Abstract
Voltage-gated sodium channels (NaV) 1.7 are highly expressed on the axons of somatic afferent neurons and are thought to play an important role in the signaling of inflammatory pain. NaV 1.7 channels are classified as tetrodotoxin (TTX)-sensitive, meaning that they are blocked by TTX concentrations of less than 300 nM. These findings prompted us to determine in decerebrated, unanesthetized rats, the role played by NaV 1.7 channels in the transmission of muscle afferent input evoking the exercise pressor reflex. We first showed that the exercise pressor reflex, which was evoked by static contraction of the triceps surae muscles, was reversibly attenuated by application of 50 nM TTX, but not 5 nM TTX, to the L4-L5 dorsal roots (control: 21 ± 1 mmHg, TTX: 8 ± 2 mmHg, recovery: 21 ± 3 mmHg; n = 6; P < 0.01). We next found that the peak pressor responses to contraction were significantly attenuated by dorsal root application of 100 nM Ssm6a, a compound that is a selective NaV 1.7 channel inhibitor. Removal of Ssm6a restored the reflex to its control level (control: 19 ± 3 mmHg, Ssm6a: 10 ± 1 mmHg, recovery: 19 ± 4 mmHg; n = 6; P < 0.05). Compound action potentials recorded from the L4 and L5 dorsal roots and evoked by single-pulse stimulation of the sciatic nerve showed that both TTX and Ssm6a attenuated input from group III, as well as group IV afferents. We conclude that NaV 1.7 channels play a role in the thin-fiber muscle afferent pathway evoking the exercise pressor reflex.
Keywords: voltage-gated sodium channels, rats, group III and IV afferents, neural control of the circulation, TTX-S, Ssm6a
the exercise pressor reflex refers to the increases in blood pressure, heart rate, and ventilation evoked by mechanical and metabolic stimuli arising in contracting muscles (5, 14, 15). The afferent arm of the reflex comprises thinly myelinated group III and unmyelinated group IV afferents (14). Group III afferents, sometimes called Aδ-fibers, are primarily mechanically sensitive, whereas group IV afferents, sometimes called C-fibers, are primarily metabolically sensitive (11–13, 16).
Voltage-gated sodium (NaV) channels are responsible for transmitting action potentials along the axons of the group III and IV afferents, evoking the exercise pressor reflex. NaV channels have been classified as either tetrodotoxin-sensitive (TTX-s) or tetrodotoxin-resistant (TTX-r). TTX-s channels include NaV 1.1, 1.2, 1.3, 1.4, 1.6, and 1.7, whereas TTX-r channels include NaV 1.5, 1.8, and 1.9 channels (22). All NaV channels except NaV 1.4 are found on dorsal root ganglion (DRG) neurons (1, 7). TTX-s channels are those that are blocked by TTX concentrations of less than 300 nM; TTX-r channels are those that are not blocked by TTX concentrations of 300 nM (21). NaV 1.7 channels, which are TTX-s, are found on both thinly myelinated, as well as unmyelinated afferents (8).
Knocking down NaV 1.7 channels in animals abolishes hyperalgesia (33). Similarly, humans without NaV 1.7 channels display a congenital indifference to pain; nevertheless, these individuals still perceive hot and cold stimuli (6, 9). On the other hand, gain-of-function mutations of NaV 1.7 channels in humans cause intolerable pain (6). Additionally, NaV 1.7 channel proteins in DRG neurons are upregulated in neuropathic pain models (3). Considered together, these studies raise the possibility that NaV 1.7 channels, which are found on Aδ- and C-fibers (8), play a large role in the afferent transmission of the exercise pressor reflex. Therefore, we were prompted to determine the role played by TTX-s NaV 1.7 channels in transmitting the exercise pressor reflex, as well as to determine whether NaV 1.7 channels were transmitting the reflex primarily through Aδ-fibers and/or C-fibers.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. Adult male rats (Sprague-Dawley, n = 27, weighing 464 ± 9 g) were used in this study.
General Surgery
Rats were initially anesthetized with a mixture of 4% isoflurane and 100% oxygen. The trachea was cannulated, after which the lungs were mechanically ventilated (Harvard Apparatus) with the anesthetic mixture. The right jugular vein and right carotid artery were cannulated. Arterial blood pressure was measured by attaching the carotid arterial catheter to a pressure transducer (model P23 XL; Statham); heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotach). Arterial blood gases and pH were measured using an automated blood-gas analyzer (model ABL 80 FLEX; Radiometer). Pco2 and arterial pH were maintained within normal physiological range by either adjusting ventilation or intravenous administration of sodium bicarbonate (8.5%). Core body temperature was monitored using a rectal temperature probe and maintained at 37–38°C using a heat lamp.
A laminectomy was performed from L1 to L5 to expose the spinal cord and the lower lumbar roots. The rats were then secured in a Kopf spinal frame by clamps placed on the pelvis and rostral lumbar vertebrae. The incised skin on the back was used to form the walls of a pool that was filled with warm (37°C) mineral oil. The dura was opened, and the L4 and L5 dorsal roots on the left side of the rat were isolated. Space for a small pool was formed by placing a longitudinally halved plastic tube (6 mm in length and 3 mm in transverse diameter) under the L4 and L5 dorsal roots. Before placing the tube under the dorsal roots, we soaked the tube in cytochrome c (100 μg/ml) overnight to prevent either TTX or Ssm6a from adhering to its sides. Both proximal and distal ends of the tube were sealed with Kwik-Sil (World Precision Instruments). Immediately after the Kwik-Sil solidified, the pool was filled with 50 μl lactated Ringer solution. The left calcaneal bone was sectioned, attached to a force transducer (FT-10, Grass), and, in turn, attached to a rack and pinion. The sciatic nerve was isolated for placement of the stimulating electrode.
