Abstract
Intrauterine growth-restricted (IUGR) fetal sheep, produced by placental insufficiency, have lower oxygen concentrations, higher lactate concentrations, and increased hepatic glucose production that is resistant to suppression by insulin. We hypothesized that increased lactate production in the IUGR fetus results from reduced glucose oxidation, during basal and maximal insulin-stimulated conditions, and is used to support glucose production. To test this, studies were performed in late-gestation control (CON) and IUGR fetal sheep under basal and hyperinsulinemic-clamp conditions. The basal glucose oxidation rate was similar and increased by 30–40% during insulin clamp in CON and IUGR fetuses (P < 0.005). However, the fraction of glucose oxidized was 15% lower in IUGR fetuses during basal and insulin-clamp periods (P = 0.05). IUGR fetuses also had four-fold higher lactate concentrations (P < 0.001) and lower lactate uptake rates (P < 0.05). In IUGR fetal muscle and liver, mRNA expression of pyruvate dehydrogenase kinase (PDK4), an inhibitor of glucose oxidation, was increased over fourfold. In IUGR fetal liver, but not skeletal muscle, mRNA expression of lactate dehydrogenase A (LDHA) was increased nearly fivefold. Hepatic expression of the gluconeogenic genes, phosphoenolpyruvate carboxykinase (PCK)1, and PCK2, was correlated with expression of PDK4 and LDHA. Collectively, these in vivo and tissue data support limited capacity for glucose oxidation in the IUGR fetus via increased PDK4 in skeletal muscle and liver. We speculate that lactate production also is increased, which may supply carbon for glucose production in the IUGR fetal liver.
Keywords: fetal, glucose oxidation, glucose production, liver, muscle
the intrauterine growth-restricted (IUGR) fetus produced by placental insufficiency is exposed to a compromised intrauterine environment. IUGR fetuses receive lower supplies of glucose, amino acids, and oxygen from the placenta (2, 32). In response to reduced nutrient supply, the IUGR fetal liver starts producing glucose (17, 33), and this glucose production rate is not suppressed by insulin (32). Despite the evidence for impaired suppression of glucose production by insulin, as well as reduced glucose and insulin concentrations compared with normal fetal sheep, IUGR fetal sheep have normal basal weight specific rates of glucose utilization (4, 17, 32, 38). IUGR fetuses also have a robust dose-dependent increase in glucose utilization in response to a hyperinsulinemic-euglycemic clamp, supporting insulin sensitivity for glucose disposal (32). However, the metabolic fate of the glucose utilized by the IUGR fetus and the substrates used to fuel hepatic glucose production are not known.
Glucose and lactate are major fuels for the ovine fetus. Their oxidation accounts for ∼50% of fetal oxygen consumption, under normal conditions in the fetal sheep (9). Normal fetal sheep also have relatively constant rates of oxygen consumption and, thus, fixed rates of oxidative metabolism, even during experimental increases in insulin or nutrient supply (1, 8, 9). Interestingly, IUGR fetuses, in our model of placental insufficiency, have relatively normal weight-specific oxygen utilization rates, despite lower oxygen concentrations (2, 32). In addition, IUGR fetuses studied under basal conditions oxidize a smaller fraction of the total glucose utilized and have a lower glucose oxidation rate (17). Furthermore, IUGR fetuses have increased lactate concentrations and decreased lactate uptake from the placenta, suggesting increased fetal lactate production (32). Therefore, we hypothesized that the IUGR fetus develops a limited capacity for glucose oxidation that results in increased lactate production (34, 42). Since the IUGR fetus also has increased hepatic glucose production, this raises the possibility that limited glucose oxidation and increased lactate production may be mechanisms to provide carbon sources for hepatic glucose production.
To test this hypothesis, we measured glucose oxidation rates in vivo and molecular pathways for glucose and lactate metabolism in skeletal muscle and liver tissues from normal and IUGR fetal sheep. We used our sheep model of placental insufficiency IUGR, which is characterized by decreased placental size, decreased blood flow, and decreased nutrient delivery to the fetus (6, 27, 36). Fetuses were studied under basal and hyperinsulinemic clamp conditions to test basal and maximal capacity for insulin-stimulated glucose oxidation. These two study conditions also allowed us to test whether increased lactate production would be active both basally and during maximal stimulation with insulin because glucose production is active during both of these conditions in the IUGR fetus (32). We also measured in vivo glucose, lactate, and oxygen concentrations and uptake rates in normal and IUGR fetal sheep. In skeletal muscle and liver tissue, we measured glycogen content and expression of genes in relevant metabolic pathways. This included genes for glucose uptake and glycolysis: insulin receptor (IRA), and IRB, glucose transporter (GLUT1), GLUT2, phosphofructokinase 1 (PFK1), and pyruvate kinase muscle isoform (PKM) and PK liver red cell isoform (PKLR); genes for pyruvate oxidation: pyruvate dehydrogenase (PDH) and PDH kinase 4 (PDK4), and lactate and pyruvate metabolism: pyruvate carboxylase (PC), lactate dehydrogenase (LDH) A and LDHB. The gluconeogenic genes, phosphoenolpyruvate carboxykinase cytosol form (PCK1) and mitochondrial form (PCK2), also were measured in the liver. Our results demonstrate that IUGR fetuses oxidize a lower fraction of the total glucose utilized under basal and insulin-stimulated conditions, despite normal basal and insulin-stimulated rates of glucose utilization. In muscle and liver tissues, our molecular data support decreased glucose oxidation via increased PDK4 mRNA expression. We also find tissue-specific adaptations in the IUGR fetus that indicate increased lactate production in liver and increased glucose-derived pyruvate in muscle. We speculate that limited glucose oxidation and increased lactate production in the liver may supply carbon for hepatic glucose production in the IUGR fetus.
MATERIALS AND METHODS
Fetal Sheep Model of IUGR
Pregnant Columbia-Rambouillet ewes were studied under regulatory compliance at the University of Colorado School of Medicine Perinatal Research Center (Aurora, CO). IUGR fetuses were created by exposing pregnant ewes to elevated humidity and temperature (40°C for 12 h, 35°C for 12 h) from ∼37 days gestation age (dGA; term = ∼147 dGA) to ∼116 dGA in an environmentally controlled room, as previously described (32). Control fetuses (CON) were from pregnant ewes exposed to normal humidity and temperatures daily (25°C) in an environmentally controlled room and pair-fed to the intake of the IUGR ewes. After treatment, ewes were exposed to normal humidity and temperatures until time of study. Fetuses were surgically instrumented with indwelling catheters placed in the umbilical vein, descending aorta, and femoral veins at ∼125 dGA using the procedures described elsewhere (11, 17). Maternal catheters were placed in the femoral artery and vein. All ewes were carrying singleton pregnancies, except for 2 in CON and 1 in IUGR groups carrying twins. In twin pregnancies, only one fetus per pregnancy was catheterized and studied, and the other fetus was used for collection of basal tissue samples and included in the basal tissue group described below.
