Abstract
The purpose of the present studies was to determine the effect of various nonhypertrophic exercise stimuli on satellite cell (SC) pool activity in human skeletal muscle. Previously untrained men and women (men: 29 ± 9 yr and women: 29 ± 2 yr, n = 7 each) completed 6 wk of very low-volume high-intensity sprint interval training. In a separate study, recreationally active men (n = 16) and women (n = 3) completed 6 wk of either traditional moderate-intensity continuous exercise (n = 9, 21 ± 4 yr) or low-volume sprint interval training (n = 10, 21 ± 2 yr). Muscle biopsies were obtained from the vastus lateralis before and after training. The fiber type-specific SC response to training was determined, as was the activity of the SC pool using immunofluorescent microscopy of muscle cross sections. Training did not induce hypertrophy, as assessed by muscle cross-sectional area, nor did the SC pool expand in any group. However, there was an increase in the number of active SCs after each intervention. Specifically, the number of activated (Pax7+/MyoD+, P ≤ 0.05) and differentiating (Pax7−/MyoD+, P ≤ 0.05) SCs increased after each training intervention. Here, we report evidence of activated and cycling SCs that may or may not contribute to exercise-induced adaptations while the SC pool remains constant after three nonhypertrophic exercise training protocols.
Keywords: muscle stem cells, Pax7, MyoD, endurance training, sprint interval training
skeletal muscle repair is largely possible due to a pool of resident muscle stem cells, referred to as satellite cells (SCs) (31). Although essential in mediating skeletal muscle regeneration (24, 30, 32, 41, 49), SCs become active after various stresses, such as eccentrically loaded muscle contractions (8, 10, 33, 38, 42) and bouts of resistance exercise (34, 37), the acute response of SCs to various exercise stimuli have been well characterized (26). Upon activation, SCs proliferate and differentiate, driving muscle repair, whereas a proportion of SCs revert to quiescence after proliferation to maintain the muscle SC pool. This process is referred to as the myogenic program and is regulated by a transcriptional network collectively referred to as myogenic regulatory factors (MRF), which includes Myf5, MyoD, Mrf4, and myogenin (2, 15, 47, 56). Shortly after activation, Myf5 is expressed by SCs, which is followed by the upregulation of MyoD through proliferation and early differentiation. After proliferation, SCs undergo differentiation, which is achieved by the upregulation of MRF4 and myogenin (5, 9).
The contribution of SCs to skeletal muscle hypertrophy after resistance training in humans has been extensively studied (20, 27, 43, 44, 53, 59). Although gains in muscle mass are often associated with an expansion of the SC pool, more recent work has demonstrated that an increase in fiber cross-sectional area (CSA) is not always associated with expansion (11). Albeit to a lesser extent, the SC response to endurance training has also been studied. An expansion of the SC pool was observed after 14 wk of interval training consisting of 1 min of high-intensity cycling interspersed by 4 min of low-intensity cycling for a total of 40–45 min (6, 61). In contrast, others have suggested that there is no change in SC content after a more traditional lower-intensity endurance training program (54). These data suggest that exercise intensity may play an important role in mediating SC pool expansion with respect to aerobic exercise. It is important to note that studies reporting an expansion in the SC pool after high-intensity cycle training also reported an increase in CSA (6, 61). We recently reported an expansion of SCs specifically associated with hybrid fibers in the absence of both an expansion of the total SC pool and muscle hypertrophy (18). Endurance exercise in rodent models has been shown to result in an increase in SC content (23, 51, 52). However, there is a paucity of information regarding the influence of SCs on exercise-induced skeletal muscle adaptations/remodeling. Our recent work suggests that SCs may support muscle adaptation and remodeling in the absence of muscle hypertrophy. We have demonstrated that a nonhypertrophic stimulus like aerobic interval training leads to an expansion of the SC pool associated with remodeling fibers (18).
Studies examining the response of SCs to various modes of exercise commonly use immunofluorescent techniques to label SCs with various markers, such as Pax7 (4, 29, 37, 38, 58) and neural cell adhesion molecule (CD56) (8, 10, 28, 29, 38, 42). Previous literature has identified SCs concomitantly with various markers of proliferation, such as Ki67, PCNA (29), or MyoD (18, 36), to further describe the SC pool and provide more indepth information on its activity status. In addition to the increase in the number of SCs associated with hybrid fibers after aerobic interval training described above, we also observed an increase in the number of active SCs associated with these fibers (18). These results suggest that enumerating the number of SCs per fiber after exercise training may not be sufficient in describing the contribution of SCs to muscle adaptations or remodeling. The present studies aimed to investigate whether the number of proliferating or differentiating SCs increased after three nonhypertrophic stimuli. We hypothesized that there would be no expansion of the SC pool; however, there would be evidence of an increase in the number of active SCs after training.
MATERIALS AND METHODS
Subjects
Study 1.
Nineteen healthy, recreationally active men (n = 16) and women (n = 3) volunteered to participate in this training study. Subjects were matched by peak O2 consumption (V̇o2peak) and assigned to either low-volume high-intensity sprint interval training (SIT-2; n = 10, age: 21 ± 2 yr, height: 175 ± 10 cm, and weight: 71 ± 17 kg) or moderate-intensity continuous exercise training (MICT; n = 9, age: 21 ± 4 yr, height: 180 ± 7 cm, and weight: 74 ± 9 kg) groups. Complete subject characteristics have been previously reported (50). Subjects were considered recreationally active and were not involved in >3 h of aerobic exercise (recreational sports, jogging, etc.) per week or involved in any structured training program within the past 6 mo.
Study 2.
Fourteen overweight/obese men (n = 7, age: 29 ± 9 yr, height: 176 ± 5 cm, and weight: 97 ± 8 kg) and women (n = 7, age: 29 ± 20 yr, height: 162 ± 8 cm, and weight: 75 ± 12 kg) performed low-volume high-intensity sprint interval training (SIT-1). Complete subject characteristics have been reported elsewhere. Subjects were considered sedentary based on their self-reported habitual physical activity, which consisted of <2 sessions/wk of structured exercise lasting <30 min.
Physiological Testing
Study 1.