A precollicular decerebration was performed by sectioning the brain less than 1 mm anterior to the superior colliculi. Dexamethasone (0.2 mg) was injected intravenously prior to the decerebration procedure to minimize brain stem edema. All neural tissue rostral to the section was removed. To minimize bleeding, small pieces of oxidized regenerated cellulose (Ethicon, Johnson and Johnson) were placed on the internal skull surface, and the cranial cavity was packed with gauze. Immediately after the precollicular transection, gas anesthesia was discontinued and the rats' lungs were ventilated mechanically with room air. After decerebration, the rats were allowed to stabilize for at least 1 h before any experimental protocol was initiated.
Experimental Protocols
Static contraction.
A shielded stimulating electrode was placed underneath the exposed sciatic nerve. Baseline tension was set between 50 and 100 g. The triceps surae muscles were statically contracted by stimulating (40 Hz, 0.01 ms, ≤2 times motor threshold) the sciatic nerve for 30 s. Ten minutes after the initial static contraction, either TTX (5 nM or 50 nM) or Ssm6a (100 nM), a selective NaV 1.7 antagonist (32), replaced the lactated Ringer solution in the pool surrounding L4 and L5 dorsal roots. Twenty minutes after TTX or five min after Ssm6a, the triceps surae muscles were statically contracted again. The drugs were then washed out by rinsing the pool several times with lactated Ringer over a period of 20–60 min. Following the washout of drugs, the triceps surae muscles were again statically contracted to determine whether the exercise pressor reflex was restored. Mean arterial pressure, heart rate, and developed tension were recorded before and during each static contraction. At the end of the protocol, the rat was paralyzed with pancuronium bromide (1 mg/kg iv), and the sciatic nerve was stimulated with the same parameters as those used to statically contract the triceps surae muscles. This procedure was done to ensure that the contraction-induced pressor responses were not evoked by electrical stimulation of group III and IV axons in the sciatic nerve.
Compound action potentials.
Compound action potentials evoked by stimulating the sciatic nerve were recorded from L4 and L5 dorsal roots. The L4 and L5 dorsal roots were sectioned at their point of entry into the spinal cord and divided into large rootlets. The distal end of one rootlet at a time was placed on one foot of a bipolar hook electrode. The other foot was grounded to the rat with a thin saline-soaked wick. The rats were paralyzed with pancuronium bromide (1 mg/kg iv followed by supplemental doses of 0.2 mg/kg every 30 min). The sciatic nerve was then stimulated using shielded hook electrodes at 0.3 Hz (square-wave pulse of 0.1–1-ms duration and supramaximal voltage). The evoked compound action potentials were passed through a high-impedance probe (model HIP 511; Grass Instruments), amplified, and filtered (0.3–1 kHz; model P511; Grass Instruments). The compound action potentials were displayed on a storage oscilloscope (model HP 54603B) and analyzed using Spike 2 software [Cambridge Electronic Design (CED)]. The Aδ- (i.e., group III afferents) and C- (i.e., group IV afferents) waves were averaged for 10 stimulus presentations, and then the areas under the waves were integrated (Spike 2). The same method of analysis was used when the areas under the Aα/β waves were assessed. The Aδ wave was defined as a compound action potential conducting between 10 and 1.6 m/s, and the C wave was defined as a compound action potential conducting between 1.5 m/s and 0.5 m/s. The signal-to-noise ratio of the C-fiber wave was low due to temporal dispersion. Next, either TTX (50 nM) or Ssm6a (100 nM) replaced the lactated Ringer in the small pool surrounding L4 and L5 dorsal roots. Compound action potentials were again evoked by stimulating the sciatic nerve using the same parameters as before. Last, 2% lidocaine replaced either TTX or Ssm6a in the small pool, and compound action potentials were again evoked using the same parameters as before. The conduction distance between the stimulating electrode on the sciatic nerve and the recording electrode on the lumbar dorsal root averaged 77 ± 2 mm (n = 10). Our calculation of conduction velocity did not take into account any changes along the path from sciatic nerve to the dorsal root (29, 30).
Data Analysis
All data were recorded continuously with a Spike 2 data acquisition system (CED) and analyzed using GraphPad Prism software. In the static contraction experiments, baselines, as well as reflex changes in mean arterial pressure, heart rate, and developed tension, were determined. Mean arterial pressure was calculated beat to beat by the Spike 2 system. The peak pressor and cardioaccelerator responses to static contraction were compared with their respective baseline levels and were analyzed using a repeated-measures, one-way ANOVA for three time points, namely before the drugs were applied to the dorsal roots (control), while the drugs were applied to the dorsal roots (TTX or Ssm6a), and after drugs were washed out from the dorsal roots (recovery). Differences among time points were identified using Tukey's post hoc analysis. Mean arterial pressure (MAP) is expressed in millimeters mercury (mmHg), and heart rate (HR) is expressed in beats per minute (bpm). The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilogram seconds (kg·s) (17).
