Abstract
Reducing blood flow to working muscles during dynamic exercise causes metabolites to accumulate within the active muscles and evokes systemic pressor responses. Whether a similar cardiovascular response is elicited with normal blood flow to exercising muscles during dynamic exercise remains unknown, however. To address that issue, we tested whether cardiovascular responses are affected by increases in blood flow to active muscles. Thirteen healthy subjects performed dynamic plantarflexion exercise for 12 min at 20%, 40%, and 60% of peak workload (EX20, EX40, and EX60) with their lower thigh enclosed in a negative pressure box. Under control conditions, the box pressure was the same as the ambient air pressure. Under negative pressure conditions, beginning 3 min after the start of the exercise, the box pressure was decreased by 20, 45, and then 70 mmHg in stepwise fashion with 3-min step durations. During EX20, the negative pressure had no effect on blood flow or the cardiovascular responses measured. However, application of negative pressure increased blood flow to the exercising leg during EX40 and EX60. This increase in blood flow had no significant effect on systemic cardiovascular responses during EX40, but it markedly attenuated the pressor responses otherwise seen during EX60. These results demonstrate that during mild exercise, normal blood flow to exercising muscle is not a factor eliciting cardiovascular responses, whereas it elicits an important pressor effect during moderate exercise. This suggests blood flow to exercising muscle is a major determinant of cardiovascular responses during dynamic exercise at higher than moderate intensity.
Keywords: integrated circulatory regulation, neural cardiovascular regulation, muscle metaboreflex
during dynamic exercise, increased skeletal muscle blood flow is essential to ensure an appropriate supply of O2 to the active muscles and to remove the metabolic byproducts and generated heat. This exercise-induced skeletal muscle hyperemia is thought to be initially elicited by local mechanical and metabolic factors and to be modulated by neural cardiovascular regulatory mechanisms (8, 41). Neural mechanisms also act to increase cardiac output (CO) and to redistribute blood flow from inactive regions to support the increase in skeletal muscle flow. This neural cardiovascular regulation is thought to be mediated by central command (41), as well as by feedback transmitted via afferent nerves (group III and IV fibers), innervating the working skeletal muscles, which are sensitive to mechanical (the so-called muscle mechanoreflex) and metabolic changes (the so-called muscle metaboreflex) (26, 33, 34, 37, 42), and are modulated via the arterial and cardiopulmonary baroreflexes (23, 41, 42).
Reducing the blood flow to working muscles during dynamic exercise causes accumulation of metabolites within the active muscles and evokes muscle metaboreflex-mediated increases in sympathetic nerve activity, heart rate (HR), and systemic blood pressure (1, 5, 12, 19, 29, 30, 35, 42). In addition, the reduced blood flow and resultant accumulation of metabolites would cause muscle fatigue so that recruitment of additional motor units would be required to perform the same amount of work, and thus central command would be increased (12, 14, 19, 30). It has been demonstrated in dogs that reducing hindlimb blood flow to ∼50% of the free flow level during mild treadmill running elicits a systemic pressor response (5, 18, 25, 51). Moreover, increasing the workload reduces the change of blood flow necessary to elicit the pressor response, so that at workloads greater than moderate, a pressor response was elicited as soon as any reduction in blood flow occurs (5, 18, 51). Similar findings have also been reported in humans (19, 30, 35). Apparently, the contribution to cardiovascular control made by metabolic feedback from an exercising muscle is dependent on both the adequacy of the muscle perfusion and the work performed by the muscle. However, the pressor responses evoked by reductions in active muscle blood flow reflect an overactivation of the pressor mechanisms not seen under free-flow conditions. Consequently, it remains unknown whether normally distributed blood flow to exercising muscle provokes the systemic cardiovascular responses observed during free-flow exercise conditions. Also unknown is whether increasing active muscle blood flow to levels higher than the free-flow exercise levels can reduce pressor responses.
One way to address these issues is to determine whether systemic cardiovascular responses are attenuated when blood flow to active muscles is increased. In the present study, therefore, we investigated systemic cardiovascular responses to increases in exercising leg blood flow (LBF) using a newly developed experimental model that noninvasively increases exercising LBF during plantarflexion exercise in humans. With this model, we assessed the effects of exercise workloads ranging from very mild to moderate on the cardiovascular responses to increases in exercising LBF. We hypothesized that, in humans, increasing exercising LBF would have no important effect on systemic cardiovascular responses during mild exercise, but it would attenuate the cardiovascular responses during exercise at higher than moderate intensity.
METHODS
Subjects.
We studied 13 healthy volunteers (12 men and 1 woman). The characteristics of the subjects are shown in Table 1. None of the subjects were receiving medication and none smoked. The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee on Human Research of Meiji University. Each subject gave informed written consent.
Table 1.
Characteristics of subjects
| Characteristic | Mean Value |
|---|---|
| Age, yr | 21 ± 1 |
| Weight, kg | 66.0 ± 3.4 |
| Height, cm | 174.2 ± 3.1 |
| Peak workload of incremental plantarflexion exercise, kg | 20.19 ± 0.82 |
Values are expressed as means ± SE.
Procedures.