Pretraining V̇o2peak was assessed by a V̇o2peak incremental ramp test to exhaustion as previously described (50). During baseline testing, subjects reported to the laboratory in the morning after an overnight fast (≥8 h). Subjects were fed a standardized breakfast [a plain bagel (190 kcal, 1 g fat, 36 g carbohydrate, and 7 g protein) with 15 g peanut butter (90 kcal, 8 g fat, 4 g carbohydrate, and 3 g protein) and 200 ml apple juice (90 kcal, 0 g fat, 22 g carbohydrate, and 0 g protein)] and rested for 1 h before a muscle biopsy was taken from the vastus lateralis muscle under superficial local anesthesia (2% lidocaine with epinephrine) using the Bergstrom needle biopsy technique (3) adapted with suction. Forty-eight hours after the muscle biopsy, participants returned to the laboratory to complete a V̇o2peak incremental ramp test to exhaustion as previously described (50). A V̇o2peak incremental ramp test to exhaustion was performed halfway through training on the first day of week 4 to adjust training loads. As a result, subjects only completed three training sessions in week 4. Posttraining testing was conducted 72 h after the last training session of week 6 and was conducted in an identical manner as the baseline testing.
Study 2.
Pretraining V̇o2peak was assessed by a V̇o2peak incremental cycling test on an electronically braked cycle ergometer to exhaustion as previously described (25). Eight days later, subjects reported to the laboratory in the morning after an overnight fast (≥10 h) for baseline testing, and, subsequently, a resting skeletal muscle biopsy was obtained from the vastus lateralis muscle under local anesthesia (1% lidocaine) using a Bergstrom needle technique adapted for suction (57). The posttraining muscle biopsy was obtained 72 h after the last training session, and the posttraining testing session was conducted 4 days later, in an identical manner as the baseline testing.
Training Intervention
Study 1.
As previously described, all subjects completed training 4 days/week for 6 wk (week 4 only had three training sessions due to the midtraining V̇o2peak test for a total of 23 sessions) (50). The SIT-2 exercise protocol was performed as previously described (19, 55). Briefly, subjects completed eight 20-s intervals at 170% of V̇o2peak separated by 10 s of rest eight times, for a total of 4 min. During rest periods, subjects cycled against no load at a self-selected cadence. The MICT exercise protocol consisted of 30 min of continuous cycling at 65% of V̇o2peak. Each exercise bout was performed at the same time of day for all subjects. A standardized warmup of descending and ascending four flights of stairs was completed before all training sessions. Training intensity was monitored by revolutions per minute (rpm) data for each training session.
Study 2.
All subjects completed 18 sessions over a 6-wk training period (Monday, Wednesday, and Friday each week). Each SIT-1 session consisted of a 2-min warmup at 50 W followed by three 20-s all-out cycling sprints against a load that corresponded to 0.05 kg/kg body mass interspersed by 2 min of low-intensity recovery cycling (50 W) with a 3-min cooldown at 50 W, for a total time commitment of 10 min.
Immunofluorescence
Muscle cross sections (7 μm) were prepared from unfixed OCT-embedded samples, allowed to air dry for 15–45 min, and stored at −80°C. Samples were stained with antibodies against Pax7 [1:1, cell supernatant from cells obtained from the Developmental Studies Hybridoma Bank (DSHB)], myosin heavy chain (MHC) type I [MHC-I; clone A4.951 (slow isoform), neat, DSHB], MHC type II (MHC-II; 1:1,000, ab91506, fast isoform, Abcam, Cambridge, MA), laminin (1:1,000, L8271, Sigma-Aldrich, Burlington, ON, Canada, and ab11575, Abcam), MyoD1 (1:30, anti-MyoD1, clone 5.8A, Dako, Burlington, ON, Canada), desmin (ab6322, 1:500, Abcam), myogenin (clone F5D, neat, DSHB), and PCNA (1:40, ab15497, Abcam).
For colormetric costaining with F5D and Pax7, a sequential colormetic reaction was completed following the Vector Labs coimmunohistochemical protocol manual (www.vectorlabs.com/protocols.aspx, Vector Canada, Burlington, ON, Canada). Briefly, slides were fixed with 2% paraformaldehyde and washed in PBS and Tween 20 for 3 × 5 min. Blocking solution containing 2% BSA, 5% FBS, 0.2% Triton X-100, 0.1% sodium azide, and 10% goat serum (GS) was used for 30 min. After being blocked, slides were incubated in Pax7 (1:1) in PBS for 60 min. Pax7 was visualized with a biotinylated secondary antibody (1:200, Vector Canada) followed by the application of the VectaStain Elite ABC-AP kit (Vector Canada) according to the manufacturer's instructions. The stain was then visualized using the Vector Black-AP kit (Vector Canada) according to the manufacturer's instructions. After completion of the reaction for Pax7 visualization, slides were washed in tap water and then blocked with an avidin/biotin blocking step (Vector Canada). Slides were then incubated in F5D (neat) overnight at 4°C. F5D was visualized with a biotinylated secondary antibody (1:200, Vector Canada) followed by the application of the VectaStain Elite ABC kit (Vector Canada) according to the manufacturer's instructions. The stain was then visualized using the diaminobenzidine reagent (Vector Canada) according to the manufacturer's instructions. Nuclei were counterstained with Mayer's hematoxylin (Sigma-Aldrich, Oakville, ON, Canada).
For immunofluorescent detection, secondary antibodies used were either goat anti-mouse IgG, goat anti-rabbit IgG, and/or goat anti-rabbit IgG based on the primary antibody used; the specific antibodies were as follows: Pax7 (Alexa Fluor goat anti-mouse IgG 594/488, 1:500, Invitrogen, Molecular Probes, Carlsbad, CA), MyoD1(biotinylated goat anti-mouse secondary antibody, 1:200, Vector Canada, and streptavidin-594 fluorochrome, 1:250, Invitrogen, Molecular Probes), laminin (Alexa Fluor goat anti-rabbit IgG 647, 1:500, Invitrogen), desmin (Alexa Fluor goat anti-mouse IgG 488, 1:500, Invitrogen, Molecular Probes), and PCNA (biotinylated goat anti-rabbit secondary antibody, 1:200, Vector Canada, and streptavidin-594 fluorochrome, 1:500, Invitrogen, Molecular Probes). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI; 1:20,000, Sigma-Aldrich) before slides were coverslipped with fluorescent mounting media (DAKO). Immunofluorescence staining methods were adapted from previously published methods (34, 36, 54). Briefly, for coimmunofluorescence staining, sections were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 min followed by multiple washes in PBS. Sections were then covered for 90 min in blocking solution containing 2% BSA, 5% FBS, 0.2% Triton X-100, 0.1% sodium azide, and 5% GS. After being blocked, sections were incubated in primary antibodies (i.e., cocktail of Pax7 and laminin) at 4°C overnight. After washes, sections were then incubated in the appropriate secondary antibodies. To prevent migration of the secondary antibodies, sections were then refixed in 4% paraformaldehyde and reblocked in 10% GS in PBS. After this, sections were incubated sequentially in secondary primary antibodies, either MHC-I and MHC-II for fiber type-specific SC quantification or MyoD1 for the quantification of quiescent, proliferating, or differentiating SCs. This was followed by incubation in the appropriate secondary antibody (see above). Nuclei were labeled with DAPI before being coverslipped. Staining procedures were verified using both positive and negative controls to ensure appropriate specificity of staining. After staining was completed, slides were viewed with a Nikon Eclipse Ti Microscope (Nikon Instruments) equipped with a high-resolution Photometrics CoolSNAP HQ2 fluorescent camera (Nikon Intruments, Melville, NY). Images were captured and analyzed using Nikon NIS Elements AR 3.2 software (Nikon Instruments). SC, CSA, and MyoD1 quantification was conducted on ≥200 muscle fibers per subject per time point, and images were obtained with a ×40 objective. Slides were masked for both age and time point.