In the compound action potential experiments, the areas under the averaged wave forms evoked by stimulating the sciatic nerve were recorded and presented as a percent change from before drug (control) values. These percent change values were analyzed using a repeated-measures one-way ANOVA for three time points, namely, before the drugs were applied to the dorsal roots (control), while TTX or Ssm6a were applied to the dorsal roots, and while lidocaine was applied to the dorsal roots. Differences among time points were identified using Tukey's post hoc analysis. All values are expressed as means ± SE. The criterion for statistical significance was set as P < 0.05.
RESULTS
Static Contraction
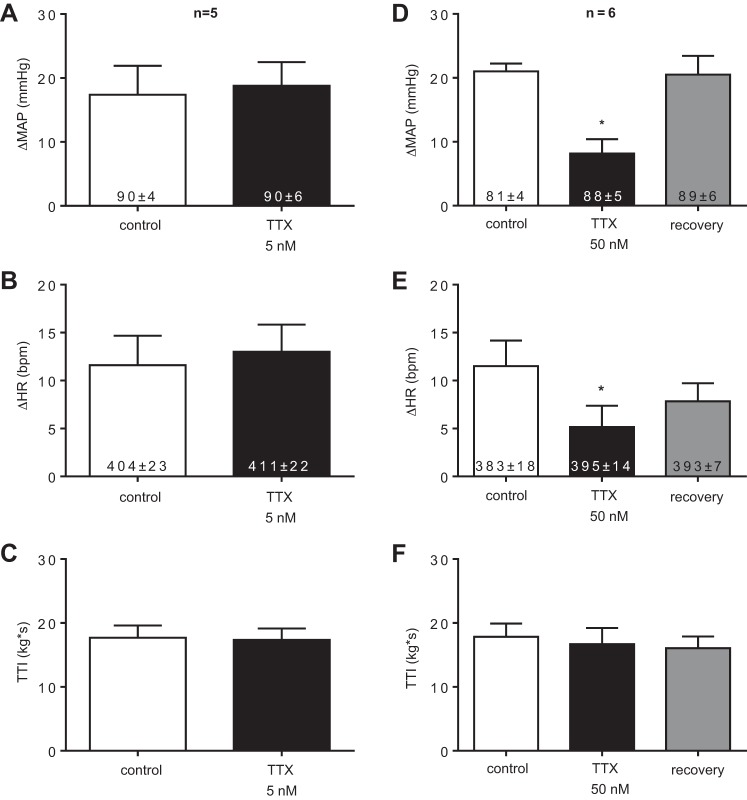
TTX (5 nM, n = 5) applied topically to the small pool bathing the L4 and L5 dorsal roots, had no effect on the peak pressor response to static contraction of the triceps surae muscles, whereas TTX (50 nM, n = 6) significantly attenuated the peak pressor response (Fig. 1, A and D, respectively). The peak pressor response was almost completely restored following the washout of TTX from the bath (Fig. 1D). TTX (5 nM) had no effect on cardioaccelerator response to static contraction, whereas TTX (50 nM) slightly, though significantly, attenuated the response (Fig. 1, B and E, respectively). The cardioaccelerator response was partially restored following the washout of TTX from the bath (Fig. 1E). TTIs were not significantly different from control contraction to TTX contraction for either 5 nM TTX or 50 nM TTX, P > 0.05 (Fig. 1 C and F, respectively).
Fig. 1.
The peak pressor and cardioaccelerator responses to static contraction before (control) or while either tetrodotoxin (TTX) 5 nM (A and B, respectively) or TTX 50 nM (D and E, respectively) was applied to the pool bathing the L4 and L5 dorsal roots, as well as those following the washout of TTX 50 nM (recovery). Tension-time indices (TTIs) were not different between the control and TTX 5-nM contractions (C). Likewise, TTIs were not different among the control, TTX 50 nM, and recovery contractions (F). Asterisks (*) represent significantly smaller (P < 0.05) responses to static contraction compared with the control contraction. The numbers inside the bars represent baseline values and are expressed as means ± SEs.
Ssm6a (100 nM, n = 6) significantly attenuated the peak pressor response to static contraction (Fig. 2A). Additionally, the peak pressor response was almost completely restored following the washout of Ssm6a from the bath (Fig. 2A). Ssm6a had no effect on the cardioaccelerator response to static contraction (Fig. 2B). TTIs were not significantly different between control contraction and Ssm6a contraction (Fig. 2C). Stimulation of the sciatic nerve after paralyzing the rats with pancuronium bromide had no effect on either arterial pressure or heart rate.
Fig. 2.
