maternal undernutrition constitutes the most common cause of intrauterine growth restriction (IUGR) in developing countries, causing significant perinatal mortality and morbidity (6) and programming of adult disease (3). Babies who were in utero during the Dutch wartime famine in the winter of 1944-45 in Holland were growth restricted and have an increased incidence of obesity and metabolic and cardiovascular disease in adulthood (49), demonstrating that maternal undernutrition during gestation has profound effects on the health of the offspring. The mechanisms linking reduced maternal nutrition to IUGR and developmental programming of adult disease remain to be elucidated.
Experiments in animal models have demonstrated alterations in placental growth, structure and function in response to reduced maternal nutrient availability, resulting in adverse pregnancy outcomes (9, 18, 35). In Western societies, IUGR has been primarily related to placental insufficiency, or impaired uteroplacental vascular remodeling, while in developing countries maternal undernutrition remains the most common cause of restricted fetal growth. However, both perturbations appear to cause similar changes in placental signaling and function (18, 46, 48, 52). Investigating the effects of maternal undernutrition is, therefore, directly relevant also for restricted placental and fetal growth in association with placental insufficiency. Decreased expression and activity of key placental amino acid transporters has been reported in IUGR in humans (13, 22, 34, 38), as well as in animal models (18, 35). It has therefore, been proposed that changes in the expression and activity of placental nutrient transporters may precede the development of IUGR (18), and that the placenta acts as a nutrient sensor regulating the delivery of nutrients to the fetus in response to changes in the maternal nutrient supply line (21).
The system A amino acid transporter is encoded by three members of the slc38 family, and all three isoforms [sodium-coupled neutral amino acid transporter 1 (SNAT1), SNAT2, and SNAT4] are known to be expressed in the placenta (10). System A functions in a sodium-dependent manner to mediate the uptake of nonessential amino acids into the cell. System A activity establishes a high intracellular concentration of nonessential amino acids, such as glycine, creating a gradient that is used to drive the exchange for extracellular essential amino acids (EAAs) via system L. System A activity is, therefore, necessary for the transport of both essential and nonessential amino acids in the placenta. The system L transporter functions as an amino acid exchanger, mediating the uptake of EAAs in a sodium-independent manner (55). It is a heterodimer comprising a light chain, either the large amino acid transporter 1 (LAT1) or LAT2, and a heavy chain, 42hc/CD98.
Placental amino acid transport is regulated by maternal hormones, such as insulin and IGF-I and mechanistic target of rapamycin (mTOR) signaling (17, 23, 26). Maternal undernutrition in humans and animal models results in reduced circulating and tissue levels of insulin and IGF-I (4, 32). mTOR is a serine/threonine kinase that plays a key role in nutrient sensing and is an important regulator of cell growth, proliferation, and metabolism in response to changes in nutrient availability. Reduced placental mTOR signaling has been observed in human and animal IUGR, including the model we employ in this study (27, 46–48).
Placental amino acid transport has not been adequately studied in women subjected to naturally occurring undernutrition, and controlled experimental undernutrition in pregnant women is not possible. The effects of maternal nutrient restriction (MNR) on placental amino acid transporter expression and activity have, therefore, been investigated utilizing rodent and ovine models. However, the placentas of these animals are not directly comparable to the human placenta (8). Studies using nonhuman primate models that are more similar to the human in terms of physiological changes during pregnancy and placentation are likely to be more informative. To this end, we recently reported that the protein expression of glucose (GLUT-1) and amino acid transporter isoforms (taurine transporter TAUT, SNAT2, and LAT1 and 2) was reduced in syncytiotrophoblast microvillous membranes isolated from placentas of MNR baboons at gestational day (GD) 165 compared with controls (27). Furthermore, the phosphorylation of proteins in the mTOR and insulin/IGF-I signaling pathways, including placental S6K, S6 ribosomal protein, protein 4E-binding protein 1, insulin receptor substrate-1, Akt, ERK-1, and glycogen synthase kinase-3 was also reduced (27). However, the impact of MNR in humans or nonhuman primates on placental transport activity is unknown. Herein, we used in vitro and in vivo approaches to test the hypothesis that MNR decreases placental amino acid transporter activity, leading to reduced transplacental transfer of amino acids.
METHODS
Animal care.
All animal procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee and conducted in facilities approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. Baboons (Papio species) were maintained in groups of 10–16 females with 1 male in outdoor metal and concrete cages at the Southwest National Primate Research Center, as described in detail previously (51).
System for controlling and recording individual feeding.
