Abstract
The brain of mammalian hibernators is naturally protected. Hibernating ground squirrels undergo rapid and extreme changes in body temperature and brain perfusion as they cycle between lengthy torpor bouts and brief periods of euthermia called interbout arousals (IBAs). Arousal from torpor to IBA occurs rapidly, but there is no evidence of brain injury accompanying this extreme physiological transition. Production of the hormone melatonin accompanies arousal, suggesting that it plays a protective role at this time. Here, we investigated mechanisms of melatonin receptor-mediated protection in the brain of the hibernating ground squirrel. We administered the competitive melatonin receptor antagonist luzindole (30 mg/kg ip) to ground squirrels at the predicted end of a torpor bout, triggering an arousal. We found that luzindole-treated animals exhibited caspase-3 activity two times higher than vehicle-treated animals in the hypothalamus at midarousal (P = 0.01), suggesting that melatonin receptor signaling is important for protection in this brain region. We also found a 30% decline in succinate-fueled mitochondrial respiration in luzindole-treated animals compared with vehicle-treated animals (P = 0.019), suggesting that melatonin receptor signaling is important for optimal mitochondrial function during arousal from torpor. The mitochondrial effects of luzindole treatment were seen only during the hibernation season, indicating that this effect is specifically important for arousal from torpor. These data provide evidence for the protective role of melatonin receptor signaling during the extreme physiological transition that occurs when a hibernating mammal arouses from torpor and provide further evidence for regional and seasonal changes in the hibernator brain.
Keywords: hibernation, luzindole, melatonin, hypothalamus, mitochondria
hibernation provides a unique opportunity to study naturally occurring neuroprotection. During deep hibernation periods called torpor bouts, thirteen-lined ground squirrels have near-freezing body temperatures and their cerebral blood flow (CBF) is reduced to ∼10% of the active rate (13). The level of brain perfusion measured in torpor would be ischemic in humans and nonhibernators, resulting in serious injury after only a few minutes (19); however, no evidence of brain damage was observed in the brains of torpid animals (13). In addition to undergoing the extreme physiological changes associated with torpor, ground squirrels also rapidly return to euthermia for short (<1 day) interbout arousals (IBAs) throughout the hibernation season, where their CBF returns to 100% (7). The arousal from torpor to IBA occurs rapidly, in an average of just 2.8 h (15). Reperfusion after an ischemic event can be extremely damaging (3), but again, no evidence of brain damage was observed in ground squirrel brains during IBA (13).
Mammals arousing from hibernation and daily torpor produce the hormone melatonin (12, 17, 33). The timing of the increase in circulating melatonin suggests that it could be playing a protective role during this extreme physiological transition (34). Melatonin, in addition to its importance in seasonal and daily rhythms, is known to be protective, both as an antioxidant and through receptor-mediated mechanisms (22, 41). Melatonin is also a key component of a hibernation-based therapy for profound blood loss and hemorrhagic shock (16). Melatonin receptor signaling has been shown to provide protection by increasing survival signaling (40), decreasing apoptotic signaling (25), and protecting mitochondria (9, 24). Melatonin can freely pass through the blood brain barrier (27) and is an excellent candidate for protection during arousal from torpor, particularly in the brain. Additionally, melatonin levels in the cerebral spinal fluid are known to be substantially higher than in the serum, further supporting a protective role of melatonin in the brain (28). Melatonin receptors are found throughout the mammalian brain and are notably present in the suprachiasmatic nucleus of the hypothalamus in nearly all mammals studied, including ground squirrels (5, 38). The hypothalamus maintains some activity throughout the physiological extremes of hibernation (6) and is thought to be very important for the control and timing of hibernation. Thus this region could be an important target for protection during arousal.
The ground squirrel brain is naturally protected during hibernation through a variety of adaptations and mechanisms (10). Previous transcriptomic work investigating seasonal gene expression in the hypothalamus of the thirteen-lined ground squirrel showed that neuroprotective signaling pathway components, including signal transducers and activators of transcription 3 (STAT3), were significantly increased during torpor and IBA (30). Additionally, there is evidence of antiapoptotic signaling during hibernation in the brain (29). Hibernating ground squirrels have even been shown to be resistant to brain damage from an unnatural source of injury (43). Here, we investigated the protective effect of melatonin receptor signaling in the thirteen-lined ground squirrel brain by administering luzindole, a competitive melatonin receptor antagonist, upon arousal from torpor, when circulating melatonin levels rise naturally. The rapid and extreme transition in CBF from torpor to IBA is arguably ischemia-reperfusion like; however, the hibernator exhibits no evidence of brain damage (13). This study purposefully manipulates a natural system known to have endogenous protective mechanisms, with the goal of gaining some insight into the control of natural neuroprotection.
METHODS
Animals
Forty thirteen-lined ground squirrels (Ictidomys tridecemlineatus) of both sexes (26 female, 14 male) were used in these experiments. The ages of these animals are not known because they were wild caught. This species is abundant and unprotected in Minnesota where they are known to damage agricultural crops (http://www.dnr.state.mn.us/mammals/thirteenlinedgroundsquirrel.html). The squirrels were live trapped in midsummer on private property with permission near Paynesville, MN by slowly pouring water into burrows and capturing the animal in a net when it emerges.
Three sets of animals were used for these experiments: HibernatingTR (n = 20; 10 luzindole and 10 vehicle), HibernatingMT (n = 10; 5 luzindole and 5 vehicle), and SummerMT (n = 10; 5 luzindole and 5 vehicle). HibernatingTR squirrels were implanted with transmitters to determine if luzindole administration had any effect on the physiological parameters of arousal from torpor. Individual brain regions were collected from this group for molecular assays. HibernatingMT and SummerMT squirrels were subjected to the same experimental protocol in winter hibernation and active summer, respectively, and brain mitochondria were isolated for respiration and membrane potential assays. Each of the three sets of animals consisted of one group that was administered luzindole and one group that was administered vehicle only. All animals were housed individually in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Animal Care Facility located in the University of Minnesota Duluth Medical School. Squirrels were kept at 21°C in a 12:12-h light-dark cycle (light period is from 7 AM to 7 PM) from their summer capture through October. During this time, they were provided with Purina rat chow and water ad libitum. From November through their experimental endpoint in mid-January to early February, the squirrels were housed in an environmental chamber and kept in hibernation conditions: constant darkness, 5–7°C ambient temperature, and no food was provided. The hibernation cycles of all animals were tracked daily using the sawdust method (23) or via implanted transmitters (see Transmitter Implantation, Monitoring, and Analysis). The SummerMT animals were wild caught in mid-May, so they did not undergo hibernation in the laboratory. The SummerMT animals were kept in captivity for ∼1 mo before the beginning of the experimental protocol. All experimental procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocols 1103A97712 and 1111A06488).
