Abstract
The endocannabinoids are lipid-derived signaling molecules that control feeding and energy balance by activating CB1-type cannabinoid receptors in the brain and peripheral tissues. Previous studies have shown that oral exposure to dietary fat stimulates endocannabinoid signaling in the rat small intestine, which provides positive feedback that drives further food intake and preference for fat-rich foods. We now describe an unexpectedly broader role for cholinergic signaling of the vagus nerve in the production of the endocannabinoid, 2-arachidonoyl-sn-glycerol (2-AG), in the small intestine. We show that food deprivation increases levels of 2-AG and its lipid precursor, 1,2-diacylglycerol, in rat jejunum mucosa in a time-dependent manner. This response is abrogated by surgical resection of the vagus nerve or pharmacological blockade of small intestinal subtype-3 muscarinic acetylcholine (m3 mAch) receptors, but not inhibition of subtype-1 muscarinic acetylcholine (m1 mAch). We further show that blockade of peripheral CB1 receptors or intestinal m3 mAch receptors inhibits refeeding in fasted rats. The results suggest that food deprivation stimulates 2-AG-dependent CB1 receptor activation through a mechanism that requires efferent vagal activation of m3 mAch receptors in the jejunum, which, in turn, may promote feeding after a fast.
Keywords: cannabinoid receptor type 1 (CB1), cholinergic receptor, endocannabinoid, gut-brain, vagus nerve
the parasympathetic and sympathetic divisions of the autonomic nervous system contribute in important ways to the regulation of energy balance (5). Studies in mice have shown that activation of central melanocortin-4 receptors, which reduce feeding and are critical for the maintenance of energy metabolism (53), inhibit cholinergic parasympathetic preganglionic neurons in the dorsal motor nucleus of the vagus (DMV) and activate sympathetic preganglionic neurons in the spinal cord (44). Evidence suggests that cholinergic signals from the efferent vagus nerve control peripheral gut-brain signaling mechanisms that regulate feeding. For example, administration of the peripherally restricted muscarinic acetylcholine receptor (mAchR) antagonist atropine methyl nitrate inhibits feeding after a fast (33), as well as sham intake of a liquid diet in rats (25). A possible interpretation of these results is that peripheral mAchRs participate in the control of food intake, but the mechanisms through which such regulation might occur remain unknown.
The endocannabinoid system is a key regulator of energy metabolism (13). A link between cholinergic neurotransmission and endocannabinoid signaling has been suggested by experiments showing that mAchRs control synaptic plasticity in various brain regions (24, 36, 38, 47, 55) by eliciting the production of endocannabinoid messengers. Moreover, recent work from our laboratory has suggested that cholinergic signaling in the gut controls the intake of dietary fat through an endocannabinoid-dependent mechanism. In those studies, oral exposure to a fat-rich liquid diet in sham-feeding rats enhanced endocannabinoid mobilization in the jejunum, and pharmacological blockade of CB1 cannabinoid receptors (CB1Rs) with a peripherally restricted antagonist blocked sham feeding (10, 11). Importantly, resection of the vagus nerve below the diaphragm completely blocked the increases in jejunal endocannabinoid signaling that occurred during sham feeding of fat. The results suggest that oral exposure to dietary fat triggers small-intestinal endocannabinoid signaling through a mechanism that is dependent on efferent vagal neurotransmission and that this peripheral signaling event provides positive feedback to the brain to drive additional food intake (9). Here, we extend these findings and show that during food deprivation, cholinergic signaling at peripheral mAchRs, possibly mediated by the efferent vagus nerve, initiates the production of the endocannabinoid, 2-AG, through the diacylglycerol lipase-alpha (DGL) pathway, which promotes feeding after a fast.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (250–300 g) were purchased from Charles River (Wilmington, MA) and housed at room temperature (22°C) in hanging wire-bottom cages to prevent coprophagia during food deprivation experiments. Animals were maintained on a 12:12-h light-dark cycle (lights on at 0630 and off at 1830) and had free access to water and standard rodent chow pellets (Harlan Teklad 2020, North America), except when noted during food deprivation experiments (all given free access to water). All experiments began at 0900. All procedures met the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Chemicals and Administration Schedule
The nonselective mAchR antagonist atropine (Sigma-Aldrich, St. Louis, MO) was administered by intraperitoneal injection at 1 mg/kg in 0.5 ml/kg DMSO (Sigma) 20 min before death. The peripherally restricted cannabinoid CB1 receptor antagonist AM6545 (Sigma) was administered by injection at 10 mg/kg ip in 0.5 ml/kg DMSO 20 min before refeeding. The diacylglycerol lipase inhibitor tetrahydrolipstatin (Cayman Chemical, Ann Arbor, MI) was administered by oral gavage at 100 mg in 1 ml mineral oil (STE Oil, San Marco, TX) 45 min before death. The selective m3 muscarinic acetylcholine receptor antagonist DAU 5884 hydrochloride (Tocris, Minneapolis, MN) or the selective m1 mAchR antagonist pirenzepine dihydrochloride (Sigma-Aldrich) were administered in separate groups of animals by intraduodenal infusion at matching molar concentrations (300 μM in 1 ml saline) 20 min before death. For refeeding experiments in animals implanted with duodenal catheters, DAU 5884 was administered by intraduodenal infusion in combination with intraperitoneal administration of AM6545, when appropriate (see Experimental Design, Feeding effects of inhibiting intestinal m3 mAchRs and CB1Rs), 20 min before presentation of chow following 24-h food deprivation. Doses of DAU 5884 were chosen on the basis of the ability of 300 nmol to significantly reduce 2-AG levels after 24 h of food deprivation and were administered intraduodenally on separate test days in 1 ml of saline. AM6545 was coadministered intraperitoneally with DAU 5884, when appropriate, at a dose (10 mg/kg ip) that significantly reduced refeeding after 24-h food deprivation. All control conditions received the same procedure, except the drug was absent from the corresponding vehicle. All drug or vehicle treatments were administered to animals after a 24-h fast, with at least 72 h between treatments.