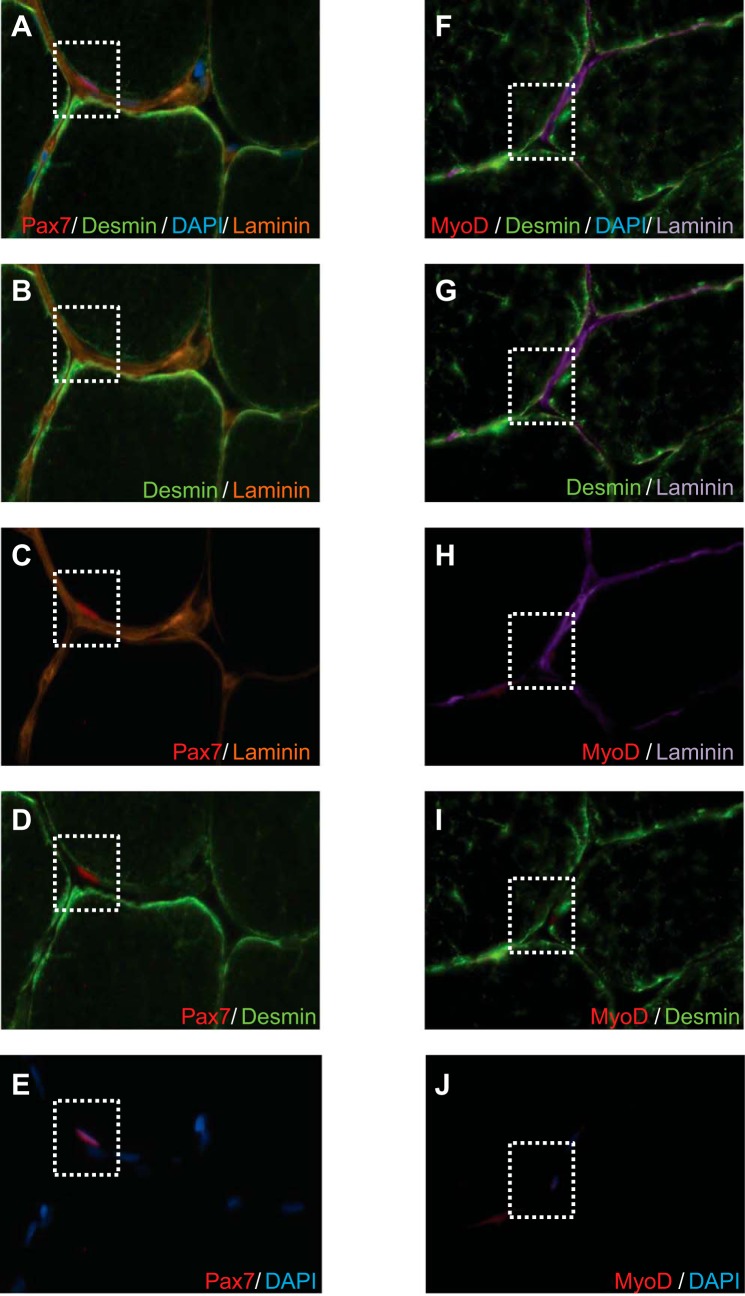
SC quantification from our costaining methods was verified against a Pax7/laminin/desmin stain that specifically demonstrates the anatomic location of the Pax7 stain (the anatomic niche between the basal lamina and sarcolemma) to ensure the validity of the Pax7 stain during the costain with multiple antigen labeling (Fig. 1, A–E). The anatomic location of a MyoD+ nuclei was also confirmed with MyoD/laminin/desmin stain. This stain confirms that MyoD+ cells were located within the SC niche, between the basal lamina and sarcolemma (Fig. 1, F–J). Numbers of myonuclei were determined in muscle sections stained for Pax7/MHC-I/MHC-II/laminin/DAPI. Myonuclei were quantified as Pax7− nuclei located beneath the basal lamina, which was identified with laminin. The number of myonuclei per myofiber was determined for ≥100 myofibers per section per subject per time point.
Fig. 1.
A: representative image of Pax7/desmin/4′,6-diamidino-2-phenylindole (DAPI)/laminin stain. B–E: costaining of desmin/laminin (B), Pax7/laminin (C), Pax7/desmin (D), and Pax7/DAPI (E). F: representative images of MyoD/desmin/DAPI/laminin. G–J: costaining of desmin/laminin (G), MyoD/laminin (H), MyoD/desmin (I), and MyoD/DAPI (J). Staining was performed to assure accurate anatomic location of Pax7+ and MyoD+ cells within the satellite cell (SC) niche.
