Abstract
Protein-rich supplements are used widely for the management of malnutrition in young and older people. Protein is the most satiating of the macronutrients in young. It is not known how the effects of oral protein ingestion on energy intake, appetite, and gastric emptying are modified by age. The aim of the study was to determine the suppression of energy intake by protein compared with control and underlying gastric-emptying and appetite responses of oral whey protein drinks in eight healthy older men (69–80 yr) compared with eight young male controls (18–34 yr). Subjects were studied on three occasions to determine the effects of protein loads of 30 g/120 kcal and 70 g/280 kcal compared with a flavored water control-drink (0 g whey protein) on energy intake (ad libitum buffet-style meal), and gastric emptying (three-dimensional-ultrasonography) and appetite (0–180 min) in a randomized, double-blind, cross-over design. Energy intake was suppressed by the protein compared with control (P = 0.034). Suppression of energy intake by protein was less in older men (1 ± 5%) than in young controls (15 ± 2%; P = 0.008). Cumulative energy intake (meal+drink) on the protein drink days compared with the control day increased more in older (18 ± 6%) men than young (1 ± 3%) controls (P = 0.008). Gastric emptying of all three drinks was slower in older men (50% gastric-emptying time: 68 ± 5 min) than young controls (36 ± 5 min; P = 0.007). Appetite decreased in young, while it increased in older (P < 0.05). In summary, despite having slower gastric emptying, elderly men exhibited blunted protein-induced suppression of energy intake by whey protein compared with young controls, so that in the elderly men, protein ingestion increased overall energy intake more than in the young men.
Keywords: aging, energy intake, gastric emptying, appetite, whey protein
the prevalence of malnutrition, both underweight/undernutrition and overweight/overnutrition, has increased over recent decades in older adults (17). Both forms of malnutrition are associated with, and lead to, reduced functional capacity and decreased quality of life in the aging population (17, 25, 27, 42). Healthy aging is associated with a reduction of appetite and energy intake, termed “the physiological anorexia of aging” (25, 51). Low intake of energy and protein predisposes older people to weight loss, particularly loss of skeletal muscle (25, 42). A growing awareness of the prevalence and adverse effects of the major muscle loss that occurs during aging, irrespective of body mass index (BMI), has led to the development of nutritional strategies designed specifically to preserve and/or restore skeletal muscle. Insufficient protein intake in the elderly is likely to exacerbate muscle loss by limiting muscle anabolism (6). Severe muscle loss leads to sarcopenia in underweight and obese older individuals, which is strongly associated with adverse outcomes (35). Strategies designed to achieve an increase in muscle mass and function include exercise programs, especially resistance exercise. However, many older adults have comorbidities and physical limitations that hamper their capacity to fully achieve the levels of exercise sufficient to fully protect the loss of muscle mass (55). As such, a common strategy for increasing energy intake and body weight/lean mass in undernourished elderly (18, 22, 23), as well as limiting energy intake, preserving lean mass, and promoting fat loss during energy restriction in overweight and obese older adults (52), is the use of protein-enriched supplements. These supplements are usually high-energy drinks rich in protein. In particular, whey protein, a major dairy protein source rich in essential amino acids, is often used. Despite the widespread use of such supplements by older people, information about their effects on energy intake is limited, and their “optimal” composition is unknown.
The rationale for using protein supplements in undernourished or obese sarcopenic older people is strengthened by evidence that aging has only minimal inhibitory effects on the capacity to synthesize muscle protein acutely after protein ingestion (13, 30, 48). A recent consensus recommendation by the PROT-AGE study group (1) stated that dietary protein intake needs to be increased from ∼0.8–1.0 g/kg body wt per day to ∼1.2–1.5 g/kg in older people (e.g., 90–112.5 g for 75 kg body wt), including a minimum of 25–30-g protein intake per meal. Older people, however, may have a significant protein portion waste of their meals (∼23–68%), resulting in low-protein intakes (∼40–64 g/day) and, therefore, may require supplementation of up to 70 g protein/day (1, 5, 29). In young adults, a protein intake of up to ∼70 g is representative of intakes during a single meal (∼250 g serving of lean steak). Accordingly, if older people can ingest sufficient protein throughout the day, it is likely to have positive anabolic effects. Protein, however, is also the most satiating of the macronutrients in young people, and high-protein energy-restricted diets are used to promote weight loss in obese young adults (44).
In older, undernourished adults, the aim is, of course, to increase, rather than reduce, overall energy intake. The effects of dietary protein on energy intake and underlying gastrointestinal mechanisms in older people are largely unknown. We have recently demonstrated that administration of 30-kcal (7.5 g), 90-kcal (22.5 g), and 180-kcal (45 g) whey-protein loads directly into the small intestine suppressed subsequent energy intake less in older men than in young controls (43). In addition, whereas cumulative energy intake (protein load plus ad libitum intake at a subsequent buffet-style meal) was reduced by the intraduodenal protein infusions in young subjects, in the older subjects there was an increase in cumulative energy intake.
Variations in the rate of gastric emptying and gastric distension are important in the regulation of appetite and energy intake, particularly in the short term after nutrient ingestion (7, 16, 19, 47). Gastric emptying is regulated primarily as a result of nutrient-mediated inhibitory feedback from the small intestine. Compared with young adults, in healthy older people, the perception of proximal gastric distension is reduced, and distension of the distal stomach, i.e., the antral area, is greater. Gastric emptying is probably slightly slower in older than young adults, particularly that of meals rich in carbohydrate and fat (7, 16, 33, 47). These differences would favor reductions in energy intake.
Older adults are less hungry and eat less than younger adults (8, 21, 36, 38, 40, 42, 47), and because of lower body weights with disproportionally lower lean mass, they require less energy to maintain their body weight (45). Therefore, a control diet containing no protein or energy is required to determine whether young and older subjects differ in their susceptibility to further suppression of energy intake by the ingestion of nutrients.
In this study, we aimed to characterize the impact of aging on feeding and gastric responses to orally ingested whey protein loads similar to (30 g) and higher than (70 g) the suggested protein intake per meal (1, 29) within a period of time (180 min) when these loads were expected to empty “completely” from the stomach (7), compared with a control drink (0 g whey protein). We hypothesized that orally administered whey protein would slow gastric emptying and reduce voluntary energy intake and perceptions of appetite, in a load-related fashion, and these suppressive effects would be less in healthy older men than in young controls.