Feeding effects of inhibiting intestinal m3 mAchRs and CB1Rs
All animals (n = 10) received vehicle treatments (i.e., intraduodenal saline at 1 ml and intraperitoneal DMSO at 0.5 ml/kg 20 min before refeeding after a 24-h fast) on the first day of testing and on the last day of testing. The mean values for 1-h refeeding intakes following each vehicle treatment, which did not statistically differ (25.1 ± 1.6 and 22.3 ± 1.9 g/kg body wt; Student's t-test, two-tailed P = 0.28), were averaged for statistical comparison vs. drug treatments. Animals were then divided into two subgroups (n = 5/group): subgroup A received DAU5884 (100 nmol id) and AM6545 (10 mg/kg ip), and subgroup B received DAU5884 (100 nmol id) and intraperitoneal DMSO vehicle. On the next day of testing, subgroup A received DAU5884 (300 nmol id) and intraperitoneal DMSO vehicle, and subgroup B received DAU5884 (300 nmol id) and AM6545 (10 mg/kg ip). On the final day of drug treatments, all animals received intraduodenal saline vehicle and intraperitoneal AM6545 (10 mg/kg ip).
Intraduodenal Infusion of Macronutrients
Rats were food-deprived for 24 h, and then they were infused at a rate of 0.5 ml/min for 10 min into the duodenum with vehicle (sterile saline), or equicaloric (10 kcal total) concentrations of Intralipid, sucrose, or Peptone. Tissues were harvested (see Tissue Processing) 30 min after infusions commenced.
Surgeries
Intraduodenal catheters.
Animals were anesthetized with an injection of ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip; Western Medical Supply, Arcadia, CA). An incision was made through the skin and abdominal muscle along the midline, and a 2-cm section of the duodenum, 1 cm distal to the pylorus, was exposed. A 2-cm Silastic tube [0.020 in. ID, 0.037 in. OD (Technical Products, Lawrenceville, GA)] was inserted, facing toward the distal end of the intestine, and 1.0 cm distal to the pylorus. This tubing (fabricated before surgery) was fitted 0.5 cm into a slightly larger Silastic tube (0.040 in. ID, 0.085 in. OD), with a small piece of mylar mesh surrounding the junction, and sealed with silicone adhesive (DAP 100% silicone). After insertion of the smaller tube into the duodenum, two small drops of skin adhesive were placed on the mesh to promote adhesion to the intestine. The larger tube, with an attached (silicone adhesive) circular piece of mesh, was carefully routed up the neck of the animal, exteriorized, then sutured to underlying tissue. A wound clip was placed to close the small opening at the site. The abdominal muscle wall was closed using plain-gut 4-0 suture (Fischer Scientific, Houston, TX), and the skin was closed using stainless-steel wound clips (World Precision Instruments, Sarasota FL). A small stainless-steel nail was placed into the end of the catheter. The catheters were flushed with saline (1 ml) daily to prevent occlusion. For pain management, animals were given buprenorphine (0.02 mg/kg ip; Western Medical Supply) following completion of surgery. Testing began following 7–10 days of recovery from surgery.
Subdiaphragmatic vagotomy.
All rats were prepared for surgery, as outlined above (see Intraduodenal catheter). The stomach and spleen were gently retracted, and the dorsal and ventral divisions of the vagus nerve below the diaphragm were isolated with fine forceps and peeled from the esophagus. Two sutures were placed 1.5 cm apart on each division of the vagus (four stitches in total), and all neural tissue between each set of stitches was isolated and removed using fine scissors. Control sham surgery animals underwent the same procedures as detailed above, except that the vagus nerve was not manipulated and was left intact. The abdominal muscle wall was closed as above (see Intraduodenal catheter). Testing began following 7–10 days of recovery from surgery.
Tissue Processing
Lipid extractions.
All animals were anesthetized with isoflurane at time of tissue harvest, and then the jejunum or other organs were rapidly removed and rinsed with PBS and snap-frozen in liquid N2. Prior to snap freezing, jejunal mucosa was separated from serosa by scraping with a glass slide. All tissues were subsequently stored at −80°C until time of processing. Frozen tissues were weighed and homogenized in 1.0 ml of methanol containing the following internal standards: [2H8]-2-AG (Cayman Chemical, Ann Arbor, MI) and dinonadecadienoin (Nu-Chek Prep, Elysian, MN). Lipids were extracted with chloroform (2 vol) and washed with water (1 vol). Organic phases were collected and fractionated by open-bed silica gel column chromatography, as previously described (3). Eluted fractions were dried under N2 and reconstituted in 0.1 ml of chloroform:methanol (1:3) for liquid chromatography/mass spectrometry (LC/MS) analyses.
Measurement of 2-AG.
We used a 1100-liquid chromatography system coupled to a 1946D-MS detector (Agilent Technologies, Palo Alto, CA) equipped with an electrospray ionization (ESI) interface. Lipids were separated on a XDB Eclipse C18 column (100 × 4.6 mm ID, 1.8 μm, Zorbax), eluted by a gradient of methanol (0.1% formic acid) in water (0.1% formic acid) (from 85% to 90% methanol in 30 min, 90–100% in 30–31 min, 100% in 31–37 min, 100-85%, in 37–38 min, and 85% in 38–40 min) at a flow rate of 0.5 ml/min. Column temperature was kept at 20°C. MS detection was in the positive ionization mode, capillary voltage was set at 3 kV, and fragmentor voltage at 30 V. Lipids were quantified with an isotope-dilution method (15), monitoring sodium adducts of the molecular ions [M+Na+] in the selected ion-monitoring mode. 2-AG can acyl-migrate to 1-AG, and thus, all values reported for 2-AG levels in tissues reflect a sum of both 1-AG and 2-AG. In addition, variability in baseline levels of 2-AG between separate experiments reflects interassay variability and does not affect comparisons and conclusions made within an individual experiment. Each individual experiment has its own control conditions for statistical comparison, and thus, no comparison is made between separate experiments. Tissue processing and endocannabinoid analysis via LC/MS of all samples within an individual experiment occur independently of other separate experiments.
Measurement of 1-stearoyl,2-arachidonoyl-sn-glycerol.
We used an Agilent 1100-LC system coupled to an MS detector Ion-Trap XCT interfaced with ESI (Agilent Technologies). Diacylglycerol (DAG) species were separated using a Poroshell C18 column (75 × 2.1 mm ID, 5 μm), eluted by a gradient of methanol (0.5% acetic acid, 5 nM ammonium acetate) in water (0.5% acetic acid, 5 nM ammonium acetate) (from 60 to 100% methanol in 6 min, 100% in 6–8 min, 100-60% in 8–9 min, and 60% in 9–10 min) at a flow rate of 1.0 ml/min. Column temperature was kept at 30°C. The capillary voltage was set at 3.5 kV, and the skimmer voltage was set at 40 V. N2 was used as the drying gas at a flow rate of 10 l/min, at a temperature of 350°C, and at a nebulizer pressure of 60 psi. Helium was used as the collision gas, and fragmentation amplitude was set at 1.3 V. DAG species were identified in the positive ionization mode, based on their retention times and MS2 properties, using authentic standards (Nu-Chek Prep) as references. Multiple reaction monitoring was used to acquire full-scan tandem MS spectra of selected 1-stearoyl,2-arachidonoyl-sn-glycerol (SAG) ions. Extracted ion chromatograms were used to quantify 1-stearoyl,2-arachidonoyl-sn-glycerol (m/z = 667.8 > 383.2) and dinonadecadienoin (m/z = 667.8 > 373.5), which was used as an internal standard.