Transmitter Implantation, Monitoring, and Analysis
The HibernatingTR ground squirrels were implanted with transmitters (PhysioTel CTA-F40; DataSciences International, St. Paul, MN) during the summer before the hibernation season. The animals were anesthetized with isoflurane in medical grade air administered by a nose cone. Anesthesia was verified before the start of the procedure and periodically throughout. The areas of incision (the ventral abdomen and neck) were shaved on an adjacent separate counter to maintain an aseptic environment. These areas were cleaned and disinfected with betadine and 70% ethanol. The transmitter was inserted intraperitoneally into a small incision on top of the intestines. The two leads were tunneled subcutaneously to their positions in the intercostal and pectoral muscles and secured in place with suture. The abdominal wall was sutured to hold the transmitter in place, and all the incisions were closed with staples. The animal was monitored for a week postsurgery. The staples were removed after 2 wk.
When hooked up to a receiver, the transmitters continually track and record core body temperature, electrocardiograms (ECGs), and activity level via the Dataquest A.R.T. acquisition software (DataSciences International). All physiological data were viewed and analyzed using Dataquest A.R.T. analysis software (DataSciences International). Heart rate was calculated from the ECG trace. The start of an arousal was determined as previously described (15), when the body temperature increased at least 0.1°C over 10 min and the average heart rate increased by at least 1 beat/min over 10 min. Eight animals were monitored on receivers simultaneously in real time. The remaining animals were monitored via the sawdust method to track occurrence of IBAs and transferred to receivers for the experimental protocol. Additionally, each animal was monitored and recorded for at least one full natural IBA before the experimental procedure to facilitate comparison of natural and experimental arousals.
Drug
The competitive melatonin receptor antagonist luzindole (Tocris Bioscience, Bristol, UK) was dissolved in 100% ethanol vehicle. Luzindole is a competitive antagonist for both the MT1 and MT2 melatonin receptors (11). Luzindole administered to hibernating animals was kept on ice to closely match the torpor body temperature of the animals. Luzindole administered to summer animals was kept at room temperature. Vehicle-treated animals served as the control and received 100% ethanol vehicle only. All animals in all groups were treated with either luzindole or vehicle in the morning, ranging from 8 AM to 12 PM.
Experimental Design
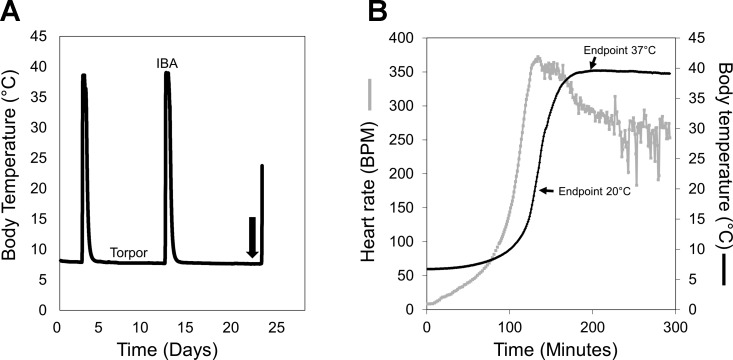
For the hibernating groups (HibernatingTR and HibernatingMT), animals were monitored throughout the hibernation season until their average torpor bout lengths were at least a week long. Using previous torpor bout lengths as a guide, we targeted animals toward the end of a torpor bout for drug administration (Fig. 1A). Each animal was weighed and verified as torpid either via rectal body temperature (HibernatingMT) or transmitter output (HibernatingTR). For the SummerMT group, animals were lightly anesthetized with isoflurane before they were weighed and rectal body temperature was taken. All animals were administered 30 mg/kg luzindole or equivalent volume of vehicle intraperitoneally during a time window of 8 AM to 12 PM. It is important to note that the hibernating groups (HibernatingTR and HibernatingMT) were in constant darkness during this time, but this timeframe translates to ∼1–5 h after lights on for the SummerMT animals. The squirrels were then returned to their home cage for undisturbed arousal. The HibernatingTR squirrels were tracked via transmitter output to one of two experimental endpoints: midarousal (transmitter output Tb = 20°C, luzindole-20 and vehicle-20) and full-arousal (transmitter output Tb = 37°C, luzindole-37 and vehicle-37; Fig. 1B). The luzindole-20 and vehicle-20 groups averaged ∼140 min to the 20°C endpoint, while the luzindole-37 and vehicle-37 groups averaged 200 min to the 37°C endpoint. These groups were euthanized at these respective endpoints. The HibernatingMT and SummerMT squirrels were euthanized 2 h after drug administration, which was the approximate time to midarousal with the more invasive rectal temperature reading.
Fig. 1.
Experimental design. A: representative trace of a ground squirrel's core body temperature during the hibernation season, showing the regular fluctuations between torpor and interbout arousals (IBAs) over ∼1 mo. This trace was obtained from a surgically implanted transmitter. Luzindole or vehicle was administered toward the end of a torpor bout, as illustrated by the black arrow. B: physiological parameters of arousal are shown, with heart rate [in beats/min (BPM)] in gray and core body temperature in black. The 2 experimental endpoints for the HibernatingTR animals are shown, midarousal at 20°C and full arousal at 37°C. The experimental endpoint for the HibernatingMT animals is also approximately midarousal.
Brain Dissection
All animals were fully anesthetized with isoflurane and then killed by decapitation. Rectal temperature (end Tb) and body weight were taken at death for all animals. The brain was removed from the skull, and the meninges and blood vessels surrounding the brain were removed. For the HibernatingMT and SummerMT animals, the whole brain collected for mitochondrial isolation. For the HibernatingTR animals, the hypothalamus, cerebral cortex, brainstem, and hippocampus were dissected out using a rodent brain atlas as a guide. All dissections were performed on ice and the dissected brain regions were rapidly frozen in liquid nitrogen. The time from decapitation to sample freezing was <10 min. Tissues were stored at −80°C until use.
Caspase-3 Assay
A colorimetric caspase-3 assay (CASP-3-C; Sigma-Aldrich) was used to determine caspase-3 activity. This assay determines the concentration of the chromophore p-nitroaniline (pNA), which is released from the hydrolysis of the substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3. Protein was purified from hypothalamus, hippocampus, cerebral cortex, and brainstem of the HibernatingTR animals for this assay. Samples were homogenized in lysis buffer (50 mM HEPES, pH 7.4, 5 mM CHAPS, and 5 mM DTT) with aprotonin (6.5 μg/ml; A1153; Sigma-Aldrich). Samples were then centrifuged for 20 min at 16,000 g at 4°C. The supernatant was collected and protein concentration was determined using a bicinchoninic acid assay (BCA; no. 23255; Thermo Fisher Scientific) according to the manufacturer's instructions.
