Abstract
Our recent study has shown that hyperventilation of humidified warm air (HWA) triggered cough and reflex bronchoconstriction in patients with mild asthma. We suggested that a sensitizing effect on bronchopulmonary C-fibers by increasing airway temperature was involved, but direct evidence was lacking. This study was carried out to test the hypothesis that HWA enhances the pulmonary C-fiber sensitivity in Brown-Norway rats sensitized with ovalbumin (Ova). In anesthetized rats, isocapnic hyperventilation of HWA for 3 min rapidly elevated airway temperature to a steady state of 41.7°C. Immediately after the HWA challenge, the baseline fiber activity (FA) of pulmonary C-fibers was markedly elevated in sensitized rats, but not in control rats. Furthermore, the response of pulmonary C-fibers to right atrial injection of capsaicin in sensitized rats was significantly higher than control rats before the HWA challenge, and the response to capsaicin was further amplified after HWA in sensitized rats (ΔFA = 4.51 ± 1.02 imp/s before, and 9.26 ± 1.74 imp/s after the HWA challenge). A similar pattern of the HWA-induced potentiation of the FA response to phenylbiguanide, another chemical stimulant of C-fibers, was also found in sensitized rats. These results clearly demonstrated that increasing airway temperature significantly elevated both the baseline activity and responses to chemical stimuli of pulmonary C-fibers in Ova-sensitized rats. In conclusion, this study supports the hypothesis that the increased excitability of these afferents may have contributed to the cough and reflex bronchoconstriction evoked by hyperventilation of HWA in patients with asthma.
Keywords: asthma, airway inflammation, cough, bronchoconstriction, TRPV1
hyperthermia can occur under normal physiological condition as the results of increased metabolic rate and/or hindered heat dissipation, such as during exercise. Hyperthermia is also found under pathophysiological conditions, for example, in patients who suffer from severe fever or heat stroke. In addition, inflammatory reaction can cause an increase of local tissue temperature. Asthma is an airway inflammatory disease; indeed, a previous study has reported that exhaled breath temperature in average was 2.7°C higher in allergic asthmatic children than in healthy children, and the increase in temperature was closely correlated with the increases in exhaled nitric oxide concentration as well as the number of eosinophils in the induced sputum (39).
In a recent study, we have reported that hyperventilation of humidified warm air (HWA) evoked an immediate and reversible bronchoconstriction (twofold increase in airway resistance) in patients with mild and stable asthma, but not in healthy subjects (15). The HWA-induced bronchoconstriction in these patients was completely prevented by pretreatment with ipratropium, indicating an involvement of cholinergic reflex. Breathing HWA also triggered coughs accompanying the bronchoconstriction in these patients, further suggesting that activation of airway sensory nerves was involved (15). However, direct evidence is still lacking.
Among the sensory nerves innervating the lung and airways, a majority (∼75%) are unmyelinated bronchopulmonary C-fibers (20). These sensory afferents exhibit distinct sensitivity to inhaled irritants (e.g., acid aerosol, sulfur dioxide, ammonia, etc.) and endogenous inflammatory mediators (e.g., hydrogen ion, eosinophil granule-derived cationic proteins, and certain metabolites of arachidonic acid, etc.) (8, 16, 22, 26, 27). Activation of these bronchopulmonary C-fiber afferents is known to elicit reflex responses such as bronchoconstriction and cough (8, 26, 27). One of the characteristic features of these afferents is the expression of transient receptor potential vanilloid type 1 receptor (TRPV1) in the nerve endings (16, 46). TRPV1 is a polymodal and nonselective cation channel (7, 38) that can be activated and sensitized by an increase in temperature within the normal physiological range (35, 36).
Furthermore, the overexpression of TRPV1 in bronchopulmonary sensory nerves was demonstrated in Brown-Norway rats actively sensitized by chronic inhalation of ovalbumin (Ova) aerosol (51), an established animal model of allergic asthma (10). In Ova-sensitized rats, acute inhalation challenge of Ova aerosol produced both early- and late-phases of bronchoconstriction, demonstrating the airway hyperreactivity to the antigen challenge. The bronchomotor responses to methacholine challenge were also markedly elevated in Ova-sensitized rats, indicating airway hyperresponsiveness to nonspecific bronchoactive challenge (50). Furthermore, accompanying the airway hyperreactivity, differential cell counts of the bronchoalveolar lavage fluid (BALF) clearly showed the inflammatory cell (eosinophils, neutrophils) infiltration in the airways of sensitized animals. Together, these pathophysiological features induced by Ova sensitization in Brown-Norway rats closely resemble the clinical observations in human allergic asthma, despite certain differences and limitations (10, 14, 42, 45).
On the basis of these findings, we hypothesize that an increase in airway temperature by hyperventilation of HWA elevates the baseline activity and excitability of vagal pulmonary C-fibers in asthmatics. Because a direct recording of bronchopulmonary C-fibers cannot be performed in asthmatic patients, we tested this hypothesis in Ova-sensitized Brown-Norway rats in the present study.
MATERIALS AND METHODS
The experimental procedures described below were in accordance with the recommendation in Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal sensitization.