RT-PCR
We extracted total RNA from jejunum mucosa and cerebellum (free-feeding rats) using a TRIzol (Invitrogen, Carlsbad, CA) and RNeasy (Qiagen, Valencia, CA) hybrid method, and synthesized first-strand complementary DNA using SuperScript II RNaseH reverse transcriptase (Invitrogen) (14, 22). All surfaces and instruments used for tissue collection were treated with an RNase inhibitor (RNase Zap) to enhance the quality of extracted RNA. Reverse transcription of total RNA (2 μg) was carried out using oligo(dT)12–18 primers for 50 min at 42°C. PCR was conducted using GoTaq DNA polymerase system (Promega) following our standard protocol (14, 22). We designed primer sets using mRNA sequences obtained from NCBI database for the following molecules: Rattus norvegicus cannabinoid receptor 1 (Cnr1), GenInfo Identifier (GI) 284055292; Rattus norvegicus cholinergic receptor, muscarinic 3 (Chrm3), GI 451172072; and Rattus norvegicus cholinergic receptor, muscarinic 1 (Chrm1), GI 18249940. Primers were synthesized through Invitrogen. The primer sequences are: Cnr1, forward, 5′-GTCTCCCATTTCAAGCAAGGAG-3′ and reverse, 5′-TGTGAGGGACAGTACAGCGA-3′; Chrm3, Forward, 5′-ATGACCTTGCACAGTAACAGTACAAC-3′ and reverse, 5′-CTACAAGGCCTGTTCCGGC-3′; and Chrm1, Forward, 5′- ATGAACACCTCAGTGCCCCCTGCTGTC -3′ and reverse, 5′- TTAGCATTGGCGGGAGGGGGTGCGGT -3′. PCR products were separated in 1% agarose gel electrophoresis and stained with ethidium bromide.
Statistical Analyses
Results are expressed as the means ± SE. The significance of differences between groups was evaluated by Student's t-test, and one-way ANOVA followed by Newman-Keuls or Dunnett's post hoc evaluation, or a two-way ANOVA followed by a Newman-Keuls or Sidak post hoc evaluation, when appropriate, for comparison of means when significant differences were found by ANOVA. Differences were considered significant if P < 0.05.
RESULTS
Feeding Regulates 2-AG Levels in the Jejunum
We focused the present work on delineating the specific molecular and neural pathways responsible for 2-AG biosynthesis in the gut, which diverge from those associated with anandamide (10). The results show that the jejunum mucosa of 24-h fasted rats contains substantially higher levels of 2-AG than does mucosa of free-feeding animals (Fig. 1A; P < 0.01; n = 4 or 5). A similar, albeit much smaller, difference was seen in the serosa (P < 0.05). By contrast, 2-AG levels in other peripheral organs and tissues (i.e., stomach, ileum, colon, liver, pancreas, spleen, blood) did not differ between feeding conditions. Time-course experiments showed that 2-AG levels began to increase after 12 h of food deprivation and reached statistical significance at 24 h (Fig. 1B; P < 0.01; n = 4). Refeeding rapidly decreased 2-AG levels (Fig. 1C; P < 0.05; n = 5 or 6). 2-AG was the primary monoacylglycerol (MAG), whose levels were affected by fasting, because no changes were observed with other MAG species, including 1(3) palmitoyl-sn-glycerol, 1(3) stearoyl-sn-glycerol, 2-oleoyl-sn-glycerol, and 2-linoleoyl-sn-glycerol (Fig. 1D). To identify individual macronutrients that might be responsible for controlling jejunal 2-AG levels, we implanted catheters in rats and infused into the duodenum separate macronutrients (equicaloric, 10 kcal) following 24 h of food deprivation. All macronutrients equally reduced 2-AG levels within 30 min of infusion, compared with vehicle infusion (Fig. 1E): fat (Intralipid, P < 0.01), carbohydrate (sucrose, P < 0.01), and protein (peptone, P < 0.05).
Fig. 1.
Feeding regulates 2-arachidonoyl-sn-glycerol (2-AG) levels in jejunum. A: effects of food deprivation on levels of 2-AG in organs of rats. FF, free feeding (denoted by open bars); FD, 24-h food deprivation (denoted by closed bars); Jej M, jejunum mucosa; Jej S, jejunum serosa; Stom, stomach; Panc, pancreas; Spl, spleen. *P < 0.05, **P < 0.01 (n = 4 or 5/condition). Student's t-tests, two-tailed. B: time course of food deprivation on levels of 2-AG in jejunum mucosa. **P < 0.01 (n = 4/condition). One-way ANOVA, Dunnett's multiple-comparisons test vs. control (FF). C: time course of refeeding after 24-h food deprivation on levels of 2-AG in jejunum mucosa. RF, refeeding (denoted by shaded bars). *P < 0.05 (n = 5 or 6/condition). One-way ANOVA, Newman-Keuls multiple-comparisons test. D: effects of food deprivation on levels of other monoacylglycerols in jejunum mucosa. 16:0, palmitoylglycerol; 18:0, stearoylglycerol; 18:1, oleoylglycerol; 18:2, linoleoylglycerol. Student's t-test, two-tailed. E: effects of intraduodenal administration of various macronutrients (closed bars) on levels of 2-AG in jejunum mucosa of 24-h food-deprived rats. V, vehicle (open bar); L, Intralipid; C, carbohydrate; P, protein. *P < 0.05, **P < 0.01 (n = 3 or 4/condition). One-way ANOVA, Dunnett's multiple-comparison test vs. control (V). Results are expressed as means ± SE.