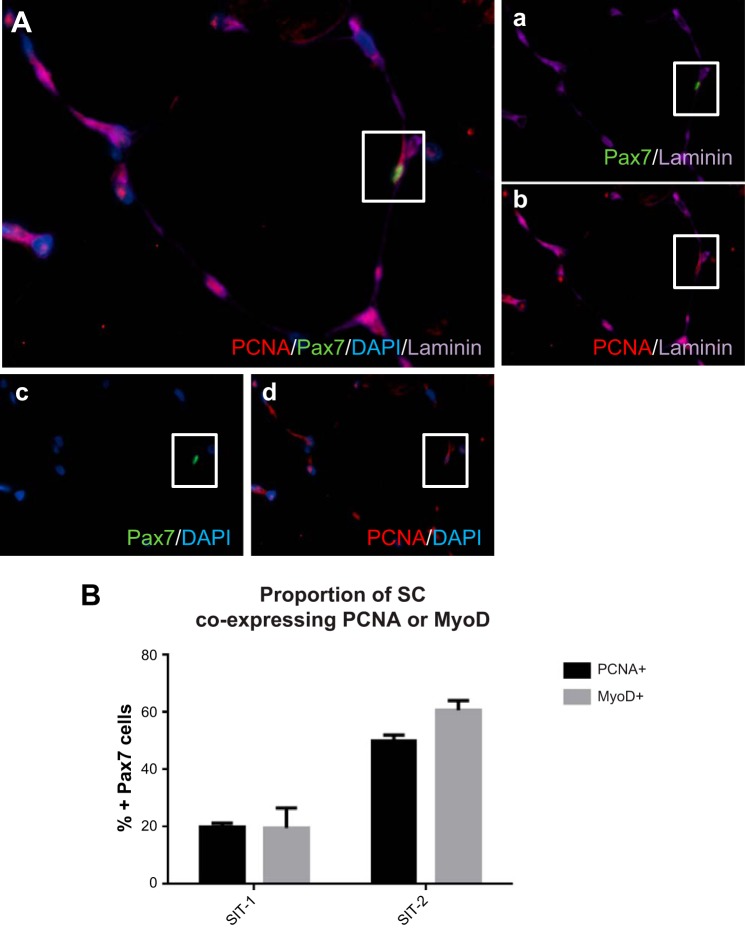
To determine whether the proportion of cells identified as Pax7+/MyoD+ (activated SCs) were similar to the number of Pax7+/PCNA+ cells, serial muscle cross sections of three subjects from both the SIT-1 and SIT-2 groups were quantified (see Fig. 5). Because of the lack of tissue, the analysis was restricted to only a small subset of subjects.
Fig. 5.
A: representative image of PCNA/Pax7/DAPI/laminin. a–d: costaining of Pax7/laminin (a), PCNA/laminin (b), Pax7/DAPI (c), and PCNA/DAPI (d) of muscle cross sections to determine the proportion of activated SCs (Pax7+/PCNA+). The proportion of Pax7+ cells coexpressing PCNA or MyoD was determined in a subset of subjects from the SIT-1 (n = 3) and SIT-2 (n = 3) groups.
To identify newly formed fibers and/or regenerating fibers, muscle cross sections were stained with neonatal MHC (nMHC1:10, VP-M666, Vector Laboratories, Burlingame, CA). Immunofluorescent staining resulted in no fibers staining positive for neonatal MHC.
Statistical Analysis
Statistical analysis was performed using Sigma Stat 3.1.0 analysis software (Systat Software, Chicago, IL). Studies 1 and 2 were run separately, and subjects in each group (SIT-1, SIT-2, and MICT) were not matched on any previous baseline physiological measures, and, therefore, between-group comparisons between the three groups were not completed. Subjects from the SIT-2 and MICT groups (study 1) were all recreationally active and V̇o2peak matched and assigned to either group. Therefore, between-group comparisons were completed between the SIT-2 and MICT groups, as were within-subject comparisons between time points, whereas only between-subject comparisons were completed in the SIT-1 group. Specifically, for study 1, two-way repeated-measures ANOVA with one factor for time (before/after training) and one factor for group (SIT-2/MICT) was conducted for all analyses. For study 2, a paired two-tailed t-test was conducted for before/after comparisons. P values of ≤ 0.05 were considered statistically significant. All results are presented as means ± SD.
RESULTS
Fiber CSA and Myonuclear Domain
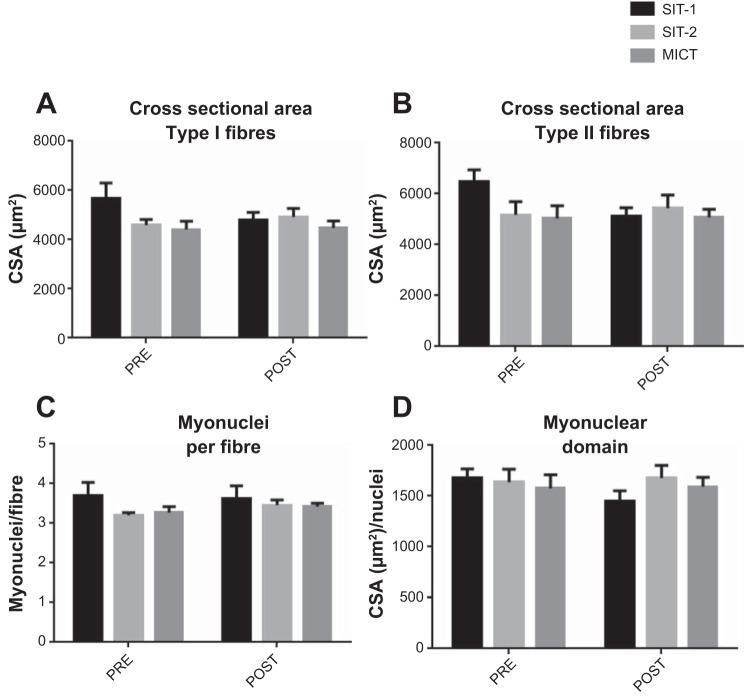
Fiber CSA for both type I and II fibers was determined based on immunoflurescent staining for MHC-I and MHC-II of muscle cross sections. Six weeks of training did not lead to a significant increase of CSA of either type I or II fibers with SIT-1 (type I fibers: 5,588 ± 1,373 μm2 for before training vs. 4,996 ± 1,138 μm2 for after training; type II: 6,202 ± 1,552 μm2 for before training vs. 5,709 ± 1445 μm2 for after training), SIT-2 (type I: 4,596 ± 689 μm2 for before training vs. 4,926 ± 1,050 μm2 for after training; type II: 5,172 ± 1,545 μm2 for before training vs. 5,439 ± 1547μm2 for after training), or MICT (type I: 4,417 ± 1,019 μm2 for before training vs. 4,480 ± 856 μm2 for after training; type II: 5,037 ± 1,500 μm2 for before training vs. 5,078 ± 933 μm2 for after training; Fig. 2, A and B).
Fig. 2.
Training does not lead to skeletal muscle hypertrophy. Skeletal muscle fiber cross-sectional areas (CSAs) of type I (A) and type II (B) fibers were measured before (pre) and after (post) training for each training intervention. The number of nuclei per fiber was determined (C), and the myonuclear domain (D) was calculated as fiber area (in μm2) per nucleus.