Fetal Hyperinsulinemic Clamp Study
A fetal hyperinsulinemic-isoglycemic clamp (insulin clamp) or saline infusion was performed at ∼132 dGA in CON and IUGR fetuses, as described previously (2, 32). In brief, each fetus was studied during a basal period and again during an insulin-clamp or saline infusion period. 3H2O tracer was infused to measure umbilical blood flow across both study periods. For the basal study period, four steady-state blood samples, ∼10–15 min apart, were drawn simultaneously from the umbilical vein and fetal artery. Next, either a saline-only (n = 8; CON and 9 IUGR) infusion or insulin clamp (n = 9 CON and 11 IUGR) was performed (3 mU·min−1·kg−1 of insulin), and variable infusion of glucose and a balanced amino acid solution (Trophamine) was used, as previously described (2, 32). After starting the insulin infusion, fetal arterial plasma glucose and branched chain amino acid (BCAA) concentrations were monitored every 10–15 min, and infusion rates were adjusted to match each fetus's own mean arterial glucose and BCAA concentration observed during the basal period to maintain glucose and amino acid concentrations in response to insulin. There were three CON and two IUGR fetuses that did not receive Trophamine during the insulin clamp but were included in all analyses, as no differences were found for variables of interest (32). After physiological and isotopic steady state was reached at ∼160 min, another set of four blood samples were drawn at 10- to 15-min intervals to characterize the insulin-clamp study period. Fetal blood was replaced isovolumetrically with heparinized maternal blood during both draw periods (40 ml per period). A total of 37 fetuses were studied.
Glucose Metabolism and Oxidation Studies
In a subset of CON (n = 4) and IUGR (n = 8) fetuses, U-[14C]-glucose tracer was infused to measure glucose metabolism during the basal and insulin-clamp study periods (32). Fetal blood concentration of radioactive glucose was measured (11, 32, 37). Labeled 14CO2 was measured as described previously (9).
Analysis of Blood Samples
Measurements were performed in all umbilical venous and arterial samples (four per period), unless indicated, and mean values were calculated per period. Blood samples were analyzed for oxygen and CO2 content using a blood gas analyzer. Plasma samples were analyzed for glucose and lactate using Yellow Springs Instrument 2700 and BCAA concentrations with spectrophotometry (1, 17, 33). Plasma arterial insulin, norepinephrine, and cortisol were measured (17, 18, 20), as well as plasma 3H2O (2, 32).
Calculations
Umbilical plasma and blood flow was determined by steady-state diffusion using 3H2O tracer (23, 37). Umbilical (net fetal) oxygen, glucose, and lactate uptake rates were calculated on the basis of the Fick principle (2, 32). In fetuses receiving glucose tracer, the fetal glucose utilization rate was calculated as described previously (10, 11, 17, 32). Total fetal glucose entry rate (GER) equals the sum of umbilical glucose uptake and glucose infusion rate (in fetuses with insulin clamp). Fetal glucose oxidation rate was calculated as glucose oxidation fraction multiplied by glucose utilization rate (9). The rate of oxygen uptake used for glucose oxidation is calculated as six times the glucose oxidation rate (9). The fraction of oxygen used for glucose oxidation is the rate of oxygen used for glucose oxidation divided by oxygen uptake rate. All results were normalized to fetal weight determined at necropsy.
Liver and Muscle Tissue Collection
After completion of blood sampling, under saline-only or insulin-clamped conditions (∼3.5 h after starting insulin), the ewe and fetus were euthanized. Fetuses and placentomes were weighed, and samples of liver and skeletal muscle (biceps femoris) were collected immediately and snap frozen in liquid nitrogen.
Gene Expression Analysis
RNA was extracted from liver and muscle tissue, reverse transcribed, and used in real-time PCR with relative standard curve quantification as described previously (32, 33). Assays for IRA, IRB, GLUT2, PFK1, PKLR, and PC were used, as previously reported (33, 35). Results were normalized to 18S rRNA expression (32, 33). Primers were developed for real-time PCR assays for the following genes: GLUT1 (F, 5′-TGGGAGGCATGATTGGTTCC-3′, R, 5′-TGAGAAGCCCATGAGCACAG-3′), GLUT4 (F, 5′-AGCAGCTGTCAGGCATCAAT-3′, R, 5′-CCGATGGTAGCATAGGCTGG-3′), PKM (F, 5′-ACCACGCAGAGACCATCAAG-3′, R, 5′-GGTCCTTTAGTGTCCAGGGC-3′), PDH (F, 5′-GTTAAGGGGGCTGCTAGGTG-3′, R, 5′-AGCCACTGCGTACTGTGAAA-3′), PDK4 (F, 5′-CCCAGAGGACCAAAAGGCAT-3′, R, 5′-GGGTCAGCTGTACAGGCATC-3′), LDHA (F, 5′-CATGGCCTGTGCCATCAGTA-3′, R, 5′-GGAAAAGGCTGCCATGTTGG-3′), LDHB (F, 5′-GAGGGAGCGATCCCAAACAA-3′, R, 5′-CAGAATGCTGATGGCACACG-3′), and PCK2 (F, 5′-GCCTGTGCTTCAGGCCCTGG-3′, R, 5′-TGCATGGCCACTGGCACACC-3′).
Glycogen Measurements
Tissue glycogen concentrations were determined in liver and skeletal muscle samples, as described previously (17).
Statistical Analysis
Data were analyzed by mixed model two-way ANOVA with fixed effects of IUGR (CON, IUGR), insulin (BASAL, INSULIN), and the interaction using SAS (V9.4, PROC MIXED). For glucose oxidation data, measurements were obtained from the same fetus for its basal and insulin-clamp period, and thus, a random effect for fetus to account for repeated measures was added into the ANOVA. When data were analyzed to include fetal sex as a variable in our ANOVA, we did not find significant differences and sex was, therefore, not included in the model. When the interaction was P < 0.15, individual post hoc test comparisons were made using PDIFF statement in SAS, and post hoc test differences are indicated when significant (P < 0.05). Correlation analyses were performed using log-transformed data in SAS (PROC CORR). Statistical significance was declared at P < 0.05.