Fasting and Refeeding Regulate 2-AG Biosynthesis via the DGL Pathway
In neurons, 2-AG is primarily produced through the phospholipase C (PLC)-dependent generation of SAG, which is subsequently hydrolyzed by diacylglycerol lipase-alpha (DGL-alpha) to yield 2-AG (1, 32, 46). We asked whether the changes in jejunal 2-AG levels caused by food deprivation and refeeding were accompanied by similar fluctuations in the levels of the 2-AG precursor, SAG. SAG was identified in lipid extracts of jejunal mucosa by liquid chromatography/tandem mass spectrometry (Fig. 2A). As seen with 2-AG (Fig. 1B), jejunal SAG levels increased following food deprivation, reaching statistical significance after 24 h, compared with free-feeding animals (Fig. 2B; P < 0.01; n = 5). Furthermore, in separate groups of animals, fasting significantly increased SAG levels by 24 h relative to controls (Fig. 2C; P < 0.05; n = 5 or 6 per condition). Such levels were rapidly reduced to free-feeding levels following 15 or 30 min refeeding (Fig. 2C; P < 0.05). The results suggest that fasting and refeeding influence SAG levels with a temporal profile similar to that of 2-AG. Confirming a role for SAG hydrolysis in 2-AG production, we found that DGL inhibition by oral gavage of the lipase inhibitor tetrahydrolipstatin (THL), which blocks 2-AG formation in rat brain (17, 28), reduced 2-AG levels in both free-feeding and food-deprived rats, compared with control rats receiving vehicle (Fig. 2D; P < 0.05 and P < 0.001, respectively; n = 4 or 5). Furthermore, food-deprived animals treated with THL failed to display significant increases in jejunal 2-AG compared with free-feeding rats treated with the inhibitor (Fig. 2D; not significant). In contrast to its effects on 2-AG (Fig. 2D), THL did not significantly affect SAG levels (Fig. 2E, not significant), which is consistent with inhibition of DGL activity. The results suggest that the DGL pathway is critical in the formation of 2-AG in the jejunum mucosa.
Fig. 2.
Diacylglycerol lipase-alpha (DGL) pathway regulates 2-AG during fasting and refeeding. A: identification of 1,stearoyl,2-arachidonoyl-sn-glycerol (SAG) in jejunum mucosa of rats by liquid chromatography/tandem mass spectrometry (LC/MS/MS). The abundant [MNa-R1COOH]+ fragment (m/z = 383.2) was selected for quantification of SAG levels in jejunum mucosa. B: time course of food deprivation on levels of SAG in jejunum mucosa. FF is denoted by open bars; FD is denoted by closed bars. *P < 0.05 (n = 5/condition). One-way ANOVA, Dunnett's multiple-comparison test vs. control (FF). C: time course of refeeding after a 24-h food deprivation on levels of SAG in jejunum mucosa. RF, refeeding (denoted by shaded bars). *P < 0.05 (n = 5 or 6/condition). One-way ANOVA, Newman-Keuls multiple-comparison test. D: effects of pharmacological blockade of diacylglycerol lipase activity with tetrahydrolipstatin (THL) by oral gavage on levels of 2-AG in jejunum mucosa. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant (n = 4 or 5/condition). Two-way ANOVA [interaction between drug and feeding conditions; F(1,16) = 10.1, P < 0.01], Newman-Keuls multiple-comparison test. E: effects of pharmacological blockade of diacylglycerol lipase activity with tetrahydrolipstatin by oral gavage on levels of SAG in jejunum mucosa. THL, tetrahydrolipstatin, 100 mg. *P < 0.05; **P < 0.01. ns, not significant (n = 4 or 5/condition). Two-way ANOVA [no interaction found between drug and feeding conditions; F(1,16) = 0.8327, P = 0.38], Newman-Keuls multiple-comparisons test. Results are expressed as mean ± SE.
Cholinergic Neurotransmission Controls Jejunal 2-AG Levels
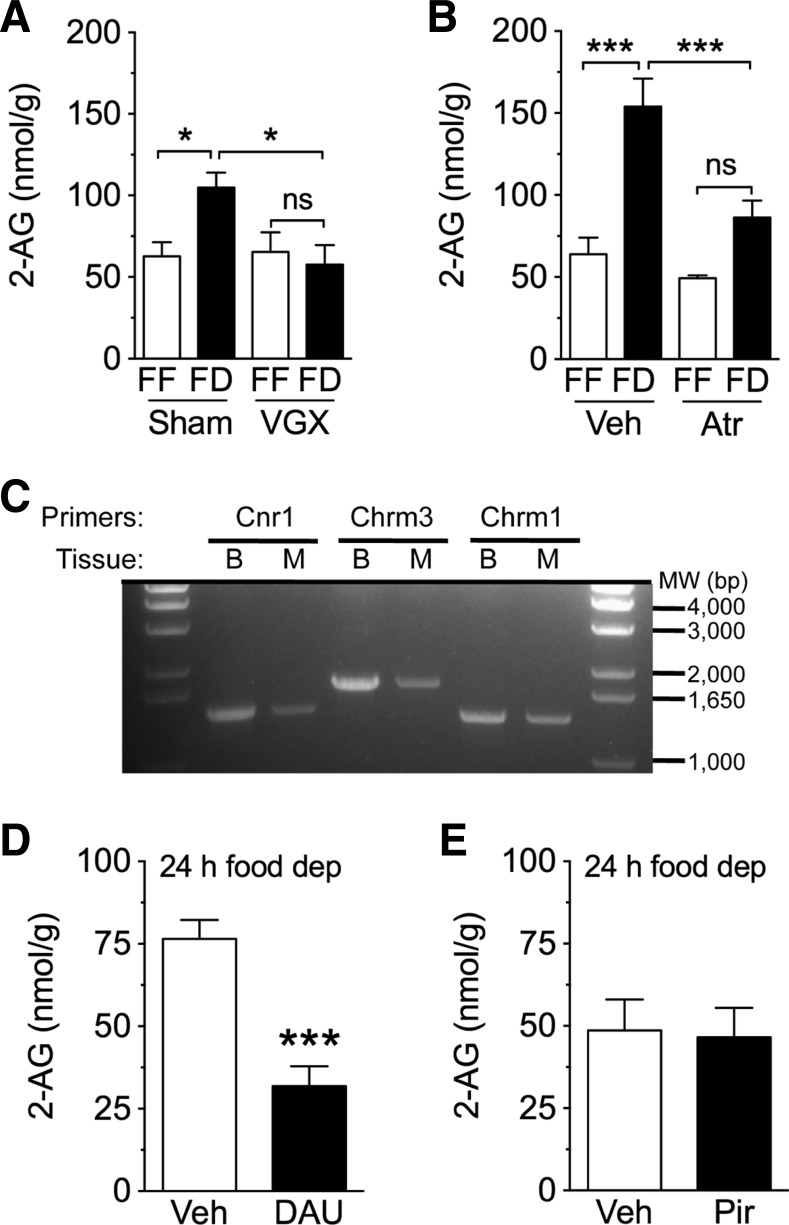
We previously reported that surgical disruption of the vagus nerve blocks increases in jejunal endocannabinoid levels in response to tasting a fatty meal (10). Therefore, we asked whether vagal fibers might also be required for fasting-induced 2-AG mobilization in the gut. To address this, rats were given either full subdiaphragmatic vagotomy or sham surgery. Control animals that received sham surgeries showed increased 2-AG levels in jejunum by 24 h after food deprivation, compared with free-feeding animals (Fig. 3A; P < 0.05; n = 4 or 5). By contrast, food-deprived vagotomized rats showed no such increase (Fig. 3A; not significant). Food-deprived vagotomized rats also had reduced levels of 2-AG in the jejunum, relative to food-deprived sham-operated controls (Fig. 3A; P < 0.05), and these levels were similar to free-feeding conditions regardless of surgical condition. Vagotomy in rodents has been shown to disrupt gastric emptying (40), an effect that possibly influences food intake and body weight. Thus, it is plausible that reductions in intake or body weight contributed to the blockade of fasting-induced elevations in jejunal 2-AG, and is a potential caveat for interpreting the results. In the present study, free-feeding