The experiments were conducted in a room maintained at 25°C on four separate days. On the first day, to determine maximum work rate, each subject performed an incremental rhythmic (30 contractions/min with a duty cycle of 1-s contraction and 1-s relaxation, metronome-paced) dynamic plantarflexion exercise to volitional fatigue. Subjects adopted a semirecumbent position and performed the plantarflexion exercise with their right leg using an ergometer (S-11046, Takei, Japan). The incremental exercise consisted of repeated 2-min exercise periods with 1-min rest intervals. The exercise was started at workload of 10 kg for male subjects and 5 kg for the female subject. The load was increased by 2.5 kg in each successive 2-min exercise period until the ratings of perceived exertion (RPEs; based on the 6 to 20 Borg scale) reached 15 (7). Thereafter, the load was increased by 1.25 kg in each successive exercise period for the rest of the test. The RPEs were obtained at the end of each exercise period. Volitional fatigue was defined as an inability to maintain the contraction frequency. The mean value of the peak workload during the incremental exercise is shown in Table 1. The main experiments were then performed on 3 other days.
After each subject adopted a semirecumbent position and all of the measuring equipment was in place, he or she was allowed to rest for at least 15 min before data collection was begun. Resting data were acquired for 3 min prior to the start of the exercise, after which the subject performed the rhythmic dynamic plantarflexion exercise for 12 min at 20%, 40%, or 60% of peak workload (EX20, EX40, and EX60, respectively) with the lower thigh area enclosed in a negative pressure box. The mean absolute workloads corresponding to 20%, 40%, and 50% of peak workload were 4.04 ± 0.16 kg, 8.08 ± 0.33 kg, and 12.12 ± 0.49 kg, respectively. Under control conditions, the box pressure was the same as the ambient air pressure. Under negative pressure conditions, beginning 3 min after the start of the exercise, the box pressure was reduced to 20, 45, and then 70 mmHg below the ambient pressure in stepwise fashion with 3-min step durations. The purpose of the negative pressure application was to raise perfusion pressure in the lower thigh via muscle pumping action to achieve an increase in blood flow to the exercising muscle. On each day, subjects performed two trials from the above-mentioned six trial conditions assigned in random order.
Measurements.
HR was monitored using a three-lead electrocardiogram (ECG). Beat-to-beat changes in blood pressure were assessed using finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, Netherlands); the monitoring cuff was placed around the middle finger of the left hand, with the forearm and hand supported, so that the cuff was aligned at the level of the heart. Stroke volume (SV) was measured beat-to-beat using impedance cardiography (Physio Flow PF05 Lab1; Manatec Biomedical, Paris, France). In addition, the subject wore a mask connected to an electric gas flow meter (AE-300s, Minato Medical Science, Japan) to measure minute ventilation (V̇e), oxygen uptake (V̇o2), and carbon dioxide production (V̇co2) breath-to-breath. The box pressure was measured using a pressure transducer mounted on the box. We measured exercising LBF using Doppler ultrasound, as previously described (20, 48). Briefly, a Doppler ultrasound system (HDI 5000; ATL Ultrasound, Bothell, WA) equipped with a hand-held transducer probe (model L12-5) with an operating frequency of 6 MHz was utilized to simultaneously measure two-dimensional common femoral artery diameter and mean blood velocity (MBV). All Doppler data were recorded continuously on S-VHS videotape (ST-120; Maxell, Osaka, Japan). The videotape record of the vessel image was digitized using a digital video board (PCI-1411; National Instruments, Austin, TX) and stored on a personal computer equipped with software for measuring vessel diameter. The femoral artery diameters related to systole (Ds; mm) and diastole (Dd; mm) were taken as the largest and smallest diameter within each cardiac cycle, respectively. The mean diameter (Dm; mm) was calculated as Dm = DS/3+2 · Dd/3. We calculated Dm at rest and at the end of each 3 min of exercise as the mean Dm from 20 consecutive beats during the final 30 s of each period. The cross-sectional area of the femoral artery (CSA) was estimated using a representative Dm as CSA = (Dm/10/2)2 ·π. MBV was continuously estimated using a computer program developed with the aid of LabVIEW (version 6.0; National Instruments), as described in detail elsewhere (20). Our system collects MBV at 100 Hz together with the analog signals representing the ECG, blood pressure waveform, and box pressure. Beat-to-beat HR, mean arterial pressure (MAP) and MBV were calculated using an off-line data-analysis program. LBF was calculated as the product of MBV (cm/s) and femoral artery CSA (cm2) and was multiplied by 60 to obtain values expressed in terms of milliliters per minute. CO was calculated from the SV and HR as CO = SV · HR. Total vascular conductance (TVC) was calculated as TVC = CO/MAP. Hemodynamic, respiratory, and metabolic values at rest were calculated by averaging the data obtained during the 3-min resting period. The values during exercise at each box pressure setting were obtained by averaging over the last minute of the each 3 min of exercise (i.e., 3rd, 6th, 9th, and 12th min of the exercise, respectively). RPEs were obtained at the end of each 3 min of exercise.
Statistical analysis.
Data are presented as means ± SE. Two-way repeated-measures ANOVA followed by the Bonferroni post hoc comparisons test were performed to compare the cardiovascular, respiratory, and metabolic responses between control and negative pressure trials during EX20, EX40, and EX60, respectively. Values of P < 0.05 were considered significant.
