Abstract
Inflammatory lung diseases (e.g., pneumonia and acute respiratory distress syndrome) are associated with hyperglycemia, even in patients without a prior diagnosis of Type 2 diabetes. It is unknown whether the lung inflammation itself or the accompanying comorbidities contribute to the increased risk of hyperglycemia and insulin resistance. To investigate whether inflammatory signaling by airway epithelial cells can induce systemic insulin resistance, we used a line of doxycycline-inducible transgenic mice that express a constitutive activator of the NF-κB in airway epithelial cells. Airway inflammation with accompanying neutrophilic infiltration was induced with doxycycline over 5 days. Then, hyperinsulinemic-euglycemic clamps were performed in chronically catheterized, conscious mice to assess insulin action. Lung inflammation decreased the whole body glucose requirements and was associated with secondary activation of inflammation in multiple tissues. Metabolic changes occurred in the absence of hypoxemia. Lung inflammation markedly attenuated insulin-induced suppression of hepatic glucose production and moderately impaired insulin action in peripheral tissues. The hepatic Akt signaling pathway was intact, while hepatic markers of inflammation and plasma lactate were increased. As insulin signaling was intact, the inability of insulin to suppress glucose production in the liver could have been driven by the increase in lactate, which is a substrate for gluconeogenesis, or due to an inflammation-driven signal that is independent of Akt. Thus, localized airway inflammation that is observed during inflammatory lung diseases can contribute to systemic inflammation and insulin resistance.
Keywords: glucose production, tumor necrosis factor, interleukin-5
lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), pneumonia, and acute respiratory distress syndrome (ARDS) feature inflammation of the airway epithelium and are associated with hyperglycemia. This hyperglycemia increases the risk of poor outcomes in hospitalized patients with lung injury (3, 6, 9, 29, 42). Hyperglycemia and insulin resistance are present almost immediately after lung injury, and before the diagnosis of ventilator-associated pneumonia (34). Even several years after an infection with pneumonia, there is an association between the disease, hyperglycemia, and increased risk of death (22). The link between hyperglycemia and pulmonary disease is poorly understood, but it is thought to be due to insulin resistance rather than β-cell dysfunction (12). The severity of inflammation during lung disease is correlated with accompanying impairments in glucose homeostasis. However, it is unclear whether a primary inflammatory event localized in the airway epithelium as opposed to accompanying comorbidities (e.g., obesity, hypoxia, steroid treatment) or the presence of a specific pathogen (e.g., bacteria or virus) can impair insulin action.
Lung diseases and diabetes are both inflammatory disorders. Lung inflammation is associated with systemic inflammation (41). However, lung inflammation also is accompanied with comorbidities (e.g., obesity) that also induce inflammation. It is unknown whether isolated lung inflammation in the absence of other comorbidities that are known to increase inflammation and diabetes risk can induce system inflammation and insulin resistance. We predicted that targeted airway inflammation would induce systemic insulin resistance. We used doxycycline to induce the airway epithelium expression of a constitutively active Iκβ kinase (IKK2), an activator of the key inflammatory regulator: nuclear factor-κB, in a transgenic mouse model. When activated, IKK2 triggers the activation and nuclear translocation of NF-κB. NF-κB activates the transcription of genes involved in inflammation, such as various cytokines and chemokines. The induction of NF-κB resulted in neutrophilic and macrophage infiltrate in the lung (10). We demonstrated that acute airway inflammation, even in the absence of hypoxemia or other commodities that are commonly seen with lung disease and that increase diabetes risk, induced systemic insulin resistance and inflammation.
METHODS
Animal care and husbandry.