For the assay, duplicate protein samples (10 μg) were incubated with Ac-DEVD-pNA for 4 h. Additionally, another set of duplicate samples were treated with a caspase-3 inhibitor and run in tandem. pNA has a high absorbance at 405 nm, which was measured with a microplate reader (SpectraMax Plus 384; Molecular Devices). The concentration of the pNA released from the substrate was calculated from a pNA calibration curve (R2 ≥ 0.98). The specific pNA concentration (μM) for each sample was determined by subtracting the nonspecific pNA concentration found in the inhibitor-treated samples. Caspase-3 activity was measured in four brain areas from the midarousal HibernatingTR ground squirrels (luzindole-20 and vehicle-20): cerebral cortex, hypothalamus, hippocampus, and brainstem. Additionally, caspase-3 activity was also measured in the hypothalamus for the luzindole-37 and vehicle-37 animals. The intra-assay coefficient of variation was 8.5%. The assay detection limit of activity is as low as 0.015 units/min or 15 ng caspase-3. One unit is defined as the amount of enzyme that will cleave 1 μmol of the substrate per minute at pH 7.4 at 25°C.
Western Blot Analysis
The remaining luzindole-20 and vehicle-20 hypothalamus protein samples were diluted further in Laemmli sample buffer and RIPA buffer with protease inhibitors (1:1,000; P8340; Sigma-Aldrich) and phosphatase inhibitors (1:1,000; P5726, P0044; Sigma-Aldrich). Samples were then boiled at 95°C for 10 min. Ten micrograms of protein were loaded onto 10% gels for SDS-PAGE. Electrophoresis was run at 180 V until the tracking dye front ran off the gel. Proteins were then transferred onto a nitrocellulose membrane at 75 V for 3 h. The membrane was blocked in 3% BSA for 1 h and then incubated with primary antibody [anti-STAT3 (phosphoY705): 1:2,000; ab76315; Abcam; and anti-STAT3: 1:2,000; ab68153; Abcam] overnight. The membrane was incubated in secondary antibody (anti-rabbit; 1:20,000; Pierce) for 1 h. The resulting Western blots were quantified using the “Analyze gels” function in ImageJ to obtain relative densities (26). Total protein stain with amido black was used as a loading control (2).
Mitochondrial Isolation
Mitochondrial isolation was performed on whole brains for the HibernatingMT and SummerMT animals. Isolation of mitochondria via differential centrifugation was adapted and modified from established protocols (21, 32). Whole brain was removed from the skull and immediately rinsed in ice-cold mitochondria isolation buffer (MIB; 250 mM sucrose, 5 mM HEPES, and 1 mM EGTA, pH 7.4 at 4°C). The brain was then minced on ice and homogenized with 10 passes in 30 ml MIB + 0.1% fatty acid free BSA using a rotating loose fitting Teflon pestle. The homogenate was filtered through three layers of gauze and centrifuged at 1,000 g for 10 min at 4°C. Floating lipid was aspirated from the supernatant, which was transferred to a new prechilled centrifuge tube and centrifuged at 500 g for 10 min at 4°C. Any additional floating lipid was aspirated from the supernatant, which was transferred to a new prechilled centrifuge tube and centrifuged at 10,500 g for 10 min at 4°C. The supernatant was decanted, and any lipid adhering to the tubes was carefully removed using Kimwipes. The pellet was resuspended, including the fluffy synaptosomal layer, in 30 ml ice-cold wash buffer (250 mM sucrose and 5 mM HEPES, pH 7.4 at 4°C) + 0.1% fatty acid free BSA and centrifuged at 12,000 g for 10 min at 4°C. The supernatant was decanted and the mitochondrial pellet was resuspended, including the fluffy synaptomosomal layer (32), in 30 ml ice-cold wash buffer and centrifuged at 12,000 g for 10 min at 4°C. The final mitochondrial pellet was transferred to a prechilled Eppendorf tube and kept on ice until assayed (maximum 6 h).
The protein concentrations of isolated mitochondria were determined by BCA protein assay (no. 23255; Thermo Fisher Scientific) according to the manufacturer's instructions, using BSA as a standard. Mitochondrial bioenergetics analyses were performed directly after mitochondrial isolation.
Mitochondrial Respiration Rate and Membrane Potential
In vitro mitochondrial respiration rates were measured using a Clark-type oxygen electrode (Hansatech Instruments) in 0.5 ml of respiration buffer (135 mM sucrose, 65 mM KCl, 5 mM KH2PO4, 5 mM HEPES, and 2.5 mM MgCl2, pH 7.2–7.4 at 4°C) at 25°C while undergoing constant stirring. Before the start of any measurements, the instrument was calibrated with dH2O to ensure accurate measurements. Unless otherwise stated, all compounds were dissolved in dH2O.
Mitochondria were added in a final concentration of 0.5 mg protein/ml. Maximal flux through various segments of the electron transport system (ETS) were determined under phosphorylating (state 3) conditions with the addition of ADP (200 nM), using specific substrates and inhibitors (8). Flux through complexes I–IV was measured using 5 mM glutamate/malate (G/M). Succinate (SUC; 5 mM) and glycerol-3-phosphate (G3P; 5 mM) were added to stimulate flux through complexes II-IV and complexes III-IV, respectively. Rotenone (2 mM, dissolved in ethanol; an ETS complex I inhibitor) was added to the mitochondrial suspension before SUC, G3P, and Asc/TMPD were introduced to prevent reverse electron flow. All substrate oxidation rates were allowed to reach both steady state 3 and steady state 4 (nonphosphorylating) respiration rates (8). To assess the integrity of the mitochondrial inner membranes of our isolated mitochondrial fractions, we measured the respiratory control ratio (RCR). RCR is the ratio between state 3 and state 4 and is an indicator of coupling efficiency between substrate oxidation and ATP synthesis (32, 42).
For the determination of proton leak kinetics, simultaneous measurements of oxygen consumption and membrane potential (ΔΨm) were required (31). Oxygen consumption was determined by the Clark-type oxygen electrode, while membrane potential was determined by a tetraphenylphosphonium (TPP+)-sensitive electrode. Initially, rotenone (2 mM) was added to inhibit complex I, and oligomycin (1 μg/ml, dissolved in ethanol; an ATPase inhibitor) was added to inhibit ATP synthesis. Mitochondria were added to the chamber at a final concentration of 0.5 mg/ml at 25°C. A TPP+-sensitive electrode (Hansatech Instruments) was inserted into the oxygen chamber to measure external [TPP+]. The TPP+ electrode was calibrated by making three additions of TPP+ (1 mM); each addition increased external [TPP+] by 1 μM. Once calibrated, SUC (10 mM) was added to stimulate approximately state 4 respiration. The kinetics of proton leak were determined by inhibiting SUC oxidation stepwise by titrating 0.3 mM malonate until a complete inhibition was obtained and then measuring this effect on ΔΨm.
