Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disorder that affects nearly 2 million people in the United States. The majority of SLE cases occur in women at an age in which the prevalence of hypertension and cardiovascular disease is typically low. However, women with SLE have a high prevalence of hypertension for reasons that remain unclear. Because immune cells and chronic inflammation have been implicated in the pathogenesis of both hypertension and SLE and because inflammation has been shown to be regulated by the autonomic nervous system, studies investigating neuroimmune mechanisms of hypertension could have direct and significant clinical implications. The purpose of this review is to introduce a recently described neuroimmune pathway and discuss its potential importance in the development of hypertension and renal injury during SLE.
Keywords: cholinergic anti-inflammatory pathway, autoimmunity, systemic lupus erythematosus, autonomic dysfunction, blood pressure
systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by aberrant immune function, including increased T cell activation and coactivation of B cells, increased T-helper cell numbers, and decreased T regulatory cells (17, 32). This abnormal T cell activity leads to the increased release of proinflammatory cytokines and differentiation of B cells into plasma cells (6, 17, 32, 37). Consequently, there is increased production of auto-antibodies [e.g., anti-double-stranded (ds) DNA autoantibodies] that form immune complexes, deposit into tissues, and initiate an inflammatory response (42). The resultant increase in inflammation can cause tissue damage in any organ system; however, the kidneys are prominently affected in the form of immune complex glomerulonephritis (4, 42, 44). Of the SLE patients that undergo renal biopsy, nearly all have evidence of a renal abnormality (e.g., glomerulonephritis), and ∼50% of these patients have impaired renal function (3, 12). Both of these can promote impaired renal hemodynamics and lead to increases in blood pressure. Indeed, the prevalence of hypertension in patients with SLE reaches as high as 74%, which contrasts sharply with normal healthy women (age ≤ 40 years), where the prevalence is typically only 2.7–14% (4, 40, 42). Despite the prevalent hypertension during SLE, the mechanisms responsible have not been elucidated.
Our laboratory is interested in determining mechanisms that may lead to the development of hypertension during SLE. In our studies, we utilize the female NZBWF1 mouse, an established model of SLE that results from mixing the genetic background of New Zealand White and New Zealand Black mice (2). We and others have demonstrated that these mice produce the anti-double-stranded (ds) DNA autoantibodies characteristic of human SLE (27). SLE mice also have increased number of splenic lymphocytes and impaired renal hemodynamics, and they exhibit renal damage in the form of albuminuria, glomerulosclerosis, and glomerulonephritis (27, 49). Most importantly, the SLE mouse is hypertensive (24–27). Because the SLE mouse has several characteristics similar to human SLE, it is an ideal model to examine the mechanisms that promote hypertension during chronic inflammatory disease.
Mechanisms Involved in the Pathogenesis of Hypertension
Hypertension, a major risk factor for cardiovascular disease, affects one out of every three adults in the United States, and the prevalence of hypertension is expected to increase by 7.2% by the year 2030 (11). The causative factor(s) in most cases of hypertension is unknown, and there is a large population of patients that are resistant to currently available hypertensive therapies. Therefore, there must be a continued effort to investigate mechanisms that may promote chronic elevations in blood pressure to reveal effective and novel therapeutic targets.
Vascular and neural mechanisms have been implicated in the pathogenesis of hypertension, and many studies focus on how these mechanisms cause alterations in the kidneys and contribute to chronic changes in blood pressure. The role of the nervous system, specifically the autonomic nervous system, is intriguing because when there is imbalance between the sympathetic and parasympathetic nervous systems, hypertension may result (36). For example, enhanced sympathetic nervous system activity, specifically through the renal sympathetic nerves, promotes chronic elevations in blood pressure both experimentally and clinically (15, 41). Conversely, reduced parasympathetic nerve activity may also promote hypertension, and there is evidence that alternative medical therapies, such as meditation and acupuncture, which both increase parasympathetic nervous system activity, may protect from chronic increases in blood pressure (19, 34). However, the mechanisms involved are unknown.