Animals were weighed and fed using a feeding system that has previously been described in detail (51). Briefly, once a day before feeding, all baboons passed along a chute, over a scale, and into an individual feeding cage. The weight of each animal was obtained as it crossed an electronic scale system (GSE 665; GSE Scale Systems, Livonia, MI) and recorded as the mean of 50 individual measurements over 30 s. Each animal was fed between either 0700 and 0900 or 1100 and 1300. Water was available ad libitum.
Baboons were fed Purina Monkey Diet 5038 (Purina, St. Louis, MO). The basic composition of each biscuit was ≥15% crude protein, ≥5% crude fat, ≤6% crude fiber, 5% ash, ≤3% added minerals, solubilized vitamin C, and other required vitamins. All animals were initially fed 60 biscuits ad libitum in the feeding tray of each individual cage. Baboons were returned to the group cage at the end of the 2-h feeding period, and biscuits that remained in the tray, floor of the cage, and pan beneath the cage were counted. Food consumption, weight, and health status of each animal were recorded daily.
Study design.
Female baboons (8–15 yr old) were selected on the basis of reproductive age, body weight, and absence of extra-genital pathological signs, and mated as described previously (51). Pregnancy was dated according to time of ovulation and changes of sex skin color, and confirmed by ultrasonography at GD 30. From that point forward, animals in the MNR group were fed 70% of the total intake of corresponding controls, calculated per kilogram body weight.
Infusion of EAAs and collection of blood and tissue samples.
Conventional cesarean sections using general anesthesia and standard sterile techniques were performed at GD 165 (term 180–184 days), as previously described (27). Animals were tranquilized with ketamine hydrochloride (10 mg/kg), intubated, and anesthetized with isoflurane (starting rate 2% with oxygen: 2 l/min).
To assess transplacental transport of EAAs, baboons (n = 5 controls, n = 4 MNR) were infused with a cocktail of nine EAAs labeled with stable isotopes 13C or 2H, as described previously in pregnant women, with modifications (12). At the time of cesarean section, a bolus infusion of EAAs diluted to 10 ml with isotonic saline solution was infused into a maternal peripheral vein over a 2-min time period. A catheter placed in the maternal femoral artery was used to obtain maternal blood samples, which were collected just before the EAA infusion, and then at ∼2-min intervals for up to 16 min after the completion of the infusion. Umbilical vein blood samples were obtained 5 min after the completion of EAA infusion. Heparinized maternal and fetal blood samples were centrifuged at 2,500 g for 15 min, and plasma was collected and stored at −80°C until further analysis. Fetuses and placentas were towel dried and weighed. Villous trophoblast tissue was processed for the isolation of syncytiotrophoblast membranes. Following cesarean section, maternal postoperative analgesia was provided (buprenorphine 0.015 mg·kg−1·day−1 as 2 doses for 3 days).
Transplacental amino acid transport in baboon was compared with previous stable isotope studies conducted in women (n = 5–7) undergoing cesarean section (12).
Isolation of trophoblast plasma microvillous membrane vesicles.
The placenta was collected, and pieces (1 cm3) of chorionic villous tissue were immediately dissected, washed in saline, and placed in buffer D (250 mM sucrose, 10 mN HEPES-Tris, and 1 mM EDTA, pH 7.4) at 4°C with protease and phosphatase inhibitors. Syncytiotrophoblast plasma microvillous membrane vesicles (MVM) were isolated as described in detail previously (16, 19), with minor modifications (27). Briefly, following initial centrifugation steps, MVM were isolated using Mg2+ precipitation, followed by further purification with differential centrifugation steps. Samples were snap frozen in liquid nitrogen and stored at −80°C. Purity of MVM was assessed using a standardized assay for alkaline phosphatase activity to determine the enrichment of alkaline phosphatase activity compared with homogenates. Microvillous plasma membrane vesicle enrichment of alkaline phosphatase activity was not significantly different between the control and MNR groups (control, 4.2 ± 0.6; MNR 3.16 ± 0.7, n = 7/group, P = 0.26). Protein content of MVM was assessed using the method of Bradford (5).
Activity of amino acid transporters in MVM.