RESULTS
Characteristics of CON and IUGR During Basal and Insulin-Clamp Study Periods
IUGR fetuses weighed 40% less than CON fetuses (Table 1). Placentome number was less, and placentome weight was ∼50% lower in the IUGR group compared with the CON group. Glucose concentrations were 30% lower in IUGR compared with CON fetuses during basal and insulin-clamp periods. Insulin concentrations were 55% lower in IUGR fetuses during the basal period (P < 0.05, IUGR vs. CON, basal period) and were increased to supraphysiological concentrations that were comparable in both groups during the insulin clamp. IUGR fetuses also had higher plasma concentrations of cortisol and norepinephrine (Table 1). Umbilical plasma flow rates, adjusted for fetal weight, were lower in IUGR fetuses. The rate of glucose uptake by the fetus during the basal period, representing uptake from the placenta, was not significantly lower in IUGR compared with CON fetuses. During the insulin clamp, the total glucose delivery rate, representing the sum of uptake from the placenta and exogenous glucose infusion, was increased nearly twofold in CON and IUGR fetuses (Table 1).
Table 1.
Fetal characteristics during metabolic studies following basal or insulin-clamp infusion periods
| CON |
IUGR |
ANOVA* |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin-Clamp | Basal | Insulin-Clamp | IUGR | Insulin | IxI | |
| n | 8 | 9 | 9 | 11 | |||
| Percent male fetuses, % | 43 | 67 | 28 | 55 | |||
| Age, days | 130.6 ± 0.7 | 133.0 ± 0.5 | 131.9 ± 0.8 | 132.9 ± 0.5 | 0.38 | <0.01 | 0.31 |
| Placentome weight, g | 428 ± 44 | 445 ± 48 | 181 ± 37 | 208 ± 30 | <0.001 | 0.59 | 0.91 |
| Placentomes, number | 76.5 ± 3.8 | 74.3 ± 5.2 | 62.7 ± 7.3 | 66.0 ± 3.9 | <0.05 | 0.92 | 0.58 |
| Weight, kg | 3.2 ± 0.1 | 3.5 ± 0.2 | 1.7 ± 0.2 | 2.0 ± 0.2 | <0.001 | 0.09 | 0.97 |
| Fetal arterial concentrations | |||||||
| Glucose, mM | 1.21 ± 0.16a | 0.96 ± 0.08a,c | 0.66 ± 0.08b | 0.82 ± 0.07b,c | <0.001 | 0.64 | <0.05** |
| Insulin, ng/ml | 0.27 ± 0.07 | 15.98 ± 2.79 | 0.12 ± 0.01 | 18.86 ± 3.91 | 0.68 | <0.001 | 0.65 |
| Oxygen content, mM | 3.0 ± 0.1 | 2.7 ± 0.2 | 1.3 ± 0.3 | 0.9 ± 0.1 | <0.001 | 0.12 | 0.96 |
| Lactate, mM | 1.8 ± 0.1 | 2.0 ± 0.3 | 7.2 ± 2.3 | 10.2 ± 1.9 | <0.001 | 0.38 | 0.43 |
| Cortisol, ng/ml | 14.2 ± 2.8 | 11.6 ± 3.8 | 29.6 ± 10.5 | 37.6 ± 7.4 | <0.05 | 0.74 | 0.52 |
| Norepinephrine, pg/ml | 325 ± 18 | 632 ± 115 | 1664 ± 170 | 1740 ± 212 | <0.001 | 0.43 | 0.64 |
| Umbilical plasma flow rates | |||||||
| Umbilical flow, pl, ml/min | 454.0 ± 6.1 | 375.0 ± 23.6 | 138.2 ± 23.2 | 155.9 ± 17.7 | <0.001 | 0.22 | 0.06 |
| Umbilical flow, pl, ml·min−1·kg−1 | 133.0 ± 1.5 | 110.5 ± 5.7 | 86.5 ± 10.8 | 79.0 ± 9.1 | <0.001 | 0.15 | 0.47 |
| Fetal nutrient uptake rates | |||||||
| Glucose, total entry, μmol·min−1·kg−1 | 28.0 ± 1.4 | 48.6 ± 2.8 | 22.6 ± 3.5 | 49.5 ± 4.9 | 0.64 | <0.001 | 0.52 |
| Lactate uptake, μmol·min−1·kg−1 | 21.8 ± 1.9 | 17.4 ± 1.7 | 9.6 ± 6.1 | 1.2 ± 4.1 | <0.05 | 0.26 | 0.71 |
Values are expressed as means ± SE.
CON, control. IUGR, intrauterine growth restricted.
Statistical significance is indicated by P value from 2 × 2 ANOVA.
Significant post hoc test group differences are indicated by letters that differ (a, b, c).
Arterial oxygen content was ∼60% lower in IUGR compared with CON fetuses (Table 1). Lactate concentrations were nearly fourfold higher in IUGR compared with CON fetuses during the basal and insulin-clamp periods (Table 1). The net fetal uptake rate of lactate from the placenta was ∼55% lower in IUGR compared with CON fetuses during the basal period. During the insulin-clamp period, net fetal lactate uptake rates decreased by ∼20% in CON fetuses. In the IUGR group, net fetal lactate uptake rates were decreased further with many fetuses becoming net producers of lactate, as evidenced by lactate output (i.e., negative uptake rates, Table 1).
Decreased Fractional Rate of Glucose Oxidation in IUGR Fetus
Metabolic studies with a glucose tracer were performed in a subset of CON and IUGR fetuses to measure glucose oxidation under basal and insulin-clamp periods (Table 2). Despite lower fetal arterial oxygenation (Table 1), CON and IUGR fetuses had similar weight-specific rates of oxygen consumption during basal and insulin-clamp study periods (Table 2).
Table 2.
Glucose oxidation in CON and IUGR fetuses during basal and insulin-clamp infusion periods
| CON |
IUGR |
ANOVAa |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin-Clamp | Basal | Insulin-Clamp | IUGR | Insulin | IxI | |
| Oxygen utilization, μmol·min−1·kg−1 | 332.3 ± 10.9 | 320.9 ± 23.7 | 308.8 ± 10.4 | 301.7 ± 12.8 | 0.27 | 0.40 | 0.84 |
| Fraction oxygen utilizaiton for glucose oxidation | 0.31 ± 0.02 | 0.42 ± 0.02 | 0.32 ± 0.01 | 0.46 ± 0.04 | 0.50 | <0.001 | 0.55 |
| Oxygen used for glucose oxidation, μmol·min−1·kg−1 | 104.6 ± 10.2 | 134.9 ± 7.2 | 99.0 ± 3.5 | 139.9 ± 11.9 | 0.97 | <0.005 | 0.54 |
Values are expressed as means ± SE.