vagotomized rats (after 10 days recovery from surgery), compared with sham controls, displayed reduced 24-h intake on the day preceding tissue harvest (vagotomized, 16.4 ± 1.8 g; sham, 26.1 ± 1.6 g; unpaired t-test, two-tailed, P = 0.007), as well as reduced body weight (vagotomized, 264.3 ± 24.1 g; sham, 355.5 ± 4.5 g; unpaired t-test, two-tailed, P = 0.001). Baseline levels of 2-AG for free-feeding controls, however, were similar to vagotomized animals (vagotomized, 65.4 ± 11.9 nmol/g; sham, 62.7 ± 8.5 g; unpaired t-test, two-tailed, P = 0.86). These data suggest that reductions in absolute food intake and body weight following vagotomy do not alter baseline 2-AG levels, and the absence of fasting-induced rises in jejunal 2-AG in vagotomized rats is likely due to a disruption in efferent vagal signaling. Given the severity of a full subdiaphragmatic vagotomy and potential confounds for interpretation of the results, further studies will be critical to more directly test the role for the efferent vagus nerve in controlling the production of 2-AG in the jejunum. These studies will include select vagotomies that more exclusively disrupt efferent signaling to the small intestine, as well as additional controls, including pair-feeding and sham groups that are matched for body weights of those subjected to vagotomy.
Fig. 3.
Cholinergic mechanisms regulate 2-AG levels in jejunum. A: effects of vagotomy on levels of 2-AG in jejunum mucosa of rats. FF is denoted by open bars, while FD is denoted by closed bars. Sham, sham surgery; VGX, full subdiaphragmatic vagotomy. *P < 0.05; n = 4 or 5/condition. Two-way ANOVA [interaction between surgery and feeding conditions; F (1,13) = 5.714, P < 0.05], Newman-Keuls multiple-comparison test. B: effects of blockade of muscarinic ACh receptors with atropine on levels of 2-AG in jejunum mucosa. Atr, atropine, 1 mg/kg ip. ***P < 0.001; (n = 4–6/condition). Two-way ANOVA [interaction between drug treatment and feeding conditions; F(1,16) = 6.798, P < 0.05], Newman-Keuls multiple-comparison test. C: expression of messenger RNA for cannabinoid receptor 1 (Cnr1), m3 muscarinic acetylcholine receptor (Chrm3), and m1 muscarinic acetylcholine receptor (Chrm1) in brain and intestine of rat. Full-length complementary DNA Cnr1, Chrm3, or Chrm1 were amplified using first-strand cDNA, which was obtained by reverse transcription of total mRNA prepared from rat brain (B, cerebellum) or jejunum mucosa (M). MW, molecular weight size marker in base pair (bp). Theoretical molecular size of the target cDNAs (bp): Cnr1, 1520; Chrm3, 1770; and Chrm1, 1383. D: effects of local blockade of m3 muscarinic acetylcholine receptors on levels of 2-AG in jejunum mucosa. DAU, DAU5884 300 nmol, intraduodenal administration. ***P < 0.001 (n = 5/condition). Student's t-tests, two-tailed. E: effects of local blockade of m1 muscarinic acetylcholine receptors on levels of 2-AG in jejunum mucosa. Pir, Pirenzepine, 300 nmol id, n = 5 or 6/condition. Student's t-tests, two-tailed. Results are expressed as means ± SE.
Acetylcholine is the major neurotransmitter of the efferent vagus nerve and activates mAchRs in the gut (5). In the mouse brain, the m3 subtype of mAchRs was found to mediate synaptic plasticity by stimulating endocannabinoid-mediated signaling (38, 55). This signaling event presumably occurs by activating PLC, which converts phosphatidylinositol-4,5-bisphosphate into arachidonic acid-containing MAGs such as SAG, and DGL-alpha, which hydrolyzes SAG to form 2-AG (32). Therefore, we tested whether cholinergic activation of mAchRs, which are found on enterocytes in villi of the rat small intestine (34, 35, 45), drive 2-AG formation in jejunal mucosa of fasting rats. Twenty-four-hour food deprivation produced the expected increase in jejunal 2-AG in vehicle-treated rats (Fig. 3B; P < 0.001). Systemic administration of the mAchR antagonist, atropine, attenuated this response (Fig. 3B; not significant; n = 4–6). Furthermore, food-deprived rats treated with atropine exhibited significantly lower 2-AG levels in jejunum, compared with food-deprived vehicle-treated rats (Fig. 3B; P < 0.001).
To localize the effects of peripheral mAchR antagonism and characterize the mAchR subtype involved in this response, we utilized a combination of surgical and pharmacological approaches. We first confirmed the presence of mRNA for CB1, m3, and m1 receptors in jejunum mucosa using RT-PCR (Fig. 3C, cerebellum was used as a positive control). Intraduodenal administration of a low dose (300 nmol) of the selective m3 antagonist, DAU 5884, decreased jejunal 2-AG levels in 24-h food-deprived rats (Fig. 3E; P < 0.001; n = 5), whereas, the selective m1 antagonist pirenzepine (300 nmol), had no such effect (Fig. 3E; n = 5). Collectively, the findings suggest that food deprivation triggers 2-AG production in the upper gut by stimulating cholinergic activation of m3 receptors.