MATERIALS AND METHODS
Subjects
The study included eight healthy young men [age (means ± SD): 25 ± 6 yr (range: 18–34 yr); body weight: 72 ± 8 kg (range: 62–86 kg); height: 1.79 ± 0.05 m, BMI (in kg/m2): 23 ± 2] and eight healthy older men [age: 73 ± 4 y (range: 69–80 yr); body weight: 77 ± 11 kg (range: 59–92 kg); height: 1.73 ± 0.05 m; BMI: 26 ± 4], who were recruited by advertisement. The body weight of the two groups did not differ significantly (P = 0.285). Height was lower and, accordingly, BMI higher in older men than in young controls (P = 0.045). On the basis of our previous work (43), we calculated that eight subjects per group would be a sufficient number to allow us to detect a difference in the suppression of energy intake by protein of 395 kcal with SDs of 316 kcal (young subjects) and 180 kcal (older subjects) (43) and in the 50% gastric emptying time of 80 min with SDs of 38 min (young subjects) and 63 min (older subjects) (7, 16, 24), between young and older subjects, with α = 0.05 and power of 80%. Exclusion criteria were smoking, alcohol abuse, diabetes, gastrointestinal surgery (apart from uncomplicated appendectomy), significant gastrointestinal symptoms (pain, reflux, diarrhea, or constipation), and use of medications known to potentially affect energy intake, appetite, or gastrointestinal motor function. For older people, additional exclusion criteria were impaired cognitive function [score <25 on Mini Mental State (11)], depression [score ≥11 on the Geriatric Depression Questionnaire (54)], and undernutrition [score <24 on the Mini Nutritional Assessment (14)]. The Royal Adelaide Hospital Human Research Ethics Committee approved the study protocol. The study was registered as a clinical trial with the Australian New Zealand Clinical Trial Registry (www.anzctr.org.au, registration number ANZCTR12612000941864). All subjects provided written informed consent prior to their inclusion in the study.
Protocol
Subjects were studied on three occasions, separated by ≥3 days, to determine the effects of two oral whey protein loads (30 g/120 kcal, and 70 g/280 kcal) and a flavored water control drink (∼0 g whey protein/∼2 kcal) on energy intake, gastric emptying, perceptions of appetite, and gastrointestinal symptoms in a randomized (using the method of randomly permuted blocks; www.randomization.com), double-blind, cross-over design.
Protein drinks (∼450 ml) were prepared by dissolving whey protein isolate (Fonterra Co-Operative Group) in varying volumes of demineralized water and diet lime cordial (Bickford's Australia, South Australia) to achieve the desired loads. Drinks were prepared on the morning of each study by a research officer (P. Fitzgerald), who was not involved in the data analysis. The drinks were served in a covered cup, so both the investigator and the subject were blinded to the treatment.
Subjects were provided with a standardized evening meal [beef lasagna (McCain Foods), ∼591 kcal] to consume on the night before each study day at ∼1900. They were instructed to fast overnight from solids and liquids and to refrain from strenuous physical activity until they attended the laboratory at the Discipline of Medicine, the University of Adelaide, Royal Adelaide Hospital, at ∼0830.
On arrival, subjects were seated in an upright position on a wooden chair, where they remained for the duration of the study. In each subject, measurements of total gastric volume and perceptions of appetite and gastrointestinal symptoms were performed immediately before (during fasting; −5 min), immediately after ingestion of the drink, and at 15-min intervals until 180 min. Subjects were instructed to consume the drink within 2 min (−2 min). Gastric volume was acquired by three-dimensional (3D) ultrasound images. Perceptions of appetite and gastrointestinal symptoms were assessed using validated visual analog scales (VAS). At 180 min, subjects were presented with a standard, cold, buffet-style meal in excess of what they were expected to consume and instructed to eat freely for up to 30 min until comfortably full (180–210 min) (10, 26). The composition of the buffet-style meal is provided in Table 1.
Table 1.
Composition of the buffet-style meal
| Food Items | Amount Served, g | Energy Content, kcal | Protein, g | Carbohydrate, g | Fat, g |
|---|---|---|---|---|---|
| Whole-meal bread, 4 slicesa | 125 | 308 | 13.4 | 53.1 | 4.7 |
| White bread, 4 slicesa | 125 | 304 | 10.8 | 59.5 | 2.6 |
| Cheese, slicedb | 85 | 346 | 21.9 | 0.9 | 28.3 |
| Ham, slicedc | 100 | 95 | 16.6 | 3.4 | 1.7 |
| Chicken, slicedd | 100 | 104 | 18.8 | 3.6 | 1.6 |
| Margarinee | 20 | 108 | 0.0 | 0.0 | 12.0 |
| Mayonnaisef | 20 | 137 | 0.4 | 0.7 | 14.7 |
| Tomato, sliced | 100 | 13 | 1.0 | 1.9 | 0.1 |
| Cucumber, sliced | 100 | 11 | 0.5 | 1.9 | 0.1 |
| Lettuce | 100 | 5 | 0.9 | 0.4 | 0.0 |
| Apple | 170 | 89 | 0.5 | 21.3 | 0.2 |
| Banana | 190 | 166 | 3.2 | 37.8 | 0.2 |
| Fruit saladg | 140 | 81 | 0.4 | 17.1 | 1.3 |
| Strawberry yogurth | 175 | 162 | 8.8 | 24.2 | 3.3 |
| Chocolate custardi | 100 | 105 | 3.2 | 16.4 | 3.0 |
| Milky Wayj | 12 | 52 | 0.3 | 8.7 | 1.8 |
| Orange juice, unsweetenedk | 300 | 117 | 1.8 | 21.9 | 2.6 |
| Iced coffeel | 375 | 254 | 12.0 | 37.1 | 6.4 |
| Water | 600 | 0 | 0.0 | 0.0 | 0.0 |
| Total | 2,457 |
Sunblest, Tiptop;
Coon Tasty Cheese slices, Australian Cooperative Foods;
KRC boneless leg ham;
Inghams chicken;
Vita-Lite canola, Peerless Holdings;
MasterFoods, Mars food;
Goulburn Valley, SPC, Ardmona Operations;
Yoplait, LD&D Foods;
Yogo, National Foods;
Mars Chocolate;
Nippy's Fruit juices;
Farmers Union, Balemar.
Measurements
Energy intake.
The amount eaten (g) was quantified by weighing the meal before and after consumption. Energy intake (kcal) at the meal and proportions of intake of protein, carbohydrate, and fat were calculated using commercially available software (Foodworks; Xyris Software). Energy intake was calculated both as the intake at the buffet meal and as the cumulative energy intake, defined as the sum of energy intake at the buffet meal and the energy content of the preload drink. Absolute (kcal) and percentage suppression/change (expressed as % of energy intake of the control day) of energy intake at the buffet meal by a given protein load compared with control was calculated.
Gastric emptying.