CON, n = 4; IUGR, n = 8.
Statistical significance is indicated by P value from 2 × 2 ANOVA.
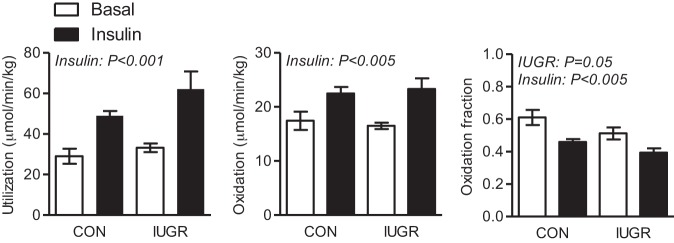
Fetal glucose utilization rates, adjusted for fetal weight, increased by ∼70% in CON fetuses and by nearly 90% in IUGR fetuses during the insulin-clamp period compared with basal period (Fig. 1) (32). The rate of glucose oxidation was similar between both groups during basal and insulin periods. In response to insulin, the glucose oxidation rate increased similarly by 40–50% during the insulin-clamp period in both CON and IUGR fetuses (Fig. 1). Despite similar rates of glucose oxidation, IUGR fetuses oxidized a lower fraction of the total glucose utilized compared with CON fetuses during basal and insulin-clamp periods. The glucose oxidation fraction decreased similarly during the insulin-clamp period in both CON and IUGR fetuses (Fig. 1). The fraction of net oxygen consumption used for glucose oxidation and rate of oxygen used for glucose oxidation was similar between CON and IUGR fetuses, yet increased by ∼40% during the insulin-clamp period (Table 2).
Fig. 1.
Glucose metabolic rates measured in late-gestation control (CON) and intrauterine growth-restricted (IUGR) fetal sheep during basal and hyperinsulinemic clamp (insulin) periods. Glucose utilization rate was measured with U-[14C]glucose tracer. Glucose oxidation rate was determined based on 14CO2 measurements. Glucose oxidation fraction represents the fraction of glucose utilized that was oxidized. Values expressed as means ± SE are shown for CON (n = 4) and IUGR (n = 8) fetuses studied during basal (white bars) and insulin-clamp periods (black bars). Significant effects from two-way ANOVA are indicated.
Coordinated Changes in Liver and Muscle Support Decreased Glucose Oxidation and Increased Lactate Production in the IUGR Fetus
Glucose uptake, glycolysis, and glycogen.
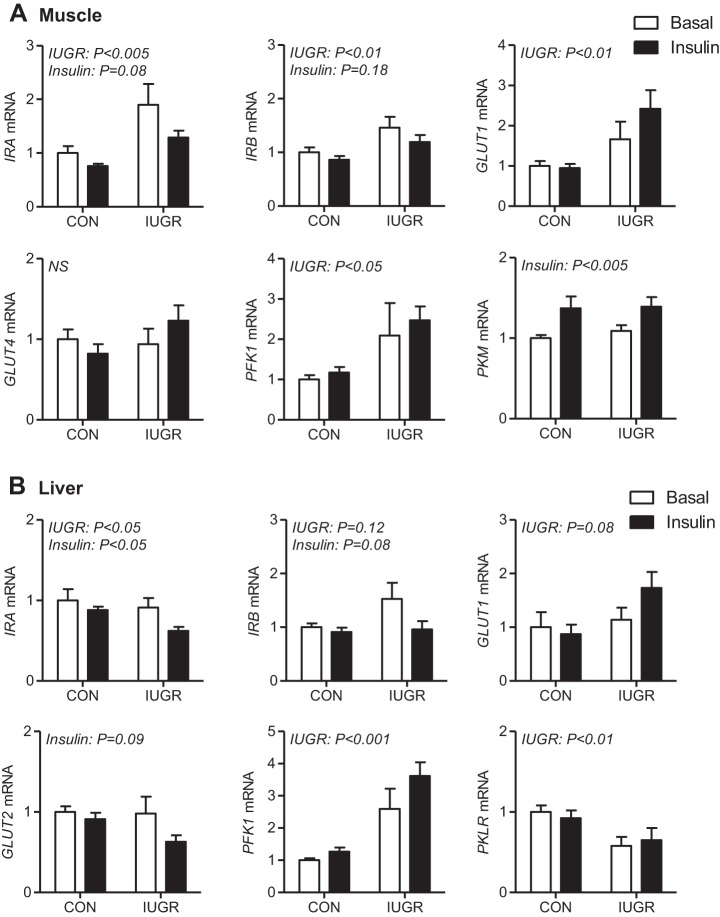
Genes involved in glucose uptake and glycolysis were measured in skeletal muscle and in the liver. In skeletal muscle, which has insulin-dependent glucose uptake, expression of insulin receptor genes (IRA and IRB) were increased ∼50% in IUGR compared with CON fetuses (Fig. 2A). Expression of the glucose transporter GLUT1 was twofold higher in IUGR compared with CON muscle and was not affected by insulin. No differences in muscle GLUT4 expression were found. Expression of PFK1, the rate-limiting enzyme in glycolysis, was increased twofold in IUGR compared with CON muscle. Expression of PKM was similar between CON and IUGR muscle and increased by ∼30% with insulin in both groups.
Fig. 2.
Expression of genes for glucose uptake and glycolysis in CON and IUGR fetal muscle and liver during basal and insulin-clamp periods. Gene expression was measured using real-time PCR in skeletal muscle for IRA, IRB, GLUT1, GLUT4, PFK1, and PKM (A) and in liver tissue samples for IRA, IRB, GLUT1, GLUT2, PFK1, and PKLR (B) from CON and IUGR fetuses under basal (white bars) and insulin-clamp (insulin, black bars) conditions. Values expressed as means ± SE are shown (n = 5–12 samples per group). Significant effects from two-way ANOVA are indicated.