More recently, immune dysfunction and inflammation have been associated with the development of hypertension. Immunosuppressive therapy, thought to reduce both T and B cells, attenuates blood pressure in hypertensive patients with underlying rheumatoid arthritis and psoriasis (14). Similarly, animals that lack T and/or B cells are protected from experimental models of hypertension (7, 13). Because immune cells and chronic inflammation may be important in the pathogenesis of hypertension, it is important to understand regulation of the immune response during the disease. It is thought that the autonomic nervous system may control immune responses based on evidence that receptors for neurotransmitters are present on immune cells and also based on the idea that immune cells can themselves produce and secrete neurotransmitters (10). Neural regulation of the immune system may be an alternative way by which the autonomic nervous system can impact renal function and ultimately blood pressure.
Cholinergic Anti-Inflammatory Pathway
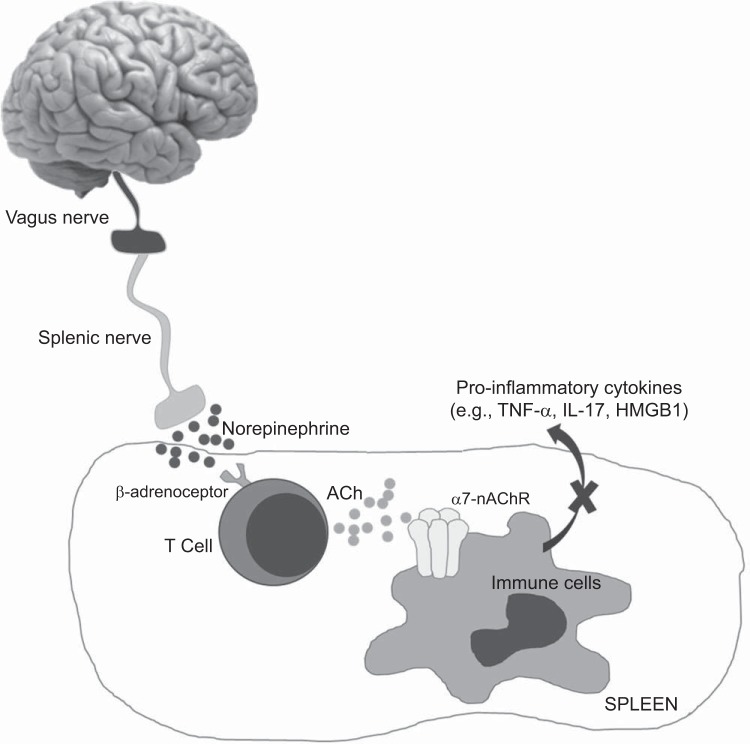
The classic inflammatory reflex is an interface between the brain and the immune system that provides a mechanism for neural inhibition of inflammation (35, 45). The afferent arm of the inflammatory reflex is activated by an unbalanced cytokine response from inflamed tissues. The normal efferent arm of this reflex, termed the cholinergic anti-inflammatory pathway, suppresses cytokine release and reduces inflammation (Fig. 1). In this pathway, once the vagus nerve is stimulated, it activates the splenic nerve that subsequently releases norepinephrine (38). Norepinephrine binds its β-adrenergic receptors on splenic ACh-producing T cells to cause ACh release (39, 47, 50), which, in turn, binds to the alpha 7 subunit of the nicotinic ACh receptor (α7-nAChR) on the same or nearby splenic T cells and macrophages through autocrine and paracrine mechanisms, respectively (35, 47, 51). This inhibits the production and release of proinflammatory mediators like TNF-α, IL-17, and high mobility group box 1 (HMGB1) from the spleen, and ultimately reduces systemic inflammation and tissue injury (47). An impaired cholinergic anti-inflammatory pathway would promote chronic inflammation, and since both SLE and hypertension are associated with chronic inflammation, it is of interest to determine whether this neuroimmune mechanism is critical.
Fig. 1.