Total gastric volume was assessed by 3D ultrasonography, a method that has been validated against the “gold standard” scintigraphy for measurement of the emptying of liquids from the stomach (12). A Logiq 9 ultrasound system (GE Healthcare Technologies, Australia) with TruScan Architecture [built-in magnetically sensored 3D positioning and orientation measurement (POM)], including a 3D sensor, attached to a 3.5C broad-spectrum 2.5–4-MHz convex transducer, and a transmitter, placed at the level of the stomach immediately behind the subject were used. As the transmitter produces a spatially varying magnetic field that is distorted by conductive metals, all metal objects were removed from the patient to minimize interference during image acquisition. The stomach was scanned by a continuous translational movement along its long axis (∼10 s). During each scan, subjects were instructed to sit still and hold their breath at the end of inspiration. If gastric contractions were observed, the acquisition was paused until the contraction wave had passed. The raw data (original scan planes) were transferred for 3D reconstructions and volume estimation using EchoPAC-3D software (GE Vingmed Sound, Horten, Norway). Gastric retentions were calculated as total gastric volumes minus baseline “empty” gastric volume at each time point expressed as a percentage of the maximal gastric volume (100%), i.e., ∼450 ml volume of the ingested drink. When ultrasound images lacked sufficient clarity to determine the volume of the stomach, data were imputed by linear interpolation. The quality of ultrasound stomach images was insufficient to determine gastric emptying in all three conditions in one older subject, and this subject was, therefore, excluded from the analysis. The time at which 50% of the preload drink had emptied from the stomach (50% gastric emptying time; T50; min) and “complete” emptying time (T100; min) of the drink, defined as the time when the residual volume of the drink in the stomach was ≤5%, was calculated for all conditions. Complete emptying time was set to 180 min when the residual volume at 180 min was ≥5%. Rate of gastric emptying was calculated as the mean of rates of emptying during each 15-min interval of the early (0–45 min) and late (45–180 min) phases, respectively, and total (0–180 min) time period.
Perceptions of appetite and gastrointestinal symptoms.
Perceptions of hunger, desire to eat, prospective consumption, fullness, nausea, and bloating were rated using a VAS questionnaire (32). The questionnaire consisted of 100-mm horizontal lines, where 0 represented that the sensation was “not felt at all” and 100 represented that the sensation was “felt the greatest.” Subjects placed a vertical mark on each horizontal line to indicate the strength of each sensation at the specified time points. Baseline fasting ratings were calculated as mean of the three study days. One young and one older subject did not comply with the guidelines of the VAS questionnaires on one or more study days and were excluded from the analyses.
Data and Statistical Analyses
Statistical analyses were performed using SPSS software (version 21; IBM, Armonk, NY). Main age and protein-load effects and interaction effects were determined by using repeated-measures ANOVA. Relations of energy intake with the rate of gastric emptying (kcal/min) and appetite were evaluated by between- and within-subject correlations (3, 4). Statistical significance was accepted at P < 0.05. All data are presented as means ± SEs.
RESULTS
The study protocol was well tolerated by all subjects.
Energy Intake
Energy intake at the buffet meal was suppressed by whey protein compared with control [mean of young and older: there was a decrease in energy intake of 134 ± 38 kcal after the 30 g (120 kcal) and 105 ± 49 kcal after the 70 g (280 kcal) protein load; main effect of protein-load P = 0.034; Fig. 1]. The main effect of age (P = 0.273) and the interaction effect of age × protein-load (P = 0.063) were not significant.
Fig. 1.
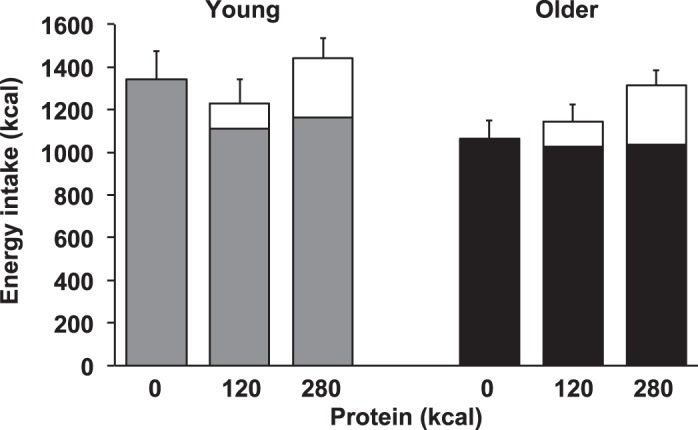
Mean (± SE) energy intake (kcal) in young (gray shading; n = 8) and older (black shading; n = 8) subjects after drinks (open bars) containing water (control) and whey protein loads of 30 g and 70 g. Main age and protein-load effects and interaction effects were determined by using repeated-measures ANOVA. The protein drinks suppressed subsequent energy intake at the buffet meal compared with control. The main effect of protein load for energy intake was significant (P = 0.034); the main effect of age (P = 0.273) and the interaction effect of age × protein-load (P = 0.063) were not significant. Cumulative energy intake (i.e., energy intake at the buffet meal plus energy content of the drink) during the protein day compared with the control day was age- and protein load-dependent. The main effects of age (P = 0.008) and protein load (P = 0.011) for cumulative energy intake during the protein days compared with the control day were significant; the interaction age × protein load was not significant (P = 0.683).
Suppression of energy intake by whey protein compared with control was less in older men than in young controls (young compared with older: 15 ± 2% compared with 1 ± 5%; main effect of age; P = 0.008; in young men 17 ± 3% by the 120-kcal and 12 ± 3% by the 280-kcal protein load compared with control [P < 0.05] and in older men 2 ± 5% and 0 ± 8% [P > 0.05]; Fig. 2). The main effect of protein load (P = 0.612) and the interaction effect of age × protein load (P = 0.683) were not significant.
Fig. 2.
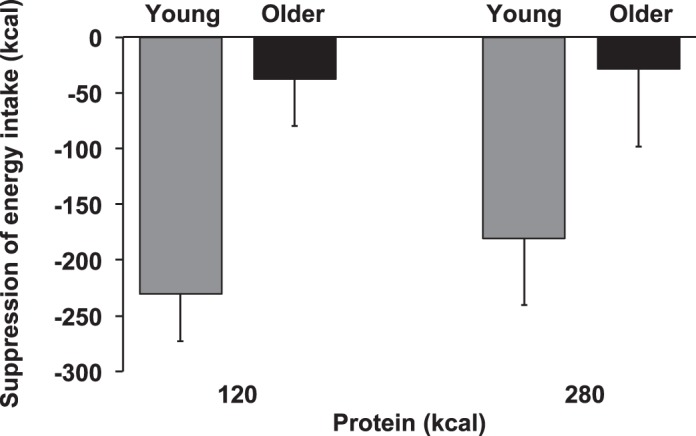
Mean (± SE) suppression of energy intake at the buffet meal (kcal) in young (gray shading; n = 8) and older (black shading; n = 8) subjects after whey protein loads of 30 g and 70 g compared with after control (0 g whey protein). Main age and protein load effects and interaction effects were determined by using repeated-measures ANOVA. Suppression of energy intake by protein was less in older than young subjects. The main effect of age for suppression of energy intake was significant (P = 0.008); the main effect of protein load (P = 0.612) and the interaction effect of age × protein-load (P = 0.683) were not significant.
