Abstract
Preeclampsia is a devastating cardiovascular disorder of late pregnancy, affecting 5–7% of all pregnancies and claiming the lives of 76,000 mothers and 500,000 children each year. Various lines of evidence support a “tissue rejection” type reaction toward the placenta as the primary initiating event in the development of preeclampsia, followed by a complex interplay among immune, vascular, renal, and angiogenic mechanisms that have been implicated in the pathogenesis of preeclampsia beginning around the end of the first trimester. Critically, it remains unclear what mechanism links the initiating event and these pathogenic mechanisms. We and others have now demonstrated an early and sustained increase in maternal plasma concentrations of copeptin, a protein by-product of arginine vasopressin (AVP) synthesis and release, during preeclampsia. Furthermore, chronic infusion of AVP during pregnancy is sufficient to phenocopy essentially all maternal and fetal symptoms of preeclampsia in mice. As various groups have demonstrated interactions between AVP and immune, renal, and vascular systems in the nonpregnant state, elevations of this hormone are therefore positioned both in time (early pregnancy) and function to contribute to preeclampsia. We therefore posit that AVP represents a missing mechanistic link between initiating events and established midpregnancy dysfunctions that cause preeclampsia.
Keywords: preeclampsia, pregnancy, vasopressin, copeptin, hypertension
preeclampsia is a disease of pregnancy annually affecting more than 6.5 million pregnancies worldwide characterized by hypertension, multiorgan dysregulation, and maternal-fetal mortality (22). Although the ultimate etiology of preeclampsia is still unknown, the concepts that 1) the fetal-placental unit represents an allogenic, transplanted tissue to the mother, and 2) that preeclampsia is initiated through a rejection-type reaction have been forwarded by leaders in the field based on significant epidemiological and basic science data (13, 15, 17, 21). Dysregulation of the normal maternal immune tolerance to the fetus has been implicated as an initiator, as the immunological changes observed in the placenta of preeclamptic pregnancies is very similar to those observed in rejected organ transplants (13). Complementary studies of immunological tolerance also support these concepts. In humans, a 30% decreased risk of preeclampsia is observed with couples having a second child compared with those who change paternity in the second pregnancy (7, 24). Mouse models demonstrate that the disruption of immune tolerance mechanisms is sufficient to replicate human preeclampsia phenotypes (8, 19). Resulting immune rejection reactions are thought to lead to poor placental implantation, poor placental perfusion, and, by mechanisms not yet clearly delineated, the clinical symptoms of preeclampsia (15).
Numerous factors have been implicated in the midgestational progression of preeclampsia, including cytokines like tumor necrosis factor-α, anti-angiogenic factors like soluble fms-like tyrosine kinase, and microparticles secreted by the syncytiotrophoblasts (9, 15). Unfortunately, although these mediators appear to be mechanistically involved in the pathogenesis of preeclampsia (and may represent therapeutic targets to treat the disorder), the dysregulation of these markers in maternal plasma is observed only shortly before the onset of clinical symptoms near the end of the second trimester of gestation (11). The delay between the initial (presumably immune rejection/poor implantation) event and the activation of other immune/angiogenic mechanisms to elicit the clinical presentation of preeclampsia implies the existence of another unidentified mechanism to link these processes across the first trimester. That uterine artery dysfunction appears already in the first trimester (14) supports the concept that a vascular modulator may be involved in this “missing link” mechanism.
Role for Arginine Vasopressin
Arginine vasopressin (AVP) is a vascular modulator, and a role for AVP in normal and abnormal pregnancies has been considered since at least the early 1950s (10). Elevated AVP secretion during preeclampsia was only recently documented, though, after assays allowing easy assessment of copeptin (a stable protein by-product of AVP synthesis and release) became commercially available (12). In 2011, Zulfikaroglu et al. (25) described for the first time an association between preeclampsia and elevated copeptin levels in the third trimester of pregnancy after symptoms were already present (25). In 2012, Foda and Abdel Aal (2) confirmed in a small cohort that at parturition (well after the onset of symptoms), copeptin is elevated in preeclamptic pregnancies. In 2014, our group (20) demonstrated that copeptin levels are elevated already by the sixth week of gestation, well ahead of the onset of clinical symptoms of preeclampsia. Later in 2014, Yeung et al. (23) also demonstrated in a large and racially/ethnically diverse population that copeptin is indeed elevated during preeclampsia in the second trimester (again, before the onset of clinical symptoms). Collectively, these studies have independently confirmed an increase in copeptin (and by extension, AVP secretion) during, and preceding, preeclampsia in various subject populations that span the globe.