In the liver, which has insulin-independent bidirectional glucose transport, expression of IRA was lower in IUGR compared with CON fetuses during the basal period and decreased during the insulin-clamp period in both groups (Fig. 2B). In contrast, IRB expression was relatively similar between groups. GLUT1 and GLUT2 expression was not different between CON and IUGR fetuses nor was it regulated by the insulin clamp. Expression of glycolytic gene PFK1 was increased twofold to threefold in IUGR compared with CON fetal livers. PKLR (pyruvate kinase liver red cell isoform) expression was ∼40% lower in IUGR fetal livers compared with CON. Expression of both hepatic PFK1 and PKLR was unchanged during the insulin clamp in CON and IUGR fetuses.
Glycogen concentrations were measured to determine whether more glucose is stored in the liver or muscle during basal or insulin-clamp conditions. Skeletal muscle glycogen concentrations were similar between CON and IUGR fetuses during basal period and insulin-clamp periods (Fig. 3A). Liver glycogen concentrations were also similar between CON and IUGR fetuses during the basal period, yet increased during the insulin clamp by ∼60% in CON and ∼30% in IUGR fetuses (Fig. 3B).
Fig. 3.
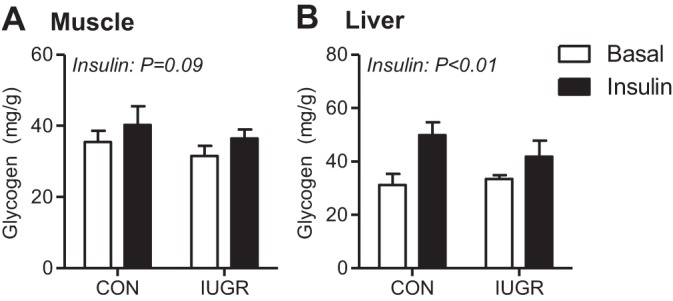
Glycogen content in CON and IUGR fetal muscle and liver during basal and insulin-clamp periods. Glycogen content was measured in skeletal muscle (A) and liver tissue (B) samples from CON and IUGR fetuses under basal (white bars) and insulin-clamp (insulin, black bars) conditions. Values expressed as means ± SE are shown (n = 5–12 samples per group). Significant effects from two-way ANOVA are indicated.
Glucose oxidation.
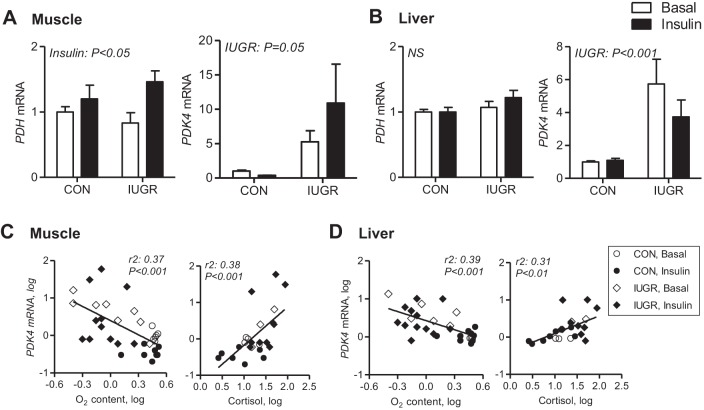
To test whether changes in fetal muscle or liver glucose oxidation patterns might be contributing to the in vivo observations, we measured genes involved in glucose oxidation. PDH produces acetyl CoA for use in the tricarboxylic acid (TCA) cycle and its activity is inhibited by phosphorylation via PDH kinase 4 (PDK4). Expression of PDH was similar between CON and IUGR fetal muscle in each period, yet increased ∼10–30% in response to insulin (Fig. 4A). PDK4 expression was increased 5- to 10-fold in IUGR compared with CON muscle during basal and insulin-clamp periods. In the liver, expression of PDH was similar between groups, yet IUGR fetal livers had approximately fourfold increase in PDK4 expression (Fig. 4B). In both skeletal muscle and liver, the expression of PDK4 was inversely correlated with fetal arterial oxygen concentrations and positively correlated with fetal plasma cortisol concentrations (Fig. 4, C and D).
Fig. 4.
Expression of genes for glucose oxidation in CON and IUGR fetal muscle and liver during basal and insulin-clamp periods. Gene expression of PDH and PDK4 was measured using real-time PCR in skeletal muscle (A) and liver (B) tissue samples from CON and IUGR fetuses under basal (white bars) and insulin-clamp (insulin, black bars) conditions. Values expressed as means ± SE are shown (n = 5–12 samples per group). Significant effects from two-way ANOVA are indicated. Correlations are shown between PDK4 mRNA expression in muscle (C) and liver (D) with fetal arterial oxygen content, and fetal plasma cortisol concentrations were analyzed following log transformation. Significance is indicated.
Pyruvate and lactate metabolism.
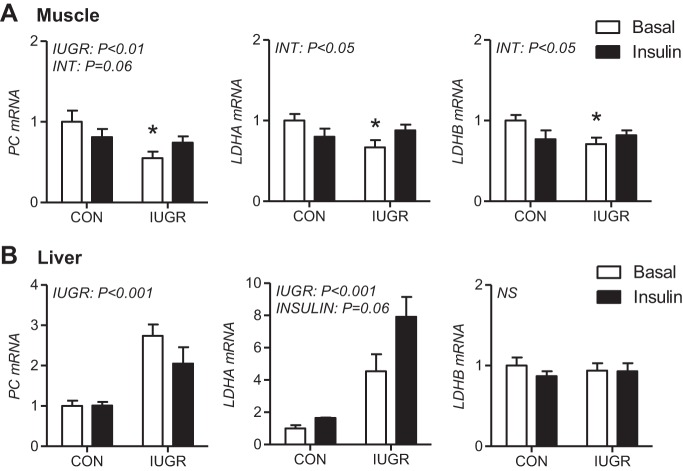
Decreased glucose oxidation combined with increased glucose uptake and glycolytic genes may increase the pyruvate pool and subsequent lactate production in muscle and liver, given the absence of an increase in oxygen consumption in the IUGR fetus (Table 2). To test this, we measured genes involved in the conversion of pyruvate to oxaloacetate (PC) and to lactate (LDHA and LDHB). In muscle, PC expression was ∼30–40% lower in IUGR fetuses (Fig. 5A). Expression of LDHA and LDHB was reduced by ∼30% only in IUGR compared with CON saline-infused fetal muscle. In contrast, hepatic expression of PC was nearly twofold higher during saline or insulin periods, supporting the supply of three-carbon substrates into the gluconeogenic pathway. (Fig. 5B). LDHA expression was fourfold in IUGR livers during the saline period and increased further in response to insulin. Hepatic LDHB expression was unchanged in IUGR fetuses or with the insulin-clamp period.