System A transporter activity was assessed by measuring the uptake of the amino acid analog methylaminoisobutyric acid (MeAIB) using modification of the protocol of Mahendran et al. (34). System L activity was studied by determining the uptake of l-leucine, as described previously with minor modifications (19, 22). Vesicles were preloaded and incubated overnight in 300 mmol/l mannitol and 10 mmol/l HEPES-Tris, pH 7.4, at 4°C. Vesicles were subsequently pelleted and resuspended in the same buffer at a protein concentration of ∼6 mg/ml. MVM were kept on ice until immediately before transport measurements, when samples were warmed to 37°C using a water bath. Vesicles were mixed rapidly with 30 μl of the appropriate incubation buffer (1:2), including [14C]MeAIB (150 μmol/l) or l-[3H]leucine (0.375 μmol/l). To determine the time course of MeAIB and leucine uptake by MVM (n = 2–3), uptake of radiolabel was terminated at 8, 12, 20, and 30 s using ice-cold PBS (pH 7.4) followed by rapid filtration. The uptake of MeAIB and leucine was rapid and time dependent, not reaching equilibrium by 30 s. Uptakes at 20 s were chosen to approximate initial rate (22) (n = 7 in each group). Vesicles were subsequently separated from the substrate medium using rapid filtration over mixed ester filters (0.45 μM pore size, Millipore, Bedford, MA) and washed using 3 × 2 ml PBS. In studies of MeAIB transport, 150 mmol/l NaCl and 150 mmol/l KCl were used in incubation buffers to measure total and sodium-independent uptake, respectively. In leucine transport experiments, nonmediated flux was studied in the presence of 20 mmol/l unlabeled l-leucine. Using BCH [2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid], a specific system L inhibitor, our laboratory has previously reported that leucine uptake in human MVM is mediated almost exclusively by system L (22), providing the justification for using unlabeled leucine to determine system L activity.
Each condition was measured in triplicate for each placenta in all uptake experiments. Filters were dissolved in 2-ml scintillation cocktail and counted. Blanks were subtracted from counts, and uptakes are expressed in terms of picomoles per milligram protein at 20 s. System A activity corresponding to the Na+-dependent uptake of MeAIB was calculated by subtracting Na+-independent uptake from total uptakes. Mediated uptake of leucine was calculated by subtracting nonmediated transport from total uptake. Protein content was measured using the Bradford assay (5).
Plasma preparation, derivatization, and analysis of amino acid derivatives.
Plasma samples were prepared as described previously (12). Briefly, 0.5 ml of plasma was mixed with norleucine (0.05 ml of 50 μg/ml), which served as an internal standard, followed by acidification with 0.15 ml of 50% acetic acid, and washed with AG-50 hydrogen form (0.05 ml). Samples were centrifuged at 4,500 g for 1 min, supernatant was discarded, and the pellet was washed four times with 1 ml of 70% isopropanol. Amino acids were released by adding 0.2 ml of 5 M ammonium hydroxide, samples were centrifuged, and supernatant was collected. Elution was repeated twice, and fractions were lyophilized.
Amino acid enrichment was carried out as previously described (12) by converting the amino acids to their respective tert-butyldimethylsilyl derivatives, by adding 0.05 ml of the silylating agent [1:1 mixture of N-(t-butyldimethylsilyl)-N-methyl-trifluoroacetamide with 1% tert-butyldimethylsilyl chloride and acetonitrile] at 65°C for 1 h. Analysis of amino acid derivatives was performed using a gas chromatograph mass spectrometer (model 5975; Agilent Technologies, Santa Clara, CA), on an HP-5-ms column (Hewlett-Packard, Palo Alto, CA). The following ions were monitored for the nine EAAs: 289/288 for valine, 303/302 for leucine and isoleucine, 323/320 for methionine, 408/404 for threonine, 337/336 for phenylalanine, 435/431 for lysine, 441/440 for histidine, and 380/375 for tryptophan (12).
The mole percent excess (MPE) is defined as a quantitative measure of the concentration of a stable isotope as a percentage of all isotopes, over and above its usual occurrence in nature. MPE of each amino acid was calculated in maternal and fetal plasma. The fetal umbilical vein/maternal artery (Fv/M) MPE was calculated as the ratio between fetal plasma amino acid enrichment over maternal plasma amino acid enrichment at the time of blood sampling (12).
Statistical analysis.
All data are expressed as means ± SE. The significance of the difference between the two groups was calculated using the unpaired Student's t-test. A P value of <0.05 was considered significant. The data were found to be normally distributed using the Kolmogorov-Smirnov test. Hence a parametric test was applied, and the data are represented as means ± SE.
RESULTS
Fetal and placental weights.