ΔΨm was calculated from external [TPP+] using a modified Nernst equation, adapted and modified from (4, 31):
[TPP+]external is the concentration of TPP+ outside of the mitochondria, and the value 0.001 represents the internal volume of the mitochondria (taken as 1.1 μl/mg protein) (21).
Because there were many different measurements performed on the mitochondrial samples, there were not always enough mitochondria to complete all of the measurements for each animal. Therefore, membrane potential was always measured first, followed by SUC-fueled respiration rates.
Data Analysis
All data are represented as means ± SE. In all cases, P < 0.05 was considered statistically significant. Differences in arousal parameters, starting body temperature, ending body temperature, torpor day, and body weight in the HibernatingTR animals were determined using a one-way ANOVA and Tukey's honestly significant difference test. Differences between treatments in these same parameters in the HibernatingMT and SummerMT animals were calculated using a t-test. For the Western blot data and the caspase-3 assay, differences between treatment groups were determined with a t-test. Differences in substrate-fueled state 3 respiration rates between treatment groups (luzindole and vehicle) were compared using t-tests.
RESULTS
We examined how melatonin receptor signaling contributes to protection in the brain during arousal from torpor, by specifically examining 1) evidence of apoptotic and survival signaling, and 2) mitochondrial function and integrity, after administration of the competitive melatonin receptor antagonist luzindole at the end of a torpor bout (Fig. 1).
Experiment 1: HibernatingTR Ground Squirrels
Transmitter output.
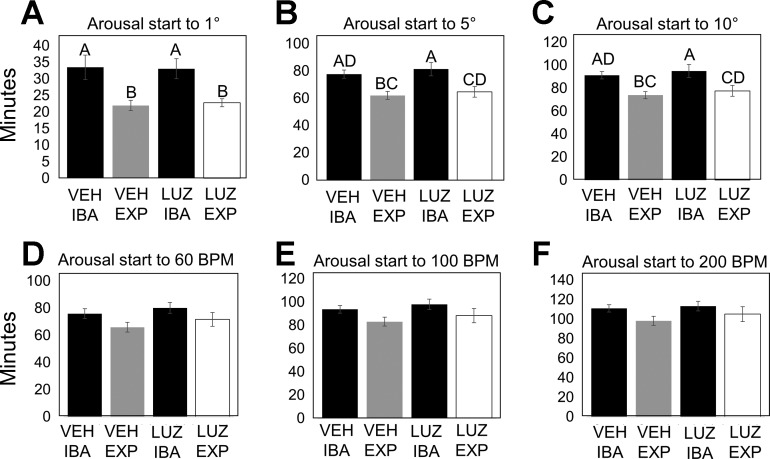
All HibernatingTR ground squirrels were monitored with surgically implanted transmitters that measured core body temperature and heart rate values in real time. All animals were torpid at the beginning of the experimental protocol with similar preceding torpor bout lengths (Table 1). The average rectal temperature for the luzindole-20 group was 15.54 ± 1.49°C, for the vehicle-20 group was 17.46 ± 1.82°C, for the luzindole-37 group was 34.67 ± 0.55°C, and for the vehicle-37 group was 34.42 ± 0.52°C (Table 1). The only significant differences in final body temperature were between the two experimental endpoints. After extensive analysis of arousal parameters, no significant differences in arousal timing of body temperature or heart rate were found between luzindole- and vehicle-treated animals (Fig. 2). However, the body temperature of both luzindole- and vehicle-treated animals rose significantly faster during early arousal than their previously recorded natural IBAs (Fig. 2, A–C, ANOVA with Tukey's honestly significance difference test). There was no significant change in how fast the heart rate increased during arousal between experimental groups and natural IBAs, although the same trend emerged, with the experimental arousal occurring faster than the arousal in natural IBA (Fig. 2, D–F).
Table 1.
HibernatingTR group parameters
| HibernatingTR | Luzindole-20 | Vehicle-20 | Luzindole-37 | Vehicle-37 | P Value |
|---|---|---|---|---|---|
| Start Tb, °C | 6.9 ± 0.13 | 7.1 ± 0.07 | 7.0 ± 0.09 | 6.8 ± 0.18 | 0.41 |
| End Tb, °C | 15.5 ± 1.49a | 17.5 ± 1.82a | 34.7 ± 0.55b | 34.4 ± 0.52b | 4E-10* |
| Arousal time, min | 143.4 ± 11.24a | 136.0 ± 5.01a | 201.8 ± 7.67b | 206.0 ± 6.69b | 4.95E-6* |
| Body weight, g | 203.4 ± 18.54 | 189.1 ± 17.96 | 155.3 ± 19.05 | 179.6 ± 6.58 | 0.24 |
| Torpor day | 7.4 ± 1.03 | 6.8 ± 0.49 | 6.8 ± 0.54 | 6.0 ± 0.54 | 0.57 |
Data are means ± SE; n = 5 for each group. Transmitter output Tb = 20 and 37°C for vehicle and luzindole is shown. For the statistical analysis, P values were obtained with an ANOVA.
P < 0.05 was considered significant. Any parameter with a significant P value from the ANOVA was then subjected to a Tukey's honestly significant difference (HSD) test. Groups on the same row labeled with different letters are significantly different according to the Tukey's HSD test.
Fig. 2.
Timing of arousal during natural and forced arousal from torpor. A–C: timing of body temperature increase from arousal initiation (see methods for details) to 1, 5, and 10°C above torpid body temperature did not differ between experimental groups [vehicle (VEH) or luzindole (LUZ); n = 10 per group], but there was a significant difference between natural and forced arousal. EXP, experimental arousal. D–F: timing of heart rate increase from arousal initiation (see methods for details) to 60, 100, and 200 beats/min did not differ between experimental groups (vehicle or luzindole) or between natural and forced arousal. Data are represented as means ± SE. For the statistical analysis, P values were obtained with an ANOVA. P < 0.05 was considered significant. Any parameter with a significant P value from the ANOVA was then subjected to a Tukey's honestly significant difference (HSD) test. Groups on the same row labeled with different letters are significantly different according to the Tukey's HSD test. Natural IBAs are shown in black, vehicle-treated animals are shown in gray, and luzindole-treated animals are shown in white.
Caspase-3 assay.