Fig. 5.
Expression of genes for pyruvate and lactate metabolism in CON and IUGR fetal muscle and liver during basal and insulin-clamp periods. Gene expression of PC, LDHA, and LDHB was measured using real-time PCR in skeletal muscle (A) and liver tissue (B) samples from CON and IUGR fetuses under basal (white bars) and insulin-clamp (insulin, black bars) conditions. Means ± SE are shown (n = 5–12 samples per group). Significant effects from two-way ANOVA are indicated. *P <0.05 vs. CON during basal conditions as determined by post hoc test comparisons given the significant interaction effect in ANOVA.
Relationship between hepatic gluconeogenesis, glucose oxidation, and lactate production.
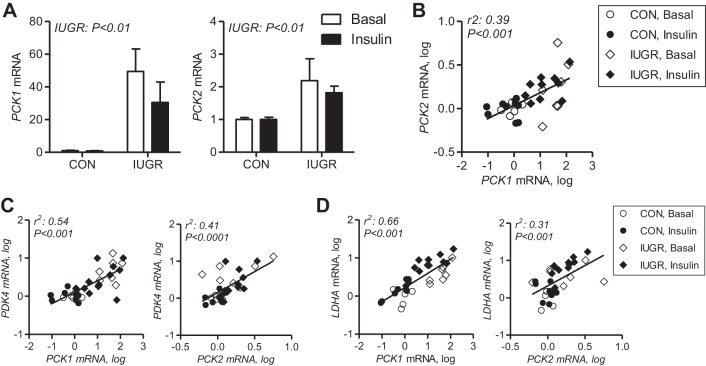
Expression of PCK1, which encodes the cytosolic form of PEPCK-C, is increased in IUGR fetal livers collected under basal conditions and during the insulin-clamp period (Fig. 6A) (32). Expression of PCK2, which encodes the mitochondrial form PEPCK-M, is increased twofold in IUGR fetal liver and remains increased during the insulin-clamp period (Fig. 6A). There was a significant correlation between hepatic PCK2 and PCK1 expression (Fig. 6B). We also observed that hepatic mRNA expression of PCK1 and PCK2 was significantly correlated with PDK4 (Fig. 6C) and LDHA expression (Fig. 6D).
Fig. 6.
Expression and correlations between hepatic genes. A: PCK1 and PCK2 gene expression was measured using real-time PCR in liver tissue samples from CON and IUGR fetuses under basal (white bars) and insulin-clamp (insulin, black bars) conditions. Values expressed as means ± SE are shown (n = 5–12 samples per group). Correlations between PCK2 (B) with PDK4 (C) and LDHA (D) and PCK1 with PCK2, and PCK1 with PDK4 and LDHA expression in liver (n = 32 samples) were analyzed following log transformation. Significance is indicated.
DISCUSSION
We hypothesized that in relatively hypoxic and hypoglycemic IUGR fetuses, glucose oxidation is limited. Further, we hypothesized that reduced glucose oxidation and increased glycolysis in the IUGR fetus would result in lactate production that would be utilized by the liver to produce glucose necessary for developing tissues. We also proposed that increased lactate production would be active both basally and during maximal stimulation with insulin because glucose production is active during both of these conditions in the IUGR fetus (32). Our in vivo tracer metabolic studies demonstrate that IUGR fetuses oxidized a lower fraction of the total amount of glucose utilized, despite maintained rates of glucose utilization under basal conditions and a robust increase in response to insulin. At the molecular level, we found increased PDK4 mRNA expression in both IUGR liver and skeletal muscle tissues, which may play an important role mechanistically in suppressing glucose oxidation. Interestingly, we found increased LDHA expression in the liver, rather than muscle, suggesting that intrahepatic lactate production during basal and insulin-stimulated conditions may provide carbon for glucose production in the IUGR fetus. Overall, our results provide novel mechanistic support for the coordinated changes in glucose metabolism that develop in IUGR fetal sheep.
In support of our hypothesis, our results demonstrate a limited capacity for glucose oxidation in the IUGR fetus under basal conditions and during maximal stimulation with insulin. Specifically, we found that IUGR fetuses oxidize a lower fraction of the total glucose utilized under basal and insulin-stimulated conditions. Consistent with this, a previous study also found that IUGR fetuses have a lower glucose oxidation fraction (17). In contrast to our results, this previous study found decreased basal glucose oxidation rates, which we speculate may be due to significantly lower fetal oxygen uptake rates in that group of IUGR fetuses [∼279 ± 43 μmol·min−1·kg−1 (17) compared with ∼308 ± 10 μmol·min−1·kg−1 in our study]. In support of these in vivo results, our data provide new mechanistic support for limited glucose oxidation capacity in the IUGR fetus, as demonstrated by increased PDK4 expression in liver and skeletal muscle tissues. We speculate that increased PDK4 expression in IUGR fetal tissues limits glucose oxidation by inhibiting PDH activity and, thus, the conversion of glucose-derived pyruvate into acetyl CoA and the entry of those glucose carbons into the TCA cycle for oxidation. Interestingly, despite a more than fourfold increase in PDK4 expression in the liver and muscle, the glucose oxidation fraction was only reduced by 10–15%, and overall rates of glucose oxidation were similar, suggesting limited capacity, rather than full inhibition of glucose oxidation.
In our data, we found that PDK4 mRNA expression was sustained during the insulin clamp in both liver and muscle tissues in the IUGR fetus. Other cell, animal, and human studies demonstrate an inhibitory effect of insulin on PDK4 transcription (3, 13, 14). Impaired insulin suppression of PDK4 in skeletal muscle of mice has been reported during acutely induced insulin-resistant states during lactate or lipid infusion (14). In addition, decreased hepatic PDK4 expression in mice improved hepatic insulin resistance (31). Insulin has been shown to inhibit both PDK4 and PCK1 transcription by inactivation of the FOXO1 transcription factor (3, 15, 19). Recently, we have shown that FOXO1 nuclear localization and phosphorylation are increased in the IUGR fetal liver during basal and hyperinsulinemic conditions (32). Thus, aberrant FOXO1 regulation in the IUGR fetal liver may be a common mechanism for insulin-resistant expression of PDK4 and PCK1. Expression of PDK4 also is induced by glucocorticoids (3, 13), and in our study, we observed a positive correlation between plasma cortisol concentrations and PDK4 expression. Interestingly, we also found that fetal oxygen concentrations were inversely related to PDK4 expression, suggesting regulation by hypoxia (16).