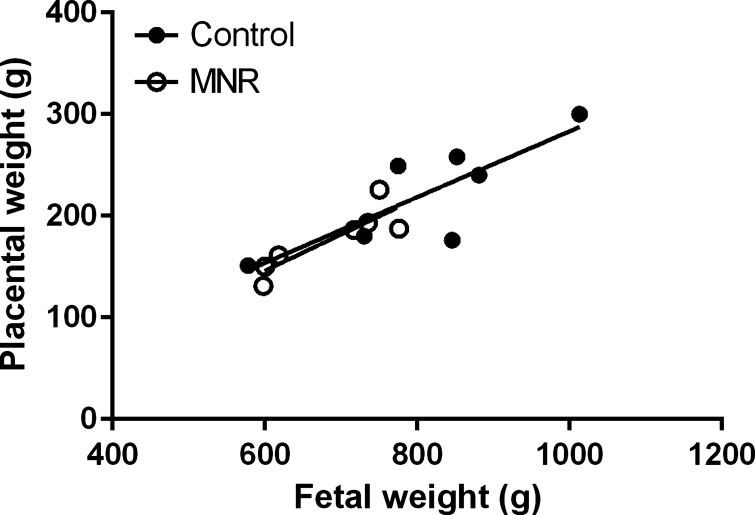
MNR significantly reduced fetal weights of the MNR group by 19% compared with controls at GD 165 (n = 7 in each group, P = 0.01, Table 1). Placental weights were reduced by 20% (n = 7 in each group, P = 0.02, Table 1). Fetal weight was positively correlated to placental weight in the control and MNR groups (control, r = 0.65, P = 0.02; MNR r = 0.75, P = 0.01, n = 7 in each group, Fig. 1).
Table 1.
Fetal and placental weights
Values are means ± SE; n, no. of animals.
MNR, maternal nutrient restriction.
P < 0.05, control vs. MNR in the baboon at gestational day 165.
Fig. 1.
Relationship between placental and fetal weights. Fetal weights were positively correlated to placental weights in the control and maternal nutrient restriction (MNR) groups in the baboon at gestational day (GD) 165. Individual Spearman's rank correlation coefficients were calculated for each slope in control and MNR baboons (control, r2 = 0.65; MNR, r2 = 0.65, P < 0.05, n = 7 in each group).
System A and system L activity in MVM.
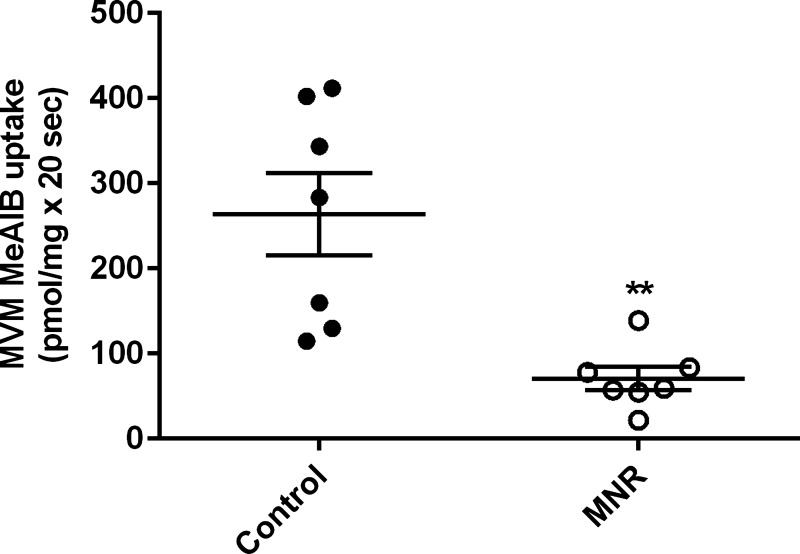
At GD 165, MVM uptake of MeAIB was reduced by 73% in MNR compared with controls (control, 263 ± 48.5 pmol/mg × 20 s; MNR, 71 ± 13.7 pmol/mg × 20 s, n = 7/group, P = 0.00071, Fig. 2).
Fig. 2.
Decreased microvillous plasma membrane vesicles (MVM) system A activity in MNR. Mediated methylaminoisobutyric acid (MeAIB) uptake into MVM isolated from control and MNR placentas of baboons at GD 165 are shown. Control and MNR groups were compared using the t-test (n = 7 in each group). Values are means ± SE. **P < 0.05.