RESULTS
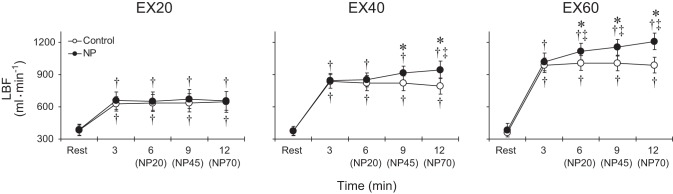
Figure 1 shows representative exercising LBF responses during EX60 in the control and negative pressure trials for one subject. Group average LBF responses in the control and negative pressure trials are shown in Fig. 2. Under control conditions, LBF increased from the resting level and then remained steady throughout the exercise at all workloads. During EX20, none of the negative pressures affected LBF. During EX40, on the other hand, application of 45 or 70 mmHg negative pressure increased LBF compared with the control condition. In addition, LBF was significantly higher during application of 70 mmHg negative pressure than at the 3rd min of exercise, a point when box pressure is the same as the ambient pressure in both the control and NP trials. During EX60, all applied negative pressures increased LBF to levels significantly higher than both control and the level at the 3rd min of exercise. Femoral artery diameter did not differ at rest among the trials, and it was unchanged during exercise at all workloads in both the control and negative pressure trials. The average femoral artery diameter in the six trials at rest was 9.5 ± 0.3 mm.
Fig. 1.
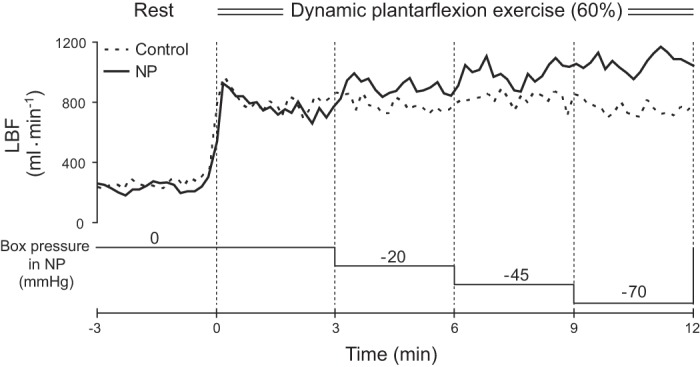
Leg blood flow (LBF) responses during exercise at 60% of peak workload in the control and negative pressure (NP) trials in a representative subject. Dashed lines indicate the exercise start time (i.e., 0 min) or the time box pressure changed in the NP trial. To better show the experimental protocol, changes in box pressure during the NP trial are shown schematically.
Fig. 2.
Group averaged LBF during exercise at 20%, 40%, and 60% of peak workload in the control and NP trials. Abbreviations: EX20, EX40 and EX60, exercise at 20%, 40% and 60% of peak workload, respectively. NP20, NP45, and NP70, 20 mmHg, 45 mmHg, and 70 mmHg negative pressure, respectively. *P < 0.05 vs. control, †P < 0.05 vs. rest, ‡P < 0.05 vs. 3rd min of exercise (i.e., when the box pressure is the same as the ambient pressure in both the control and NP trials).
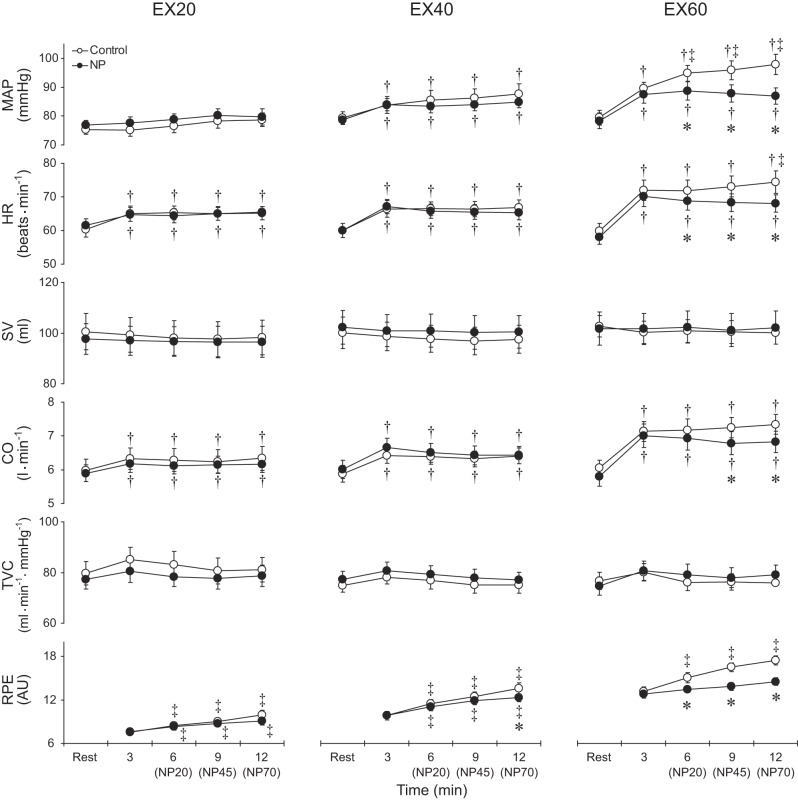
Figure 3 shows group average responses for MAP, HR, SV, CO, TVC, and RPE in the control and negative pressure trials. During EX20, HR, and CO increased from rest and then remained steady in both the control and negative pressure trials, whereas MAP, SV, and TVC did not significantly change from the resting values. During EX40, MAP, HR, and CO increased from their resting levels and then remained steady throughout the exercise periods in both the control and negative pressure trials. SV and TVC did not significantly change from the resting levels in either the control or negative pressure trials. During EX60 in both the control and negative pressure trials, MAP, HR, and CO increased from their resting levels, while SV and TVC did not significantly change. In addition, in the control trial, MAP and HR gradually increased over time throughout the exercise. RPE increased progressively throughout the exercise at all intensities in the control trials.