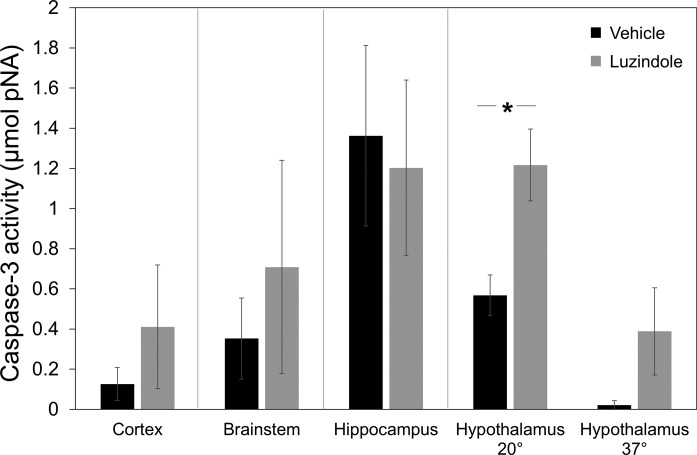
Caspase-3 activity was used as an indicator of apoptotic activity. Luzindole-treated ground squirrels had significantly higher caspase-3 activity in the hypothalamus during the midarousal endpoint (Fig. 3, t-test; n = 5; P = 0.01). However, there was no longer a significant difference in this brain region at the full-arousal endpoint (Fig. 3, t-test; n = 5; P = 0.13). No significant differences between groups were seen in the cerebral cortex, hippocampus, or brainstem; Fig. 3, t-tests; n = 5 per group; P = 0.40 (cerebral cortex); P = 0.55 (brainstem); and P = 0.81 (hippocampus)].
Fig. 3.
Caspase-3 activity in ground squirrel brain. Caspase-3 activity [μmol; p-nitroaniline (pNA)] at the midarousal endpoint after vehicle (black) or luzindole (gray) treatment in cortex, brainstem, hippocampus, and hypothalamus (20 and 37°C). Luzindole-treated animals had significantly higher caspase-3 activity in the hypothalamus at the 20°C endpoint (*P = 0.01, t-test; n = 5 per group). Error bars represent means ± SE.
Survival signaling.
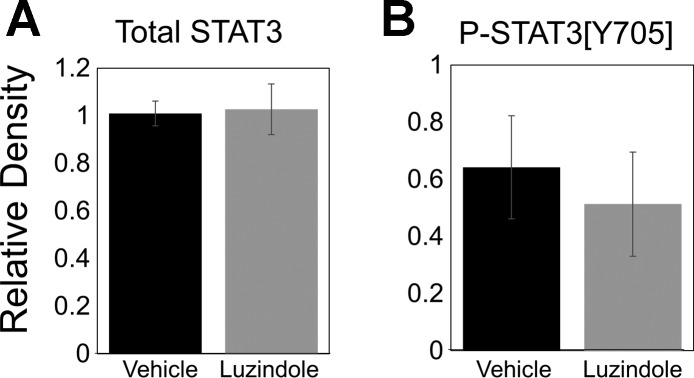
Previous work has shown that STAT3 phosphorylation on tyrosine-705 (P-STAT3[Y705]) is involved in protective melatonin receptor signaling (40). Since the midarousal hypothalamus samples showed evidence of protection in the caspase-3 assay, we analyzed the luzindole-20 and vehicle-20 hypothalamus samples further for evidence of changes in STAT3 signaling. However, no significant differences were found in levels of P-STAT3[Y705] (t-test; n = 5; P = 0.62) or total STAT3 (t-test; n = 5; P = 0.88) between groups (Fig. 4), suggesting that this signaling pathway is not involved in melatonin receptor-mediated protection during arousal from torpor.
Fig. 4.
Expression of total signal transducers and activators of transcription 3 (STAT3) and phosphorylated STAT3 [Y705] in ground squirrel brain. There was no significant difference between groups in total STAT3 (A) or P-STAT3 [Y705] (B; n = 5 per group). The bar graphs represent quantification of Western blots. Error bars represent means ± SE.
Experiment 2: HibernatingMT and SummerMT Ground Squirrels
Mitochondrial respiration.
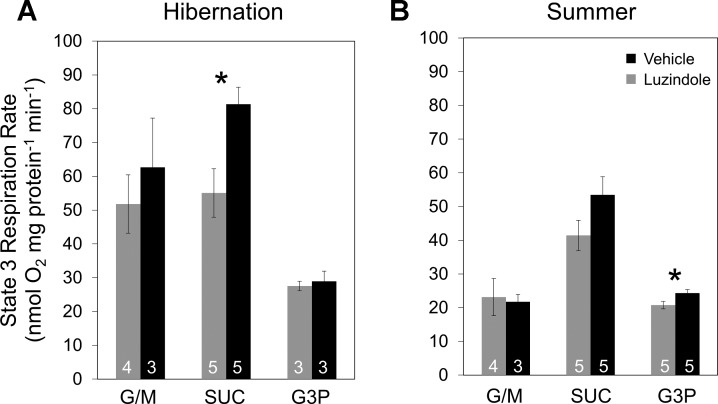
In this set of experiments, we investigated the effect of luzindole administration on brain mitochondrial function during hibernation and in summer, a time when the ground squirrels are not hibernating and melatonin levels during the day would be essentially undetectable. Mitochondria were isolated from whole brains for these experiments. State 3 respiration rates were measured using three different substrates (G/M, SUC, and G3P) specifically designed to parse out different aspects of the ETS (see methods for details). Mitochondria from luzindole-treated animals had significantly lower state 3 respiration rates using SUC as a fuel during hibernation (Fig. 5A, t-test; n = 5; P < 0.05), but not in the summer (Fig. 5B, t-test; n = 5; P = 0.12). There were no significant differences between groups using G/M or G3P as a fuel in the HibernatingMT animals. State 3 respiration rates with G3P were significantly higher in the vehicle-treated animals in the SummerMT group (Fig. 5B). Additionally, RCRs, used as an indicator of mitochondrial quality, were calculated for each group after respiration assay analysis. Luzindole-treated animals tended to have lower RCRs for all fuels during hibernation, but the effect was not significant (Table 2).
Fig. 5.
State 3 respiration rates in brain mitochondria. A: state 3 respiration rates at midarousal from hibernation in brain mitochondria from animals treated with luzindole (gray) or vehicle (black). Luzindole-treated animals had significantly lower respiration rates with succinate (SUC; *P = 0.019, t-test). G/M, glutamate/malate. B: state 3 respiration rates during summer in brain mitochondria from animals treated with luzindole (gray) or vehicle (black). Vehicle-treated animals had significantly higher respiration rates with glycerol-3-phosphate (G3P; *P = 0.0496, t-test). Number of animals (n) for each group is listed at the bottom of the respective bar. Error bars represent means ± SE.
Table 2.
Respiratory control ratios of brain mitochondria during arousal from hibernation (HibernatingMT)
| Luzindole | Vehicle | P Value | |
|---|---|---|---|
| G/M | 3.5 ± 0.58 (n = 4) | 5.2 ± 0.69 (n = 3) | 0.13 |
| Succinate | 2.8 ± 0.26 (n = 5) | 3.8 ± 0.42 (n = 5) | 0.08 |
| G3P | 2.7 ± 0.43 (n = 3) | 3.3 ± 1.05 (n = 3) | 0.63 |
Respiratory control ratios are calculated as the state 3 respiration rate divided by the state 4 respiration rate. Data are means ± SE and n for each group is provided. G/M, glutamate/malate; SUC, succinate; G3P, glycerol-3-phosphate. P < 0.05 with a Student's t-test was considered significant. There was no statistical significance between groups.