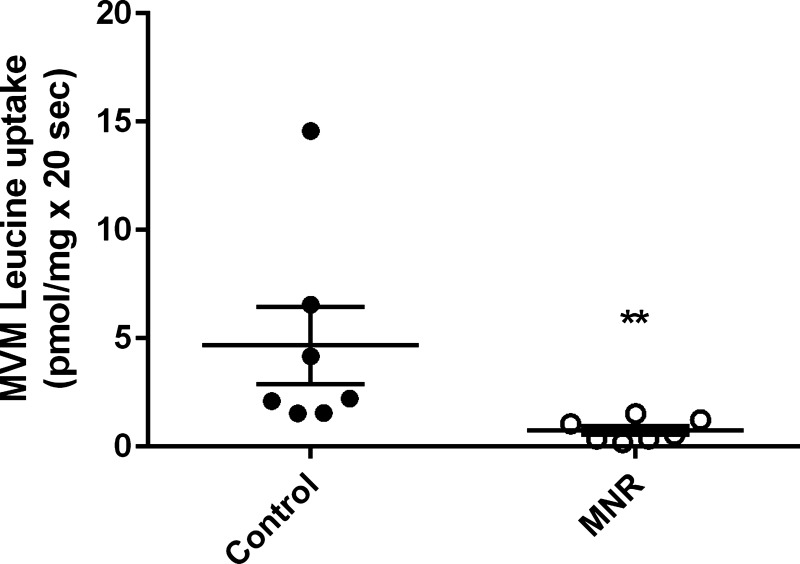
Mediated MVM l-leucine uptake, indicative of system L activity, was reduced by 84% in MNR compared with controls (control, 4.6 ± 1.7 pmol/mg × 20 s; MNR, 0.7 ± 0.1 pmol/mg × 20 s, n = 7/group, P = 0.0006, Fig. 3) at GD 165. Out of n = 7 in each group, three fetuses were female and four were male.
Fig. 3.
Decreased MVM system L activity in MNR. Mediated l-leucine uptake into MVM isolated from control and MNR placentas of baboons at GD 165 are shown. Control and MNR groups were compared using the t-test (n = 7 in each group). Values are means ± SE. **P < 0.05.
Fv/M MPE ratios.
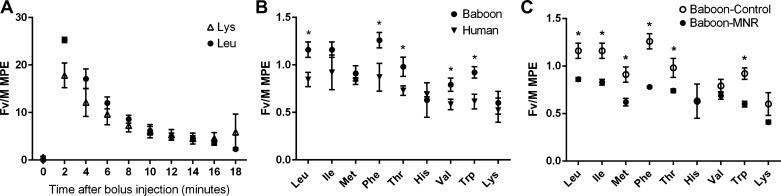
Fv/M MPE ratios were calculated for the nine amino acids that were infused in pregnant baboons and pregnant women at cesarean section (12, 43). In the baboon at GD 165, leucine and lysine were rapidly cleared from the maternal circulation in an exponential manner that was similar for the two amino acids (Fig. 4A). This decrease in isotope enrichment in the maternal circulation is representative of all nine amino acids studied.
Fig. 4.
In vivo transplacental transfer of essential amino acids (EAAs). A: mole percent excess (MPE) ratios of stable isotope labeled leucine (Leu) and lysine (Lys) in maternal arterial blood after intravenous injection of a mixture of labeled EAAs to the pregnant baboon at GD 165 (n = 5). B: fetal vein/maternal artery (Fv/M) MPE ratios of nine EAAs in baboons (n = 5) and pregnant women (n = 5–7). Ile, isoleucine; Met, methionine; Phe, phenylalanine; Thr, threonine; His, histidine; Val, valine; Trp, tryptophan. C: Fv/M MPE ratios of nine EAAs in MNR baboons (n = 4) compared with controls (n = 5) at GD 165. Values are means ± SE. *P < 0.01.
Fv/M MPE ratios for isoleucine, methionine, histidine and lysine were comparable between pregnant women (n = 5–7) at term and control baboons at GD 165 (n = 5) (Fig. 4B). In contrast, the MPE ratios were significantly higher (P < 0.01) for leucine, phenylalanine, threonine, valine, and tryptophan in the baboon compared with the human, which is consistent with the possibility that the baboon placenta has a higher transport capacity for some EAAs. In general, MPE ratios for the branched-chain amino acids leucine and isoleucine were higher than for tryptophan and lysine in both species, indicating a higher placental capacity to transport leucine and isoleucine.
Fv/M MPE levels of the EAAs leucine, isoleucine, methionine, phenylalanine, threonine, and tryptophan were reduced in the MNR group (n = 4) compared with controls (n = 5) at GD 165 in the baboon (P < 0.01, Fig. 4C).