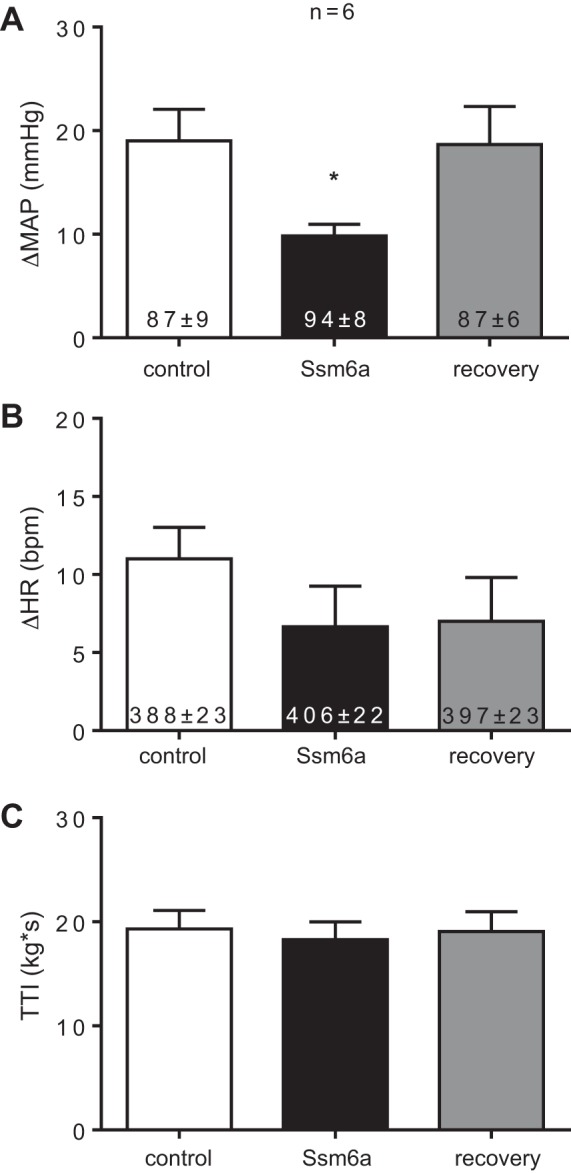
The peak pressor (A) and cardioaccelerator (B) responses to static contraction before (control), while (Ssm6a), and after (recovery) Ssm6a (100 nM), was applied to the pool bathing the L4 and L5 dorsal roots. TTIs were not different among the control, Ssm6a, and recovery contractions (C). Asterisks (*) represent significantly smaller (P < 0.05) responses to static contraction compared with the control contraction. The numbers inside the bars represent baseline values and are expressed as means ± SE.
Compound Action Potentials
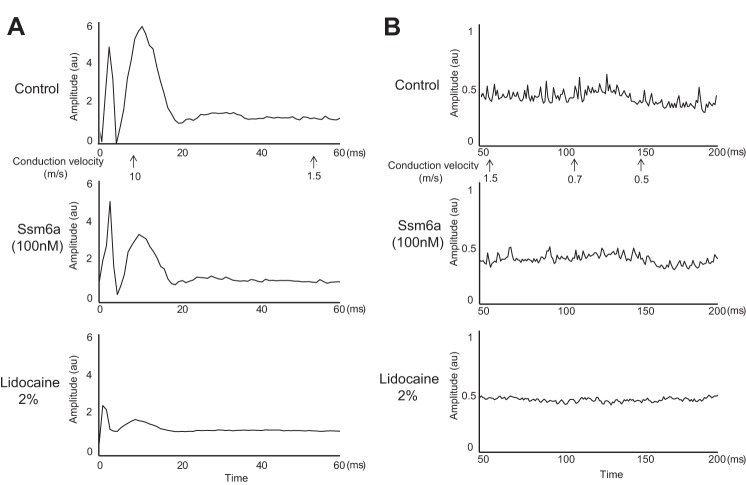
The effect of TTX (50 nM) on the compound action potential evoked by single-pulse supramaximal stimulation of the sciatic nerve was determined in five rats. Both TTX (50 nM) and lidocaine (2%) blocked the Aδ-wave and C-wave in each of the five rats tested (Fig. 3). On average, TTX (50 nM) blocked 47 ± 16% (P < 0.05) of the action potentials arising from group III afferents (Aδ-wave; Fig. 4B), and 37 ± 16% (P < 0.05) of the action potentials arising from group IV afferents (C-wave; Fig. 4C). Additionally, lidocaine (2%) almost completely abolished (P < 0.05) the remaining group III and IV action potentials following TTX (50 nM) in each of the four rats tested (Fig. 4, A and B).
Fig. 3.