M3 Receptors Mediate Orexigenic 2-AG Signaling in the Gut
To investigate the role of local m3 mAchRs in feeding, we infused low doses of DAU5884 directly into the duodenum of 24-h food-deprived animals and monitored 1-h intake upon refeeding. Local treatment with 100 nmol of DAU5884 did not significantly affect intake compared with vehicle (Fig. 4; n = 5 or 10); however, increasing the dose to 300 nmol, the same dose that decreased jejunal 2-AG levels in fasting animals (Fig. 3C), inhibited refeeding (Fig. 4; P < 0.01) to a similar level found after blocking peripheral CB1Rs with AM6545 (Fig. 4, P < 0.001; ID DAU5884 300 nmol vs. IP AM6545, not significant). To test whether the feeding inhibitory effects of blocking small intestinal m3 and CB1Rs are mediated through the same mechanisms (i.e., activation of small intestinal m3 mAchRs and subsequent production of 2-AG, which signals at local CB1Rs and drives feeding), we coadministered DAU5884 along with AM6545 before refeeding. Simultaneous inhibition of small intestinal m3 mAchRs and CB1Rs failed to produce any discernable additive effects compared with each treatment individually (Fig. 4; not significant). We interpret the results to suggest that cholinergic neurotransmission at m3 muscarinic receptors in the proximal gut controls local 2-AG signaling at CB1Rs, which then promotes refeeding in fasted animals.
Fig. 4.
CB1 and m3 mAch receptors control deprivation-induced feeding. Effects of pharmacological blockade of peripheral cannabinoid CB1 receptors with AM6545 and local small intestinal m3 muscarinic acetylcholine receptors with DAU5884 on 1-h refeeding intakes after a 24-h fast. (Veh, 1 ml id of saline and 0.5 ml/kg ip DMSO; ID DAU 100, intraduodenal DAU5884, 100 nmol; ID DAU 300, DAU5884, 300 nmol; IP AM6545, intraperitoneal AM6545; DAU 100+AM, intraduodenal DAU5884, 100 nmol and intraperitoneal AM6545; DAU 300+AM, intraduodenal DAU5884 300 nmol and intraperitoneal AM6545). One-way ANOVA, Newman-Keuls multiple-comparison test. **P < 0.01, ***P < 0.001; n = 5/10 per condition. Results are expressed as means ± SE.
DISCUSSION
This study describes four new findings that advance our understanding of gut-brain physiology and hunger signaling: first, feeding status controls 2-AG signaling in the jejunum mucosa; second, fasting and refeeding regulate 2-AG biosynthesis in the jejunum through the DGL pathway; third, cholinergic neurotransmission—possibly carried by the efferent vagus nerve—controls 2-AG biosynthesis in the jejunum; fourth, m3 mAchRs in the jejunum might control 2-AG-mediated orexigenic signaling at local CB1Rs (Fig. 5).
Fig. 5.

Cholinergic control of endocannabinoid signaling in the gut. The efferent vagus nerve is thought to release acetylcholine onto m3 muscarinic acetylcholine receptors in the rat jejunum. In turn, during a fast, the activation of m3 muscarinic acetylcholine receptors leads to increases in the production of 2-AG through the diacylglycerol lipase pathway. 2-AG activates local cannabinoid CB1 receptors and drives feeding, and thus, is suggested to be a peripheral hunger signal. SI, small intestine.
Endocannabinoid signaling in the upper small intestine promotes food intake (12). We previously reported that tasting dietary fats, but not proteins or carbohydrates, initiates production of the endocannabinoids, 2-AG, and anandamide, in the jejunum of sham-feeding rats (10). Oral exposure to monounsaturated or diunsaturated free fatty acids (i.e., oleic acid and linoleic acid, respectively) increases jejunal endocannabinoid levels, which, in turn, activate local CB1Rs and drive further fat intake (10, 11). Indeed, intraduodenal administration of the CB1R antagonist rimonabant or intraperitoneal administration of the peripherally restricted CB1R antagonists URB447 (26) and AM6545 (7) inhibited sham feeding of fat (10, 11). Importantly, surgical disruption of the vagus nerve prevented increases in endocannabinoid levels during sham feeding of fat, thereby suggesting that cholinergic neurotransmission controls the production of endocannabinoids in the jejunum, which, in turn, generates positive feedback to the brain to drive the intake of a fatty meal.
In the present report, we extend those findings and describe a broader role for gut-brain endocannabinoid signaling in the control of feeding behavior. We focused on delineating the specific biochemical, molecular, and neural pathways responsible for 2-AG biosynthesis in the gut, which diverge from those associated with anandamide (10, 31). Prior studies have found that fasting increases levels of anandamide (16, 20) and 2-AG (20) in the rat duodenum, which suggests that endocannabinoids in the small intestine promote food intake. We now show that 2-AG levels in rat jejunum mucosa are elevated in proportion to the time since the onset of fasting, reaching levels that are twofold higher than free-feeding controls after a 24-h fast. Furthermore, refeeding lowers 2-AG to baseline levels within 15 min. This biochemical event is highly organ-specific, because 24 h of food deprivation do not affect 2-AG mobilization in other organs and tissues, and is specific to 2-AG because levels of other MAG species are not affected by fasting. Furthermore, intraduodenal administration of fat, carbohydrate, or protein in fasted rats suppresses fast-induced 2-AG mobilization equally well, which suggests that food entering the small intestine terminates local 2-AG signaling, irrespective of macronutrient content. We next asked whether fasting causes the production of 2-AG in the jejunum mucosa by increasing levels of its precursor, SAG. Parallel to 2-AG, SAG levels increase temporally from the start of fasting, and return to baseline levels within 15 min after refeeding. In the brain, 2-AG is formed following the hydrolysis of SAG by DGL (21). Similar to the brain, we found that inhibiting DGL activity in the small intestine with THL blocks the production of 2-AG in the jejunum under all feeding conditions, without affecting levels of SAG. These results suggest that the DGL pathway is critical for the production of 2-AG in the jejunum of fasted rats and is the primary source of 2-AG biosynthesis.