Similar to the previous experiment, all HibernatingMT animals were torpid at the beginning of the experimental protocol and had similar preceding torpor bout lengths (Table 3). Body temperature at collection (luzindole: 20.28 ± 2.30°C; vehicle: 18.02 ± 2.19°C) indicated that all HibernatingMT animals were midarousal at collection and there were no significant body temperature differences between groups (t-test; n = 5 per group; P = 0.49). Similarly, SummerMT animals had comparable starting and ending body temperatures (Table 3).
Table 3.
HibernatingMT and SummerMT group parameters
| Parameter | Luzindole | Vehicle | P Value |
|---|---|---|---|
| HibernatingMT | |||
| Start Tb, °C | 5.0 ± 0.14 | 5.1 ± 0.20 | 0.57 |
| End Tb, °C | 20.3 ± 2.30 | 18.0 ± 2.19 | 0.49 |
| Body weight, g | 169.0 ± 9.66 | 162.6 ± 6.11 | 0.59 |
| Torpor day | 7.6 ± 0.40 | 6.0 ± 1.30 | 0.27 |
| SummerMT | |||
| Start Tb, °C | 37.2 ± 0.09 | 36.5 ± 0.27 | 0.04* |
| End Tb, °C | 36.9 ± 0.28 | 36.8 ± 0.30 | 0.96 |
| Body weight, g | 218.0 ± 13.27 | 202.7 ± 7.45 | 0.34 |
| Torpor day | N/A | N/A | N/A |
Data are means ± SE n = 5 for each group. For the statistical analysis, P values were obtained with a Student's t-test between the 2 groups.
P < 0.05 was considered significant.
Mitochondrial membrane potential.
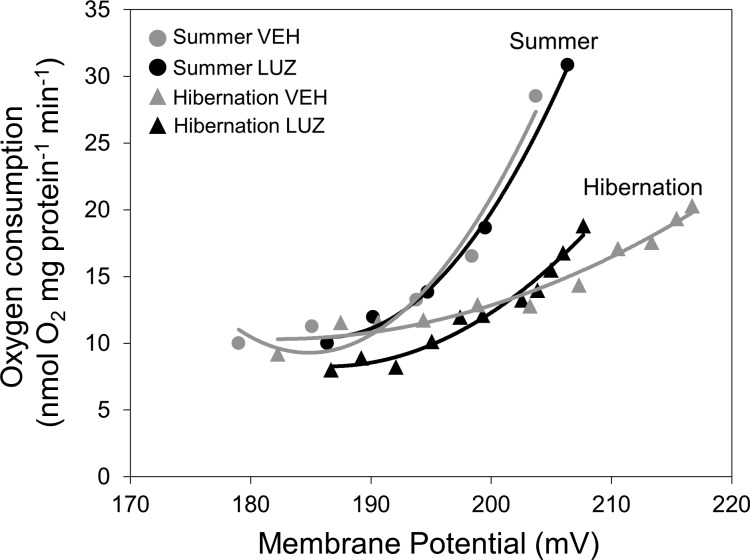
The kinetics of proton leak were also determined for isolated brain mitochondria by the simultaneous measurement of membrane potential (ΔΨm) and state 4 respiration rate. There was no significant change in maximum ΔΨm or state 4 respiration rate between groups in either hibernation or summer, but the trend in hibernation was that luzindole-treated animals had a lower maximum ΔΨm than vehicle-treated animals (Table 4; Fig. 6). The highest ΔΨm shared between the two groups during hibernation was ∼207 mV (Fig. 6), and at this shared ΔΨm, proton leak is higher in luzindole-treated animals compared with vehicle-treated animals. However, there was no significant change in proton leak rate at any of the shared ΔΨm values, suggesting that proton permeability of the mitochondrial membrane did not change between luzindole and vehicle-treated animals in hibernation. The proton leak curves of the two experimental groups in summer look virtually identical (Fig. 6), indicating that luzindole treatment had no effect. However, there does appear to be a clear seasonal change in proton kinetics between hibernation and summer in the brain (Fig. 6).
Table 4.
Bioenergetics of brain mitochondria
| Luzindole | Vehicle | P Value | |
|---|---|---|---|
| Hibernation state 4 ΔΨm, mV | 207.7 ± 3.53 | 216.7 ± 1.97 | 0.056 |
| Hibernation state 4 respiration, nmol O2·mg protein−1·min−1 | 18.8 ± 2.00 | 20.3 ± 1.40 | 0.56 |
| Summer state 4 ΔΨm, mV | 206.4 ± 4.35 | 203.7 ± 8.05 | 0.78 |
| Summer state 4 respiration, nmol O2·mg protein−1·min−1 | 30.9 ± 4.00 | 28.5 ± 5.55 | 0.74 |
Data are means ± SE; n = 5 for each group. ΔΨm, Mitochondrial membrane potential. For the statistical analysis, P values were obtained with a Student's t-test between the 2 groups. P < 0.05 was considered significant.
Fig. 6.
Kinetic response of proton leak according to mitochondrial membrane potential (ΔΨm) in ground squirrel brain mitochondria during hibernation (triangles) and summer (circles). Luzindole-treated animals are shown in black and vehicle-treated animals are shown in gray (n = 5 per group). No significant effect of treatment is seen in either season, but luzindole-treated animals tended to have lower membrane potential during hibernation. Error bars were omitted for clarity.
DISCUSSION
This work investigated the effect of the competitive melatonin receptor antagonist luzindole on the physiological parameters of arousal, regulation of survival and apoptotic signaling, and optimal mitochondrial function in a hibernating mammal during arousal from torpor. Additionally, we investigated luzindole's effect on mitochondrial function in the summer, after completion of the hibernation season.
Melatonin Receptor Signaling Promotes Neuroprotection in Hypothalamus
In this work, we show an increase in caspase-3 activity in the hypothalamus during midarousal with luzindole treatment (Fig. 3), indicating that melatonin receptor signaling is important to help quench apoptotic signaling during this time. Previous work showed that melatonin reduced caspase-3 activity through receptor-mediated mechanisms in cultured cells after induction of apoptosis (24, 25). Another study showed that melatonin was protective against cytotoxicity in cultured cells, indicated by a reduction in caspase-3 activation and an enhancement in STAT3 phosphorylation (40). Luzindole attenuated these protective effects in both cases, indicating that melatonin receptor signaling played an important role in orchestrating the protection. While we found no effect of luzindole treatment on levels of phosphorylated STAT3 (Fig. 4), our midarousal caspase-3 activity results support this previous work. Interestingly, there is no longer a difference in caspase-3 activity between treatment groups in the hypothalamus at the full arousal endpoint (Fig. 3). Overall caspase-3 activity levels are lower in the hypothalamus at the full arousal point, which is at the end of the physiological transition of arousal and could translate to a period of lower damage risk. It is possible that the longer amount of time to reach full arousal could have allowed enough time for the clearance of luzindole from the system, resulting in no difference between groups. It is also possible and likely that there are other protective mechanisms in place in the hypothalamus to help account for the missing melatonin receptor signaling.