Fig. 3.
Group averaged mean arterial pressure (MAP), heart rate (HR), stroke volume (SV), cardiac output (CO), total vascular conductance (TVC) and ratings of perceived exertion (RPE) during exercise at 20%, 40%, and 60% of peak workload in the control and NP trials. AU, arbitrary unit. Other abbreviations are the same as in Fig. 2. *P < 0.05 vs. control; †P < 0.05 vs. rest; ‡P < 0.05 vs. 3rd min of exercise (i.e., when the box pressure is the same as the ambient pressure in both the control and NP trials).
Application of negative pressure did not alter the hemodynamic responses or RPE during EX20. During EX40, however, although we detected no significant differences in any of the hemodynamic parameters between the control and negative pressure trials, RPE was lower during application of 70-mmHg negative pressure than under control conditions. During EX60, all levels of negative pressure reduced MAP and HR, and 45 and 70 mmHg negative pressure also reduced CO compared with control. As a result, the gradual increases in MAP and HR observed under control conditions were almost completely eliminated in the negative pressure trials. In addition, RPE was lower than control during application of all levels of negative pressure.
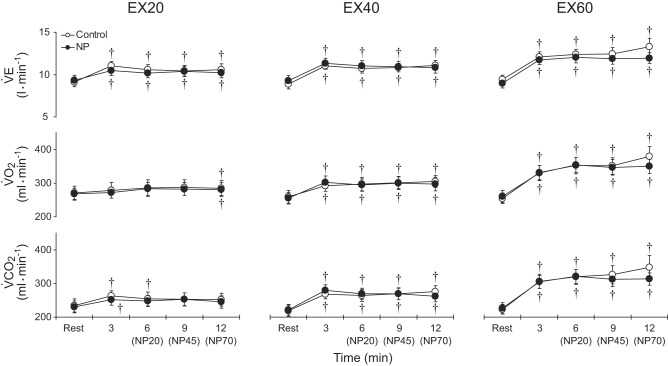
Effects on V̇e, V̇o2, and V̇co2 are presented in Fig. 4. During EX20 in both the control and negative pressure trials, V̇e was higher than the resting level at all time points. Significant increases in V̇o2 were observed only at the 12th min of exercise in both trials. V̇co2 was higher than the resting level at the 3rd and 6th min in the control trials and only at the 3rd min in the negative pressure trials. During EX40 and EX60, V̇e, V̇o2, and V̇co2 all increased from their resting levels, but then did not significantly change over time in either the control or negative pressure trials. Thus, application of negative pressure to the leg did not alter the V̇e, V̇o2, or V̇co2 responses during exercise at any workload.
Fig. 4.
Group averaged V̇e, V̇o2, and V̇co2 during exercise at 20%, 40%, and 60% of peak workload in the control and NP trials. Abbreviations are the same as in Fig. 2. †P < 0.05 vs. rest.
DISCUSSION
To the best of our knowledge, this is the first study examining the effects of increasing blood flow to exercising muscle on systemic cardiovascular, respiratory, and metabolic responses during dynamic exercise at workloads ranging from very mild to moderate in humans. The major finding of this investigation is that the increase in exercising LBF induced through application of negative pressure to the lower thigh had no significant effect on systemic cardiovascular responses during EX40, but it greatly attenuated the pressor responses induced by EX60. These results indicate that during EX40, normal blood flow to exercising muscles is not a factor eliciting systemic cardiovascular responses, but systemic pressor responses are elicited under the same conditions during EX60. This suggests that blood flow to the exercising muscle becomes inadequate relative to the work being done by the muscle at an exercise intensity between 40% and 60% of peak workload and that this insufficiency contributes to the systemic pressor responses elicited during exercise at levels higher than that threshold intensity. Importantly, the increase in LBF during EX60 almost completely eliminated the gradual increase in MAP and HR observed under control conditions, suggesting that normal levels of blood flow to exercising muscles are a major factor contributing to the gradual induction of pressor responses during dynamic exercise at moderate intensity.