Mechanistic Links
We recently demonstrated that chronic low-dose infusion of AVP into wild-type mice throughout gestation is sufficient to induce all of the cardinal maternal and fetal symptoms of preeclampsia (20), and ongoing work is aimed at optimizing the model and identifying the receptors involved. In humans, four major classes of mechanisms have been implicated in the midgestational pathogenesis of preeclampsia: vascular, immune, angiogenic, and renal. In the nonpregnant state, vasopressin has been associated with each of these mechanisms through actions at its four receptor subtypes (V1A, V1B, V2, and CUL5) (1, 3, 4, 16, 18). Furthermore, the rs4606 single nucleotide polymorphism in the regulator of G protein signaling-2 (RGS2) gene, which acts as an endogenous brake on AVP signaling, results in decreased RGS2 function and correlates with human preeclampsia and its sequelae (5, 6). Thus AVP is sufficient to initiate preeclampsia symptoms in mice, and its receptors are known to interact with the identified major midgestational mechanisms of preeclampsia in human patients.
Hypothesis and Questions Moving Forward
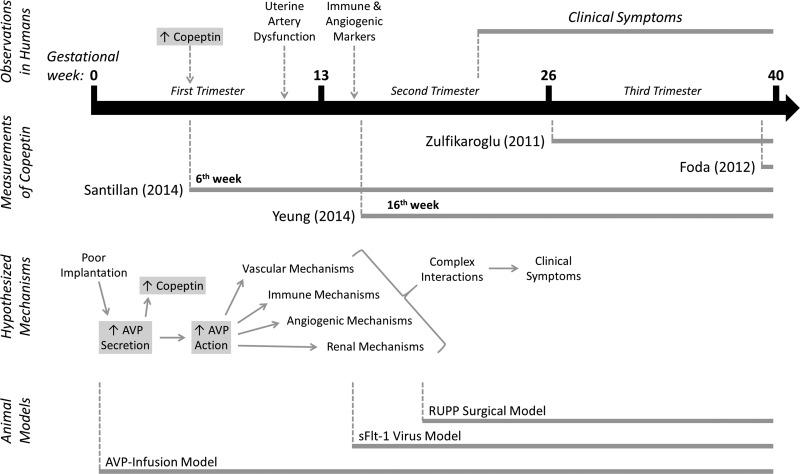
We now know that 1) AVP hypersecretion precedes preeclampsia symptoms, 2) AVP infusion is sufficient to initiate these symptoms, and 3) in the nonpregnant state AVP interacts with all of the mechanisms implicated in midgestational preeclampsia. We therefore hypothesize that AVP may represent a critical mechanistic link in the early pathogenesis of this disorder (Fig. 1).
Fig. 1.
Hypothesized role for vasopressin in the early pathogenesis of preeclampsia. Multiple groups have demonstrated elevated copeptin throughout pregnancies that develop preeclampsia, both before and after the onset of clinical symptoms. We hypothesize that poor implantation leads to the increased secretion of arginine vasopressin (AVP), which then acts through some combination of as-yet unidentified receptors and target tissues to initiate other later-appearing mechanisms. AVP infusion now represents a simple, third, clinically relevant, nongenetic model of preeclampsia.
We propose five groupings of ongoing questions: 1) Is copeptin a useful biomarker for the very-early pregnancy diagnosis of preeclampsia in all populations? What other factors (history of preeclampsia, comorbidities) alter this relationship? 2) Why is AVP secretion increased during preeclampsia? Is this solely from the brain? Does poor placentation stimulate AVP? If so, does it work via an osmotic or nonosmotic stimulus? Does inhibition of AVP secretion prevent preeclampsia? 3) Where, and through which receptors, does AVP act to initiate the phenotypes of preeclampsia? Are effects of AVP (vascular, immune, angiogenic, renal) the result of a single common mechanism, or through distinct mechanisms? 4) When does AVP act to initiate preeclampsia phenotypes? When would interference with AVP signaling be therapeutically beneficial? 5) Does the copeptin peptide itself have any pathological effect during pregnancy, either through a receptor-based mechanism, or via an immune response?