DISCUSSION
In this study, we demonstrate that global MNR from GD 30 to GD 165 in the baboon is associated with 1) reduced activity of the placental nutrient transport systems A and L in isolated MVM; and 2) reduced transplacental transfer of EAAs. This is the first report in the nonhuman primate determining placental nutrient transport activity in vitro and in vivo in the same individuals, and our data provide strong validation for previous measurements of amino acid transport in isolated syncytiotrophoblast plasma membranes (11–14). Reduced activity of placental amino acid transporters has been reported in human IUGR due to placental insufficiency in a number of studies and may be correlated with severity of IUGR (13, 18, 22, 34, 35). Downregulation of system A in the rat before the development of IUGR has been demonstrated in response to a low-protein diet, suggesting that reduced placental amino acid transport may directly contribute to, rather than be a consequence of, IUGR (18). However, the rodent placenta differs from that of humans and other primates in terms of structure, function, and physiology (8). The use of this well-established nonhuman primate model, which shares similarity to the human in terms of physiological changes of pregnancy and placentation (8), lends clinical relevance to this study. Furthermore, this type of dietary manipulation and the investigation of changes in placental amino acid transport before term cannot be conducted in pregnant women. However, mothers who experience food insecurity (39) may be exposed to comparable levels of calorie restriction, resulting in a similar degree of fetal growth restriction.
Our laboratory reported previously that the protein expression of SNAT2 as well as LAT1 and 2 isoforms mediating system A and system L transport, respectively, is reduced in MVM isolated from nutrient-restricted baboons at GD 165 (27). In the present study, we show that the downregulation of protein expression of system A and L isoforms in MVM results in a reduced amino acid uptake capacity mediated by systems A and L in vitro. In addition, we demonstrate that the in vivo fetal-to-maternal plasma enrichment ratios for a number of EAAs are lower in MNR compared with control. These data, together with the lower circulating levels of EAAs in the MNR fetus (27), strongly suggest that transplacental transport of amino acids is impaired in MNR. The activity and expression of placental nutrient transporters in the placenta are regulated by the nutrient sensor mTOR, which, in turn, is influenced by growth factor signaling and the levels of nutrients, oxygen, and ATP. Our laboratory has previously demonstrated a decreased activity in placental insulin/IGF-I and mTOR signaling pathways in response to MNR in the baboon (27) and in pregnant rats subjected to protein restriction (48). Collectively, these observations are consistent with the model that downregulation of placental amino acid transporters, mediated by inhibition of growth factor and mTOR signaling pathways, may directly contribute to decreased fetal nutrient availability and restricted fetal growth in response to MNR.
This is the first study to assess transplacental transport of EAAs in vivo in the nonhuman primate. We have shown that the pattern of transplacental amino acid flux is similar in normal term pregnant women and control late gestation baboons, indicating that amino acid transport likely occurs via similar mechanisms in both species. This is further supported by the observation that the human and baboon placenta express the same glucose and amino acid transporter isoforms with comparable subcellular localization (27). Furthermore, the Fv/M MPE ratios for the amino acids leucine, isoleucine, methionine, phenylalanine, threonine, and tryptophan were reduced in MNR compared with control baboons at GD 165. We, therefore, provide direct evidence that the downregulation of system A and system L activity in MVM isolated from the placental villi of MNR baboons leads to decreased placental amino acid transport and, consequently, reduced amino acid concentrations in the fetal circulation (24). In a previous study, fetal amino acid concentrations were determined in baboons at GD 90 (0.5 of gestation) and were found to be similar in control and MNR animals (37), and it was proposed that impaired placental amino acid transport may be a feature of late gestation, when increasing fetal demand cannot be matched by maternal nutrient supply. The results of the present study are consistent with this hypothesis.
Numerous studies in the human and in different animal models have demonstrated that IUGR due to placental insufficiency is associated with reduced placental amino acid transport and decreased fetal levels of EAAs. For example, using stable isotopes, it has been reported that in vivo transplacental transport of leucine and phenylalanine is decreased in human IUGR, with decreased fetomaternal enrichment ratios of leucine correlating with disease severity (36, 42). In vivo studies of placental amino acid transport in sheep have shown that transplacental flux of leucine (50) and threonine (1) is decreased in a model of IUGR. In vitro studies of amino acid transport in MVM isolated from human IUGR placentas show decreased leucine and lysine uptake (22). Furthermore, placental amino acid transport is impaired in placental insufficiency subsequent to reduction of uteroplacental blood flow in the guinea pig (20). Collectively, these studies suggest that there are common placental responses, which includes downregulation of amino acid transport, to placental insufficiency and maternal undernutrition, and that these responses are conserved from rodents to humans.