During EX60, increasing LBF through application of negative pressure to the lower thigh reduced HR and CO, but TVC was not significantly affected. This means that the attenuation of the blood pressure response seen with increases in exercising LBF is due mainly to a reduction in CO, not peripheral vasodilation, and that the decrease in CO was the net result of a decrease in HR with no change in SV. Application of negative pressure to the entire lower body (i.e., lower body negative pressure; LBNP) during dynamic leg exercise increases perfusion pressure in the exercising muscles (13, 36), but LBNP also reduces central blood volume by causing blood to pool in the lower body (13, 28, 36, 43). As a result, CO and arterial blood pressure fall due to a significant reduction in SV, while unloading of the arterial and cardiopulmonary baroreceptors work to increase HR and peripheral vascular resistance (9, 13, 21, 28, 36). Thus, the mechanism underlying the decrease in CO and arterial blood pressure observed in the present study differs from that during application of LBNP. In our experimental model, we applied negative pressure to only a small tissue mass (i.e., lower thigh) in an effort to avoid pooling of nonnegligible amounts of blood and the resultant reduction in SV. Indeed, the observed reduction in HR in the face of decreased arterial blood pressure indicates that application of negative pressure to the lower thigh did not evoke baroreflex responses. This is particularly important for evaluating the effect of increasing blood flow to exercising muscle on the systemic pressor response, as baroreflex responses would counteract and obscure the attenuation of pressor responses. In addition, application of negative pressure did not significantly affect any measured variables during EX20. These results indicate that application of negative pressure to the lower thigh does not, by itself, affect hemodynamic responses, which is further evidence that the attenuation of pressor responses observed with application of negative pressure during EX60 is secondary to the increase in blood flow to the exercising muscle.
Although we do not know for certain the mechanism(s) responsible for the attenuation of cardiovascular responses elicited by increasing blood flow to exercising muscles, it is likely that two major neural cardiovascular regulatory mechanisms are involved: the muscle metaboreflex and central command. Recent studies by Dempsey and colleagues (2, 11) demonstrated in humans that partial blockade of group III and IV muscle afferent input into the spinal cord significantly attenuates cardiovascular and respiratory responses during mild- to moderate-intensity cycling exercise. Their results provide strong evidence of the participation of skeletal muscle afferents in normal cardiorespiratory responses to exercise in humans. The functional relationship between reductions in exercising muscle blood flow and the resultant pressor responses has been examined in both animals and humans to characterize muscle metaboreflex-mediated pressor responses (5, 18, 19, 24, 30, 35, 51). It has been shown that at low workloads, a substantial reduction in exercising muscle blood flow (∼50% of free flow) is needed to evoke a pressor response, but that increases in the workload reduce the change in blood flow necessary to evoke the reflex pressor response. Our results are consistent with those earlier studies and further show the contribution of the exercising muscle blood flow to cardiovascular responses under free-flow conditions, which could not be examined in earlier studies relying on reduced blood flow to exercising muscle. The nonsignificant effects of the increase in exercising LBF on the pressor responses during EX40 indicate that the muscle metaboreflex is not activated at mild workloads with normal exercising muscle blood flow, most likely because flow remains sufficient to prevent accumulation of metabolites within the active muscles. It follows then that the observed attenuation of the pressor responses elicited by increases in exercising LBF during EX60 could be due to inhibition of the muscle metaboreflex, reflecting the elimination of metabolite accumulation. These results also suggest that the muscle metaboreflex is tonically active during exercise at a moderate workload, reflecting conditions in which normal blood flow to exercising muscles is not sufficient to prevent the accumulation of metabolites.
Alternatively, or in addition, central command may be reduced by the increase in exercising muscle blood flow. It has been shown that subjects must make a greater effort to perform the same amount of work when blood flow to exercising muscles is restricted (14, 19, 30). In such situations, the reduced blood flow and resultant accumulation of metabolites would cause muscle fatigue, and recruitment of additional motor units would be required to maintain the same force. Thus, central command would be increased. It seems likely that the opposite reaction occurs when blood flow to exercising muscles is increased by negative pressure. More specifically, the increased blood flow would reduce the accumulation of metabolites within exercising muscles, thereby reducing fatigue. This concept is supported by our observations that 1) decreased exertion was only observed when blood flow to exercising muscle was increased (i.e., RPE was lower in the negative pressure trials during EX40 and EX60, but not EX20), and 2) the decrease in RPE was more apparent during higher-intensity exercise that likely induced greater accumulation of metabolites under normal blood flow conditions. Central command primarily alters HR in humans via modulation of parasympathetic tone (41). During small muscle mass exercise, such as rhythmic plantarflexion, it does not increase sympathetic outflow to muscles until the effort is nearly maximal (47). In the present study, effort was moderate at the end of EX60 under control conditions. At that intensity, the reduction in central command during the negative pressure trials may not have influenced sympathetic activity to the resistance vessels. Therefore, less central command may account for part of the reduction in HR seen in the negative pressure trials during EX60. Still, the mechanism(s) mediating the attenuation of cardiovascular responses via increases in blood flow to exercising muscles remains to be elucidated.
In contrast to the apparent inhibition of pressor responses, the increase in exercising LBF had no significant effect on ventilatory responses. This indicates that normal blood flow to exercising muscle does not provoke exercise hyperpnea during rhythmic plantarflexion exercise up to moderate intensity. In addition, our results suggest that the cardiovascular responses are more susceptible to alterations in the balance between exercising muscle blood flow and the metabolic demands of the active muscle than are the ventilatory responses. These results agree well with earlier findings showing that whereas metabolic feedback evokes important pressor responses from working muscles, it has little effect on respiration (27, 40, 46). It is noteworthy, however, that according to the aforementioned studies by Dempsey and colleagues (2, 11), activation of skeletal muscle afferents plays an important role in the normal respiratory responses to cycling exercise. But the contribution of neural control of ventilatory and cardiovascular responses during exercise would depend importantly on the mass of the contracting muscle (42). The nonsignificant effect of the increase in exercising muscle blood flow on exercise hyperpnea in the present study may, thus, reflect the small muscle mass engaged during the plantarflexion exercise. Further studies are clearly needed to better understand the relationship between blood flow to exercising muscle and control of ventilation during dynamic exercise.