The cholinergic anti-inflammatory pathway: The cholinergic anti-inflammatory pathway is a vagally mediated neuroimmune reflex that suppresses the release of inflammatory mediators from the spleen. Current understanding of the mechanism suggests that upon activation, the vagus nerve stimulates the splenic nerve to release norepinephrine. Norepinephrine then binds to its β-adrenergic receptors on T cells in the spleen. These T cells produce and secrete acetylcholine (ACh), which binds to its alpha 7 subunit of the nicotinic ACh receptor (α7-nAChR) on immune cells in the spleen, which inhibits the production and release of proinflammatory mediators, such as tumor necrosis factor (TNF)-α, interleukin (IL)-17 and high-mobility group box 1 (HMGB1) from the spleen. As a result, there is a reduction of systemic inflammation.
Role of the Cholinergic Anti-Inflammatory Pathway in SLE Hypertension
Autonomic dysfunction is common in SLE patients, and studies reveal impaired parasympathetic (vagal) tone during SLE (18, 22, 30, 43). Reduced vagal tone may suggest impairment of the cholinergic anti-inflammatory pathway, and this has been proposed as a possible mechanism leading to systemic inflammation and tissue injury in chronic autoimmune disorders (20, 21) and sepsis (38), as well as essential hypertension (16) and experimental models of hypertension (1, 9, 21). The cholinergic anti-inflammatory pathway could potentially be compromised at several locations: at the level of the vagus nerve (i.e., autonomic/vagal dysfunction), at the level of the cholinergic receptors (i.e., altered numbers of receptors), or at the level of the immune cells (i.e., over-responsive/overactive immune cells). Since both SLE and hypertension are associated with autonomic dysfunction and increased immune cell activation, we hypothesize that the cholinergic anti-inflammatory pathway is impaired during hypertension in the setting of SLE.
The cholinergic anti-inflammatory pathway requires an active vagus nerve; therefore, decreased vagal nerve activity is consistent with an impaired cholinergic anti-inflammatory pathway. We have collected preliminary data that suggest impaired vagal tone in hypertensive SLE mice (28). Additional unpublished data from our laboratory demonstrate splenic α7-nAChR expression is increased in hypertensive SLE mice (23), which may also indicate an impaired cholinergic anti-inflammatory pathway. Others have shown that the α7-nAChR is reduced in experimental models of hypertension in rats (5, 21); however, we propose that any alteration or unbalanced regulation of the receptor may be critical.
The cholinergic anti-inflammatory pathway may be an important target in hypertension research in future years. Stimulation of this pathway has already been shown to be protective in diseases of chronic inflammation (9, 20, 21, 33, 46, 48), and my unpublished preliminary data indicate that pharmacological stimulation of the cholinergic anti-inflammatory pathway via subcutaneous nicotine (2 mg·kg−1·day−1, 7 days), a nonselective agonist of the α7-nAChR, reduces splenic and renal inflammation and protects from the development of hypertension in SLE mice (29). These findings have been reproduced in other preliminary studies that utilize a selective agonist to stimulate the cholinergic anti-inflammatory pathway at the level of the α7-nAChR.
In summary, the cholinergic anti-inflammatory pathway may be important in the pathogenesis of hypertension during SLE. More studies are needed to determine whether reduced vagal tone and alterations in cholinergic receptors, as well as splenic immune cells, contribute to the development of chronic inflammation via an impaired cholinergic anti-inflammatory pathway and whether this increase in inflammation promotes impaired renal hemodynamics and hypertension during SLE.
Perspectives and Significance
SLE is an autoimmune disease that predominantly affects women in 90% of all cases. There is prevalent autonomic dysfunction, immune dysfunction, and hypertension in SLE; therefore, this disease model could be useful in determining the link between neuroimmune mechanisms and hypertension. The cholinergic anti-inflammatory pathway has been implicated in diseases of chronic inflammation; however, the association between this pathway and SLE has not been adequately explored (8). While manipulation of this pathway is promising in other diseases of chronic inflammation like rheumatoid arthritis, the mechanisms of action remain unclear and are the focus of much debate (31). Studies investigating the role of the cholinergic anti-inflammatory pathway in SLE will aid the understanding of how neuroimmune interactions may promote alterations in renal function and lead to hypertension. This insight can potentially lead to novel therapeutic targets for patients with SLE and essential hypertension.