We previously reported that surgical disruption of the vagus nerve in rats blocks increases in jejunal endocannabinoid levels that are normally found after tasting a fatty meal (10), which suggests that efferent vagal cholinergic neurotransmission controls endocannabinoid biosynthesis in the jejunum. The vagus nerve contains separate fibers that communicate information bidirectionally between the brain stem and gut. The efferent arm of the vagus, with preganglionic cell bodies located in the DMV in the medulla, is thought to serve several functions associated with feeding and energy balance, including setting the gain for afferent vagal neurotransmission and the regulation of intestinal motility and resulting rate of nutrient absorption by enteroendocrine cells (5). In contrast to the efferent vagus, the afferent limb—or sensory vagus—carries neurotransmission from the gut to the nucleus of the solitary tract (NST) in the caudal brain stem regarding satiation/satiety [e.g., cholecystokinin (43)] and hunger signaling [e.g., ghrelin (8)]. The NST dynamically integrates gustatory, autonomic, and circulating energy status signals (e.g., leptin), and reciprocally communicates with forebrain structures (18). We now show that in animals subjected to surgical transection of the vagus nerve below the diaphragm, deprivation-induced increases in jejunal 2-AG levels are absent. One interpretation of the results is that the efferent vagus controls 2-AG biosynthesis in the gut during food deprivation. Accordingly, intraduodenal administration of the selective m3 mAchR antagonist DAU5884 inhibited 2-AG production in fasting rats; however, equimolar concentrations of the selective m1 mAchR antagonist pirenzepine failed to elicit the same response. These findings are congruent with studies showing that m3 mAchRs facilitate synaptic plasticity in the mouse brain through an endocannabinoid-dependent mechanism (38, 55). Collectively, the results suggest that cholinergic neurotransmission—possibly carried by the efferent vagus—activates m3 mAchRs in the jejunum mucosa, which, in turn, drives 2-AG biosynthesis through the DGL pathway. We cannot rule out the possibility, however, that the activation of jejunal m3 mAchRs and production of 2-AG occur via an alternate mechanism to that of the vagus nerve, including enteric signaling and changes in peristalsis.
Early studies support the hypothesis that a peripheral mechanism contributes to feeding and is driven by cholinergic signals carried by the efferent vagus nerve. In these studies, inhibiting peripheral cholinergic signaling at mAchRs with atropine nitrate (a brain-impenetrant derivative of atropine) attenuated refeeding in fasted animals (33) and blocked sham feeding of liquid nutrients in rats, but not the sham intake of water (25). Furthermore, vagal stimulation induces c-Fos expression in myenteric and submucosal plexus of rat proximal small intestine (6, 56), which suggests that the efferent vagus may activate peripheral signaling pathways to regulate feeding. Several groups have reported roles for peripheral CB1Rs in mediating feeding (4, 7, 10, 16, 26, 37, 49, 50). To investigate whether efferent vagal cholinergic neurotransmission at m3 mAchRs in the jejunum drives feeding in fasted rats through an endocannabinoid-mediated mechanism, we administered into the duodenum the m3 mAchR antagonist DAU5884 and evaluated food intake after a 24-h fast. DAU5884—at a dose that inhibited fasting-induced increases in 2-AG levels in jejunum—decreased 1-h refeeding in fasted rats by the same magnitude as inhibiting peripheral CB1Rs with AM6545. In congruence with the hypothesis that m3 mAchRs contribute to feeding, the Wess group (54) reported that mice lacking m3 mAchRs are hypophagic. Together, the data suggest that efferent vagal cholinergic activity at m3 mAchRs in the jejunum drives 2-AG signaling at local CB1Rs and promotes feeding in hungry rats. Further supporting this hypothesis, coadministration of AM6545 or DAU5884, at exact doses that each readily reduced intakes, failed to decrease intakes further than when either drug was administered alone. A lack of additive effects on inhibiting refeeding after a 24-h fast for administration of both compounds strongly supports a signaling event in the jejunum that is mediated through common cholinergic and endocannabinoid pathways. Further studies are required to identify the precise physiological role for this signaling; however, the results do suggest that 2-AG in the gut controls refeeding after a fast and may serve as a hunger signal. It is noteworthy that anticholinergics are reported to induce conditioned taste aversions in rodents (39), which is a potential confound for using these agents as tools to investigate food intake. Therefore, despite the low doses used for intraduodenal administration of DAU5884 during our feeding studies (Fig. 4), we cannot completely rule out potential conditioned taste aversions to this compound. This is not likely under our conditions, however, because administration of DAU5884 in combination with AM6545 reduced intakes to the same degree as AM6545 treatment alone, which is reported not to elicit any discernable taste aversions in rats (7).
The specific molecular and neural pathways by which jejunal CB1Rs signal to the brain to drive feeding remain to be determined. Evidence suggests that the endocannabinoids might not directly interact with CB1Rs on afferent vagus because mice null for CB1Rs selectively on vagal fibers showed no changes in body weight (51). However, 2-AG might indirectly interact with the vagus nerve through an intermediate messenger, such as CCK. It is plausible that endocannabinoids might inhibit CCK release from the intestine, which would functionally delay meal termination. Indeed, enteroendocrine I cells—which produce and secrete CCK—express mRNA for CB1Rs (48), and inhibiting mAchRs with atropine enhances the release of CCK in response to feeding in rats (29). Furthermore, similar to 2-AG in the intestine, circulating levels of ghrelin increase in proportion to time since the last meal in humans and rodents (2, 23). Importantly, gastric cells that produce and secrete ghrelin contain CB1Rs, and inhibiting CB1Rs with rimonabant reduces ghrelin release in rats, but only after food deprivation (41).
Reports differ on the effectiveness of vagotomy alone for weight reduction in rodents and humans (19, 30, 42, 52). Consequently, further studies are critical to determine roles for specific branches of the vagus in the control of body weight, and potential differences between rodents and humans in this response. Nonetheless, humans that underwent laproscopic gastric banding in combination with truncal vagotomy reported an absence of subjective feelings of hunger despite no changes in body weight gain compared with subjects with gastric band alone (27). This result suggests that vagotomy may reduce the activity of hunger-promoting pathways in humans through a mechanism that includes decreased endocannabinoid signaling in the gut; however, a test of this hypothesis remains to be run. Furthermore, potential caveats in interpretation of the results for vagotomy experiments in this article should be noted given the known disruptions in gastric emptying, food intake, and body weight that occur following full subdiaphragmatic vagotomy in rodents.
Perspectives and Significance
The results presented here reveal that, similar to tasting dietary fats (10, 11), fasting stimulates the production of 2-AG in the jejunum mucosa and drives feeding. This suggests that 2-AG-mediated endocannabinoid signaling in the gut may be a general hunger signal that is initiated under several behavioral and metabolic conditions. Importantly, our findings also imply that cholinergic neurotransmission—possibly controlled by the efferent vagus nerve—participates in the control of feeding through a mechanism that requires activation of m3 mAchRs in the gut, local 2-AG mobilization, and 2-AG-dependent activation of CB1Rs. Thus, endocannabinoid signaling in the gut may have been selected by evolution due to its prosurvival properties during times of famine, which may in turn, promote overeating and obesity in modern times of abundance (13). As such, the present investigation advances our understanding of gut-brain endocannabinoid signaling and suggests potential new pharmacological avenues for appetite control.