Outside of the hypothalamus, luzindole treatment did not have a significant effect on caspase-3 activity in any other brain region. This suggests that the hypothalamus could be a specific target of melatonin receptor-mediated protection. The hypothalamus continues to show some level of activity throughout the transitions between torpor and IBA in hibernation (6) and therefore could be at more risk of damage, particularly during the arousal period. Melatonin-mediated neuroprotection in the hypothalamus may have specifically evolved due to the importance of the hypothalamus in regulating body temperature, food intake, and physiological rhythms, all important for the hibernation phenotype. It is also possible that other regions of the brain are protected through different mechanisms, like synaptic plasticity (37), and might not be prone to the same risk of injury. Previous work from our laboratory has shown regional differences in gene expression between the hypothalamus and the cerebral cortex (30), particularly in terms of protection and survival signaling, supporting the possibility that protective melatonin receptor signaling could be localized to certain regions.
Another possibility for regional differences in protection could potentially be from the availability of luzindole in the brain. While melatonin levels in the cerebral spinal fluid are known to be high (28), the luzindole administered in these experiments was intraperitoneal, and it is unknown whether luzindole would have reached all areas of the brain equally. However, previous effects of luzindole in the brain have been reported in mice administered intraperitoneally with a lower dose of luzindole (14, 36). Additionally, our results from mitochondria isolated from the whole brain (Figs. 5 and 6) also support widespread availability of luzindole in the brain.
Melatonin Receptor Signaling Is Important For Optimal Mitochondrial Function
In addition to specifically protecting the hypothalamus during arousal from torpor, melatonin receptor signaling also appears to be important for optimal mitochondrial function in the whole brain during this time. Here, we report that disruption of melatonin receptor signaling during arousal from torpor results in decreased mitochondrial function, specifically including a significant decrease in the state 3 respiration rate of luzindole-treated animals when using SUC as a fuel. We also see a decrease in mitochondrial membrane potential with luzindole treatment, although this trend is not significant, potentially due to our small sample size.
Melatonin is known to protect mitochondria (1), which is particularly evident in research in animal models of neurological disorders. Treatment with melatonin ameliorated mitochondrial dysfunction associated with Alzheimer's disease by restoring membrane potential and respiration rates in young mice (9). Luzindole partially blocked the ability of melatonin to restore mitochondrial function, indicating that melatonin receptors play a role in mitochondrial protection. Melatonin also slowed disease progression in a mouse model of Huntington's disease, and this study also showed that melatonin receptor expression decreased in Huntington's disease mice, suggesting that depletion of melatonin receptor signaling enhances the disease phenotype (39). Additionally, previous work has shown that melatonin protects mitochondria by antagonizing apoptotic signaling, as mentioned previously, an effect that is reversed by luzindole (24). Our work in the hibernator, which produces melatonin naturally during arousal from hibernation, supports this work by functionally demonstrating that disrupting melatonin receptor signaling upon arousal from torpor reduces mitochondrial function.
It is important to state that the effect of luzindole is seen in mitochondria isolated from the whole brain, not just the hypothalamus. It is possible that the effect of melatonin receptor signaling on whole brain mitochondrial respiration and membrane potential is not necessarily just to protect the mitochondria but to actually enhance and improve mitochondrial function during arousal in the brain, effectively allowing the hibernator's mitochondria to perform better than the mitochondria of a nonhibernating mammal in the same conditions. This idea is supported in part by the summer experiments, showing both that luzindole administration has no effect on mitochondrial function compared with vehicle and that the summer respiration rates and membrane potentials overall are lower than what is seen in hibernation (Figs. 5 and 6; Table 4). This is also supported by the ability of melatonin to restore mitochondrial function in a mouse model of Alzheimer's disease (9). Additionally, previous work showed that melatonin administration stimulated mitochondrial respiration in rat brain (20); however, it is not known whether this effect is receptor mediated. Arousal is a very energy costly event (18), requiring a lot of energy generated by the mitochondria. It is possible that melatonin production upon arousal from torpor functions to help the brain mitochondria work harder and more efficiently during a period of extreme energy need.
Natural vs. Forced Arousal in Hibernation
It is important to note that this experimental design, while using a natural model of extreme physiology and neuroprotection, promotes arousal from torpor in a relatively unnatural way. Every effort was made to make this experimental paradigm as natural as possible, for example, administering the treatments at the predicted end of a torpor bout when the animal would arouse naturally, keeping the temperature of the luzindole and vehicle treatments near the body temperature of the animal, and returning treated animals to their home cages and their normal laboratory environment for the arousal period. However, this arousal-type distinction is important. It is possible that a forced arousal could influence the molecular mechanisms of neuroprotection differently than a natural arousal. Importantly though, both luzindole- and vehicle-treated groups followed the same experimental design, so that any effect of forced arousal would be the same in both groups.
We were interested in whether treatment would have an effect on arousal timing, specifically in how fast body temperature and heart rate increased. While there was no treatment effect on these physiological parameters of arousal, there was a significant difference in timing between the experimental forced arousals and natural arousals to IBA (Fig. 2). The experimental arousals reported here occurred faster than natural arousals recorded in the same animals, in contrast to previous reports in another hibernating ground squirrel species (35). However, the experimental protocol reported here involved intraperitoneal administration of luzindole or vehicle, rather than mild handling to trigger arousal reported previously, which could account for the difference in arousal time.
Perspectives and Significance
This work provides evidence for the importance of melatonin receptor signaling in neuroprotection and promotion of optimal brain function in hibernating mammals arousing from torpor. The protective effects of melatonin have been shown in many disease models, but this study provides an example of melatonin's protective role in a natural system. This provides important groundwork for future studies, particularly in investigating how this system is seasonally controlled and other specific signaling pathways that could be involved.
GRANTS
This study was funded by National Institute of Neurological Disorders and Stroke Grant 5F32NS077643 (to C. Schwartz), U.S. Army Medical Research and Materiel Command Grant W81XWH-11-1-0409 (to M. T. Andrews), and a University of Minnesota McKnight Foundation Professorship (to M. T. Andrews).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.S., M.A.B., and M.T.A. conception and design of research; C.S. and M.A.B. performed experiments; C.S. and M.A.B. analyzed data; C.S., M.A.B., and M.T.A. interpreted results of experiments; C.S. and M.A.B. prepared figures; C.S. drafted manuscript; C.S., M.A.B., and M.T.A. edited and revised manuscript; C.S., M.A.B., and M.T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Margarita L. Dubocovich for guidance in the experimental design.