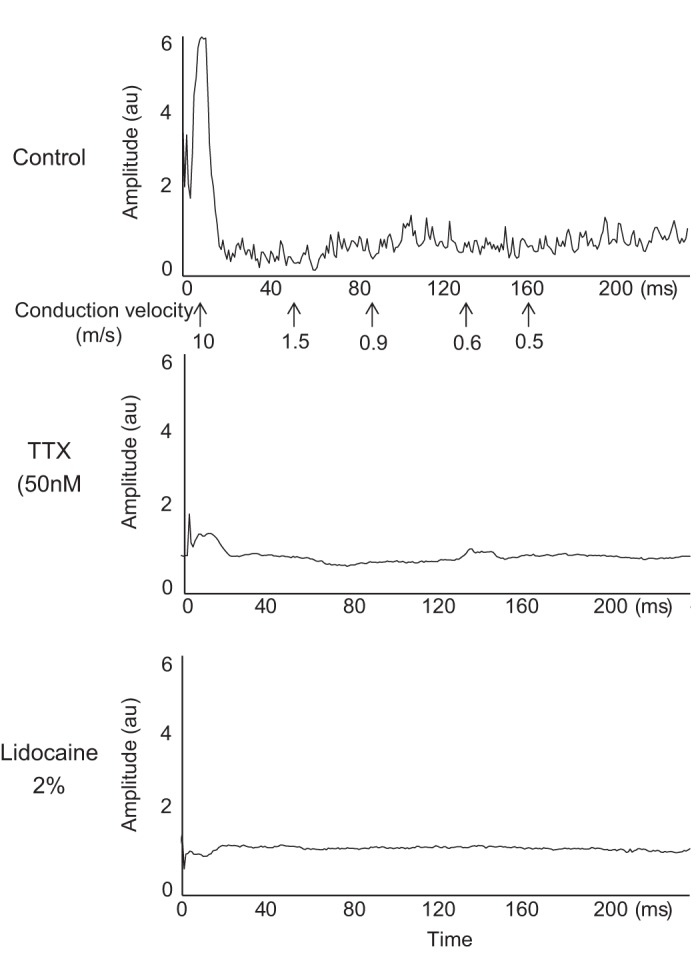
The compound action potential evoked by a single stimulus presentation to the sciatic nerve. The Aδ wave conducted between 10 and 1.5 m/s, whereas the C-wave conducted between 1.5 and 0.5 m/s. For this filament, which was dissected from the L5 dorsal root, both the Aδ wave (peak around 10 m/s) and C-wave (peak between 0.9 m/s and 0.6 m/s) were reduced when TTX (50 nM) was applied to the pool and abolished when lidocaine (2%) was applied to the pool.
Fig. 4.
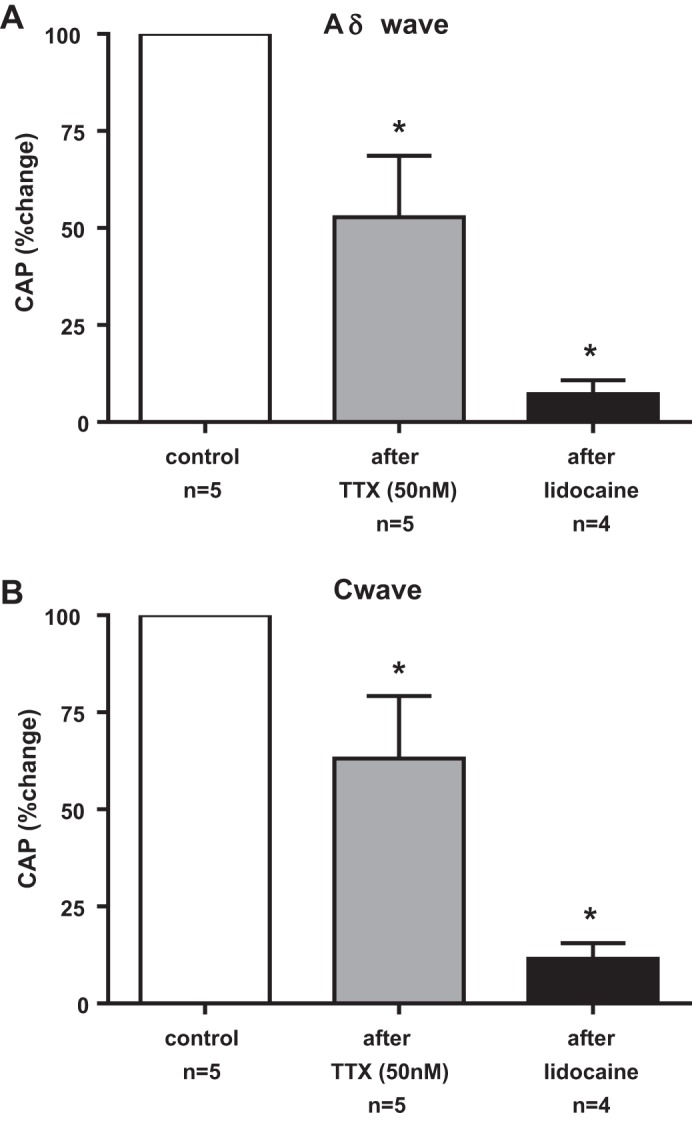
Summary data of the effects of TTX (50 nM) and lidocaine (2%) applied to the pool bathing the dorsal roots on the compound action potentials [CAP evoked by single-pulse electrical stimulation of the sciatic nerve: Aδ-wave (A) and C-wave (B)]. The effects of TTX and lidocaine were expressed as percentages of the control area. Asterisks (*) represent a significant decrease (P < 0.05) in the areas of the compound action potential from their respective control values.
The effect of Ssm6a (100 nM) on the compound action potential evoked by supramaximal stimulation of the sciatic nerve was determined in an additional five rats. Both Ssm6a (100 nM) and lidocaine (2%) significantly blocked the Aδ-wave (Fig. 5A) and C-wave (Fig. 5B) in each of the five rats tested. On average, Ssm6a blocked 54 ± 4% (P < 0.05) of the action potentials arising from group III afferents (Aδ-wave; Fig. 6B) and 63 ± 12% (P < 0.05) of the action potentials arising from group IV afferents (C-wave; Fig. 6C). Additionally, lidocaine (2%) almost abolished (P < 0.05) the remaining group III and IV action potentials following Ssm6a in each of the five rats tested.
Fig. 5.