Limitations and methodological considerations.
We assume that application of negative pressure raises the perfusion pressure in the lower thigh via muscle-pumping action and leads to an increase in exercising muscle blood flow. However, because we did not measure leg venous pressure, we could not assess changes in perfusion pressure in the leg and provide a definitive explanation for the increase in blood flow. It has been reported, however, that externally applied negative pressure is well transmitted to calf tissues and that ∼80% of the applied negative pressure is even transmitted as much as 4 cm deep into the muscle tissue (39, 50). Although these results were observed under resting conditions, it would seem reasonable to assume that ∼80% of the negative pressure was transferred to the calf muscle tissue, at least during the relaxation phase of the exercise. The reduction in tissue pressure would be expected to lead to distention of the veins, lowering venous pressure, and, in turn, greater filling of the venous segments during the relaxation periods than would occur at normal pressure. Contractions of the leg muscles during application of negative pressure would then lead to intermittent emptying of the well-filled veins, and hence, the mean flow in the leg will be increased (i.e., muscle pumping action). For this reason, effective muscle pumping action is essential for continuous elevation of the perfusion pressure and facilitation of LBF upon application of negative pressure. We found that the application of negative pressure to the lower thigh increased LBF during EX40 and EX60, but not during EX20. We think that during EX20, the force of the muscle contraction was too weak to pump out a sufficient volume of blood from the well-filled veins to reduce venous pressure. As a consequence, the perfusion pressure may not increase enough to facilitate flow into the veins during the phasic relaxation periods. This scenario is supported by the results of an earlier study demonstrating that application of negative pressure to a resting leg induces a rapid rise in leg blood flow within 30 s, after which the flow declines to a level below the baseline within 60 s (32). It is likely that negative pressure application at rest increases blood flow only during the initial venous distension, as venous pressure returns to baseline once the veins are fully distended (4).
In addition, without leg venous pressure measurements, we were unable to evaluate changes in vascular conductance in the exercising leg. If the leg vascular conductance (LVC) increased with application of negative pressure, it might be a factor contributing to the reduction in MAP observed during the EX60 trials. When we calculated LVC as LBF/MAP, which does not take the change in leg perfusion pressure into account, it was significantly higher than control during application of negative pressure in EX40 and EX60. The largest increase in LVC was observed with application of 70 mmHg negative pressure during EX60. We estimate that this increase in LVC reduced MAP by ∼4 mmHg, as follows. Making the assumption that CO and vascular conductance elsewhere in the body (i.e., TVC − LVC) did not differ from those observed under control conditions, we estimated MAP using the following formula: MAP = CO observed in control/(TVC in control − LVC in control + LVC in negative pressure conditions). However, the estimated reduction in MAP (i.e., MAP observed in control − estimated MAP) accounted for less than 40% of the actual change in MAP, which means the attenuated MAP responses observed during the negative pressure trials are not explained by the increase in LVC. Importantly, assuming that perfusion pressure in the exercising leg increased during application of negative pressure, the actual LVC might not increase or may be lower than that calculated as LBF/MAP. Therefore, the influence of an increase in LVC on the MAP response should also be smaller than what we estimated as above. Moreover, as mentioned, the major cause of the lower MAP was a reduction in CO, as there was no significant change in TVC. It is noteworthy that HR and CO would not decline during application of negative pressure if the reduction in MAP resulted solely from an increase in LVC, rather than deactivation of pressor mechanism(s) via increased LBF.
To evaluate blood flow to the calf muscles, flow measurements at the popliteal artery would be preferable to measurements at the common femoral artery. However, to sustain the negative pressure at specified levels, our subjects had to wear a Neoprene leg seal that covered the area around the knee to prevent leakage of air into the box. In addition, a sidewall of the negative pressure box was situated around the popliteal region. These technical features made it impossible to position the ultrasonograph probe at the proper location to measure blood flow in the popliteal artery. Therefore, we decided to assess LBF in the common femoral artery.
The LBF measured in the common femoral artery reflects the whole-leg tissue blood flow. At rest, blood flow to the skin and nonmuscle tissues accounts for 40% to 50% of the total LBF (45). During exercise, however, the relative fractions of flow to the skin and nonmuscle tissues greatly decline due to a marked rise in blood flow to the exercising muscles. For example, the contribution of skin blood flow to total femoral blood flow is reportedly less than 10% during bicycle exercise at ∼50% of maximum oxygen consumption (45), and the flow to nonmuscle tissues is even lower than to the skin. Thus, it appears safe to assume that a large majority of the increase in LBF during plantarflexion exercise reflects blood flow to exercising muscles. Moreover, because the rise in LBF during application of negative pressure is induced through muscle pumping action, most of that increase in blood flow would be expected to perfuse exercising muscles. Consistent with that idea, application of LBNP reportedly reduces leg skin blood flow (49).