The protocol of Ova sensitization used in this study was identical to that reported in details in our recent studies (50, 51). Adult male Brown-Norway rats (age: 3∼4 mo) were randomly divided into control and sensitized groups. Sensitized rats received an initial intraperitoneal injection of a solution containing 2 mg Ova in 1 ml Imject Alum as an adjuvant. Three days later, the sensitized rats were exposed to Ova aerosol for 15 min each time, three times a week for 3 wk. During exposure, the conscious rat was placed in a Plexiglas restrainer (University of Kentucky, Center for Manufacturing) and breathed spontaneously and continuously through a nose cone connected to a free stream of air-aerosol mixture under a negative-pressure exhaust hood. Ova solution (wt/vol concentration: 1.25% in isotonic saline) was nebulized and delivered by an ultrasonic nebulizer (model 099HD; DeVilbiss, Somerset, PA) at a droplet size ranging 0.5–5 μm. Control rats received the intraperitoneal injection of Imject Alum alone and aerosol inhalation of the vehicle (isotonic saline) aerosol following the identical procedures.
Animal preparation.
One day after the last inhalation exposure to Ova aerosol, rats were initially anesthetized with an intraperitoneal injection of α-chloralose (100 mg/kg) and urethane (500 mg/kg) dissolved in a 2% borax solution; supplemental doses (one-tenth of the initial dose) of the same anesthetics were injected intravenously to maintain abolition of pain reflexes elicited by pinching the tail. One femoral artery was cannulated for recording the arterial blood pressure (ABP) with a pressure transducer (model P23AC; Statham, Hato Rey, Puerto Rico). For administration of pharmacological agents, a catheter was inserted into the left jugular vein and advanced until its tip was positioned just above the right atrium. A short tracheal cannula was inserted just below the larynx via a tracheotomy. Tracheal pressure (Ptr) was measured by a transducer (MP45-28; Validyne, Northridge, CA) via a side port of the tracheal cannula. Body temperature was maintained at ∼36°C by means of a heating pad placed under the animal lying in supine position.
Electrophysiological recording of pulmonary C-fiber activity.
Single-unit fiber activities of vagal pulmonary C-fibers were recorded in anesthetized, closed-chest rats, and the lung was artificially ventilated with a respirator (model 7025; UGO Basile, Comerio-Varese, Italy). Tidal volume (VT) and frequency were set at 7∼8 ml/kg and 60 breaths/min, respectively, to mimic those of unilaterally vagotomized rats (43). The right cervical vagus nerve was separated from right carotid artery. The caudal end of the cut right vagus nerve was placed on a small dissecting platform and immersed in a pool of mineral oil. A thin filament was teased away from the desheathed nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified by a preamplifier (model P511K; Grass Technologies, Warwick, RI) and monitored by an audio monitor (model AM8RS; Grass Technologies). The thin filament was further split until the afferent activity arising from a single unit was electrically isolated. The afferent activity of a single C-fiber was first searched by hyperinflation of the lung (3∼4 times VT) and then identified by the immediate (delay <1 s) response to a bolus injection of capsaicin (Cap, 1.0 μg/kg) into the right atrium. Pulmonary C-fibers typically exhibited a rapid and intense response to Cap injection, but low sensitivity to lung inflation (16); these combined characteristics distinguished them from the high-threshold A-δ pulmonary afferents (49). Finally, at the end of experiment, a midline thoracotomy was made, and the general receptor field of the pulmonary C-fiber was identified by its responses to gentle pressing of the lungs with a blunt-ended glass rod. All physiological signals were recorded on a thermal writer and analyzed by a computer and a data acquisition system (model TS-100; Biocybernetics, Taipei, Taiwan) in 1-s intervals.
HWA challenge.
HWA was delivered to the rat's lung using the same preparation that was described in detail previously (17, 29). Briefly, HWA was generated by connecting the outlet of the respirator inspiratory line to an air stone and immersing it in distilled water contained in a bottle that had been placed in a heated water bath. HWA was then delivered directly into the lung via the tracheal tube by the respirator. During HWA challenge, VT and frequency were set at 14∼16 ml/kg and 150 breaths/min, respectively, for 3 min. To prevent arterial hypocapnia and alkalosis, a gas mixture containing 3.5∼4.0% CO2, 21% O2, and balance N2 was administered during hyperventilation. We did not measure the end-tidal CO2 concentration in this study, but our previous studies carried out under identical conditions have shown that both the end-tidal CO2 and arterial blood pH were maintained unchanged from the baselines during the HWA hyperventilation (17, 29). To continuously measure the temperature in the tracheal lumen (Ttr) before, during, and after HWA challenge, we inserted a miniature thermistor (model IT-18; Physitemp, Clifton, NJ, time constant: 0.1 s) to near the distal tip of tracheal tube. Ttr was raised to 41∼42°C by maintaining the heated water bath temperature at 80°C. The amount of water content in HWA measured in this study was 226 ± 12 mg/l of air.
Experimental protocol and data analysis.