The compound action potential evoked by a single stimulus presentation to the sciatic nerve. For this filament, which was dissected from the L5 dorsal root, the Aδ-wave (A; peak around 10 m/s), and C-wave (B; peak between 0.7 m/s and 0.5 m/s) were reduced by Ssm6a (100 nM), and were almost completely abolished by lidocaine (2%).
Fig. 6.
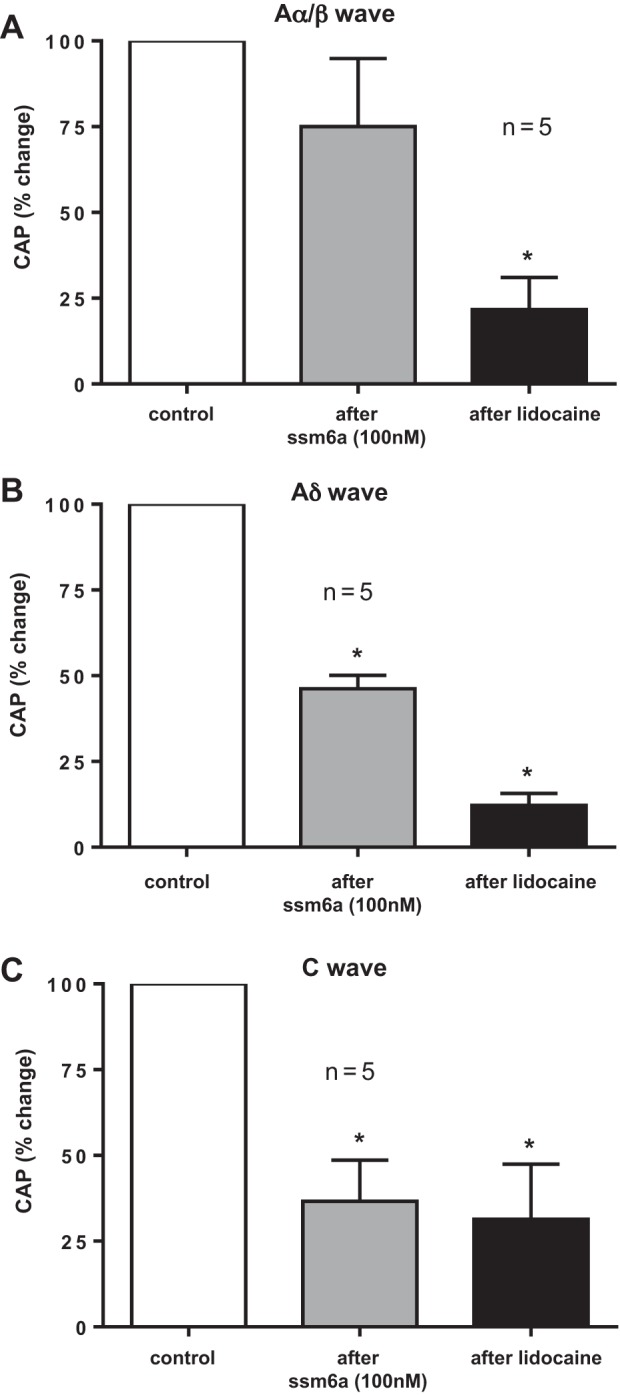
Summary data of the effects of Ssm6a (100 nM) and lidocaine (2%) applied to the pool bathing the dorsal roots on the compound action potentials evoked by single-pulse electrical stimulation of the sciatic nerve: Aα/β-wave (A), Aδ-wave (B), and C-wave (C). The effects of Ssm6a and lidocaine were expressed as percentages of the control area. Asterisks (*) represent a significant decrease (P < 0.05) in the areas of the compound action potentials from their respective control values.
The effect of Ssm6a on the Aα/β component (Fig. 6C) of the compound action potential evoked by supramaximal stimulation of the sciatic nerve was also determined in the five rats in which Ssm6a was topically applied to the dorsal roots. In two rats, the Aα/β-wave increased after application of Ssm6a, in two others, the Aα/β-wave decreased and in the remaining one, the wave was unchanged. On average (n = 5), Ssm6a decreased the Aα/β-wave by 25 ± 20%, an amount that did not approach statistical significance (P = 0.49).
DISCUSSION
Previous work from this laboratory has shown that application of 1 μM TTX to the L4 and L5 dorsal roots of the decerebrated unanesthetized rat attenuated the exercise pressor reflex that was evoked by static contraction of the triceps surae muscles (28). The upper limit of the concentration of TTX that blocks only TTX-s channels is 300 nM (21); consequently, our previous work offers no information about the roles played by TTX-s channels and, more specifically, NaV 1.7 channels in transmitting the afferent information that evokes the exercise pressor reflex. Our present work, in contrast, provides evidence consistent with the hypothesis that NaV 1.7 channels play a role in transmitting information arising from the contraction-induced stimulation of group III and IV afferent endings in the triceps surae muscles. Specifically, we found that TTX in a concentration of 50 nM, which is obviously well below the upper limit of 300 nM, substantially attenuated the exercise pressor reflex. In addition, we found that a NaV 1.7 channel antagonist, Ssm6a, also attenuated the reflex.