Training did not result in an increase in the number of nuclei per fiber for either SIT-1 (before training: 3.7 ± 1.2 μm2 and after training: 3.6 ± 1.2 μm2), SIT-2 (before training: 3.2 ± 0.2 μm2 and after training: 3.5 ± 0.3 μm2) or MICT (before training: 3.3 ± 0.4 μm2 and after training: 3.4 ± 0.2 μm2) groups (P > 0.05; Fig. 2C). Additionally, the myonuclear domain, defined as the CSA per myonuclei, was determined, and consistent with CSA and nuclei per fiber, it remained unchanged after training for all three groups (SIT-1: 1,575 ± 275 for before training vs. 1,474 ± 276 for after training, SIT-2: 1,638 ± 359 for before training vs. 1,677 ± 352 for after training, and MICT 1,576 ± 407 for before training vs. 1,593 ± 281 for after training, P > 0.05; Fig. 2D).
Fiber Type-Specific SC Response to Training
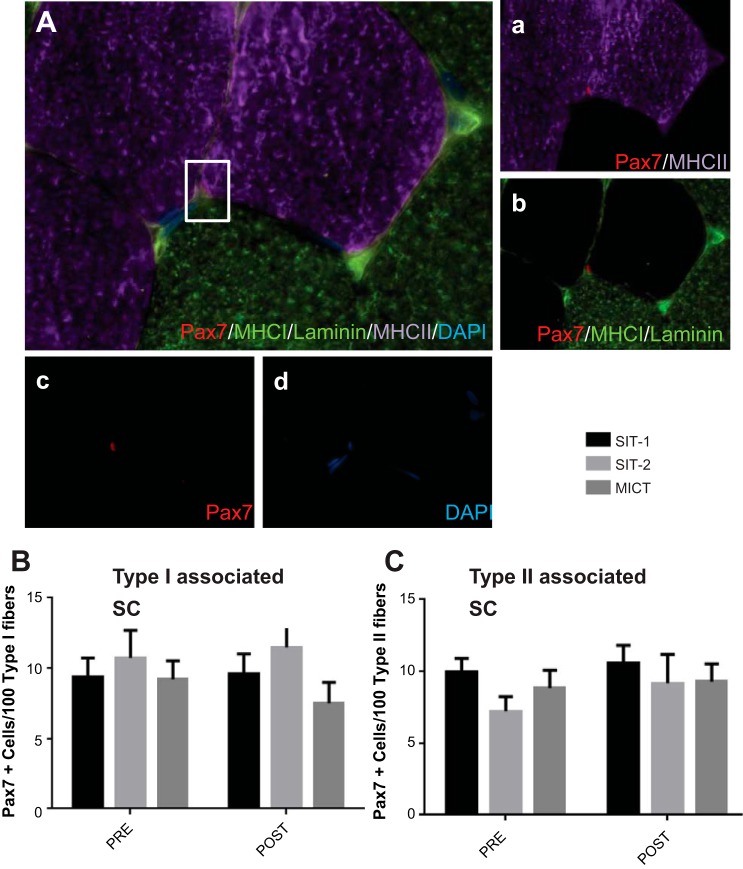
SCs were quantified based on immunofluorescent staining of muscle cross sections with MHC-I and MHC-II to determine fiber type, with Pax7 as a marker of SCs and DAPI and laminin to assure accurate anatomic location (Fig. 3A). Six weeks of training did not increase the number of SCs associated with either type I fibers (SIT-1: 9.4 ± 5.1 Pax7+ cells/100 type I fibers for before training vs. 9.6 ± 5.8 Pax7+ cells/100 type I fibers for after training, SIT-2: 10.7 ± 5.5 Pax7+ cells/100 type I fibers for before training vs. 11.5 ± 6.7 Pax7+ cells/100 type I fibers for after training, and MICT: 9.2 ± 3.7 Pax7+ cells/100 type I fibers for before training vs. 7.5 ± 4.2 Pax7+ cells/100 type I fibers for after training, P > 0.05; Fig. 3B) or type II fibers (SIT-1: 10.0 ± 3.4 Pax7+ cells/100 type II fibers for before training vs. 10.6 ± 5.0 Pax7+ cells/100 type II fibers for after training, SIT-2: 7.2 ± 2.8 Pax7+ cells/100 type II fibers for before training vs. 9.2 ± 5.7 Pax7+ cells/100 type II fibers for after training, and MICT: 8.8 ± 3.5 Pax7+ cells/100 type II fibers for before training vs. 9.3 ± 3.5 Pax7+ cells/100 type II fibers for after training, P > 0.05; Fig. 3C).
Fig. 3.
Fiber type-specific SC responses to training. A: representative image of a Pax7/laminin/myosin heavy chain (MHC) type I (MHC-I)/MHC type II (MHC-II)/nuclei immunofluorescent stain on a muscle cross section. a: MHC-II and Pax7; b: Pax7, MHC-I, and laminin; c: Pax7; d: nuclei (DAPI). B and C: SC responses of type I (B) and type II (C) fiber-associated SCs before and after very low-volume high-intensity sprint interval training (SIT)-1, SIT-2, and moderate-intensity continuous exercise training (MICT) expressed per 100 fibers. Values are means ± SE.