In both control and Ova-sensitized rats, the baseline fiber activity (FA) and the response to chemical stimulation were determined before, at 1 min, and 15 min after HWA challenge. Cap, a selective TRPV1 agonist (0.75 μg/kg) and phenylbiguanide [PBG, a selective 5-hydroxytryptamine type 3 (5-HT3) receptor agonist; 5 μg/kg] were selected as the chemical activators of pulmonary C-fiber afferents. The experiments studying C-fiber responses to Cap and PBG after the HWA challenge were carried out in two separate groups of rats. The volume of each injection was 0.1 ml, which was first injected slowly into the catheter (dead space 0.15 ml) and then flushed into right atrium by an injection of 0.3-ml bolus of saline. FA was continuously recorded and analyzed for 20 s before and 60 s after each injection. The baseline FA was averaged over the 10-s period immediately preceding the injection; and the peak responses were determined by the maximum 2-s and 4-s averages of the FA after the injections of Cap and PBG, respectively. The change in FA (ΔFA) was calculated as the difference between the peak response and baseline FA.
Pharmacological agents.
The Ova solution was prepared daily at the concentration described earlier. Stock solutions of Cap (250 μg/ml) were prepared in 10% Tween 80, 10% ethanol, and 80% saline, and that of PBG (1 mg/ml) was prepared in saline. Both solutions were stored at −20°C and prepared daily for injection at the desired concentrations based on the animal's body weight by dilution with isotonic saline. All chemical agents were purchased from Sigma-Aldrich (St. Louis, MO), except Imject Alum (Pierce Biotechnology, Rockford, IL).
Statistical analysis.
Data were analyzed with the one-way or two-way repeated-measures ANOVA. For the latter, one factor was the treatment effect of Ova, and the other factor was the effect of HWA challenge. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A value of P < 0.05 was considered significant. Data are reported as means ± SE.
RESULTS
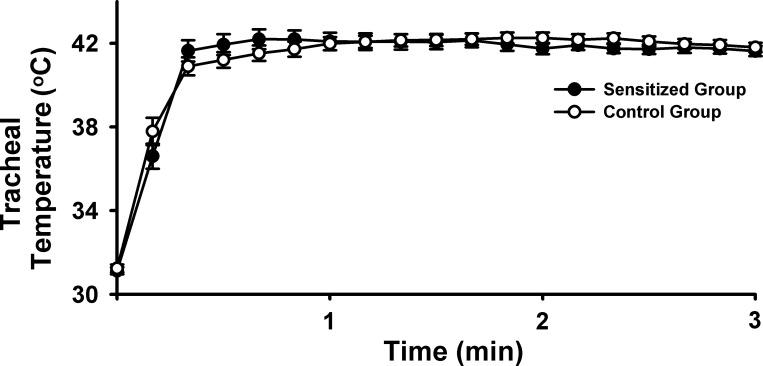
A total of 32 pulmonary C-fibers were studied in 30 rats in this study; when more than one fiber was recorded in the same animal, the responses were averaged and counted as a single measurement. The average body weight of control rats (299.9 ± 4.8 g, n = 15) was significantly higher than the sensitized rats (277.7 ± 6.9 g, n = 15; P < 0.05) of the same age. Hyperventilation with HWA increased the Ttr rapidly from 31.2 ± 0.1 to 41.7 ± 0.2°C (n = 22; n = 11 in each group); there was no difference in the peak Ttr between control and sensitized groups (Fig. 1). The peak Ptr during tidal breathing increased significantly from 5.95 ± 0.20 cmH2O before HWA to 7.16 ± 0.17 cmH2O (n = 6; P < 0.05) immediately after the HWA challenge in control rats; and from 5.84 ± 0.10 cmH2O before to 7.74 ± 0.13 cmH2O (n = 5; P < 0.05) after the HWA challenge in Ova-sensitized rats (e.g., Fig. 2, middle). These increases in Ptr gradually returned toward the baseline after 30 min.
Fig. 1.
Change in tracheal temperature during the 3-min hyperventilation with humidified warm air (HWA) in control (open circles) and ovalbumin (Ova)-sensitized Brown-Norway rats (filled circles) that were anesthetized and mechanically ventilated (see text for more details). Data are means ± SE; n = 11 in each group.