Cumulative energy intake during the protein days compared with the control day increased more in older men than young controls (young compared with older: 1 ± 3% compared with 18 ± 6%; main effect of age: P = 0.008) and increased more during the 70-g than the 30-g protein day (30- compared with 70-g protein load: 1 ± 4% compared with 19 ± 6%; main effect of protein load P = 0.011). The interaction age × protein load was not significant (P = 0.683).
Macronutrient proportions at the buffet meal did not differ between the age groups at baseline, nor on any of the other study days, and were not affected by either of the protein treatments (P > 0.05).
Gastric Emptying
Gastric emptying parameters are detailed in Table 2. Baseline gastric volumes were not different between young and older men (31 ± 6 ml compared with 39 ± 3 ml; P = 0.282). The control drink (water) emptied in a nonlinear pattern, whereas the pattern of the 280-kcal protein drink was more linear, particularly in the older men: age appears to affect the initial rate of gastric emptying (Fig. 3). After ingestion of all three study drinks, gastric emptying was slower in older men than young controls, with T50 times (mean of three study days young compared with older of 36 ± 5 min compared with 68 ± 5 min; main effect of age: P = 0.007), equating to an emptying rate of energy of 1.0 ± 0.0 kcal/min in the young subjects compared with 0.8 ± 0.0 kcal/min in the older subjects on the two protein days (effect of age P = 0.022), and higher measures of gastric retention (main effect of age P = 0.003). Ingestion of the protein drinks resulted in a dose-dependent slowing of gastric emptying, of comparable magnitude in both age groups, with T50 more than a doubling from the control to the 120-kcal day (mean of all men; 17 ± 2 to 41 ± 5 min), with a further similar increase from the 120-kcal to the 180-kcal day (mean of all men; 41 ± 5 to 96 ± 11 min; main effect of protein load; P < 0.001). Consequently, rates of energy emptying (kcal/min) did not differ between the 120-kcal and 280-kcal days. The age × protein-load interactions for T50 (P = 0.083) and gastric retention (P = 0.218) were not significant.
Table 2.
Gastric emptying rate, T50, T100, and gastric retention (area under the curve; 0–180 min) of water (control) and protein drinks in young and older men
| Young Men (n = 8) |
Older Men (n = 7) |
|||||
|---|---|---|---|---|---|---|
| 0 g | 30 g | 70 g | 0 g | 30 g | 70 g | |
| T50, min1,2 | 12 ± 13,4 | 25 ± 43,4 | 72 ± 133,4 | 23 ± 33,4 | 59 ± 53,4 | 123 ± 133,4 |
| T100, min1,5 | 60 ± 73,4 | 126 ± 143,4 | 171 ± 64 | 109 ± 133,4 | 174 ± 63,4 | 174 ± 6 |
| Amount emptied at 180 min, %6 | 100 ± 0 | 98 ± 1 | 86 ± 5 | 99 ± 0 | 89 ± 2 | 70 ± 5 |
| Gastric retention, %2 | 173 ± 10 | 337 ± 31 | 591 ± 45 | 271 ± 24 | 544 ± 32 | 807 ± 39 |
| Rate of gastric emptying, 0–180 min; kcal/min6 | 0.7 ± 0.0 | 1.3 ± 0.1 | 0.6 ± 0.0 | 1.1 ± 0.1 | ||
| Early phase of rate of gastric emptying, 0–45 min; kcal/min5,6 | 1.8 ± 0.33,4 | 2.6 ± 0.23,4 | 1.2 ± 0.03 | 1.4 ± 0.12 | ||
| Late phase of rate of gastric emptying (45–180 min; kcal/min)6,7 | 0.3 ± 0.0 | 0.9 ± 0.1 | 0.4 ± 0.0 | 1.0 ± 0.1 | ||
All values are expressed as means ± SE. Main age and protein-load effects and interaction effects were determined by using repeated-measures ANOVA.
50% emptying time (T50; min). Complete emptying time (T100; min) of the drink was defined as the time when the residual volume of the drink in the stomach was ≤5% and T100 was set to 180 min when the residual volume at 180 min was ≥5%.
The main effects of age and protein-load for gastric retention (AUC from baseline to 180 min; P = 0.003; P < 0.001) and T50 (P = 0.007; P < 0.001) were significant. The age (young, older) x protein-load (0, 120, 280 kcal) interaction for gastric retention (AUC from baseline to 180 min; P = 0.218) and T50 (P = 0.083) was not significant.
P < 0.05; between age groups.
P < 0.05; between protein conditions.
The age × protein-load interaction and main effects of age and protein-load for T100 (P = 0.048, P = 0.027; P < 0.001) and early phase rate of gastric emptying (0–45 min; P = 0.003, P = 0.001; P = 0.001) were significant.
Rate of gastric emptying was calculated as the mean of rates of emptying during each 15-min interval of the early (0–45 min) and late (45–180 min) phase, respectively, and total (0–180 min) time period.
The main effect of protein-load for the amount emptied at 180 min (%; P < 0.001), rate of gastric emptying (0–180 min; P < 0.001) and late phase of rate of gastric emptying (45–180 min; P < 0.001) were significant. The age × protein-load interaction and main effect of age for the amount emptied at 180 min (%; P = 0.173; P = 0.063), rate of gastric emptying (0–180 min; P = 0.135; P = 0.079), and late phase of rate of gastric emptying (45–180 min; P = 0.870; P = 0.227) were not significant.
Fig. 3.