Recent studies by Sinoway and colleagues (10, 32) showed that distension of veins in the limbs can induce reflex vasoconstriction and systemic pressor responses. If this response occurred during application of negative pressure in the present study, it could offset the decrease in systemic pressor responses during exercise elicited by increases in LBF. However, those studies also suggest that substantial venous congestion, distension of the veins, and/or tissue deformation within the leg is needed to induce systemic pressor responses. For example, Cui et al. (10) showed that application of −100 mmHg to an arterially occluded leg increases muscle sympathetic nerve activity and induces a ∼9 mmHg rise in MAP. In addition, Lott et al. (32) demonstrated that application of −25 mmHg or −50 mmHg to one leg without occlusion has no influence on MAP, while application of −75 mmHg or −100 mmHg to the leg without occlusion evokes small increases in MAP (∼2 mmHg with application of −75 mmHg and ∼3 mmHg with application of −100 mmHg). Application of negative pressure to an exercising leg may induce less venous congestion than during resting conditions, as the muscle pump would facilitate venous blood flow toward the heart. Hence, it is unlikely that the application of negative pressure to the exercising lower thigh in the present study caused the substantial venous congestion needed to elicit important systemic pressor responses. Therefore, we think that venous distention in the leg had little, if any, effect on cardiovascular responses in the present study.
Systemic V̇o2 and V̇co2 did not significantly change during application of negative pressure, which indicates the net metabolic rate in the body as a whole did not change. Nonetheless, our results do not preclude the possibility that increases in LBF influence the metabolism of exercising muscles. For example, increasing blood flow to exercising muscles reportedly leads to a rise in oxygen consumption by the working muscles (6, 29) and reduces venous metabolite concentrations (29). However, we found that subjects performed the same amount of work with less sensation of fatigue when LBF was increased through application of negative pressure. This suggests that under negative pressure conditions, fewer muscle fibers were recruited to maintain the same force than under control conditions. If that was the case, oxygen consumption by the exercising leg may not have increased or may have even decreased (i.e., there was an increase in exercise efficiency) with increased in LBF. Further studies investigating metabolism in exercising muscles (e.g., oxygen consumption and production of metabolites) will be important for elucidation of the mechanisms underlying the decreases in cardiovascular responses associated with increases in blood flow to exercising muscles.
Perspectives and Significance
The results obtained in this study provide new insight into the cardiovascular regulation during dynamic exercise in humans. We have shown that during mild exercise, the normal blood flow to the exercising muscles is not a contributing factor affecting the observed cardiovascular responses. During moderate exercise under the same conditions, however, normal muscle blood flow elicits an important pressor response. It has been shown that blood flow to active skeletal muscles increases linearly with increasing workload from very mild to severe (3, 41). Above a moderate workload, however, the rise in blood flow may not be sufficient to meet the metabolic demand of the muscle, and the resultant blood flow deficit may gradually enlarge at heavier workloads. This insufficient blood supply to exercising muscles may then be a cause of the exponential rise in sympathetic nerve activity observed during dynamic exercise with increasing workloads above moderate levels (22, 44). In addition, because even normal blood flow to exercising muscle elicits pressor responses, impairment of blood flow in pathological conditions such as heart failure (18, 25, 38) and arteriosclerosis obliterans in limbs (31), as well as conditions such as hyperthermia and dehydration (15, 16, 17), would exaggerate sympathoexcitation and cardiovascular responses during exercise. Furthermore, our findings should also contribute to a better understanding of exercise-induced fatigue. We found that increasing blood flow to exercising muscles enables subjects to perform the same amount of work with less effort. Therefore, the normal blood flow to exercising muscles would be an important factor contributing to the development of fatigue during dynamic exercise.
In conclusion, we found that increasing blood flow to exercising muscles had no significant effect on systemic cardiovascular responses during EX40, but it greatly attenuated the pressor responses elicited during EX60. It is noteworthy that increasing blood flow to the exercising muscle during EX60 almost completely eliminated the gradual rise in MAP and HR otherwise observed under control conditions. These results suggest that the blood flow normally distributed to exercising muscle is a major determinant of the cardiovascular responses seen during dynamic exercise at higher than moderate intensities.