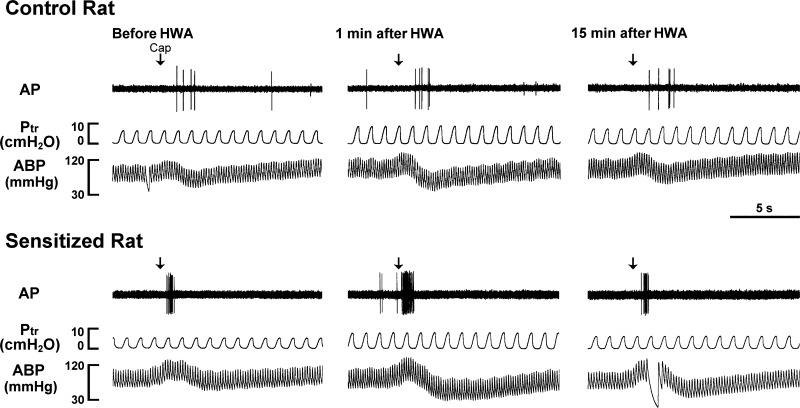
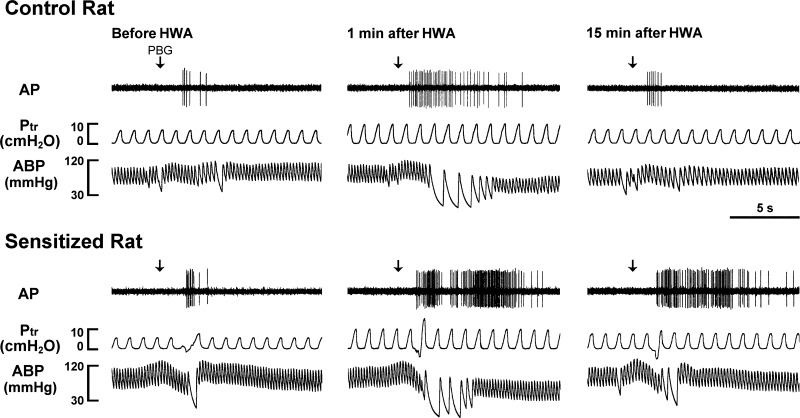
Fig. 2.
Experimental records illustrating the effects of the HWA challenge on pulmonary C-fiber responses to capsaicin (Cap, 0.75 μg/kg) in anesthetized, closed-chest, and artificially ventilated rats. Cap solution (0.1 ml) was slowly injected into the catheter (volume 0.15 ml), and then flushed (at arrow) into the right atrium by a bolus of 0.3 ml saline. From left to right: responses to Cap before, at 1 min, and 15 min after HWA challenge, respectively. AP, action potential; Ptr, tracheal pressure, ABP, arterial blood pressure. Receptor location in the control rat (290 g): right upper lobe. Receptor location in the sensitized rat (260 g): right accessory lobe.
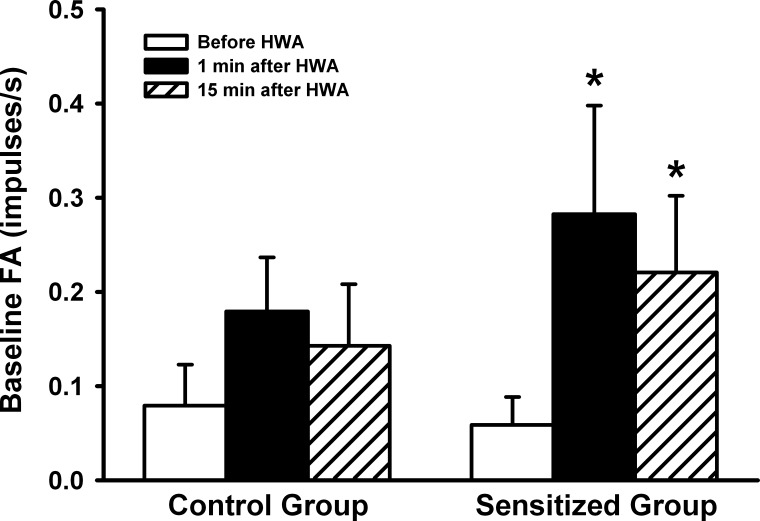
Pulmonary C-fibers showed either no or low and irregular discharge at baseline in both control (baseline FA = 0.08 ± 0.04 imp/s, n = 15) and Ova-sensitized rats (0.06 ± 0.03 imp/s, n = 15) before the HWA challenge (e.g., Figs. 2 and 3). In sensitized rats, the baseline FA increased markedly at 1 and 15 min after the termination of HWA challenge, reaching 0.28 ± 0.12 imp/s (n = 15; P < 0.05) and 0.22 ± 0.08 imp/s (n = 15; P < 0.05), respectively (Fig. 3). In comparison, the same HWA challenge did not cause any significant increase in the baseline FA in control rats (Fig. 3). The same C-fibers also showed very mild responses to lung inflation (Ptr = 30 cmH2O for 10 s) in both control (0.99 ± 0.45 imp/s, n = 15) and sensitized rats (0.93 ± 0.26 imp/s, n = 15).
Fig. 3.
A comparison of the average baseline fiber activities (FA) before, at 1 min, and 15 min after the HWA challenge between control and sensitized rats. Data were collected from the fibers that are also reported in Figs. 4 and 6. Baseline FA was averaged over 10 s in each fiber. Data are means ± SE; n = 15 in each group. *P < 0.05, significantly different from before HWA.