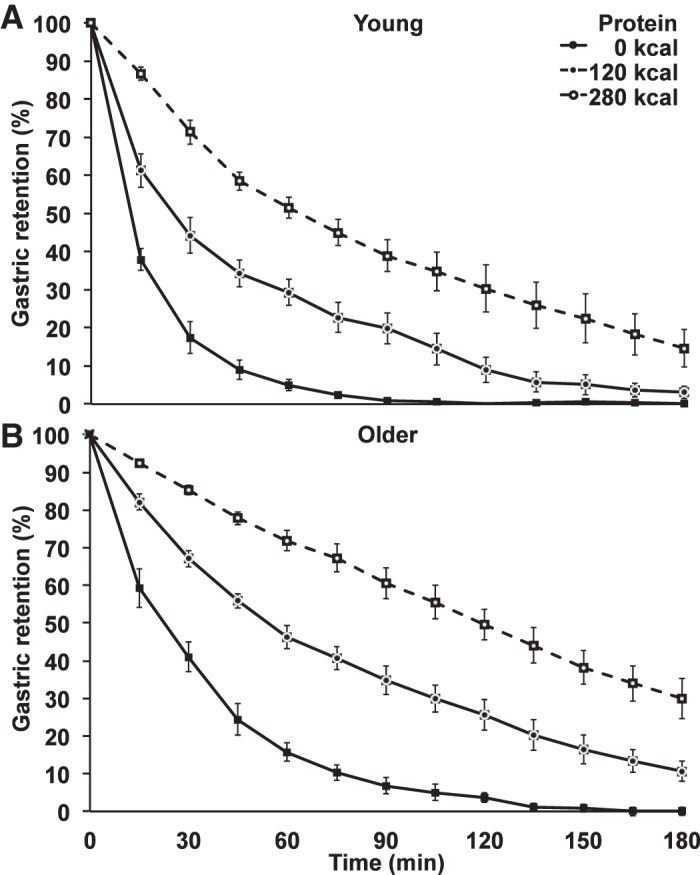
Mean (± SE) gastric retention (%) in young (n = 8) and older (n = 7) subjects after drinks containing water (control) and whey protein loads of 30 g or 70 g. Main age and protein load effects and interaction effects were determined by using repeated-measures ANOVA. Gastric emptying of the water and protein drinks was slower in older than young men. The main effects of age and protein load for the 50% gastric emptying time (T50; min; P = 0.007; P < 0.001) and gastric retention (%; area under the curve; P = 0.003; P < 0.001) were significant; the age × protein load interactions for T50 (P = 0.083) and gastric retention (P = 0.218) were not significant.
Perceptions of Appetite and Gastrointestinal Symptoms
Appetite.
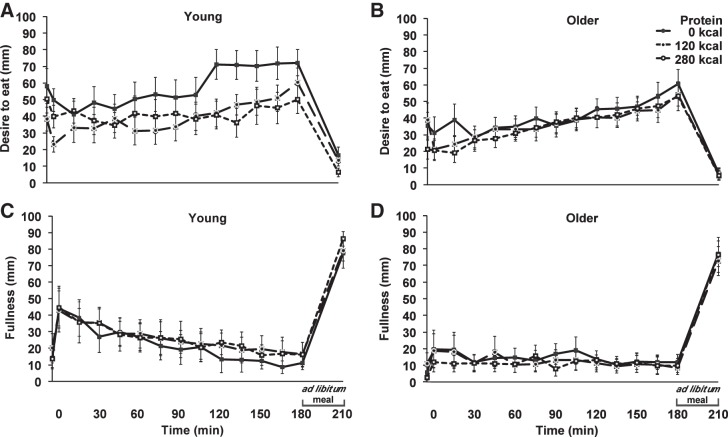
Baseline ratings of desire to eat (young compared with older; 49 ± 8 compared with 32 ± 6 mm; P = 0.109), prospective food consumption (54 ± 8 compared with 43 ± 5 mm; P = 0.245), hunger (43 ± 8 compared with 34 ± 6 mm; P = 0.373), and fullness (17 ± 4 compared with 7 ± 3 mm; P = 0.093) were not statistically different between young and older men. Ratings of desire to eat, prospective food consumption, and hunger differed from baseline over time (0–180 min) during all study days in both age groups (P < 0.05); fullness increased from baseline in young (P < 0.05), but not in older (P > 0.05), subjects (Fig. 4).
Fig. 4.
Mean (± SE) visual analog scores (mm; 0–180 min) of desire to eat (A and B) and fullness (C and D) in young (n = 7) and older (n = 7) subjects after whey protein loads of 30 g and 70 g and a control drink (water), and after the ad libitum buffet meal (210 min). Time (0–180 min) effects were determined by using repeated-measures ANOVA. Ratings of desire to eat changed from baseline during all study days in both age groups (P < 0.05); fullness was increased from baseline in young (P < 0.05), but not in older (P > 0.05), subjects.
The main effect of age for area under the curve (AUC) ratings of desire to eat (mean of three study days; young: a decrease of 647 ± 910 mm from baseline; older: an increase of 1,027 ± 458 mm from baseline; P = 0.024) and prospective food consumption (mean of 3 study days; young: a decrease of 1,616 ± 706 mm from baseline; older: an increase of 119 ± 370 mm from baseline; P = 0.021) was significant, while the main effect of age for ratings of hunger (P = 0.477) and fullness (P = 0. 587) was not. The main effect of protein-load and the age × protein load interaction for ratings of desire to eat, prospective food consumption, hunger and fullness were not significant (Table 3).
Table 3.
Hunger, desire to eat, prospective food consumption, fullness, nausea, and bloating (area under the curve) after water (control) and protein drinks (0–180 min) in young and older men
| Young Men (n = 7) |
Older Men (n = 7) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 g | 30 g | 70 g | Mean Change by Protein Compared with Control | 0 g | 30 g | 70 g | Mean Change by Protein Compared with Control | P1 | |
| Desire to eat, mm2 | −86 ± 485 | 186 ± 835 | −1534 ± 1181 | −588 ± 860 | 933 ± 678 | −400 ± 1311 | 2454 ± 690 | 94 ± 421 | 0.490 |
| Prospective food consumption, mm2 | 231 ± 876 | −1422 ± 1082 | −1811 ± 1228 | −1848 ± 643 | 257 ± 638 | −793 ± 846 | 1030 ± 1095 | −138 ± 921 | 0.154 |
| Hunger, mm | 1376 ± 945 | 28 ± 582 | −553 ± 1168 | −1638 ± 1065 | 167 ± 719 | −173 ± 1804 | 2030 ± 1405 | 761 ± 862 | 0.105 |
| Fullness, mm | 1453 ± 1756 | 1521 ± 602 | 2366 ± 1086 | 490 ± 1502 | 1670 ± 697 | 275 ± 1525 | 1655 ± 790 | −705 ± 1007 | 0.521 |
| Nausea, mm | 1021 ± 1093 | 274 ± 1717 | 2059 ± 1160 | 145 ± 1210 | 334 ± 308 | 175 ± 181 | 204 ± 126 | −145 ± 209 | 0.817 |
| Bloating, mm | 2186 ± 1328 | 1311 ± 2023 | 1581 ± 1331 | −739 ± 913 | 342 ± 376 | 618 ± 475 | 595 ± 366 | 265 ± 166 | 0.301 |
All values are expressed as means ± SE. Main age and protein-load effects and interaction effects were determined by using repeated-measures ANOVA.
Age effect of mean change by protein (30 g and 70 g) compared with control (0 g) (ANOVA).
The main effect of age for ratings of desire to eat (P = 0.024) and prospective food consumption (P = 0.021) was significant. The age (young, older) × protein-load (0, 120, 280 kcal) interaction for ratings of desire to eat (P = 0.104), prospective food consumption (P = 0.603), hunger (P = 0.326), fullness (P = 0.827), nausea (P = 0.468) and bloating (P = 0.338) was not significant. The main effect of age for ratings of hunger (P = 0.477), fullness (P = 0. 587), nausea (P = 0.193) and bloating (P = 0.344) was not significant. The main effect of protein-load for ratings of desire to eat (P = 0.460), prospective food consumption (P = 0.417), hunger (P = 0.735), fullness (P = 0. 638), nausea (P = 0.670), and bloating (P = 0.542) was not significant.