GRANTS
The unpublished preliminary work referred to in this review was supported by a research grant to K. W. Mathis from the American Heart Association (14SDG18320033).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: K.W.M. prepared figures; K.W.M. drafted manuscript; K.W.M. edited and revised manuscript; K.W.M. approved final version of manuscript.
REFERENCES
- 1.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 59: 755–762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. J Exp Med 148: 1198–1215, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boumpas D, Austin H, Fessler B, Balow J, Klippel J, Lockshin M. Systemic lupus erythematosus: emerging concepts. Part 1: Renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med 122: 940–950, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Budman D, Steinberg A. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007, 1976. [PubMed] [Google Scholar]
- 5.Chen JK, Zhao T, Ni M, Li DJ, Tao X, Shen FM. Downregulation of alpha7 nicotinic acetylcholine receptor in two-kidney one-clip hypertensive rats. BMC Cardiovasc Disord 12: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol 6: 317–325, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowley S, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das UN. Can vagus nerve stimulation halt or ameliorate rheumatoid arthritis and lupus. Lipids Health Dis 10: 19–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling O, Rochelson B, Way K, Al-Abed Y, Metz C. Nicotine inhibits cytokine production by placenta cells via NF-κB: Potential role in pregnancy-induced hypertension. Mol Med 13: 576–583, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco R, Pacheco R, Lluis C, Ahern G, O'Connell P. The emergence of neurotransmitters as immune modulators. Trends Immunol 28: 400–407, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Go A, Mozaffarian D, Roger V, Benjamin E, Berry J, Borden W, Bravata D, Dai S, Ford E, Fox C, Franco S, Fullerton H, Gillespie C, Hailpern S, Heit J, Howard V, Huffman M, Kissela B, Kittner S, Lackland D, Lichtman J, Lisabeth L, Magid D, Marcus G, Marelli A, Matcher D, McGuire D, Mohler E, Moy C, Mussolino M, Nichol G, Paynter N, Schreiner P, Sorlie P, Stein J, Turan T, Virani S, Wong N, Woo D, and Turner MB; on behalf of the American Heart Association Statistics Subcommittee and Stroke Statistics Committee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127: e6–e245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Q, Lu X, Miao L, Wu M, Lu S, Luo P. Analysis of clinical manifestations and pathology of lupus nephritis: a retrospective review of 82 cases. Clin Rheumatol 29: 1175–1180, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17: 218–225, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Iliescu R, Yanes L, Bell W, Dwyer T, Baltatu O, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol 290: R341–R344, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Julius S. Autonomic nervous dysfunction in essential hypertension. Diabetes Care 14: 249–259, 1991. [DOI] [PubMed] [Google Scholar]
- 17.La Cava A. Lupus and T cells. Lupus 18: 196–201, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Lagana B, Tubani L, Maffeo N, Vella C, Makk E, Baratta L, Bonomo L. Heart rate variability and cardiac autonomic function in systemic lupus erythematosus. Lupus 5: 49–55, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Lai X, Wang J, Nabar N, Pan S, Tang C, Huang Y, Hao M, Zhou SF. Proteomic response to acupuncture treatment in spontaneously hypertensive rats. PLoS One 7: e44216, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leib C, Goser S, Luthje D, Ottl R, Tretter T, Lasitschka F, Zittrich S, Pfitzer G, Katus HA, Kaya Z. Role of the cholinergic anti-inflammatory pathway in murine autoimmune myocarditis. Circ Res 109: 130–140, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Li DJ, Evans RG, Yang ZW, Song SW, Wang P, Ma XJ, Liu C, Xi T, Su DF, Shen FM. Dysfunction of the cholinergic anti-inflammatory pathway mediated organ damage in hypertension. Hypertension 57: 298–307, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Louthrenoo W, Ruttanaumpawan P, Aramrattana A, Sukitawut W. Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. Q J Med 92: 97–102, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Mathis Keisa W. Evidence of an impaired neuorimmune pathway in autoimmune-associated hypertension. FASEB J 29: Suppl 811.13, 2015. [Google Scholar]