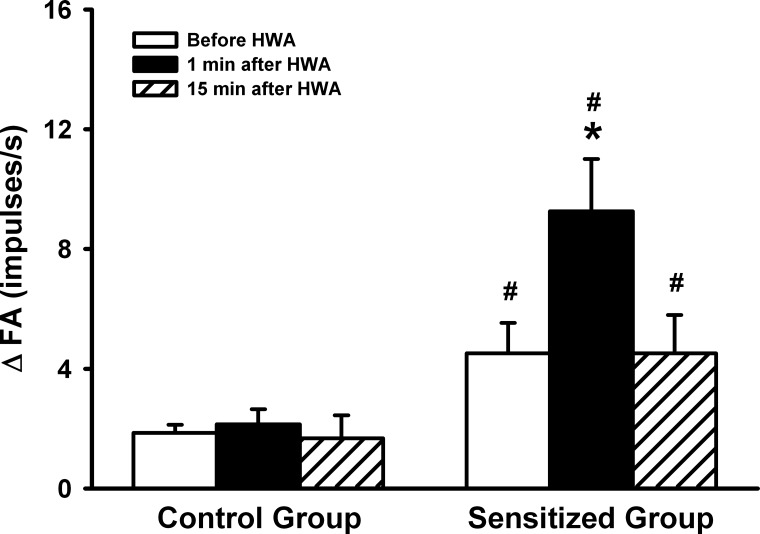
Before the HWA challenge, the response of pulmonary C-fibers to Cap was significantly greater in the Ova-sensitized rats (ΔFA = 4.51 ± 1.02 imp/s, n = 8) than in control rats (ΔFA = 1.86 ± 0.27 imp/s, n = 7; P < 0.05) (Figs. 2 and 4). At 1 min after the HWA challenge, the C-fiber response to Cap was further amplified in the sensitized rats (ΔFA = 9.26 ± 1.74 imp/s, n = 8; P < 0.05), and this potentiation gradually declined and returned to pre-HWA control at 15 min after the HWA challenge (ΔFA = 4.51 ± 1.28 imp/s, n = 8; P > 0.05; Figs. 2 and 4). In a sharp contrast, the HWA did not affect the C-fiber response to Cap in control rats (n = 7; P > 0.05; Figs. 2 and 4).
Fig. 4.
A comparison of the effects of the HWA challenge on the average peak response of pulmonary C-fibers to right atrial bolus injection of capsaicin (0.75 μg/kg) between control and sensitized rats. ΔFA, difference between the peak response of FA (average over 2-s interval) and the baseline FA (average over 10-s interval) in each fiber. Responses were tested before, at 1 min, and 15 min after the HWA challenge in both control and sensitized rats. Data are means ± SE; n = 7 in the control group and n = 8 in the sensitized group. *P < 0.05, significantly different from before HWA. #P < 0.05, significant difference when corresponding data between control and sensitized groups were compared.
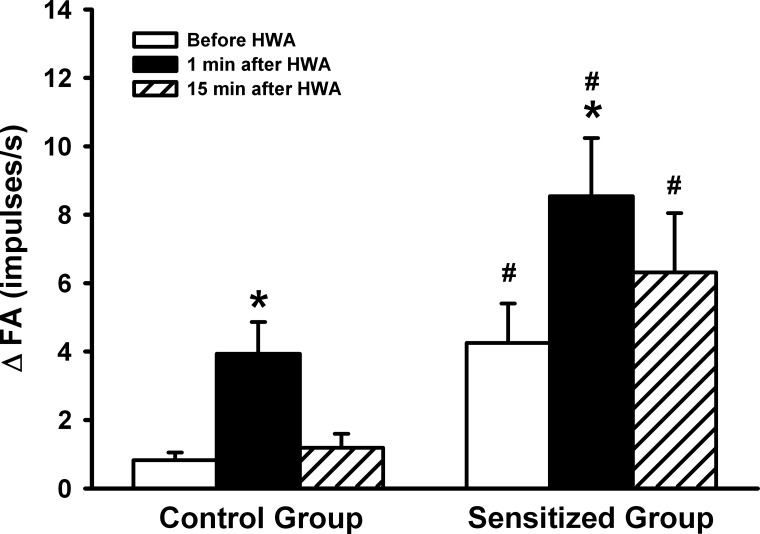
Before the HWA challenge, the response of pulmonary C-fibers to PBG was significantly greater in the Ova-sensitized rats (ΔFA = 4.25 ± 1.15 imp/s, n = 7) than in control rats (ΔFA = 0.83 ± 0.22 imp/s, n = 8; P < 0.05) (Figs. 5 and 6). At 1 min after the HWA challenge, the C-fiber response to PBG was increased pronouncedly in both control and sensitized rats, but the response was significantly higher in sensitized rats (ΔFA = 8.54 ± 1.70 imp/s, n = 7) than in control rats (ΔFA = 4.10 ± 0.88 imp/s, n = 8; P < 0.05) (Figs. 5 and 6). In sensitized rats, this increased response to PBG sustained and remained significantly higher than that in control rats at 15 min after the HWA challenge (Figs. 5 and 6).
Fig. 5.
Experimental records illustrating the effects of the HWA challenge on pulmonary C-fiber responses to phenylbiguanide (PBG, 5 μg/kg). From left to right: responses to PBG before, at 1 min, and 15 min after HWA challenge, respectively. Receptor location in the control rat (320 g): right upper lobe. Receptor location in the sensitized rat (271 g): right upper lobe. For detailed descriptions of symbols, see Fig. 2.