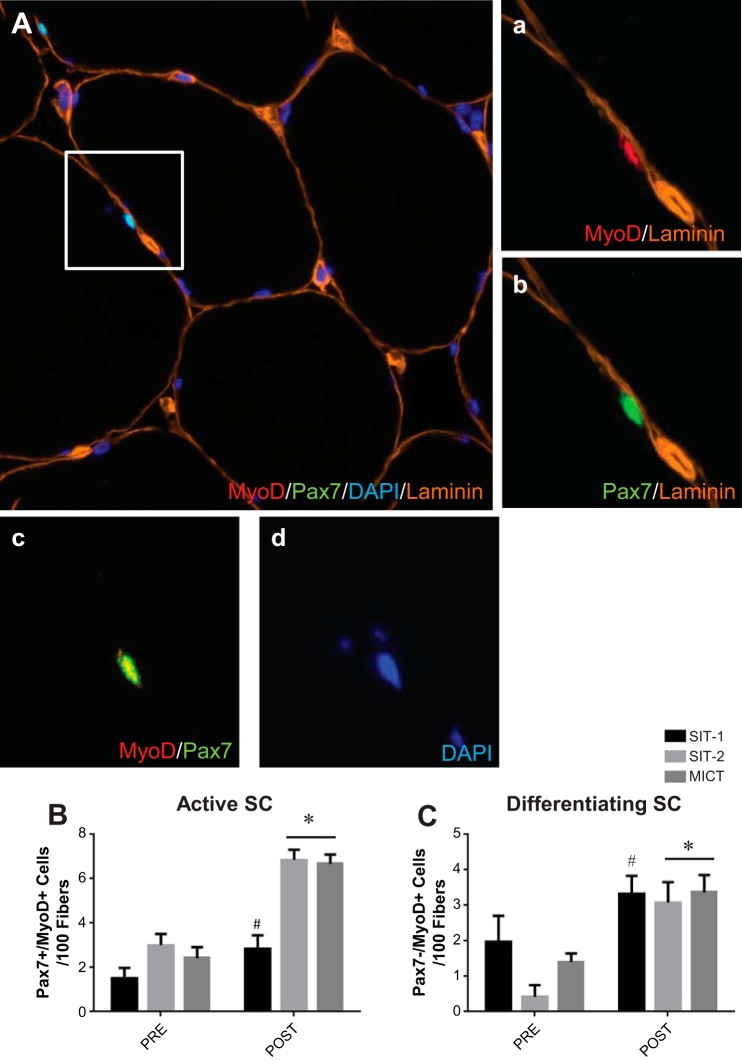
SC Activity After Training
To further describe the SC pool, we determined the proportion of active SCs at baseline and after training. Muscle cross sections were stained with Pax7, MyoD, laminin, and DAPI (Fig. 4A). Activated SCs were identified as expressing both Pax7 and MyoD (Pax7+/MyoD+), whereas differentiating SCs were identified as SCs only expressing MyoD (Pax7−/MyoD+). A significant increase in activated SCs was observed after SIT-1 (before training: 1.5 ± 1.7 Pax7+/MyoD+ cells/100 fibers and after training: 2.9 ± 2.2 Pax7+/MyoD+ cells/100 fibers), SIT-2 (before training: 3.0 ± 1.4 Pax7+/MyoD+ cells/100 fibers and after training: 6.9 ± 1.3 Pax7+/MyoD+ cells/100 fibers), and MICT (before training: 2.4 ± 1.4 Pax7+/MyoD+ cells/100 fibers and after training: 6.7 ± 1.9 Pax7+/MyoD+ cells/100 fibers, P ≤ 0.05; Fig. 4B). Additionally, a significant increase in the number differentiating SCs was observed after 6 wk of either SIT-1 (before training: 2.0 ± 2.7 Pax7−/MyoD+ cells/100 fibers and after training: 3.3 ± 1.9 Pax7−/MyoD+ cells/100 fibers), SIT-2 (before training: 0.4 ± 0.9 Pax7−/MyoD+ cells/100 fibers and after training: 3.1 ± 1.6 Pax7−/MyoD+ cells/100 fibers), and MICT (before training: 1.4 ± 0.7 Pax7−/MyoD+ cells/100 fibers and after training: 3.4 ± 1.4 Pax7−/MyoD+ cells/100 fibers, P ≤ 0.05; Fig. 4C).
Fig. 4.
MyoD response of SCs to training. A: representative image of a Pax7/MyoD1/laminin/nuclei immunofluorescent stain on a muscle cross section. a: MyoD1 and laminin; b: Pax7 and laminin; c: MyoD1 and Pax7; d: nuclei (DAPI). B and C: Pax7/MyoD1 responses to training. Shown are active (Pax7+/MyoD1+; B) and differentiating (Pax7−/MyoD+; C) cells before and after SIT-1, SIT-2, and MICT expressed per 100 fibers. Values are means ± SE. *P ≤ 0.05, main effect for time by two-way repeated-measures ANOVA; #P ≤ 0.05 different from before training by a paired t-test.
To verify that the proportion of MyoD+/Pax7+ cells was similar to the proportion of PCNA+/Pax7+ cells, serial muscle cross sections were stained for either Pax7/MyoD/laminin or Pax7/PCNA/laminin in a small subset of subjects (SIT-1: n = 3 and SIT-2: n = 3; Fig. 5A, a–d). In the subset of subjects from the SIT-1 group, the proportion of Pax7+ cells expressing MyoD (Pax7+/MyoD+) was 19.6%, whereas the proportion of Pax7+ cells expressing PCNA (Pax7+/PCNA+) was 20.0% (Fig. 5B). In the subset of subjects from the SIT-2 group, the proportion of Pax7+ cells expressing MyoD (Pax7+/MyoD+) was 60.8% and the proportion of Pax7+ cells expressing PCNA (Pax7+/PCNA+) was 50.1%. Although the following stain was completed in a small subset of subjects, it did confirm that the proportion of Pax7+ cells identified as activated in serial sections with either MyoD or PCNA was similar.
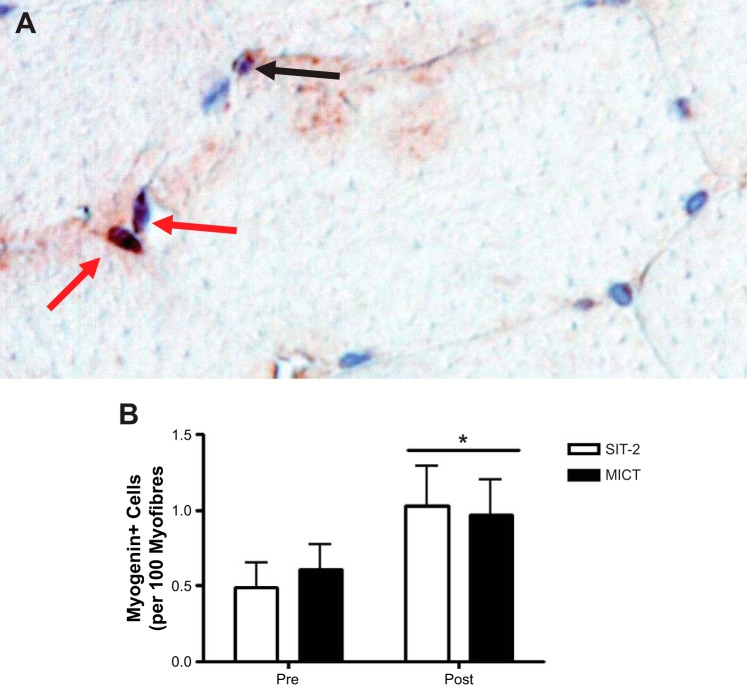
Muscle sections of the SIT-2 and MICT groups were also stained for myogenin (Fig. 6A), a MRF expressed during terminal differentiation (Fig. 6B). In accordance with the increase in differentiating SCs as assessed with MyoD staining, an increase in the number of cells stained positive for myogenin was observed after SIT-2 (before training: 0.5 ± 0.4 and after training: 1.0 ± 0.7) and MICT (before training: 0.6 ± 0.5 and after training: 1.0 ± 0.6).