Male mice on a C57BL/6J (Figs. 2–7) or BALB/c (Fig. 1) background that express an inducible constitutively active Iκβ kinase (IKK2) in the airway epithelium were used (10). The mice that expressed constitutively active IKK2 were generated after mating two different transgenic mouse lines. The first group of transgenic mice expressed a constitutively active IKK2 under the control of a tet-O promoter (cIKK2) and a gene for tetracycline transcriptional silencer (tTS) that is driven by a CC10 promoter, which was specifically expressed in Clara cells of the lung epithelia (31). The double transgenic mouse line, was crossed with an additional transgenic mouse line that expresses a gene for reverse transactivator (rtTA) that is under the control of the CC10 promoter. This cross-generated a triple transgenic mouse line that expressed constitutively active IKK2 when exposed to doxycycline (denoted IKTA). Inclusion of doxycycline (1.0 g/l) in the drinking water for the control mice and the IKTA mice induced the expression of the constitutive IKK2 only in lung epithelial cells of the IKTA mice. Splenda (2.0 g/l) was also added to drinking water. Control littermates carried only the CC10-rtTA component. The mice were maintained in microisolator cages on a 12:12-h light-dark cycle with free access to food and water. All experiments were performed on mice that were ∼3 mo of age. The Vanderbilt University Institutional Animal Care and Use Committee approved all procedures performed.
Fig. 2.
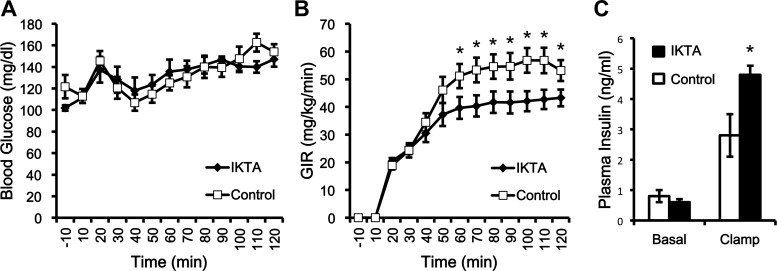
Lung inflammation decreases the amount of glucose required to maintain euglycemia, even though mice with lung inflammation have more circulating insulin during the clamp. The doxycycline-inducible mice (IKTA; n = 11) and controls (TA; n = 9) underwent a hyperinsulinemic-euglycemic clamp to measure whole body insulin action after receiving doxycycline for 5 days (C57BL/6J). Mice were treated with doxycycline to induce NF-κB expression, induce proinflammatory cytokine production, and lung inflammation in IKTA mice. A: blood glucose concentrations during the clamp. B: glucose infusion rate (GIR) during the clamp. C: plasma insulin concentrations during the basal period prior to the clamp and during the clamp. Data are expressed as means ± SE. *Significant difference, P < 0.05.
Fig. 7.
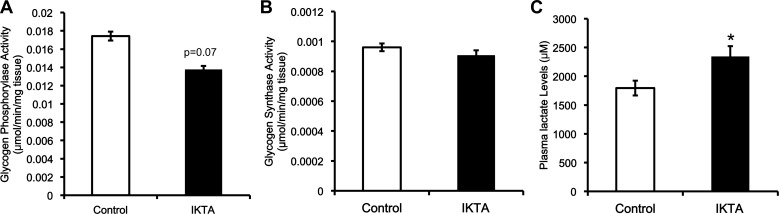
The increased glucose production in the liver that results from lung inflammation does not arise from increased glycogen breakdown and is likely due to an increase in substrate supply to gluconeogenesis. A: glycogen phosphorylase activity was normalized to milligram of tissue per reaction. B: glycogen synthase activity was normalized to milligrams of tissue per reaction. C: arterial plasma lactate concentration. Data are expressed as means ± SE. *Significant difference, P < 0.05.
Fig. 1.