Fig. 6.
A comparison of the effects of the HWA challenge on the average peak response of pulmonary C-fibers to right atrial bolus injection of phenylbiguanide (PBG, 5 μg/kg) between control and sensitized rats. ΔFA, difference between the peak response of FA (average over 4-s interval) and the baseline FA (average over 10-s interval) in each fiber. Responses were tested before, at 1 min, and 15 min after the HWA challenge in both control and sensitized rats. Data are means ± SE; n = 8 in the control group and n = 7 in the sensitized group. *P < 0.05, significantly different from before HWA. #P < 0.05, significant difference when corresponding data between control and sensitized groups were compared.
DISCUSSION
Results of this study showed that isocapnic hyperventilation of HWA for 3 min markedly elevated the baseline FA of pulmonary C-fibers in Ova-sensitized rats (Fig. 3). Despite the same increase in Ttr during the HWA challenge (Fig. 1), there was no significant increase in the baseline FA in control rats (Fig. 3), indicating a heightened stimulatory effect of HWA on pulmonary C-fibers in sensitized rats. Furthermore, the pulmonary C-fiber response to right atrial injection of the same dose of Cap was significantly higher in Ova-sensitized rats than control rats before the HWA challenge, and this enhanced sensitivity to Cap was further amplified after the HWA challenge. A similar pattern of the HWA-induced potentiation in the response to PBG was also observed in sensitized rats. These results clearly demonstrated that increasing airway temperature significantly elevated both the baseline activity and sensitivities to chemical stimuli of pulmonary C-fibers in Ova-sensitized rats. The enhanced C-fiber excitability in Ova-sensitized rats gradually declined and returned to the initial level 15 min after the termination of HWA challenge, suggesting that the effect was not caused by irreversible tissue damage or injury.
In a recent study, we (15) reported that hyperventilation of HWA for 4 min immediately evoked coughs and an increase in airway resistance in patients with mild and stable asthma; at first glance, this finding appeared to be contradictory to the existing knowledge that cold dry air, not warm humid air, triggered bronchoconstriction in asthmatics (3). However, a more in-depth review will reveal that these two seemingly opposite responses are mediated through distinctly different mechanisms. It is known that the primary cause of cold air-induced bronchoconstriction is the injury of airway mucosa, resulting the release of various bronchoconstrictive mediators such as leukotrienes (3). Thus, the airway constriction usually developed slowly, and the response sustained for a much longer duration (1, 3). In contrast, the HWA-induced bronchoconstriction occurred rapidly and was completely prevented by pretreatment with ipratropium, suggesting that it was mediated through cholinergic reflex triggered by activation of airway sensory nerves (15). However, definitive evidence was lacking because a direct recording of bronchopulmonary sensory nerve activity was not feasible in human subjects. Thus the observation in the current study has provided the first direct evidence in support of the hypothesis that an increase in airway temperature by HWA hyperventilation induces both stimulatory and sensitizing effects on the pulmonary C-fibers in allergen-sensitized airways. Indeed, stimulation of these afferents is known to elicit centrally mediated reflex responses, which include bronchoconstriction and mucus hypersecretion via the cholinergic pathway, accompanied by airway irritation and urge to cough (8, 26, 27). Although the mechanisms underlying the sensitizing effect of HWA on pulmonary C-fiber afferents in Ova-sensitized rats are not fully understood, an increase in the expression and/or excitability of TRPV1 is probably a contributing factor (51).
TRPV1 is considered as a biomarker for the C-fiber sensory nerves due to its selective and abundant expression in these neurons. Endogenous TRPV1 activators such as hydrogen ion and certain lipoxygenase metabolites are consistently detected in the BALF, sputum, and/or exhaled breath condensate of patients with airway inflammatory diseases (19, 32). Recent studies further revealed an increase in sensitivity and/or expression of the TRPV1 channel in bronchopulmonary sensory nerves in patients with certain chronic airway diseases (9, 12). The important role of the temperature sensitivity of TRPV1 in regulating airway functions is gaining increasing recognition (11, 24, 38, 40). A previous study carried in our lab has demonstrated that vagal bronchopulmonary sensory neurons isolated in primary culture exhibit distinct thermal sensitivity in whole cell patch-clamp electrophysiological recording experiments (35). Increasing temperature within the normal physiological range evoked inward currents (in voltage-clamp mode), and membrane depolarization and action potentials (in current-clamp mode) in these neurons (35). Furthermore, when the temperature was raised from normal (∼36°C) to hyperthermic (∼40.6°C) level of the rat body temperature and held constant, the inward current evoked by Cap was significantly increased (36). This potentiating effect was clearly present even at a moderate level of hyperthermia (∼39°C). However, it was largely attenuated by selective TRPV1 antagonists capsazepine or AMG 9810 (36) and completely absent in pulmonary nodose/jugular neurons isolated from TRPV1-null mice (37), indicating the potentiating effect of hyperthermia on the TRPV1 chemosensitivity.