Fig. 6.
Myogenin responses to training. A: representative image of a myogenin/Pax7 immunohistochemical stain. The black arrow shows perinuclear staining; red arrows show myogenin-positive cells. B: myogenin responses before and after SIT-2 and MICT. *P ≤ 0.05, main effect for time by two-way repeated-measures ANOVA.
DISCUSSION
These studies demonstrate, for the first time, that a nonhypertrophic training stimulus leads to an increase in SC activity without an appreciable expansion of the SC pool in humans. More specifically, we describe an increase in the proportion of active SCs after three different types of aerobic exercise despite different exercise intensities and fitness status of subjects. Similar increases in each group despite different training protocols and baseline fitness measures further support the likelihood that SCs may play a role in skeletal muscle adaptation beyond increases in fiber size. Our results suggest that describing the contribution of SCs to a training stimulus, such as resistance and/or endurance (traditional or sprint interval) exercise, via enumeration of the SC pool alone may not be sufficient to fully appreciate the contribution of SCs to muscle adaptations. General practice has been to describe the expansion of the SC pool to determine whether SCs contributed to adaptations in response to a given stimulus; however, the data in the present report provides evidence for the presence of activated SCs while the SC pool remained constant. This may reflect a slow and consistent contribution of SCs to muscle fibers where proliferation and differentiation are virtually matched so that no appreciable expansion can be detected. Alternatively, it is possible that the SC pool remains constant because of a loss in activated SCs that fail to fuse to existing myofibers, as previously shown in denervated rodent models (13). The inclusion of additional acute muscle biopsies after the exercise stimuli may have provided further information on the progression of SCs through the myogenic program. Determining whether intensity of the stimulus or the individual's training status may impact the initial expansion of the SC pool immediately after exercise or a change in activity status of the SC pool has yet to be determined.
Consistent with other reports, we did not observe an increase in CSA of either type I or II fibers after any of the interventions (Fig. 2, A and B) (7, 18, 23, 54). Additionally, training interventions did not lead to nuclear accretion (Fig. 2C). In accordance, the myonuclear domain, defined as the cytoplasmic volume governed by each nucleus, remained unchanged (Fig. 2D). Taken together, these data suggest that all three training interventions were nonhypertrophic regardless of training intensity, volume, or subject pool.
Several studies have examined expansion of the SC pool after various training interventions in humans. An increase in SC content after resistance training is well documented, which is usually associated with an increase in lean mass (21, 28, 43, 44, 59). The data regarding SC pool expansion after endurance training is equivocal with some studies reporting an increase in the SC pool (6, 61), whereas others reported no change (18, 54). Importantly, the studies reporting an expansion in the SC pool after aerobic training also reported an increase in muscle fiber CSA, suggesting that the intensity of exercise was sufficient to be hypertrophic in nature or that the subject pool was sufficiently sedentary to require a hypertrophic adaptation in response to the endurance training protocol. Taken together with our data, this suggests that for an expansion of the quiescent SC pool to occur, an increase in fiber size is required. However, the observed increase in SC pool activity may support skeletal muscle adaptations observed during endurance exercise interventions regardless of exercise intensity or training status of the individual.
Sprint interval training, much like endurance training, leads to numerous skeletal muscle adaptations (12). Although an expansion of the SC pool was not observed after the different training modalities used here, it is possible that SCs play a role in the nonhypertrophic muscle remodeling that occurs with this type of training. We found that all training interventions led to an increase in activated and differentiating SCs despite no appreciable expansion in the SC pool. It is well established that MyoD is an important regulator of myoblast proliferation (22, 40); however, the presence of MyoD in SCs may not be sufficient to identify a cell as proliferating since MyoD is simply a transcritpion factor and not a bona fide marker of proliferation. Other specific markers of cell proliferation, such as Ki67 or PCNA, have been used to specifically identify proliferating SCs (29). Even among these markers, however, there exist discrepancies in the proportion of SCs expressing Ki67 and PCNA 24 h after eccentric exercise in muscle cross sections. These discrepancies are likely explained by the difference in protein half-life of Ki67 versus PCNA (4). We determined the proportion of Pax7+/MyoD+ cells and the number of Pax7+/PCNA+ cells in corresponding serial sections in a subset of subjects. Although the analysis was completed in a small number of subjects, due to limited tissue samples, we observed a similar proportion of Pax7+ cells coexpressing either MyoD or PCNA. These data suggest that the identification of a cell expressing both Pax7 and MyoD may be classified as an activated/proliferating SC as the proportion of PCNA+ cells is similar to the proportion of MyoD+ cells in serial muscle cross sections (Fig. 5B). Furthermore, as discussed by Rudnicki et al. (47), MyoD remains upregulated, whereas Pax7 is downregulated, at the onset of differentiation. On this basis, we indicated that the cells expressing both Pax7 and MyoD (Pax7+/MyoD+) were activated SCs, whereas cells expressing only MyoD (Pax7−/MyoD+) were cells that originally expressed Pax7 but initiated the process of differentiation. To ensure that the MyoD+ cells identified were in fact SCs and not existing myonuclei, we qualitatively assessed the anatomic location of MyoD+ nuclei within muscle cross sections (Fig. 1, F–J). Several muscle cross sections were stained accordingly, and MyoD+ nuclei were consistently found to reside within the SC niche (between the basal lamina and sarcolemma). Several rodent studies have reported that myonuclei and not only SCs express MyoD (16, 17). Based on our observations that MyoD+ nuclei were almost exclusively located within the SC niche, we suggest that nuclei expressing MyoD but not Pax7 were differentiating SCs and not myonuclei based on the anatomic localization of MyoD+ nuclei.
No change in fiber type distribution was observed after 6 wk of SIT-1, however, a greater proportion of type I fibers was observed after both SIT-2 and MICT, which is consistent with a previous report (50). Irradiation studies have demonstrated that when SCs are ablated, a fiber type shift can still be achieved, suggesting that SCs may not be necessary for fiber type shifting (45, 46). However, while not necessary, SCs may be sufficient to induce fiber type transitions, as we have previously shown that after training, hybrid fibers were associated with a greater number of SCs compared with either type I or II fibers (18). The increase in mitochondrial content and transition in MHC protein expression often observed after nonhypertrophic aerobic training may require a contribution of SCs, as evidenced by an increase in active SCs after three distinct types of aerobic training (Fig. 4, B and C).