The limited glucose oxidation capacity in the IUGR fetus is unlikely due to limited glucose uptake since IUGR fetuses have normal rates of glucose utilization under basal conditions and a robust increase in response to either an insulin clamp or a glucose clamp (17, 32). Importantly, we previously observed a dose-dependent relationship between the insulin infusion rate and glucose utilization rate (during the insulin clamp) in IUGR fetuses, but not CON fetuses. This suggests that IUGR fetuses may have increased sensitivity for insulin-stimulated glucose disposal compared with CON fetuses. In addition, two other studies in sheep models of IUGR also demonstrate increased peripheral sensitivity for glucose disposal (25, 38). At the tissue level, both skeletal muscle and liver gene expression profiles in IUGR fetuses support maintained capacity for basal and insulin-stimulated glucose uptake and glycolysis. In skeletal muscle, we found increased mRNA expression of IRA, IRB, GLUT1, and PFK1, and previously, we reported increased IR-β protein expression in IUGR fetuses, suggesting increased capacity for insulin signaling, glucose uptake, and glycolysis (33). Increased IR-β protein expression in fetal muscle also has been found in another sheep model of placental insufficiency produced by carunclectomy (24). By comparison, in liver, we only found increased PFK1 expression. Despite the maintenance of glucose utilization and decreased glucose oxidation capacity, glycogen concentrations were not increased in the IUGR skeletal muscle, nor increased or decreased in the IUGR liver, which also has active glucose production. These observations suggest that glucose carbons are being metabolized or stored in another form in these tissues. In response to insulin, glycogen content increased in the liver, consistent with maintained insulin sensitivity for glucose utilization.
IUGR fetuses have relatively normal, or slightly reduced, weight-specific rates of oxygen consumption under basal or stimulated conditions (2, 17, 27, 32, 33). Thus, given the absence of a concomitant increase in oxygen uptake to parallel increases in insulin-stimulated glucose utilization, these data support a limited capacity for glucose oxidation in the IUGR fetus, even during maximal stimulation with insulin. Further, IUGR fetuses have increased lactate concentrations, which may result from lower fetal oxygen concentrations, increased glycolysis, and reduced glucose oxidation capacity (2, 32). Thus, we hypothesized that increased lactate produced from glucose-utilizing tissues (skeletal muscle), as a result of limited glucose oxidation, is utilized by the liver to fuel glucose production. In support of this, we found increased LDHA gene expression in liver, but not skeletal muscle. LDHA encodes the M protein subunit of the LDH enzyme and favors lactate and NAD+ formation in liver (5). We speculate that there is sufficient LDH activity, given the absence of a decrease in LDHB expression, to convert lactate back to pyruvate for use in the gluconeogenic pathway. We also found increased PC expression, supporting the conversion of pyruvate into PEP for use in gluconeogenesis. Thus, our results suggest that increased intrahepatic lactate production may supply carbon for glucose production, rather than the “classic” interorgan lactate shuttle defined by the Cori cycle.
The increase in glucose utilization rates, lower glucose oxidation fraction, and increased lactate production in the IUGR fetus may be important coordinated changes in metabolism to support the energy and carbon requirements for glucose production. First, hepatic expression of PDK4 and LDHA was both correlated with PCK1 and PCK2 expression. Second, the suppression of glucose oxidation, via PDK4, may spare substrates such as pyruvate, lactate, and alanine from oxidative metabolism and allow these carbon substrates to be used as gluconeogenic precursors (12, 31). Third, increased hepatic lactate production itself, as a result of increased glycolysis and incomplete glucose oxidation, may provide lactate as a carbon source for hepatic glucose production in the IUGR fetus. Fourth, our data suggest that increased glucose production (32) and gluconeogenic gene expression (PCK1, PCK2) may occur simultaneously with increased glycolysis (PFK1 gene expression), creating a potential futile cycle in glucose metabolism in the IUGR fetal liver. In terms of energetics, the increase in LDHA and lactate production would be predicted to produce a concomitant increase in NAD+, which is necessary to sustain a high glycolytic flux. A high glycolytic flux rate may occur to generate ATP needed for gluconeogenesis or to compensate for lower ATP production as a result of decreased glucose-derived pyruvate oxidation. Lastly, our data demonstrate increased gene expression of both the cytosolic (PCK1) and mitochondrial (PCK2) forms of PEPCK enzyme. Emerging data in rodents and isolated cells indicate that both of these forms of PEPCK are important for glucose production and that each form may have unique roles in coordinating substrate preference and mitochondrial metabolism (21, 22, 28–30, 41). Increased gluconeogenic gene expression has been found in our model of IUGR (17, 32, 33) and also in another sheep model of placental insufficiency and during fetal hypoglycemia (7, 35). Furthermore, PCK1 and PCK2 expression is reduced in a sheep model of maternal overnutrition (26).
In IUGR fetal muscle, our results suggest increased glucose-derived pyruvate, rather than lactate production. First, expression of LDHA/LDHB in IUGR muscle was modestly lower, rather than increased, compared with the CON fetus under basal conditions and did not increase during the insulin clamp. Indeed, in normally grown fetal sheep, studies using catheters across the hindlimb (skeletal muscle) demonstrate that acute hyperinsulinemia increases glucose uptake but does not change lactate output (39, 40). The effect of insulin on pyruvate flux has not been reported across the hindlimb of the fetal sheep. Second, we found no increase in glycogen contents, supporting that the increased glucose taken up by the muscle is metabolized rather than stored. Third, we found decreased PC expression, supporting decreased anaplerosis for TCA cycle activity. Thus, we speculate that the glucose-derived intracellular pyruvate pool is increased in skeletal muscle. The functional significance of this pool of pyruvate remains unknown.
In our study, we report combined results for male and female fetuses. We found no differences between male and female fetuses when we included sex in our analysis, which may be limited by the sample size in each of the four treatment groups. Furthermore, our study included data on three sets of twins. The data for these twin fetuses were indistinguishable from the data on the singleton fetuses in this study and in our previous study (32).