- 24.Mathis KW, Venegas-Pont M, Flynn E, Williams J, Maric-Bilkan C, Dwyer T, Ryan M. Hypertension in an experimental model of systemic lupus erythematosus occures independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol 305: R711–R719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathis KW, Venegas-Pont M, Masterson C, Stewart N, Wasson K, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59: 673–679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis KW, Venegas-Pont M, Masterson C, Wasson K, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Physiol Regul Integr Comp Physiol 301: R1281–R1285, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathis KW, Wallace K, Flynn E, Maric-Bilkan C, LaMarca B, Ryan M. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 64: 792–800, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathis KW. Nicotine reduces blood pressure in mouse model of systemic lupus erythematosus (Abstract). FASEB J 27, 1116.2. 2013. [Google Scholar]
- 29.Mathis KW. Stimulation of a neuroimmune pathway protects from hypertension (Abstract). FASEB J 28 Suppl 1: 860.22 2014. [Google Scholar]
- 30.Maule S, Quadri R, Mirante D, Pellerito A, Marucco E, Marinone C, Vergani D, Chiandussi L, Zanone M. Autonomic nervous dysfunction in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA): possible pathogenic role of autoantibodies to autonomic nervous structures. Clin Exp Immunol 110: 423–427, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAllen R, Cook A, Khiew H, Martelli D, Hamilton J. The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res Ther 17: 87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy G, Koncz A, Perl A. T- and B-cell abnomalities in systemic lupus erythematosus. Crit Rev Immunol 25: 123–140, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol 183: 6681–6688, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann NY Acad Sci 1172: 172–180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248: 188–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oparil S, Zaman M, Campochiaro PA. Pathogenesis of hypertension. Annals Intern Med 139: 761–776, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 358: 929–939, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholienrgic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA 105: 11,008–11,013, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosas-Ballina M, Olofsson PS, Ochani M, Ochani M, Valdes-Ferrer S, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neual signals in a vagus nerve circuit. Science 334: 98–101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabio J, Vargas-Hitos J, Navarrete-Navarrete N, Mediavilla J, Jimenez-Jaimez J, Diaz-Chamarro A, Jimenez-Alonso J. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: Novel implications for an old concept. Hypertension 54: 1–6, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 37: 1075–1082, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Stojanovich L, Milovanovich B, de Luka S, Popovich-Kuzmanovich D, Bisenich V, Djukanovich B, Randjelovich T, Krotin M. Cardiovascular autonomic dysfunction in systemic lupus, rheuamtoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus 16: 181–185, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Swaak AJ, van den Brink HG, Smeenk RJT, Manger K, Kalden JR, Tosi S, Marchesoni A, Domljan Z, Rozman B, Logar D, Pokorny G, Kovacs L, Kovacs A, Vlachoyiannopoulous PG, Moutsopoulos HM, Chwalinska-Sadowska H, Dratwianka B, Kiss E, Cikes N, Branimir A, Schneider M, Fischer R, Bombardieri S, Mosca M, Graninger W, Smolen JS. Systemic lupus erythematosus: Clinical features in patients with a disease duration of over 10 years, first evaluation. Rheumatology 38: 953–958, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Tracey K. Reflex control of immunity. Nat Rev Immunol 9: 418–428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Disc 4: 673–684, 2005. [DOI] [PubMed] [Google Scholar]
- 48.van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 60: 114–122, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover P, Ryan M. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 301: R1286–R1292, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vida G, Pena G, Kanashiro A, del Rocio Thompson-Bonilla M, Palange D, Deitch EA, Ulloa L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J 25: 4476–4485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor a7 subunit is an essential regulator of inflammation. Nature 421: 384–387, 2003. [DOI] [PubMed] [Google Scholar]