Skeletal muscle is a highly plastic tissue. Muscle fibers are able to change in size (atrophy/hypertrophy) and alter their metabolic characteristics when challenged with appropriate stimuli. Adaptation of skeletal muscle occurs through changes in the translational capacity of resident myonuclei. It is commonly believed that nuclei are reprogrammed to modulate the transcriptional potential of muscle fibers. It is, however, also possible that nuclei are eliminated from the muscle fiber through enucleation or a process similar to pyknosis and karyorrhexis and replaced with new nuclei to further support muscle remodeling: the theory of myonuclear turnover (39). Here, we report an increase in proliferating and differentiating SCs (Fig. 4, B and C) without an appreciable expansion of the SC pool (Fig. 3, B and C). Additionally, an increase in the number of nuclei per fiber was not observed (Fig. 2C), suggesting, in combination with an increase in SC activity (Fig. 4, B and C), that there may be an elimination of myonuclei throughout the training process and an addition of new myonuclei. A study (1) using rodent models has described a reduction in the number of nuclei per fiber and an increase in apoptotic nuclei in various atrophic conditions. Although this notion has been challenged (14), this suggests that the nuclear content in skeletal muscle may fluctuate in response to different stimuli.
It is generally believed that SCs contribute to muscle hypertrophy induced through resistance exercise training via nuclear addition, but here we demonstrate that when faced with a nonhypertrophic stimuli, SCs may still play a role, albeit likely different than that associated with resistance training, as training did not lead to an increase in nuclear content. Additionally, our results highlight the importance of considering the activation status of the SC pool when describing their contribution to various stimuli in humans, as simply enumerating SC content using various markers of SCs, such as Pax7 or neural cell adhesion molecule, may not be sufficient in fully describing the response of the SC to a given stimulus.
GRANTS
This study was funded by a Natural Sciences and Engineering Research Council Discover Grant (to G. Parise).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J., B.R.M., T.D.S., B.J.G., J.B.G., M.J.G., and G.P. conception and design of research; S.J., B.R.M., J.P.N., T.D.S., B.J.G., J.B.G., and M.A.T. performed experiments; S.J., B.R.M., and J.P.N. analyzed data; S.J., B.R.M., J.P.N., and G.P. interpreted results of experiments; S.J. prepared figures; S.J. and G.P. drafted manuscript; S.J., B.R.M., J.P.N., T.D.S., B.J.G., J.B.G., M.J.G., M.A.T., and G.P. edited and revised manuscript; S.J., B.R.M., J.P.N., T.D.S., B.J.G., J.B.G., M.J.G., M.A.T., and G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The Pax7 hybridoma cells developed by Dr.A. Kawakami, the A4.951 cells developed by Dr. H. Blau, and F5D cells developed by Dr. W. E. Wright were obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development and maintained at Department of Biology of The University of Iowa (Iowa City, IA).
REFERENCES
- 1.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22: 1350–1360, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224: 7–16, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Van Loon LJC. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 45: 230–237, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chargé SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Charifi N, Kadi F, Féasson L, Denis C. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28: 87–92, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cocks M, Shaw CS, Shepherd SO, Fisher J, Ranasinghe AM, Barker TA, Tipton KD, Wagenmakers AJ. High intensity interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol 3: 641–656, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crameri RM, Langberg H, Magnusson P, Jensen CH, Schrøder HD, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558: 333–340, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15: 666–673, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fiber type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibala M. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab 34: 428–432, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Grounds MD, White JD, Rosenthal N, Bogoyevitch MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 50: 589–610, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol 586: 2675–2681, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin Cell Dev Biol 16: 575–84, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hyatt JPK, Roy RR, Baldwin KM, Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol 285: C1161–C1173, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ishido M, Kami K, Masuhara M. Localization of MyoD, myogenin and cell cycle regulatory factors in hypertrophying rat skeletal muscles. Acta Physiol Scand 180: 281–289, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky a M, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27: 4596–605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma JK, Scribbans TD, Edgett BA, Boyd JC, Simpson CA, Little JP, Gurd BJ. Extremely low-volume, high-intensity interval training improves exercise capacity and increases mitochondrial protein content in human skeletal muscle. Open J Mol Integr Physiol 03: 202–210, 2013. [Google Scholar]
- 20.Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol 111: 189–195, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113: 99–103, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142: 1447–59, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurosaka M, Naito H, Ogura Y, Machida S, Katamoto S. Satellite cell pool enhancement in rat plantaris muscle by endurance training depends on intensity rather than duration. Acta Physiol 205: 159–166, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macaluso F, Myburgh KH. Current evidence that exercise can increase the number of adult stem cells. J Muscle Res Cell Motil 33: 187–198, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Mackey AL, Andersen LL, Frandsen U, Sjøgaard G. Strength training increases the size of the satellite cell pool in type I and II fibres of chronically painful trapezius muscle in females. J Physiol 589: 5503–5515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackey AL, Esmarck B, Kadi F, Koskinen SOA, Kongsgaard M, Sylvestersen A, Hansen JJ, Larsen G, Kjaer M. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports 17: 34–42, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40: 455–465, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110: 16474–16479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay BR, De Lisio M, Johnston APW, O'Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLos One 4: e6027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 304: C717–C728, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. [DOI] [PubMed] [Google Scholar]
- 37.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. [DOI] [PubMed] [Google Scholar]
- 38.McKay BR, Toth KG, Tarnopolsky MA, Parise G. Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol 588: 3307–3320, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve 29: 707–715, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10: 1173–1183, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Petrella JK, Kim J, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 73: 2538–2543, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflügers Arch 423: 255–264, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Scribbans TD, Edgett a B, Vorobej K, Mitchell AS, Joanisse SD, Matusiak JBL, Parise G, Quadrilatero J, Gurd BJ. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLos One 9: e98119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J 280: 4063–4073, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLos One 5: e13307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285: E197–E205, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Snijders TIM, Verdijk LB, Hansen D, Dendale P, Van Loon LJC. Continuous endurance-type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve 43: 393–401, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M, Yamamoto K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and V̇o2 max. Med Sci Sports Exerc 28: 1327–1330, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr Opin Genet Dev 13: 413–422, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction-modified Bergström muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve 43: 717–25, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Toth KG, McKay BR, de Lisio M, Little JP, Tarnopolsky MA, Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLos One 6: e17392, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg HHCM, Dendale P, Van Loon LJC. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol Ser A Biol Sci Med Sci 64: 332–339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verney J, Kadi F, Charifi N, Féasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 38: 1147–1154, 2008. [DOI] [PubMed] [Google Scholar]