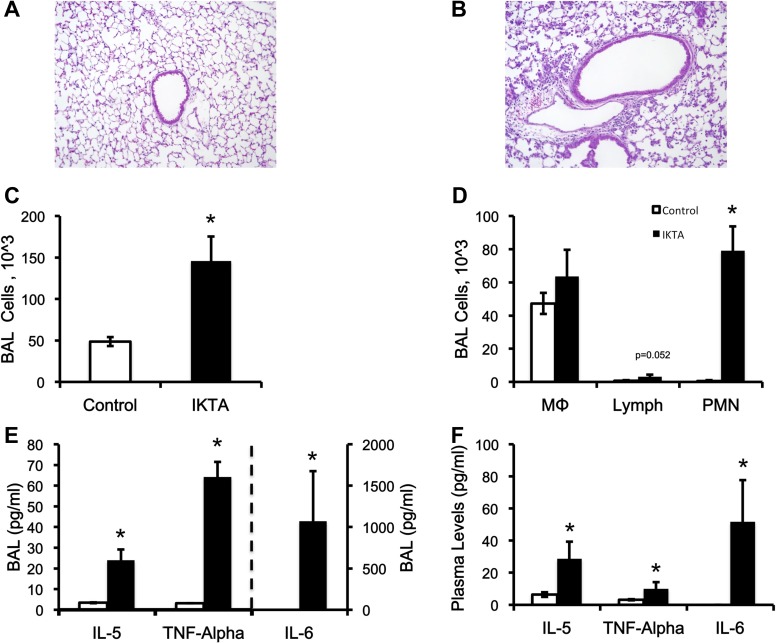
The induction of NF-κB-dependent lung inflammation increases circulating cytokines in the plasma. A: lung section stained with hematoxylin and eosin (H&E) in control mice (BALB/c). B: lung section stained with H&E in IKTA mice (a mouse line that expresses constitutively active IKK2 when exposed to doxycycline). C: total number of cells in the bronchoalveolar lavage (BAL) fluid. D: total number of macrophages, lymphocytes, and polymorphonuclear neutrophils in the BAL fluid. E: concentration of cytokines in the BAL fluid. F: concentration of cytokines in the plasma. Data are expressed as means ± the SE. *Significant difference, P < 0.05.
Surgical procedures.
The surgical procedures for implanting chronic catheters have been described previously (1, 2, 37). Six days before a metabolic study, mice were anesthetized with isoflurane. The left common carotid artery and right jugular vein were catheterized, allowing for sampling and infusing, respectively. The free ends of the catheters were tunneled subcutaneously to the back of the neck and attached via stainless-steel connectors to Micro-Renathane (0.033 in OD) tubing. The tubing was exteriorized and then plugged. After surgery, the animals were individually housed, and body weight was allowed to recover to within 10% of presurgical weight.
In vivo metabolic experiments.
Five days prior to metabolic experiments, chronically catheterized IKTA (C57BL/6J) mice and controls were given access to water that contained (1.0 g/l) doxycycline (Dox). IKK2 transgene induction specifically within airway epithelium has been previously shown to induce NF-κB activation and lung inflammation in IKTA, but not control mice The impact on systemic inflammation was not assessed (10). After a 5-h fast, a hyperinsulinemic-euglycemic clamp (goal glucose: 130 mg/dl) was performed to assess insulin action in control (n = 9) and IKTA (n = 11) mice. A bolus of 1.0 μCi [3-3H]-d-glucose was given (t = −120 min) followed by a constant 0.05 μCi/min infusion for 120 min until the initiation of the clamp. Clamp onset was defined as the initiation of an infusion of insulin (4 mU·kg−1·min−1), of glucose containing [3-3H]-d-glucose, and of reconstituted red blood cells from a donor mouse (t = 0 min). Arterial blood samples were taken every 10 min to determine blood glucose levels, and the glucose infusion rate was adjusted to maintain euglycemia. After a 120-min clamp period, a 5.0 μCi bolus of [14C-2]-deoxyglucose ([2-14C] DG) was given into the jugular vein. Blood samples for tracer analysis were collected at t =−10, 0, 80, 90, 100, 120, 122, 125, 130, 135, and 145 min. At the end of the clamp, the mice were terminally anesthetized with pentobarbital sodium. The soleus, gastrocnemius, superficial vastus lateralis (SVL), liver, heart, and brain were excised, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. Plasma glucose levels, plasma glucose specific activity ([3-3H]-d-glucose and [14C-2]-deoxyglucose), and tissue accumulation of phosphorylated [2-14C] DG were assessed. Tracer-determined glucose flux and tissue glucose uptake (Rg) were calculated, as previously described (1, 35).
H&E staining, BAL cell collection, and counting.
Mice were euthanized after 5 days of Dox. At the time of death, lungs were lavaged (1.0 ml sterile normal saline) and then perfused and fixed in formalin for 24 h. After fixation, lungs were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin (H&E). The bronchoalveolar lavage (BAL) fluid was centrifuged at 300 g for 10 min to separate cells from the supernatant. Total and differential cell counts were assessed as previously described (45). Cytokine (TNF-α, IL-1β, IL-5, and IL-6) content in BAL samples and plasma was assessed using multiplex technology (MAGPIX, Millipore Billerica, MA).