The present study demonstrated that this potentiating effect of hyperthermia on the pulmonary C-fiber sensitivity was further enhanced in Ova-sensitized rats. In a previous study, we have reported that chronic airway inflammation induced by Ova sensitization enhanced Cap sensitivity resulting from an increased expression of TRPV1 in these sensory nerves (51). Furthermore, Ova sensitization triggered a phenotypic switch in myelinated bronchopulmonary afferents and upregulated their sensitivity to Cap (50). Neurotrophins such as brain-derived neurotrophic factor and nerve growth factor have been shown to upregulate the expression and sensitivity of TRPV1 in sensory neurons (41, 48); and the synthesis and release of these neurotrophins are known to increase in allergic airways and BALF (4, 31, 47). In addition, other inflammatory mediators released in asthmatic airways (e.g., prostaglandins, protease, etc.) do not activate the TRPV1 directly but can lower its activation threshold (23, 24, 34). Moreover, these inflammatory mediators are also known to cause more generalized hypersensitivity of pulmonary C-fibers to other non-TRPV1 activators (27).
Our results revealed that other non-TRPV1 ion channels were also involved in the HWA-induced sensitizing effect on pulmonary C-fibers in Ova-sensitized rats. The C-fiber response to PBG, a selective agonist of 5-HT3 receptor, was also significantly augmented in Ova-sensitized rats, and the heightened response was further amplified after the HWA challenge (Figs. 5 and 6). As reported in our previous study, the pulmonary C-fiber response to PBG is not mediated by activation of TRPV1 and cannot be blocked by the TRPV1 antagonists (25). An increase in the tissue temperature can increase the metabolic rate and production of CO2 and hydrogen ion locally in the airway and lung tissue, which may induce the nonspecific hypersensitivity of pulmonary C-fibers (13). Acute hyperthermia can also elevate the levels of several inflammatory mediators such as arachidonic acid metabolites [e.g., prostaglandin E2 (PGE2)] (6) and pro-inflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α)] in the tissue and blood (5); and the sensitizing effects of PGE2 and TNF-α on bronchopulmonary C-fibers are well documented (18, 23, 30). Furthermore, it has been shown that increasing temperature to ∼42°C shifts the TRPV1 channel activation curve from a nonphysiological positive voltage range toward the negative potential in a physiologically relevant voltage range (44). Thus this shift of the voltage-dependent activation curve with a relatively small gating charge may play an important role in the hyperthermia-induced hypersensitivity of these TRPV1-expressing pulmonary sensory neurons.
In conclusion, this study has provided direct evidence in support of the hypothesis that increasing airway temperature elevated the sensitivity of bronchopulmonary C-fibers in the animals sensitized with allergen. This finding explains, at least in part, the observation that breathing hot humid air-triggered coughs and reflex bronchoconstriction in patients with asthma (15) and vigorous cough responses in patients with allergic rhinitis (21). It should be noted that several recent epidemiological and environmental studies have reported a close link of high ambient air temperature to acute asthma exacerbation and airway dysfunction (2, 28, 33). Some of the symptoms reported in those studies, such as cough and dyspnea, are probably related to activation of bronchopulmonary C-fibers (27). However, whether and to what extent the hypersensitivity of bronchopulmonary C-fibers is involved in the airway dysfunction observed in those patients remains to be determined.
Perspectives and Significance
This study has provided the first direct evidence that an increase in airway temperature induces both stimulatory and sensitizing effects on vagal pulmonary C-fibers in an animal model of allergic asthma. This finding offers support to our hypothesis that activation of these sensory nerves is responsible for the cough and reflex bronchoconstriction triggered by hyperventilation of humidified warm air in patients with mild asthma observed in our recent study (15). Taken together, these studies suggest that exercise-induced hyperventilation in hot humid environment may be a risk factor for asthmatics due to the possibility of triggering dyspnea, cough, bronchospasm, and other symptoms resulting from elevated activity of these sensory nerves.
GRANTS
This study was supported in part by US Department of Defense DMRDP/USAMRMC Award W81XWH-10-2-0189, National Heart, Lung, and Blood Institute Grant HL-96914 and National Center for Advancing Translational Sciences Grant UL1TR0000117.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.-J.L., M.K., and L.-Y.L. conception and design of research; Y.-J.L. and R.-L.L. performed experiments; Y.-J.L., R.-L.L., and L.-Y.L. analyzed data; Y.-J.L., R.-L.L., M.K., and L.-Y.L. interpreted results of experiments; Y.-J.L., R.-L.L., and L.-Y.L. prepared figures; Y.-J.L., R.-L.L., M.K., and L.-Y.L. drafted manuscript; Y.-J.L., R.-L.L., M.K., and L.-Y.L. edited and revised manuscript; Y.-J.L., R.-L.L., M.K., and L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Reyno Tapia and Charles Shelton for technical assistance.