The maternal plasma amino acid profile influences amino acid delivery to the fetus, as highlighted by maternal amino acid supplementation studies (24, 25, 44, 45, 56). However, although elevating the maternal concentration of EAAs in human IUGR increased the umbilical uptake of some amino acids, no changes in valine, phenylalanine, lysine, histidine, and threonine umbilical uptake was observed, suggesting that competition for the same transporter may be occurring in the placenta (45). In the present study, the Fv/M MPE ratios of valine, histidine, and lysine were unchanged in nutrient-restricted baboons compared with controls following EAA infusion, suggesting that similar alterations in placental amino acid transport mechanisms occur in human and baboon IUGR. Although EAAs such as valine and histidine are transported by other transporters than system L, they may compete for binding to this transporter in the placental barrier. It is possible to speculate that a preference for certain amino acids by the system L transporter may explain the finding that the in vivo transport of some, but not all, EAAs was decreased in MNR, despite decreased transporter protein expression (27) and leucine transport activity in response to MNR. However, the exact mechanisms are likely to be complex and remain to be elucidated.
IUGR is associated with increased incidence of obesity, insulin resistance, reduced lean body mass, diabetes, and neurodevelopmental impairment in adulthood (2, 3, 9, 28, 58). Reduced nutrient supply leads to alterations in the intrauterine environment, and fetal adaptations to this environment ensure maintenance of basal metabolic functions at the cost of fetal growth (15, 53). A reduction in the transplacental transport of amino acids could, therefore, impact a wide variety of fetal tissues in utero, resulting in developmental programming of adult disease in IUGR. Insulin is a primary fetal growth factor, which also acts via IGF-I to promote fetal growth (11, 14, 31, 40, 57). A reduction in the transport of these amino acids could, therefore, impact fetal insulin and IGF-I signaling, and indeed moderate nutrient restriction in the baboon causes reduced IGF-I expression in the placenta (30) and fetal frontal cortex (58), as well as the development of insulin resistance in juvenile offspring (9). Methionine plays a role in DNA methylation and folate metabolism (33). Nutrient restriction in the baboon alters fetal kidney and brain methylation patterns at 0.5 and 0.9 of gestation (54), consistent with an epigenetic mechanism for the developmental programming of adult diseases in IUGR (41). An alteration in folate metabolism during pregnancy is associated with anxiety and altered learning patterns in offspring (29), and offspring of MNR baboons exhibit more variations in emotional behavior compared with controls (28).
Perspectives and Significance
Maternal supplementation with amino acids has been proposed to be an attractive option to increase fetal growth in IUGR pregnancies. In this study, we report reduced transplacental amino acid transport in vivo and fetal growth restriction following moderate nutrient restriction at 0.9 of gestation in the baboon, a model that shares extensive similarities with human pregnancy. However, it is important to thoroughly understand the mechanisms of transplacental transport of amino acids, as well as the impact of individual amino acids on placental metabolism and fetal growth, before new treatments for IUGR can be developed. This study represents the first step in understanding transplacental amino acid flux in IUGR in a nonhuman primate model.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant P01-HD-21350.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.P., F.J.R., A.C., and C.L. performed experiments; P.P., F.J.R., A.C., and C.L. analyzed data; P.P., F.J.R., A.C., P.W.N., T.L.P., and T.J. interpreted results of experiments; P.P., F.J.R., and A.C. prepared figures; P.P. drafted manuscript; P.P., F.J.R., M.N., A.C., P.W.N., T.L.P., H.L.G., C.L., and T.J. edited and revised manuscript; P.P., F.J.R., M.N., A.C., P.W.N., T.L.P., H.L.G., C.L., and T.J. approved final version of manuscript; F.J.R., M.N., P.W.N., T.L.P., H.L.G., C.L., and T.J. conception and design of research.