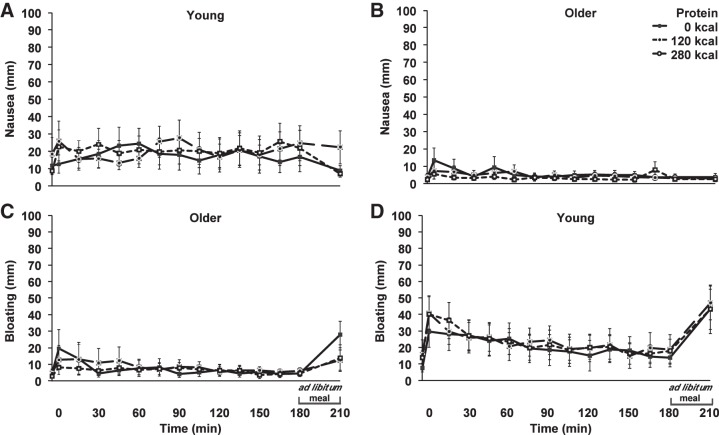
Nausea and bloating.
Baseline ratings of nausea (young compared with older; 13 ± 4 compared with 3 ± 1 mm, P = 0.040) were lower in older men than young controls, while ratings of bloating were not (8 ± 4 compared with 3 ± 1 mm, P = 0.287). Ratings of bloating increased in young (P < 0.05), but not in older (P > 0.05), subjects (Fig. 5). The main effects of age and protein load and age × protein load interaction for ratings of nausea and bloating were not significant (Table 3).
Fig. 5.
Mean (± SE) visual analog scores (0–180 min; mm) of nausea (A and B) and bloating (C and D) in young (n = 7) and older (n = 7) subjects after whey protein loads of 30 g and 70 g and a control drink (water), and after the ad libitum buffet meal (210 min). Time effects (0–180 min) were determined by using repeated-measures ANOVA. Ratings of nausea were not different from baseline in either age group (P > 0.05); ratings of bloating were increased from baseline in young (P < 0.05), but not in older (P > 0.05), subjects.
Relations Between Energy Intake with Gastric Emptying and Perceptions of Appetite
Energy intake (kcal) at the buffet meal was, within subjects, inversely related to gastric retention (AUC from baseline to 180 min; %) in young (r = −0.54, P = 0.026), but not older (r = −0.16, P = 0.570), men (all men; r = −0.35, P = 0.051); i.e., the slower the study drink emptied from the stomach (0–180 min) within a young subject—70 g < 30 g < 0 g—the lower the subsequent energy intake (180–210 min).
Suppression of energy intake at the buffet meal by protein compared with control was, between subjects, directly related to suppression of prospective food consumption by protein compared with control (r = 0.60; P = 0.024) in all men. There was no association between fullness ratings at 180 min (i.e., just before the buffet meal) and energy intake, in the young (r = 0.04; P = 0.917), older (r = 0.35, P = 0.397), or combined subjects (r = 0.18; P = 0.501).
Suppression of energy intake by protein was, irrespective of age, directly related to energy intake during the control day (young: r = 0.88; P = 0.004; older: r = 0.71; P = 0.048; combined subjects: r = 0.82; P < 0.001). The age effect on the suppression of energy intake at the buffet meal by protein was still significant (P = 0.001), taking energy intake during the control day into account as a covariate.
DISCUSSION
This study examined the influence of aging on the acute effects of oral whey protein consumption on suppression of energy intake, appetite, and gastric emptying. The major finding was that suppression of energy by protein was less in healthy older men (1%) than young controls (15%), so that the cumulative energy intake (buffet meal plus preload drink) was increased more by the protein ingestion in older men (18%) than young controls (1%). These observations are consistent with our recent finding that the suppression of subsequent energy intake by intraduodenal infusions of whey protein was less in healthy older men (∼1%) than young controls (∼19%) (43). They are also consistent with reports of reduced responsiveness in older people to the suppressive effects of oral mixed macronutrient meals on energy intake (34, 36) and extend these to show that these age differences also apply when protein is ingested on its own.
The oral whey protein consumptions decreased the ratings in young of desire to eat and prospective food consumption compared with the baseline (after overnight fasting), while they were increased in the healthy older males. The ratings of prospective food consumption reflected subsequent energy intake in both age groups. The greater the reduction in these ratings after protein consumption, the greater the reduction in subsequent energy intake. Thus, while older people are less hungry and eat less than younger adults (8, 21, 36, 38, 40, 42, 47), they appear also to be less susceptible to further suppression of appetite and eating behavior by ingestion of energy and nutrients, including protein.
The finding of an age-related reduction in the satiating effects of protein is potentially important. In young adults protein is the most satiating macronutrient when ingested orally (44), and there is evidence that high-protein diets promote satiety and facilitate deliberate weight loss during energy restriction diets in overweight, younger adults (41). While beneficial in those circumstances, protein-enriched nutritional supplements given to older people for management of undernutrition, could have unintended adverse effects, if the satiating effects of protein are undiminished, or increased, by age. The use of high-protein supplements by older people for this purpose is widespread and increasing. This is partly in response to greater awareness of the prevalence of undernutrition and sarcopenia in older people, and evidence that protein supplementation may increase both muscle mass and function (13, 20). The age-related reduction in suppression of appetite and feeding responses to oral protein, and to protein directly infused into the small intestine (43), suggest that if timing and preparation are optimized, it may be possible to give sufficient protein to older people to preserve, or increase muscle mass and function, without suppressing energy intake. The optimum composition for nutritional supplements for management of undernutrition in older people is not known. The nutritional supplements are probably best given in liquid form between meals (50), as suppression of energy intake by energy-containing beverages are less compared with isoenergetic solid loads (9).
In the young men, suppression of energy intake by the 70 g whey protein was comparable with the 30 g whey protein, suggesting that there may be a threshold of maximum suppression in energy intake. This observation is consistent with comparable suppression of energy intake by protein-rich meals 3 h after whey protein ingestion of 15 and 30 g (49) and 4 h after oral protein intakes of 24, 44, and 80 g (2).
Cumulative energy intake was increased most by the highest protein dose (70 g), a substantial increase of 19% or 175 kcal. Although only a limited number of studies have examined the effects of the state of nutrition in older people on the regulation of appetite, there is persuasive evidence of substantial differences between undernourished and healthy older people, which may potentially be an outcome of and/or contribute to the undernourished state. In particular, suppression of energy intake by a mixed-nutrient preload was less in undernourished older women compared with healthy older women (46). These findings raise the possibility that appropriately designed protein supplements might even act to increase overall energy intake in undernourished people by meaningful amounts.