Western blot analysis.
Liver protein samples were homogenized using an extraction buffer (50 mM Tris, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100 at pH 7.5 with 1 mM DTT, 1 mM PMSF, 5 μg/ml protease inhibitor cocktail, 10 μg/ml trypsin inhibitor, 50 mM NaF, and 5 mM NaPP added before homogenization). After homogenization, tissues were centrifuged for 10 min at 4°C. Protein extracts were separated by electrophoresis on an SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA). Proteins were transferred to a PVDF transfer membrane (Bio-Rad, Hercules, CA) by electroblotting. Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline-Tween (TBS-T) (Sigma, St. Louis, MO) and then incubated overnight with antibodies to total Akt, phospho-Akt (Ser-473), β-actin (Cell Signaling, Danvers, MA), binding immunoglobulin protein (BiP; Cell Signaling), C/EBP homologous protein (CHOP; Santa Cruz, Dallas, TX), and phosphoenolpyruvate carboxykinase (PEPCK; Abcam, Cambridge, MA). After primary incubation, the blots were incubated with the appropriate peroxidase-conjugated secondary antibody (Cell Signaling) and were analyzed using chemiluminescence (GE Healthcare, Piscataway, NJ). Bands were quantified via ImageJ software.
Real-time PCR.
Total mRNA was extracted from liver, skeletal muscle, and brain using TRIzol. The cDNA was synthesized from 2 μg RNA with the high-capacity cDNA transcription kit. PCR amplification was carried out using the Bio-Rad CFX system with TaqMan probes, and the ΔΔCt method was used to quantify mRNA levels. Data are represented using the Rq value, which is the normalization relative to the rtTA (control) group or Rq = 2−ΔΔCt [ΔCt = Ct (target)–Ct (GAPDH); ΔΔCt = ΔCt (sample)–ΔCt (rtTA)]. Gene expression was normalized against the expression of GAPDH. All PCR products were retrieved from Life Science Technologies (Grand Island, NY).
Glycogen phosphorylase and glycogen synthase assays.
Liver samples were homogenized (∼10 mg in 500 μl) in buffer (50 mM Tris:pH 6.8, 100 mM NaF, 10 mM EDTA, 5.0 mM dithioerythritol, and 0.5% glycogen). Glycogen synthase activity was measured after incubating the homogenate with the synthase reaction medium (50 mM Tris: pH 7.5, 1.5 mM UDP-glucose, 5.0 mM EDTA, 1% glycogen, 5.0 μCi [14C] UDP-glucose, and 3.0 mM glucose-6-phosphate). Glycogen phosphorylase activity was measured after incubating the homogenate with phosphorylase reaction medium (150 mM NaF, 15 mM glucose-1-phosphate, 1.5% glycogen, 5.0 μCi [14C]glucose-1-phosphate, and 7.5 mM AMP). Both reactions were incubated at 37°C, and 50-μl aliquots were spotted onto chromatography filter paper every 15 min for 30 min. The spotted filter paper was washed in 70% ethanol, and radioactivity was measured. Total glycogen synthase activity was calculated by the activity in the presence of 3.0 mM glucose-6-phosphate per milligram of tissue. The activity ratio was calculated by dividing the activity of glycogen synthase in the absence of glucose-6-phosphate and by the activity in the presence of glucose-6-phosphate. Total glycogen phosphorylase activity was calculated as the activity in the presence of 7.5 mM AMP per milligram of tissue. The activity of glycogen synthase represents the rate of glucose-6-phosphate incorporation into glycogen. The activity of glycogen phosphorylase is calculated as the rate of incorporation of glucose-1-phosphate into glycogen.
Lactate assay.
Plasma samples from the basal period of the clamp (t = −10 min and 0 min) were deproteinized with 4% perchloric acid. Lactate levels were determined in the supernatant by an enzymatic (lactate dehydrogenase) assay, where the fluorescence of NADH was assessed on a 96-well plate fluorometer, as previously described (26).