REFERENCES
- 1.Anderson AH, Fennessey PV, Meschia G, Wilkening RB, Battaglia FC. Placental transport of threonine and its utilization in the normal and growth-restricted fetus. Am J Physiol Endocrinol Metab 272: E892–E900, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci U S A 108: 3011–3016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81: 3419–3423, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med 19: 307–319, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 3: 428–444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter AM. Animal models of human placentation–a review. Placenta 28, Suppl A: S41–S47, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol 301: R757–R762, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol 290: C305–C312, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Eremia SC, de Boo HA, Bloomfield FH, Oliver MH, Harding JE. Fetal and amniotic insulin-like growth factor-I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology 148: 2963–2972, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Galan HL, Marconi AM, Paolini CL, Cheung A, Battaglia FC. The transplacental transport of essential amino acids in uncomplicated human pregnancies. Am J Obstet Gynecol 200: 91.e1–e7, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 42: 514–519, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman PD, Butler JH, Comline R, Fowden A. The effects of pancreatectomy on the plasma concentrations of insulin-like growth factors 1 and 2 in the sheep fetus. J Dev Physiol 9: 79–88, 1987. [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta 1029: 218–226, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 88: 1205–1211, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576: 935–946, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 51: 2214–2219, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Jansson T, Persson E. Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatr Res 28: 203–208, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? A review. Placenta 27, Suppl A: S91–S97, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res 44: 532–537, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes 59: 1161–1170, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jozwik M, Teng C, Battaglia FC, Meschia G. Fetal supply of amino acids and amino nitrogen after maternal infusion of amino acids in pregnant sheep. Am J Obstet Gynecol 180: 447–453, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Jozwik M, Teng C, Wilkening RB, Meschia G, Battaglia FC. Reciprocal inhibition of umbilical uptake within groups of amino acids. Am J Physiol Endocrinol Metab 286: E376–E383, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Karl PI, Alpy KL, Fisher SE. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol Cell Physiol 262: C834–C839, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 28: 1294–1305, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keenan K, Bartlett TQ, Nijland M, Rodriguez JS, Nathanielsz PW, Zurcher NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: evidence from a translational primate model. Am J Clin Nutr 98: 396–402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konycheva G, Dziadek MA, Ferguson LR, Krageloh CU, Coolen MW, Davison M, Breier BH. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr Res 31: 790–804, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Levitz M, Hubbard GB, Jenkins SL, Han V, Ferry RJ Jr, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta 28: 1200–1210, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci 31: 89–98, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, Hsu JL, Janke SM, Pham TD, Lane RH. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics 18: 43–50, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol Cell Physiol 271: C295–C303, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res 46: 114–119, 1999. [DOI] [PubMed] [Google Scholar]
- 37.McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30% nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. Br J Nutr 109: 1382–1388, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res 44: 233–238, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Nord M, Coleman-Jensen A, Andrews M, Carlson S. Household Food Security in the United States in 2010. Economic Research Report No. 125. September 2011 Washington, DC: US Dept. of Agriculture, Economic Research Services, 2011. [Google Scholar]
- 40.Oliver MH, Harding JE, Breier BH, Gluckman PD. Fetal insulin-like growth factor (IGF)-I and IGF-II are regulated differently by glucose or insulin in the sheep fetus. Reprod Fertil Dev 8: 167–172, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Padmanabhan N, Jia D, Geary-Joo C, Wu X, Ferguson-Smith AC, Fung E, Bieda MC, Snyder FF, Gravel RA, Cross JC, Watson ED. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 155: 81–93, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 86: 5427–5432, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res 73: 602–611, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronzoni S, Marconi AM, Cetin I, Paolini CL, Teng C, Pardi G, Battaglia FC. Umbilical amino acid uptake at increasing maternal amino acid concentrations: effect of a maternal amino acid infusate. Am J Obstet Gynecol 181: 477–483, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Ronzoni S, Marconi AM, Paolini CL, Teng C, Pardi G, Battaglia FC. The effect of a maternal infusion of amino acids on umbilical uptake in pregnancies complicated by intrauterine growth restriction. Am J Obstet Gynecol 187: 741–746, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449–459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 152: 1119–1129, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas 70: 141–145, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol Endocrinol Metab 270: E491–E503, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 33: 117–126, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res 58: 827–832, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr.. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unterberger A, Szyf M, Nathanielsz PW, Cox LA. Organ and gestational age effects of maternal nutrient restriction on global methylation in fetal baboons. J Med Primatol 38: 219–227, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch 447: 532–542, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Wilkes PT, Meschia G, Teng C, Zhu Y, Wilkening RB, Battaglia FC. The effect of an elevated maternal lysine concentration on placental lysine transport in pregnant sheep. Am J Obstet Gynecol 189: 1494–1500, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335: 1363–1367, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Xie L, Antonow-Schlorke I, Schwab M, McDonald TJ, Nathanielsz PW, Li C. The frontal cortex IGF system is down regulated in the term, intrauterine growth restricted fetal baboon. Growth Horm IGF Res 23: 187–192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]