In summary, AVP and copeptin may represent the “missing link” between poor implantation and other later-appearing and well-recognized mechanisms of the pathogenesis of preeclampsia. We propose that new, extremely early gestation diagnostics to predict the development of preeclampsia will likely come from increased understanding of the regulation and role of AVP in this disorder. Preventative, and possibly corrective, therapeutics for preeclampsia may also therefore result from increased understanding of the actions of AVP and copeptin during early pregnancy.
GRANTS
The authors were supported by fellowships from the National Institutes of Health (T32 GM-067795 to J. A. Sandgren and T32 AI-007260 to S. M. Scroggins) and grants from the American Heart Association (14IRG18710013, 13SDG143400012, 15SFRN23480000, 15SFRN23730000, 15SFRN23760002, 15SFRN23860007), National Institutes of Health (HL-098276, HL-084207, HL-062984, HL-048058, AG043722), American Diabetes Association (1-14-BS-079), Shelly Bridgewater Dreams Foundation, Swift Family Foundation, University of Iowa Center for Hypertension Research, and Roy J. Carver Trust.
DISCLOSURES
J. L. Grobe and M. K. Santillan have submitted patent applications on the topic of using copeptin as a diagnostic biomarker to predict preeclampsia and to target the vasopressin system as a therapeutic approach to preeclampsia.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.S. and J.L.G. prepared figures; J.A.S. drafted manuscript; J.A.S., S.M.S., D.A.S., E.J.D., K.N.G.-C., G.L.P., C.D.S., M.K.S., and J.L.G. edited and revised manuscript; J.A.S., S.M.S., D.A.S., E.J.D., K.N.G.-C., G.L.P., C.D.S., M.K.S., and J.L.G. approved final version of manuscript.
REFERENCES
- 1.Buchwalter A, Van Dort C, Schultz S, Smith R, Le IP, Abbott JL, Oosterhouse E, Johnson AE, Hansen-Smith F, Burnatowska-Hledin M. Expression of VACM-1/cul5 mutant in endothelial cells induces MAPK phosphorylation and maspin degradation and converts cells to the angiogenic phenotype. Microvasc Res 75: 155–168, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Foda AA, Abdel Aal IA. Maternal and neonatal copeptin levels at cesarean section and vaginal delivery. Eur J Obstet Gynecol Reprod Biol 165: 215–218, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Germer A, Enzmann VV, Drossler K. Arginine-vasopressin influences the mitogen-induced development of T and B effector cells. Endocr Regul 30: 13–17, 1996. [PubMed] [Google Scholar]
- 4.Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev 92: 1813–1864, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet 15: 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Oian P, Andersgaard AB, Dechend R, Staff AC. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension 61: 655–661, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Li DK, Wi S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am J Epidemiol 151: 57–62, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Varea A, Pellicer B, Perales-Marin A, Pellicer A. Relationship between maternal immunological response during pregnancy and onset of preeclampsia. J Immunol Res 2014: 210241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 57: 1r–7r, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Mc Cartney CP, Vallach FJ, Pottinger RE. Further studies on the inactivation of pitressin antidiuretic effect by the blood of pregnant women. Am J Obstet Gynecol 63: 847–853, 1952. [DOI] [PubMed] [Google Scholar]
- 11.McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 207: 407 e401–407, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Internat 2014: 297397, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–543, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Ripoll GV, Garona J, Pifano M, Farina HG, Gomez DE, Alonso DF. Reduction of tumor angiogenesis induced by desmopressin in a breast cancer model. Breast Cancer Res Treat 142: 9–18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 30, Suppl A: S32–S37, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JA, Walley KR. Vasopressin and its immune effects in septic shock. J Innate Immunol 2: 446–460, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, Gibson-Corley KN, Scroggins SM, Grobe JL, Yang B, Hunter SK, Sigmund CD. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep 3: e12257, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 64: 852–859, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reproduct Immunol 101–102: 120–126, 2014. [DOI] [PubMed] [Google Scholar]
- 22.The Preeclampsia Foundation. http://www.preeclampsiaorg/, 2014. [Google Scholar]
- 23.Yeung EH, Liu A, Mills JL, Zhang C, Mannisto T, Lu Z, Tsai MY, Mendola P. Increased levels of copeptin before clinical diagnosis of preelcampsia. Hypertension 64: 1362–1367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Patel G. Partner change and perinatal outcomes: a systematic review. Paediatr Perinat Epidemiol 21, Suppl 1: 46–57, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res 37: 1198–1202, 2011. [DOI] [PubMed] [Google Scholar]