We found that the attenuating effects of both TTX and Ssm6a on the exercise pressor reflex were reversible (Ref. 28 and present findings). The significance of this finding is that it offers incontrovertible proof that the increases in arterial pressure that were evoked by static contraction of the triceps surae muscles were reflex in origin. Frequently, the reflex origin of the exercise pressor reflex is demonstrated by showing that sectioning the dorsal roots—the afferent pathway of the reflex—abolishes or greatly reduces it. Although sectioning of the dorsal roots is an acceptable method of demonstrating that the contraction-induced pressor and cardioaccelerator responses to contraction are reflex in origin, sectioning does not exclude the possibility that these cardiovascular responses to contraction would lessen as the decerebrate preparation deteriorates over time.
Any conclusions drawn from our findings must be limited to the site of application of TTX and Ssm6a, namely the lumbar dorsal roots. NaV 1.7 channels have been found on the axons of rat Aδ and C fibers, as they travel through the dorsal roots, the site where we applied both TTX and Ssm6a (2). In addition, NaV 1.7 channels have been found on cutaneous sensory endings, as well as on their terminals in the spinal dorsal horn (2, 18). Therefore, we think that it is reasonable to speculate that these TTX-s channels would also be found on both the sensory and spinal endings of the thin-fiber muscle afferents whose stimulation by contraction evokes the exercise pressor reflex. As of yet, no in vivo method is available that enables one to apply TTX and Ssm6a in concentrations known to be selective for NaV 1.7 channels onto these spinal or sensory endings while hind limb muscles are contracted. Although application of known concentrations of these sodium channel blockers might be possible in an ex vivo preparation (10), statically contracting skeletal muscles that are not perfused with hemoglobin would most likely result in severe tissue hypoxia, thereby distorting the findings.
Our use of 50 nM TTX enabled us to conclude that TTX-s channels on the dorsal roots played a major role in transmitting the afferent input evoking the exercise pressor reflex. Nevertheless, our use of 50 nM TTX did not allow us to identify the particular TTX-s channel that was playing this role. To shed light on this issue, Ssm6a, an antagonist of NaV 1.7 channels, was applied to the dorsal roots (32). When tested in human embryonic kidney 293 (HEK 293) cells transfected for the nine sodium channels found in humans, Ssm6a was found to be at least 150 times more selective for the NaV 1.7 channel than for any of the other sodium channels except for the NaV 1.2 channel, for which the selectivity of Ssm6a was more than 32-fold (32). The NaV 1.2 channel is embryonic (22) and probably does not play a role in afferent transmission in the adult rat. Moreover, the NaV 1.2 channel, as well as the NaV 1.1 and NaV 1.3 channels, has been recently shown to play no role in the A-fiber compound action potential evoked by electrical stimulation of the rat sciatic nerve (31).
We were particularly interested in comparing the IC50s of Ssm6a for the NaV 1.6 and 1.7 channels, both of which have been localized on DRG cells innervating the triceps surae muscles of rats (8, 19). The NaV 1.6 channel is highly concentrated at the node of Ranvier (4) and, therefore, could play an important role in transmitting information arising from group III afferents, which are thinly myelinated. The IC50s for Ssm6a for the NaV 1.7 channel in HEK 293 cells were 25.4 nM, whereas the IC50 for the NaV 1.6 channel was 15.2 μM (32). In other words, Ssm6a in HEK 293 cells was almost 600 times more selective for the NaV 1.7 channel than for the NaV 1.6 channel. To the extent that one can extend information obtained from transfected HEK 293 cells to an in vivo preparation, our findings suggest that Ssm6a blocked NaV 1.7 channels rather than NaV 1.6 channels.
Evidence exists indicating that both thinly myelinated and unmyelinated afferents possess TTX-s NaV 1.7 channels (2) (8). In our experiments, we, therefore, needed to determine whether TTX and Ssm6a attenuated the exercise pressor reflex by reducing group III and/or group IV afferent input to the dorsal horn of the spinal cord. To make this determination, we recorded the compound action potential that was evoked by single-pulse electrical stimulation of the sciatic nerve before and after placing TTX or Ssm6a onto the L4 and L5 dorsal roots. The Aδ component of the compound action potential represented activity evoked from group III muscle afferents, but it also included activity evoked from skin and joint afferents, with the same range of conduction velocities as that of group III muscle afferents. Likewise, the C component of the compound action potential represented activity evoked from group IV muscle afferents, as well as activity evoked from skin and joint afferents with the same range of conduction velocities. We found that both TTX (50 nM), as well as Ssm6a (100 nM), applied to the L4 and L5 dorsal roots, attenuated both the Aδ- and C-fiber components of the compound action potential. This finding leads us to conclude that NaV 1.7 channels on the axons of both group III and IV muscle afferents play an important role in transmitting afferent information evoking the exercise pressor reflex. Comparisons determining whether NaV 1.7 channels play more of a role in transmitting impulses along the axons of group III afferents than in transmitting impulses along the axons of group IV afferents are probably best accomplished by recording the impulse activity of single fibers rather than recording compound action potentials, which in our experiments were not specific to muscle afferents and whose amplitudes are dependent on filament thickness.