The regulation of appetite and energy intake is dependent on the precise coordination of interrelated “intragastric” and “small intestinal” sensory and motor mechanisms, including variations in gastric emptying (on average 1–4 kcal/min) (15) and gastric distension (19). Gastric emptying of ingested nutrients results in a relatively constant rate of energy delivery of the ingested nutrient load from the stomach to the duodenum. Slower gastric emptying results in greater distension of the stomach at a given time after food ingestion. This can, in turn, lead to greater fullness and a consequent reduction in subsequent energy intake. The suppressive effect of gastric distension on energy intake has led to successful attempts to reduce energy intake and induce weight loss in obese people by implanting gastric balloons (53). While slower gastric emptying prolongs retention of food in the stomach, favoring satiation, it also delays the onset of powerful satiety signals initiated by the interaction of nutrients with the small intestine (31).
In the present study, gastric emptying of water and both protein drinks was slower in older men than young controls, consistent with results of previous studies (16, 24, 28). The water drink emptied slower in older men than young controls, which implies an “intragastric” etiology. There was a dose-dependent slowing of gastric emptying by protein to a similar degree in both age groups, with 50% gastric emptying time more than doubling from the control to the 30 g protein drink day, and from the 30 g to the 70 g protein drink day. The older subjects had a slower gastric emptying of the protein drinks than the young subjects (0.8 kcal compared with 1.0 kcal/min on average over 180 min), especially during the initial phase of emptying. Therefore, the older men had greater intragastric volumes at all time points between protein ingestion and the buffet meal than the young men, Despite the latter observation, fullness increased from baseline in young but not in older subjects and the protein-induced suppression of subsequent energy intake was less in the older men compared with the young controls. This finding, and the lack of an association between fullness ratings at the start of the buffet meal and energy intake at that meal, suggests that age-related slowing of gastric emptying rate is not a major mediator of the age-related differences in protein-induced satiety at 3 h after ingestion. It should, however, be recognized that to evaluate “intragastric” factors, the buffet meal has to be given much earlier. At the start of the meal, more than 85% of all drinks had emptied from the stomach, except the 70-g protein drink in the older subjects. Also, all drinks, except the 70-g protein drink in the older subjects, emptied in a nonlinear pattern, so once gastric emptying has started, both intragastric volume and small-intestinal feedback were relevant. In older people, however, the perception of proximal gastric distension is diminished (33), which may explain why their slower gastric emptying had little, if any, suppressive effect on subsequent energy intake in this study. More likely, age-related differences in the satiating effects of orally ingested protein are mediated predominantly by mechanisms activated after the protein passes into the small intestine, e.g., gut hormone secretion (CCK, glucagon-like peptide-1, peptide tyrosine tyrosine and gastric inhibitory peptide) (37) and gut motility (39), rather than intragastric mechanisms, or by central mechanisms of amino acids, e.g., leucine, which is abundant in whey and acts directly in the central nervous system, to reduce intake. This is consistent with our previous finding of greater suppression of energy intake by protein in young than older men when protein is infused directly into the small intestine, thus removing any influence of differences in gastric emptying (43), implying that both intragastric and small intestinal mechanisms, which act to suppress energy intake, are attenuated in healthy elderly. Further studies are needed to determine the nature of these mechanisms.
This study has several limitations. The subject numbers were relatively small. We studied only men, as they appear to have the greatest ability to regulate energy intake in response to energy manipulation (36). The results do not, therefore, necessarily apply to the effects of aging in women. Further studies are needed to determine this, and also, the effects of protein when administered as part of a mixed macronutrient supplement in both undernourished and obese older people. Nevertheless, our findings support the use of protein supplements to increase energy intake in older people.
Perspectives and Significance
The significant finding was that despite having slower gastric emptying, older men exhibited blunted protein-induced suppression of energy intake by oral whey protein compared with young controls, so that in the older men compared with the young controls, protein ingestion increased overall energy intake more. The observations support the use of protein supplements in undernourished older people, but suggest that their use as a strategy to decrease energy intake in older obese individuals may not be effective. Future studies are needed to characterize the effects of protein and carbohydrate and fat in isolation and as a mixture, both in a liquid and solid form, in malnourished, i.e., underweight and obese, elderly and, thereby, provide comprehensive insights into the underlying mechanisms, and lead to improved, evidence-based, strategies for the use of supplements to increase energy intake in older undernourished individuals or to decrease energy intake as part of a weight-loss diet strategy in the obese elderly.
GRANTS
The research was funded by a G-TRAC Resthaven Grant. S Soenen was supported by a Royal Adelaide Hospital Mary Overton Early Career Research Fellowship, K. L Jones was supported by an NHMRC Senior Clinical Career Development Award, N. D. Luscombe-Marsh is supported by an NHMRC New Investigator Project Grant, C. Feinle-Bisset was supported by an NHMRC Senior Research Fellowship, A. T. Hutchison was supported by a Faculty of Health Sciences Postgraduate Scholarship, and L. G. Trahair was supported by an Australian Postgraduate Award and a Dawes scholarship from the Royal Adelaide Hospital. Whey protein was kindly donated by Fonterra Research Centre, Palmerston North, New Zealand. Fonterra, G-TRAC Resthaven, and the Royal Adelaide Hospital Research Foundation, did not have any input in the design, implementation, analysis, or interpretation of the data.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.G., L.G.T., R.S.R., A.T.H., and S.S. performed experiments; C.G. and S.S. analyzed data; C.G., T.H., K.L.J., M.H., I.M.C., and S.S. interpreted results of experiments; C.G. and S.S. prepared figures; C.G. drafted manuscript; C.G., L.G.T., R.S.R., A.T.H., C.F.-B., N.D.L.-M., T.H., K.L.J., M.H., I.M.C., and S.S. approved final version of manuscript; L.G.T., R.S.R., A.T.H., C.F.-B., N.D.L.-M., T.H., K.L.J., M.H., I.M.C., and S.S. edited and revised manuscript; C.F.-B., N.D.L.-M., M.H., I.M.C., and S.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Fonterra Research Centre, Palmerston North, New Zealand, for providing the whey protein, and Penelope Fitzgerald, Kylie Lange, Judith Wishart, and Scott Standfield of the National Health and Medical Research Council of Australia Centre of Clinical Research Excellence in Translating Nutritional Research to Good Health, Discipline of Medicine, Royal Adelaide Hospital, The University of Adelaide, for assistance with studies (PF), statistical support (KL) and performance of biochemical assays (JW, SS).