GRANTS
The authors gratefully acknowledge support from the U.S. National Institute on Drug Abuse Grant K99/R00 DA-034009 to N. V. DiPatrizio and from the U.S. National Institute of Diabetes and Digestive and Kidney Disorders Grant DK-073955 to D. Piomelli.
DISCLOSURES
The authors report the following conflicts of interest. D. Piomelli is a cofounder of Thesan Pharmaceuticals, a biopharmaceutical company dedicated to the development of innovative therapies for skin diseases. D. Piomelli owns equity in the company and his laboratory received research support unrelated to the work presented here.
AUTHOR CONTRIBUTIONS
Author contributions: N.V.D., M.I., K.-M.J., and D.P. conception and design of research; N.V.D., M.I., V.N., C.M., J.G., and A.R. performed experiments; N.V.D., M.I., K.-M.J., and D.P. analyzed data; N.V.D., M.I., K.-M.J., and D.P. interpreted results of experiments; N.V.D. and M.I. prepared figures; N.V.D. and D.P. drafted manuscript; N.V.D. and D.P. edited and revised manuscript; N.V.D., M.I., V.N., C.M., J.G., A.R., K.-M.J., and D.P. approved final version of manuscript.
REFERENCES
- 1.Aaltonen N, Riera Ribas C, Lehtonen M, Savinainen JR, Laitinen JT. Brain regional cannabinoid CB1 receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase-deficient mice. Eur J Pharm Sci 51: 87–95, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2755–2767, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, Cannich A, Delamarre A, Haring M, Martin-Fontecha M, Vega D, Leste-Lasserre T, Bartsch D, Monory K, Lutz B, Chaouloff F, Pagotto U, Guzman M, Cota D, Marsicano G. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc Natl Acad Sci USA 110: 4786–4791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec 262: 29–40, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161: 629–642, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120–1128, 2002. [DOI] [PubMed] [Google Scholar]
- 9.DiPatrizio NV. Is fat taste ready for primetime? Physiol Behav 136: 145–154, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA 108: 12,904–12,908, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPatrizio NV, Joslin A, Jung KM, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J 27: 2513–2520, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPatrizio NV, Piomelli D. Intestinal lipid-derived signals that sense dietary fat. J Clin Invest 125: 1–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci 35: 403–411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem 282: 1518–1528, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem 280: 87–93, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22: 9612–9617, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregg LC, Jung KM, Spradley JM, Nyilas R, Suplita RL 2nd Zimmer A, Watanabe M, Mackie K, Katona I, Piomelli D, Hohmann AG. Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-alpha initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci 32: 9457–9468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg 24: 2145–2151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol 158: 451–461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol 72: 612–621, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun 3: 1080, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 288: R716–R722, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10,182–10,191, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenz D, Nardi P, Smith GP. Atropine methyl nitrate inhibits sham feeding in the rat. Pharmacol Biochem Behav 8: 405–407, 1978. [DOI] [PubMed] [Google Scholar]
- 26.LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Stella N, Xu C, Tarzia G, Piomelli D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett 19: 639–643, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin MB, Earle KR. Laparoscopic adjustable gastric banding with truncal vagotomy: any increased weight loss? Surg Endosc 25: 2522–2525, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci 24: 10,707–10,715, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano I, Tawil T, Spannagel AW, Liddle RA, Green GM. Atropine enhances food-stimulated CCK secretion in the rat. Pancreas 5: 621–625, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH, Linden BC, Lautz DB. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes Res Clin Pract 9: 274–280, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Piomelli D. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 76 Pt B: 228–234, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci 8: 743–754, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Pradhan SN, Roth T. Comparative behavioral effects of several anticholinergic agents in rats. Psychopharmacologia 12: 358–366, 1968. [DOI] [PubMed] [Google Scholar]
- 34.Przyborski SA, Levin RJ. Cholinergic modulation of electrogenic ion transport in different regions of the rat small intestine. J Pharm Pharmacol 49: 691–697, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Przyborski SA, Levin RJ. Enterocytes on rat jejunal villi but not in the crypts posses m3 mRNA for the M3 muscarinic receptor localized by in situ hybridization. Exp Physiol 78: 109–112, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, Watanabe M, Katona I, Patel S. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron 81: 1111–1125, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, Salamone JD. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav 97: 179–184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldo L, Hansel C. Muscarinic acetylcholine receptor activation blocks long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses via cannabinoid signaling. Proc Natl Acad Sci USA 110: 11,181–11,186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano JA, King JM. Conditioned taste aversion and cholinergic drugs: pharmacological antagonism. Pharmacol Biochem Behav 27: 81–85, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol Regul Integr Comp Physiol 264: R630–R637, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Senin LL, Al-Massadi O, Folguiera C, Pardo M, Barja-Fernandez S, Roca-Rivada A, Amil M, Criujeiras AB, Garcia-Caballero T, Gabellieri E, Leis R, Dieguez C, Pagotto U, Casanueva FF, Seoane LM. The gasric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One 8: e80339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shikora S, Toouli J, Herrera MF, Kulseng B, Zulewski H, Brancatisano R, Kow L, Pantoja JP, Johnsen G, Brancatisano A, Tweden KS, Knudson MB, Billington CJ. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes 2013: 245683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213: 1036–1037, 1981. [DOI] [PubMed] [Google Scholar]
- 44.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 612–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadelmann AM, Walgenbach-Telford S, Telford GL, Koch TR. Distribution of muscarinic receptor subtypes in rat small intestine. J Surg Res 80: 320–325, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773–778, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol 578: 773–785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One 7: e42373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF, Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16: 167–179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vianna CR, Donato J Jr, Rossi J, Scott M, Economides K, Gautron L, Pierpont S, Elias CF, Elmquist JK. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J Neurosci 32: 10,331–10,337, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Liu J. Combination of bypassing stomach and vagus dissection in high-fat diet-induced obese rats-a long-term investigation. Obes Surg 20: 375–379, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15: 1350–1355, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410: 207–212, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci 31: 3158–3168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng H, Berthoud HR. Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am J Physiol Gastrointest Liver Physiol 279: G73–G81, 2000. [DOI] [PubMed] [Google Scholar]