GRANTS
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Overseas Outreach Program of Meiji University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.I., T.K.I., N.K., and T.N. conception and design of research; M.I. and T.K.I. performed experiments; M.I. and T.K.I. analyzed data; M.I., T.K.I., N.K., and T.N. interpreted results of experiments; M.I. and T.K.I. prepared figures; M.I., T.K.I., N.K., and T.N. drafted manuscript; M.I., T.K.I., N.K., and T.N. edited and revised manuscript; M.I., T.K.I., N.K., and T.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank the volunteer subjects.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aratow M, Fortney SM, Watenpaugh DE, Crenshaw AG, Hargens AR. Transcapillary fluid responses to lower body negative pressure. J Appl Physiol 74: 2763–2770, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 8.Calbet JA, Joyner MJ. Disparity in regional and systemic circulatory capacities: do they affect the regulation of the circulation? Acta Physiol (Oxf) 199: 393–406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Convertino VA, Ludwig DA, Cooke WH. Stroke volume and sympathetic responses to lower-body negative pressure reveal new insight into circulatory shock in humans. Auton Neurosci 111: 127–134, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Blaha CA, Herr MD, Drew RC, Muller MD, Sinoway LI. Limb suction evoked during arterial occlusion causes systemic sympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol 309: R482–R488, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempsey JA, Blain GM, Amann M. Are type III-IV muscle afferents required for a normal steady-state exercise hyperpnoea in humans? J Physiol 592: 463–74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–45, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Eiken O. Effects of increased muscle perfusion pressure on responses to dynamic leg exercise in man. Eur J Appl Physiol Occup Physiol 57: 772–776, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JP, Adlan AM, Shantsila A, Secher JF, Sørensen H, Secher NH. Muscle metaboreflex and autonomic regulation of heart rate in humans. J Physiol 59: 3777–3788, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation 107: 824–830, 2003. [DOI] [PubMed] [Google Scholar]
- 16.González-Alonso J, Calbet JAL, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol 513: 895–905, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol 586: 45–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Ichinose M, Delliaux S, Watanabe K, Fujii N, Nishiyasu T. Evaluation of muscle metaboreflex function through graded reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am J Physiol Heart Circ Physiol 301: H609–H616, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Ichinose M, Nishiyasu T. Muscle metaboreflex modulates the arterial baroreflex dynamic effects on peripheral vascular conductance in humans. Am J Physiol Heart Circ Physiol 288: H1532–H1538, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol 576: 947–958, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586: 2753–2766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol 544: 939–948, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichinose M, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinose M, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, Fano D, O'Leary DS. Dynamic cardiac output regulation at rest, during exercise, and muscle metaboreflex activation: impact of congestive heart failure. Am J Physiol Regul Integr Comp Physiol 303: R757–R768, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichinose M, Watanabe K, Fujii N, Kondo N, Nishiyasu T. Muscle metaboreflex activation speeds the recovery of arterial blood pressure following acute hypotension in humans. Am J Physiol Heart Circ Physiol 304: H1568–H1575, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Iellamo F, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Role of muscular factors in cardiorespiratory responses to static exercise: contribution of reflex mechanisms. J Appl Physiol 86: 174–180, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974. [DOI] [PubMed] [Google Scholar]
- 29.Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol 263: H1078–H1083, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Joyner MJ. Does the pressor response to ischemic exercise improve blood flow to contracting muscles in humans? J Appl Physiol 71: 1496–1501, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. [DOI] [PubMed] [Google Scholar]
- 32.Lott ME, Hogeman C, Herr M, Bhagat M, Kunselman A, Sinoway LI. Vasoconstrictor responses in the upper and lower limbs to increases in transmural pressure. J Appl Physiol 106: 302–310, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JH, Schmibt RF. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. In: Handbook of Physiology: The Cardiovascular System, sect. 2, vol. III, part 2, Bethesda, MD: American Physiological Society, 1983, p. 623–658. [Google Scholar]
- 34.Mitchell JH. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990. [PubMed] [Google Scholar]
- 35.Nishiyasu T, Nagashima K, Nadel ER, Mack GW. Human cardiovascular and humoral responses to moderate muscle activation during dynamic exercise. J Appl Physiol 88: 300–307, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Nishiyasu T, Shi X, Mac GW, Nadel ER. Forearm vascular responses to baroreceptor unloading at the onset of dynamic exercise. J Appl Physiol 75: 979–985, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol 77: 2778–2783, 1994. [DOI] [PubMed] [Google Scholar]
- 38.O'Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Olsen H, Länne T. Reduced venous compliance in lower limbs of aging humans and its importance for capacitance function. Am J Physiol Heart Circ Physiol 275: H878–H886, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol 41: 693–701, 1976. [DOI] [PubMed] [Google Scholar]
- 41.Rowell LB, O'Leary DS, Kellogg DL. Integration of cardiovascular control systems in dynamic exercise. In: Handbook of Physiology: Exercise: Regulation and Integration of Multiple Systems. sect. 12, chapt. 17, Bethesda, MD: American Physiological Society, 1996, p. 770–838. [Google Scholar]
- 42.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflex and mechanoreflexes. J Appl Physiol 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Rowell LB. Human Circulation. New York: Oxford University Press, 1986. [Google Scholar]
- 44.Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol 75: 663–667, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol 64: 649–657, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Scott AC, Francis DP, Davies LC, Ponikowski P, Coats AJS, Piepoli MF. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol 529: 863–870, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe K, Ichinose M, Tahara R, Nishiyasu T. Individual differences in cardiac and vascular components of the pressor response to isometric handgrip exercise in humans. Am J Physiol Heart Circ Physiol 306: H251–H260, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Watenpaugh DE, Breit GA, Buckley TM, Ballard RE, Murthy G, Hargens AR. Human cutaneous vascular responses to whole-body tilting, Gz centrifugation, and LBNP. J Appl Physiol 96: 2153–2160, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wolthuis RA, Bergman SA, Nicogossian AE. Physiological effects of locally applied reduced pressure in man. Physiol Rev 54: 566–595, 1974. [DOI] [PubMed] [Google Scholar]
- 51.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]