Data analysis.
Data are presented as the means ± SE. Paired comparisons were performed with the two-tailed Student's t-test or ANOVA with repeated measures. The significance level was set at P < 0.05.
RESULTS
The activation NF-κβ in the lung airway epithelium causes lung inflammation and systemic inflammation.
It has previously been demonstrated that the treatment of IKTA mice with Dox results in activation of NF-κB in the airway epithelium, and the consequent neutrophilic inflammation in the airways and alveoli (10). After 5 days of Dox, immune cell infiltration was increased in the IKTA mice (Fig. 1, A and B). In IKTA mice, the total number of cells in BAL fluid increased (Fig. 1C), largely due to an increase in polymorphonuclear neutrophils (Fig. 1D). Additionally, proinflammatory cytokines IL-5, TNF-α, and IL-6 were increased in the BAL fluid in the IKTA vs. control mice (Fig. 1E). Lastly, circulating levels of these same cytokines were increased in the plasma of the IKTA mice (Fig. 1F). IL-1β in both BAL and plasma was at or below the limit of detection (3.2 pg/ml; data not reported) in both groups.
Lung airway inflammation impairs the action of insulin in vivo.
After the 5 days of Dox treatment, C57BL6/J background mice underwent hyperinsulinemic-euglycemic clamps to evaluate insulin action in vivo (Fig. 2). During the clamp, the glucose levels were matched between the groups (Fig. 2A), but the glucose infusion rate (GIR) was lower in the IKTA group (Fig. 2B). The decreased GIR indicates that the IKTA mice had decreased insulin sensitivity. This decrease occurred despite IKTA mice having higher plasma insulin concentrations during the clamp (1.72-fold higher than control; Fig. 2C), which is consistent with insulin resistance.
Lung inflammation impairs hepatic insulin action and decreases peripheral tissue glucose uptake.
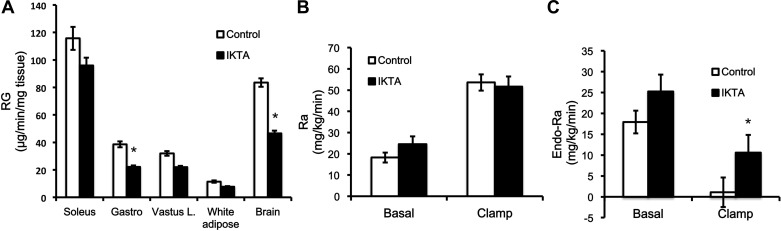
The ability of insulin to enhance peripheral glucose uptake and to suppress endogenous glucose production was assessed in C57BL/6 background mice. Despite higher insulin levels in IKTA mice, the induction of lung inflammation in IKTA mice significantly decreased tissue glucose uptake (Rg) in gastrocnemius muscle and the brain. The Ra (rate of appearance of glucose) was not significantly different between groups (Fig. 3B). Lastly, insulin's ability to suppress endogenous glucose production (Endo-Ra) was blunted in the IKTA mice (Fig. 3C).
Fig. 3.
Lung inflammation decreases glucose uptake. Mice with lung inflammation are not able to shut off the endogenous production of glucose. A: tissue glucose uptake (Rg). B: rate of glucose appearance during the clamp (Ra). C: rate of endogenous glucose production (Endo-Ra). Data are expressed as means ± the SE. *Significant difference, P < 0.05.
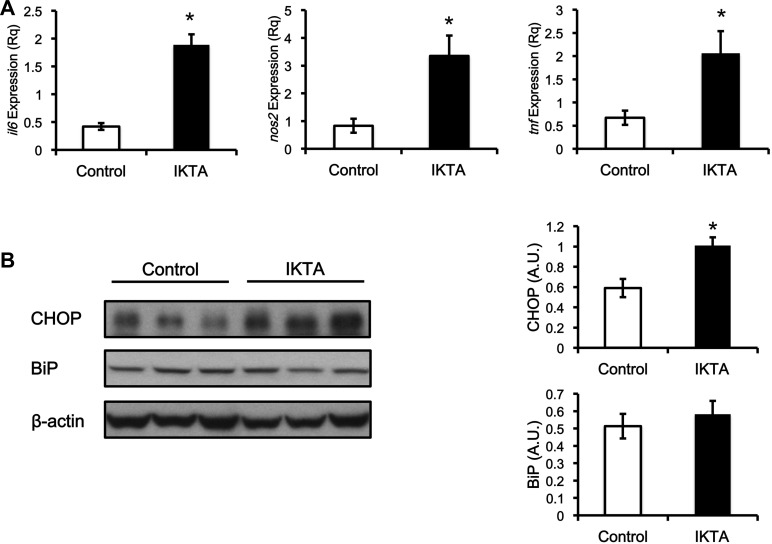
Lung airway inflammation triggered systemic and liver inflammation.