REFERENCES
- 1.Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ. Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res 30: 65–74, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Meth 172: 250–254, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab 17: 1048–1056, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Barger JL, Brand MD, Barnes BM, Boyer BB. Tissue-specific depression of mitochondrial proton leak and substrate oxidation in hibernating arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 284: R1306–R1313, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bittman EL, Thomas EM, Zucker I. Melatonin binding sites in sciurid and hystricomorph rodents: studies on ground squirrels and guinea pigs. Brain Res 648: 73–79, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bratincsak A, McMullen D, Miyake S, Toth ZE, Hallenbeck J, Palkovits M. Spatial and temporal activation of brain regions in hibernation: c-fos expression during the hibernation bout in thirteen-lined ground squirrel. J Comp Neurol 505: 443–458, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chance B, Williams G. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217: 383–394, 1955. [PubMed] [Google Scholar]
- 9.Dragicevic N, Copes N, O'Neal-Moffitt G, Jin J, Buzzeo R, Mamcarz M, Tan J, Cao C, Olcese JM, Arendash GW. Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res 51: 75–86, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Drew K, Rice M, Kuhn T, Smith M. Neuroprotective adaptations in hibernation: therapeutic implications for ischemia-reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med 31: 563–573, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dubocovich ML, Markowska M. Functional MT1 MT2 melatonin receptors in mammals. Endocrine 27: 101–110, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Florant GL, Rivera M, Lawrence AK, Tamarkin L. Plasma melatonin concentrations in hibernating marmots: absence of a plasma melatonin rhythm. Am J Physiol Regul Integr Comp Physiol 247: R1062–R1066, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Frerichs K, Kennedy C, Sokoloff L, Hallenbeck J. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 14: 193–205, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Gressens P, Schwendimann L, Husson I, Sarkozy G, Mocaer E, Vamecq J, Spedding M. Agomelatine, a melatonin receptor agonist with 5-HT 2C receptor antagonist properties, protects the developing murine white matter against excitotoxicity. Eur J Pharmacol 588: 58–63, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hampton M, Nelson BT, Andrews MT. Circulation and metabolic rates in a natural hibernator: an integrative physiological model. Am J Physiol Regul Integr Comp Physiol 299: R1478–R1488, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein AH, Wendroth SM, Drewes LR, Andrews MT. Small-volume D-β-hydroxybutyrate solution infusion increases survivability of lethal hemorrhagic shock in rats. Shock 34: 565–572, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Larkin JE, Yellon SM, Zucker I. Melatonin production accompanies arousal from daily torpor in Siberian hamsters. Physiol Biochem Zool 76: 577–585, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Lyman CP, Willis J, Malan A, Wang L. Hibernation and Torpor in Mammals and Birds. New York: Academic, 1982. [Google Scholar]
- 19.Markus H. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry 75: 353–361, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M, Macias M, Escames G, Reiter R, Agapito M, Ortiz G, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 28: 242–248, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Palmeira CM, Rolo AP. Mitochondrial membrane potential (ΔΨ) fluctuations associated with the metabolic states of mitochondria. In: Mitochondrial Bioenergetics. New York: Springer, 2012, p. 89–101. [DOI] [PubMed] [Google Scholar]
- 22.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 85: 335–353, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Pengelley ET, Fisher KC. Rhythmical arousal from hibernation in the golden-mantled ground squirrel, Citellus lateralis tescorum. Can J Zoolog 39: 105–120, 1961. [Google Scholar]
- 24.Radogna F, Cristofanon S, Paternoster L, D'Alessio M, De Nicola M, Cerella C, Dicato M, Diederich M, Ghibelli L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl2. J Pineal Res 44: 316–325, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Radogna F, Paternoster L, Albertini MC, Cerella C, Accorsi A, Bucchini A, Spadoni G, Diamantini G, Tarzia G, Nicola MD. Melatonin antagonizes apoptosis via receptor interaction in U.937 monocytic cells. J Pineal Res 43: 154–162, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Rasband WS. ImageJ (Online) Bethesda, MD: National Institutes of Health; http://imagej.nih.gov/ij/ [1997]. [Google Scholar]
- 27.Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res 181: 127–151, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan DX, Kim SJ, Cruz MH. Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct Funct 219: 1873–1887, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Rouble AN, Hefler J, Mamady H, Storey KB, Tessier SN. Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. PeerJ 1: e29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz C, Hampton M, Andrews MT. Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS One 8: e58427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serviddio G, Sastre J. Measurement of mitochondrial membrane potential and proton leak. In: Advanced Protocols in Oxidative Stress II. New York: Springer, 2010, p. 107–121. [DOI] [PubMed] [Google Scholar]
- 32.Silva AM, Oliveira PJ. Evaluation of respiration with Clark type electrode in isolated mitochondria and permeabilized animal cells. In: Mitochondrial Bioenergetics, New York: Springer, 2012, p. 7–24. [DOI] [PubMed] [Google Scholar]
- 33.Stanton TL, Craft CM, Reiter RJ. Pineal melatonin: circadian rhythm and variations during the hibernation cycle in the ground squirrel Spermophilus lateralis. J Exp Zool 239: 247–254, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Tan D, Manchester LC, Sainz RM, Mayo JC, Leon J, Reiter RJ. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production. Endocrine 27: 149–157, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Utz JC, van Breukelen F. Prematurely induced arousal from hibernation alters key aspects of warming in golden-mantled ground squirrels, Callospermophilus lateralis. J Therm Biol 38: 570–575, 2013. [Google Scholar]
- 36.Valero N, Nery A, Bonilla E, Espina LM, Chacin-Bonilla L, Añez F, Maldonado M, Meleán E. Antagonistic effect of luzindole in mice treated with melatonin during the infection with the Venezuelan equine encephalomyelitis virus. Neurochem Res 34: 268–273, 2009. [DOI] [PubMed] [Google Scholar]
- 37.von der Ohe CG, Garner CC, Darian-Smith C, Heller HC. Synaptic protein dynamics in hibernation. J Neurosci 27: 84–92, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309: 151–162, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Sirianni A, Pei Z, Cormier K, Smith K, Jiang J, Zhou S, Wang H, Zhao R, Yano H. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J Neurosci 31: 14496–14507, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Liu D, Wang J, Liu S, Gao M, Ling EA, Hao A. Cytoprotective effects of melatonin on astroglial cells subjected to palmitic acid treatment in vitro. J Pineal Res 52: 253–264, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57: 131–146, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Nuebel E, Wisidagama DR, Setoguchi K, Hong JS, Van Horn CM, Imam SS, Vergnes L, Malone CS, Koehler CM. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc 7: 1068–1085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol 158: 2145–2151, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]