We need to stress that our conclusion concerning the role played in NaV 1.7 channels in evoking the exercise pressor reflex does not exclude a role for other sodium channels. Particular attention should be paid to both NaV 1.6, which is TTX-s, and NaV 1.8, which is TTX-r. The concentration of TTX needed to block the NaV 1.6 channel is similar to that needed to block the NaV 1.7 channel, and as a result caused us to use Ssm6a. The NaV 1.6 channel is significant because it is thought to be responsible for a resurgent current, which, in turn, can cause repetitive discharge in myelinated afferents (23). The NaV 1.8 channel is significant because it is likely to be responsible for much of the current generating action potentials in the cell bodies of group IV muscle afferents (19, 20).
Any interpretation of our findings needs to be done with two limitations in mind. The first concerns the effect of Ssm6a on NaV 1.6 channels. Although Ssm6a was found in HEK 293 cells to be almost 600 times more effective in blocking NaV 1.7 channels than in blocking NaV 1.6 channels, Ssm6a, in the concentration used in our in vivo experiments, decreased the Aα/β component of the compound action potential evoked by sciatic nerve stimulation in two of five rats tested. This finding is relevant because NaV 1.7 channels are not present on thickly myelinated dorsal root axons (2), whose electrical activity comprises the Aα/β component of the compound action potential. Consequently, we cannot completely rule out an effect of Ssm6a on NaV 1.6 channels as a contributing factor in the reduction of the exercise pressor reflex that we observed. Nevertheless, we would like to emphasize that the overall effect of Ssm6a on the Aα/β component of the compound action potential evoked by sciatic nerve stimulation did not approach significance (i.e., P < 0.49).
A second limitation is that our experiments do not clearly distinguish between the contraction-induced activation of nociceptors responding to either ischemia or noxious mechanical distortion of their receptive fields and the contraction-induced activation of afferents responsive to nonnoxious mechanical and metabolic stimuli. Nevertheless, some recent findings from our laboratory in decerebrated rats with freely perfused triceps surae muscles (26) might enable us to shed some speculative light on this issue. We found that group III afferents appeared to be more responsive to 30 s of static contraction than were group IV afferents to 30 s of static contraction. Moreover, group III afferents were more responsive to nonnoxious mechanical stimuli, such as tendon stretch and probing their receptive fields, than were group IV afferents (26). Our electrophysiological findings concerning the group III and IV afferents' responses to the same stimulus as that used in the present experiments to evoke the exercise pressor reflex raise the possibility that for the most part, we were stimulating afferents that were not nociceptors.
Perspectives and Significance
We have shown that NaV 1.7 channels on the dorsal root axons of both group III and IV muscle afferents play an important role in the transmission of information evoking the exercise pressor reflex in healthy rats with freely perfused muscles. This reflex is known to be exaggerated in several pathophysiological conditions, including simulated peripheral artery disease (27), heart failure (24), and hypertension (25), and over time may be at least, in part, responsible for adverse cardiac events, including fibrillation. Part of this exaggeration may be caused by an increase in NaV 1.7 channels on group III and IV muscle afferents.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.J.S. and M.P.K. conception and design of research; A.J.S. and S.W.C. performed experiments; A.J.S. analyzed data; A.J.S. and M.P.K. interpreted results of experiments; A.J.S. prepared figures; A.J.S. and M.P.K. drafted manuscript; A.J.S., S.W.C., and M.P.K. edited and revised manuscript; A.J.S., S.W.C., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Joyce S. Kim for excellent technical assistance.
REFERENCES
- 1.Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res 43: 117–131, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Black JA, Frezel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108: 237–247, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci USA 97: 5616–5620, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coote JH, Hilton SM, and Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci 33: 325–347, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci 14: 49–62, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Djouhri L, Newton R, Levinson SR, Berry CM, Carruthers B, Lawson SN. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J Physiol 546: 565–576, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes SB, Wang MY. Na(V)1.7 pain control: a novel target. Neurosurgery 73: N16, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: 160–165, 1987. [PubMed] [Google Scholar]
- 13.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 14.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Gonzalez JF. Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-861981. [PubMed] [Google Scholar]
- 18.Persson AK, Gasser A, Black JA, Waxman SG. Nav1.7 accumulates and co-localizes with phosphorylated ERK1/2 within transected axons in early experimental neuromas. Exp Neurol 230: 273–279, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol 108: 2230–2241, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629–640, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol 511: 771–789, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579: 1–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav16, expressed in mouse spinal sensory neurones. J Physiol 564: 803–815, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone AJ, Copp SW, McCord JL, Kaufman MP. Femoral artery ligation increases the responses of thin-fiber muscle afferents to contraction. J Neurophysiol 113: 3961–3966, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 300: H652–H663, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol 76: 1924–1941, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Waddell PJ, Lawson SN, McCarthy PW. Conduction velocity changes along the processes of rat primary sensory neurons. Neuroscience 30: 577–584, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MJ, Yoshikami D, Azam L, Gajewiak J, Olivera BM, Bulaj G, Zhang MM. mu-Conotoxins that differentially block sodium channels NaV1.1 through 18 identify those responsible for action potentials in sciatic nerve. Proc Natl Acad Sci USA 108: 10,302–10,307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, Xiao Y, Kang D, Liu J, Li Y, Undheim EA, Klint JK, Rong M, Lai R, King GF. Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci USA 110: 17,534–17,539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, Wilson SP. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther 16: 271–277, 2005. [DOI] [PubMed] [Google Scholar]