Overall, these results provide new insights into the adaptive mechanisms that occur for glucose metabolism and insulin sensitivity in the IUGR fetus and the coordinated responses between liver and muscle. Our data demonstrate that IUGR fetal sheep have limited capacity for glucose oxidation, which may be due to increased PDK4 gene expression, despite increased capacity for glucose utilization in liver and muscle fetal tissues. Identification of these limitations in glucose oxidation by the IUGR fetus is important because therapeutic strategies to increase glucose delivery to the IUGR fetus may fail to improve growth if IUGR tissues have developed permanent adaptations that limit glucose oxidation and ultimately energy production and growth. Thus, reduced glucose oxidation in the IUGR fetus may be a contributing mechanism that limits fetal energy production and ultimately fetal growth. These data also provide new evidence supporting the concept that reduced glucose oxidation and intrahepatic production of lactate may be a mechanism to produce substrates that fuel glucose production in the IUGR fetus.
GRANTS
Research support was provided from National Institutes of Health Grants K01-DK-090199 to S. R. Thorn-Wesolowski, K12-HD-057022 (Building Interdisciplinary Research Careers in Women's Health) to L. D. Brown, R01-DK-088139, K08-HD-060688 to P. J. Rozance, and T32-HD-07186 to W. W. Hay Jr.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.D.B., P.J.R., J.E.F., W.W.H.J., and S.R.W. conception and design of research; L.D.B., J.L.B., and S.R.W. performed experiments; L.D.B. and S.R.W. analyzed data; L.D.B. and S.R.W. interpreted results of experiments; L.D.B., P.J.R., J.L.B., J.E.F., W.W.H.J., and S.R.W. edited and revised manuscript; L.D.B., P.J.R., J.L.B., J.E.F., W.W.H.J., and S.R.W. approved final version of manuscript; S.R.W. prepared figures; S.R.W. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank David Caprio, Karen Trembler, Gates Roe, and Dan LoTurco for assistance with studies (all from the University of Colorado Anschutz Medical Campus).
REFERENCES
- 1.Brown LD, Hay WW Jr. Effect of hyperinsulinemia on amino acid utilization and oxidation independent of glucose metabolism in the ovine fetus. Am J Physiol Endocrinol Metab 291: E1333–E1340, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW Jr. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 303: E352–E364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol 315: 159–167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4: 727–732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galan HL, Hussey MJ, Chung M, Chyu JK, Hobbins JC, Battaglia FC. Doppler velocimetry of growth-restricted fetuses in an ovine model of placental insufficiency. Am J Obstet Gynecol 178: 451–456, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Gentili S, Morrison JL, McMillen IC. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod 80: 1121–1127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay WW Jr, DiGiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol Endocrinol Metab 256: E704–E713, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Hay WW Jr, Myers SA, Sparks JW, Wilkening RB, Meschia G, Battaglia FC. Glucose and lactate oxidation rates in the fetal lamb. Proc Soc Exp Biol Med 173: 553–563, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Hay WW Jr, Sparks JW, Battaglia FC, Meschia G. Maternal-fetal glucose exchange: necessity of a three-pool model. Am J Physiol Endocrinol Metab 246: E528–E534, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Hay WW Jr, Sparks JW, Quissell BJ, Battaglia FC, Meschia G. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am J Physiol Endocrinol Metab 240: E662–E668, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Herbst EA, MacPherson RE, LeBlanc PJ, Roy BD, Jeoung NH, Harris RA, Peters SJ. Pyruvate dehydrogenase kinase-4 contributes to the recirculation of gluconeogenic precursors during postexercise glycogen recovery. Am J Physiol Regul Integr Comp Physiol 306: R102–R107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J 36: 328–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes 55: 2311–2317, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-α inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes 53: 899–910, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Kim EJ, Kim DK, Lee JM, Park SB, Lee IK, Harris RA, Lee MO, Choi HS. Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRgamma. PLoS One 7: e46324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 18: 388–395, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab 302: E1483–E1492, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendez-Lucas A, Duarte JA, Sunny NE, Satapati S, He T, Fu X, Bermudez J, Burgess SC, Perales JC. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J Hepatol 59: 105–113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez-Lucas A, Hyrossova P, Novellasdemunt L, Vinals F, Perales JC. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. J Biol Chem 289: 22,090–22,102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meschia G, Cotter JR, Breathnach CS, Barron DH. Simultaneous measurement of uterine and umbilical blood flows and oxygen uptake. Q J Exp Physiol 52: 1–8, 1966. [Google Scholar]
- 24.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens JA, Gatford KL, De Blasio MJ, Edwards LJ, McMillen IC, Fowden AL. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol 584: 935–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rattanatray L, Muhlhausler BS, Nicholas LM, Morrison JL, McMillen IC. Impact of maternal overnutrition on gluconeogenic factors and methylation of the phosphoenolpyruvate carboxykinase promoter in the fetal and postnatal liver. Pediatr Res 75: 14–21, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 28: 714–723, 2007. [DOI] [PubMed] [Google Scholar]
- 28.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol 20: 6508–6517, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark R, Guebre-Egziabher F, Zhao X, Feriod C, Dong J, Alves TC, Ioja S, Pongratz RL, Bhanot S, Roden M, Cline GW, Shulman GI, Kibbey RG. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J Biol Chem 289: 7257–7263, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark R, Kibbey RG. The mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M) and glucose homeostasis: has it been overlooked? Biochim Biophys Acta 1840: 1313–1330, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao R, Xiong X, Harris RA, White MF, Dong XC. Genetic inactivation of pyruvate dehydrogenase kinases improves hepatic insulin resistance induced diabetes. PLoS One 8: e71997, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorn SR, Sekar SM, Lavezzi JR, O'Meara MC, Brown LD, Hay WW Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol Regul Integr Comp Physiol 263: R578–R585, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Van Veen LC, Hay WW Jr, Battaglia FC, Meschia G. Fetal CO2 kinetics. J Dev Physiol 6: 359–365, 1984. [PubMed] [Google Scholar]
- 38.Wallace JM, Milne JS, Aitken RP, Hay WW Jr. Sensitivity to metabolic signals in late-gestation growth-restricted fetuses from rapidly growing adolescent sheep. Am J Physiol Endocrinol Metab 293: E1233–E1241, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Wilkening RB, Boyle DW, Teng C, Meschia G, Battaglia FC. Amino acid uptake by the fetal ovine hindlimb under normal and euglycemic hyperinsulinemic states. Am J Physiol Endocrinol Metab 266: E72–E78, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Wilkening RB, Molina RD, Battaglia FC, Meschia G. Effect of insulin on glucose/oxygen and lactate/oxygen quotients across the hindlimb of fetal lambs. Biol Neonate 51: 18–23, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem 284: 27,025–27,029, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yates DT, Green AS, Limesand SW. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep. J Pregnancy 2011: 740408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]