REFERENCES
- 1.Aitken ML, Marini JJ. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. Am Rev Respir Dis 131: 357–361, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GB, Dominici F, Wang Y, McCormack MC, Bell ML, Peng RD. Heat-related emergency hospitalizations for respiratory diseases in the Medicare population. Am J Respir Crit Care Med 187: 1098–1103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol 106: 453–459, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA 93: 10955–10960, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchama A, Parhar RS, el-Yazigi A, Sheth K, al-Sedairy S. Endotoxemia and release of tumor necrosis factor and interleukin 1 α in acute heatstroke. J Appl Physiol (1985) 70: 2640–2644, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood SK, Bornstein B, Farnum EK, Stevenson MA. Heat shock stimulates the release of arachidonic acid and the synthesis of prostaglandins and leukotriene B4 in mammalian cells. J Cell Physiol 141: 325–333, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55: 643–649, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elwood W, Barnes PJ, Chung KF. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic brown-Norway rats. Int Arch Allergy Immunol 99: 91–97, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207–214, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170: 1276–1280, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Gu Q, Lee LY. Alveolar hypercapnia augments pulmonary C-fiber responses to chemical stimulants: role of hydrogen ion. J Appl Physiol (1985) 93: 181–188, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Haczku A, Macary P, Haddad EB, Huang TJ, Kemeny DM, Moqbel R, Chung KF. Expression of Th-2 cytokines interleukin-4 and -5 and of Th-1 cytokine interferon-gamma in ovalbumin-exposed sensitized Brown-Norway rats. Immunology 88: 247–251, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes D Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 185: 1190–1196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CC, Lin RL, Lin YS, Lee LY. Bronchoconstriction induced by increasing airway temperature in ovalbumin-sensitized rats: role of tachykinins. J Appl Physiol (1985) 115: 688–696, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-α in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 299: L483–L492, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Khosravi M, Collins PB, Lin RL, Hayes D Jr, Smith JA, Lee LY. Breathing hot humid air induces airway irritation and cough in patients with allergic rhinitis. Respir Physiol Neurobiol 198: 13–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol 555: 115–123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong K, Lee LY. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol (1985) 93: 1419–1428, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9: 243–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol (1985) 76: 1848–1855, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Baker PJ, Jalaludin BB, Guo Y, Marks GB, Denison LS, Williams GM. Are childrens asthmatic symptoms related to ambient temperature? A panel study in Australia. Environ Res 133: 239–245, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Lin RL, Hayes D Jr, Lee LY. Bronchoconstriction induced by hyperventilation with humidified hot air: role of TRPV1-expressing airway afferents. J Appl Physiol (1985) 106: 1917–1924, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RL, Lin YJ, Geer MJ, Kryscio R, Lee LY. Pulmonary chemoreflex responses are potentiated by tumor necrosis factor-α in mice. J Appl Physiol (1985) 114: 1536–1543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, Schulte-Herbruggen O, Gill H, Schuff-Werner P, Virchow JC. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am J Respir Crit Care Med 171: 115–120, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Lundstrom SL, Yang J, Kallberg HJ, Thunberg S, Gafvelin G, Haeggstrom JZ, Gronneberg R, Grunewald J, van Hage M, Hammock BD, Eklund A, Wheelock AM, Wheelock CE. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLos One 7: e33780, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mireku N, Wang Y, Ager J, Reddy RC, Baptist AP. Changes in weather and the effects on pediatric asthma exacerbations. Ann Allergy Asthma Immunol 103: 220–224, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1: 3, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291: R541–R550, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Ni D, Lee LY. Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 294: L563–L571, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Ni D, Lee LY. Lack of potentiating effect of increasing temperature on responses to chemical activators in vagal sensory neurons isolated from TRPV1-null mice. Am J Physiol Lung Cell Mol Physiol 295: L897–L904, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, Boner AL. Exhaled air temperature in asthma: methods and relationship with markers of disease. Clin Exp Allergy 37: 415–419, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Preti D, Szallasi A, Patacchini R. TRP channels as therapeutic targets in airway disorders: a patent review. Expert Opin Ther Pat 22: 663–695, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol 86: 2931–2938, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Spahn JD. Asthma biomarkers in sputum. Immunol Allergy Clin North Am 27: 607–622; vi, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Vizek M, Fialova E, Palecek F. Participation of the Breuer-Hering inflation reflex in regulation of respiration frequency in anaesthetized rats. Physiol Bohemoslov 24: 29–33, 1975. [PubMed] [Google Scholar]
- 44.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430: 748–754, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Waserman S, Olivenstein R, Renzi P, Xu LJ, Martin JG. The relationship between late asthmatic responses and antigen-specific immunoglobulin. J Allergy Clin Immunol 90: 661–669, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe T, Fajt ML, Trudeau JB, Voraphani N, Hu H, Zhou X, Holguin F, Wenzel SE. Brain derived neurotrophic factor (BDNF) expression in asthma: association with severity and type-2 inflammatory processes. Am J Respir Cell Mol Biol. In press. [DOI] [PMC free article] [PubMed]
- 48.Winter J. Brain derived neurotrophic factor, but not nerve growth factor, regulates capsaicin sensitivity of rat vagal ganglion neurones. Neurosci Lett 241: 21–24, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang G, Lin RL, Wiggers ME, Lee LY. Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C fibers in rats. J Appl Physiol (1985) 105: 128–138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]