REFERENCES
- 1.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Directors Assoc 14: 542–559, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Belza A, Ritz C, Sorensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr 97: 980–989, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1—Correlation within subjects. Br Med J 310: 446, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2—Correlation between subjects. Br Med J 310: 633, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boirie Y, Morio B, Caumon E, Cano NJ. Nutrition and protein energy homeostasis in elderly. Mech Ageing Dev 136–137: 76–84, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda C, Dolnikowski GG, Dallal GE, Evans WJ, Crim MC. Protein turnover and energy metabolism of elderly women fed a low-protein diet. Am J Clin Nutr 62: 40–48, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol Regul Integr Comp Physiol 272: R243–R248, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Cook CG, Andrews JM, Jones KL, Wittert GA, Chapman IM, Morley JE, Horowitz M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am J Physiol Regul Integr Comp Physiol 273: R755–R761, 1997. [DOI] [PubMed] [Google Scholar]
- 9.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 24: 794–800, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 287: R524–R533, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein S, McHugh PR. Mini-Mental-State: a practical method for grading cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Gentilcore D, Hausken T, Horowitz M, Jones KL. Measurements of gastric emptying of low- and high-nutrient liquids using 3D ultrasonography and scintigraphy. Neurogastroenterol Motil 18: 1062–1068, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Groen BB, Res PT, Pennings B, Hertle E, Senden JM, Saris WH, van Loon LJ. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am J Physiol Endocrinol Metab 302: E52–E60, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Guigoz Y, Garry JP. Mini nutritional assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol 15–59, 1994.
- 15.Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 254: G671–G679, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin Sci (Lond) 67: 213–218, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Jahangir E, De Schutter A, Lavie CJ. Low weight and overweightness in older adults: risk and clinical management. Prog Cardiovasc Dis 57: 127–133, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MA. Strategies to improve diet in older adults. Proc Nutr Soc 72: 166–172, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Jones KL, Doran SM, Hveem K, Bartholomeusz FD, Morley JE, Sun WM, Chatterton BE, Horowitz M. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr 66: 127–132, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr 139: 1707–1713, 2009. [DOI] [PubMed] [Google Scholar]
- 21.MacIntosh CG, Horowitz M, Verhagen MA, Smout AJ, Wishart J, Morris H, Goble E, Morley JE, Chapman IM. Effect of small intestinal nutrient infusion on appetite, gastrointestinal hormone release, and gastric myoelectrical activity in young and older men. Am J Gastroenterol 96: 997–1007, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Directors Assoc 14: 10–17, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev CD003288, 2009. [DOI] [PMC free article] [PubMed]
- 24.Moore JG, Tweedy C, Christian PE, Datz FL. Effect of age on gastric emptying of liquid—solid meals in man. Dig Dis Sci 28: 340–344, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Silver AJ. Anorexia in the elderly. Neurobiol Aging 9: 9–16, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Nair NS, Brennan IM, Little TJ, Gentilcore D, Hausken T, Jones KL, Wishart JM, Horowitz M, Feinle-Bisset C. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br J Nutr 101: 1094–1102, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc 49: 1309–1318, 2001. [DOI] [PubMed] [Google Scholar]
- 28.O'Donovan D, Hausken T, Lei Y, Russo A, Keogh J, Horowitz M, Jones KL. Effect of aging on transpyloric flow, gastric emptying, and intragastric distribution in healthy humans—impact on glycemia. Dig Dis Sci 50: 671–676, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care 17: 5–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Parker BA, Chapman IM. Food intake and ageing—the role of the gut. Mech Ageing Dev 125: 859–866, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr 58: 212–218, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rayner CK, MacIntosh CG, Chapman IM, Morley JE, Horowitz M. Effects of age on proximal gastric motor and sensory function. Scand J Gastroenterol 35: 1041–1047, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Roberts SB, Fuss P, Heyman MB, Evans WJ, Tsay R, Rasmussen H, Fiatarone M, Cortiella J, Dallal GE, Young VR. Control of food intake in older men. JAMA 272: 1601–1606, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care 14: 15–21, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr 62: 923–931, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M, and Luscombe-Marsh ND. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 96: 474–482, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Schneider SM, Al-Jaouni R, Caruba C, Giudicelli J, Arab K, Suavet F, Ferrari P, Mothe-Satney I, Van Obberghen E, Hebuterne X. Effects of age, malnutrition and refeeding on the expression and secretion of ghrelin. Clin Nutr 27: 724–731, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Seimon RV, Lange K, Little TJ, Brennan IM, Pilichiewicz AN, Feltrin KL, Smeets AJ, Horowitz M, Feinle-Bisset C. Pooled-data analysis identifies pyloric pressures and plasma cholecystokinin concentrations as major determinants of acute energy intake in healthy, lean men. Am J Clin Nutr 92: 61–68, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Serra-Prat M, Palomera E, Clave P, Puig-Domingo M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am J Clin Nutr 89: 1410–1417, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Soenen S, Bonomi AG, Lemmens SG, Scholte J, Thijssen MA, van Berkum F, Westerterp-Plantenga MS. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol Behav 107: 374–380, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Soenen S, Chapman IM. Body weight, anorexia, and undernutrition in older people. J Am Med Directors Assoc 14: 642–648, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Soenen S, Giezenaar C, Hutchison AT, Horowitz M, Chapman I, Luscombe-Marsh ND. Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am J Clin Nutr 100: 1108–1115, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Soenen S, Westerterp-Plantenga MS. Proteins and satiety: implications for weight management. Curr Opin Clin Nutr Metab Care 11: 747–751, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Speakman JR, Westerterp KR. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am J Clin Nutr 92: 826–834, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Sturm K, MacIntosh CG, Parker BA, Wishart J, Horowitz M, Chapman IM. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J Clin Endocrinol Metab 88: 3747–3755, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Sturm K, Parker B, Wishart J, Feinle-Bisset C, Jones KL, Chapman I, Horowitz M. Energy intake and appetite are related to antral area in healthy young and older subjects. Am J Clin Nutr 80: 656–667, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 86: 451–456, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 96: 675–682, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Wilson MM, Purushothaman R, Morley JE. Effect of liquid dietary supplements on energy intake in the elderly. Am J Clin Nutr 75: 944–947, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wurtman JJ, Lieberman H, Tsay R, Nader T, Chew B. Calorie and nutrient intakes of elderly and young subjects measured under identical conditions. J Gerontol 43: B174–B180, 1988. [DOI] [PubMed] [Google Scholar]
- 52.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 96: 1281–1298, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Yasawy MI, Al-Quorain AA, Hussameddin AM, Yasawy ZM, Al-Sulaiman RM. Obesity and gastric balloon. J Fam Commun Med 21: 196–199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: 37–49, 1982. [DOI] [PubMed] [Google Scholar]
- 55.Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U. S older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc 59: 132–137, 2011. [DOI] [PubMed] [Google Scholar]