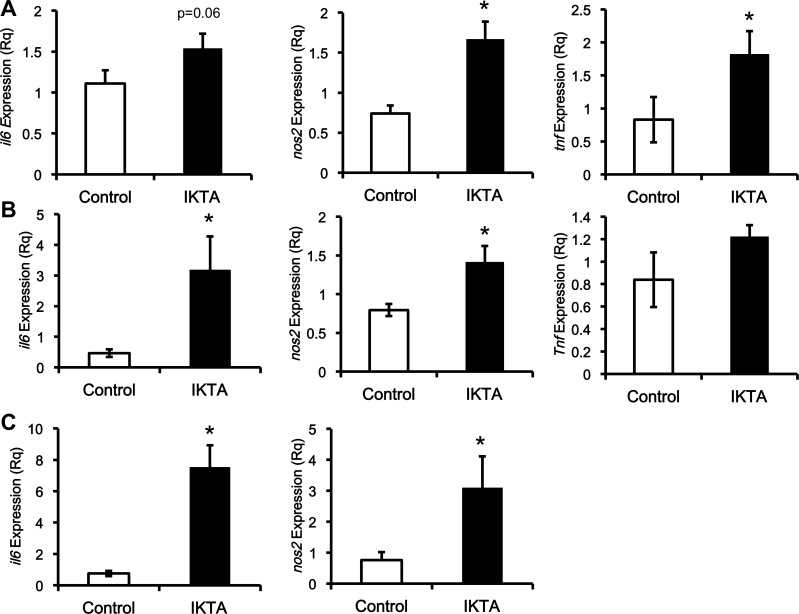
Hypoxemia from airway inflammation could lead to systemic insulin resistance. Hypoxemia was not present as arterial Po2 on the day of the clamp study was 93 ± 9 vs. 101 ± 5 mmHg in IKTA and control littermates, respectively. Airway inflammation, however, triggered systemic inflammation. The mRNA expression of Il6 (IL-6), Nos2 (iNOS), and Tnf (TNF-α) in the brain, superficial vastus lateralis muscle, gastrocnemius muscle, and the liver were increased in the IKTA mice (Figs. 4 and 5). In addition, the expression of CHOP protein was also increased (approximately twofold) in the liver, which could indicate an increase in ER stress (Fig. 5B). However, another marker of endoplasmic reticulum (ER) stress, BiP was unchanged (Fig. 5B).
Fig. 4.
Lung inflammation increases proinflammatory cytokines in peripheral tissues. The mRNA expression of markers for inflammation is induced by lung inflammation. A: expression of proinflammatory cytokines in brain tissue. B: expression of inflammatory cytokines in superficial vastus lateralis muscle. C: expression of proinflammatory cytokines in gastrocnemius muscle. The mRNA expression of il6, nos2, and tnf is corrected against the expression of gapdh (Rq). Data are expressed as the means ± SE. *Significant difference, P < 0.05.
Fig. 5.
Liver inflammation and endoplasmic reticulum (ER) stress marker C/EBP homologous protein (CHOP), but not binding immunoglobulin protein (BiP), are increased in mice with lung inflammation. The mRNA expression of markers for inflammation, and ER stress are all induced by lung inflammation. The mRNA expression of il6, nos2, and tnf is normalized against the expression of gapdh (Rq). B: protein expression of CHOP and BiP is normalized against β-actin (AU). Data are expressed as means ± SE. *Significant difference, P < 0.05.
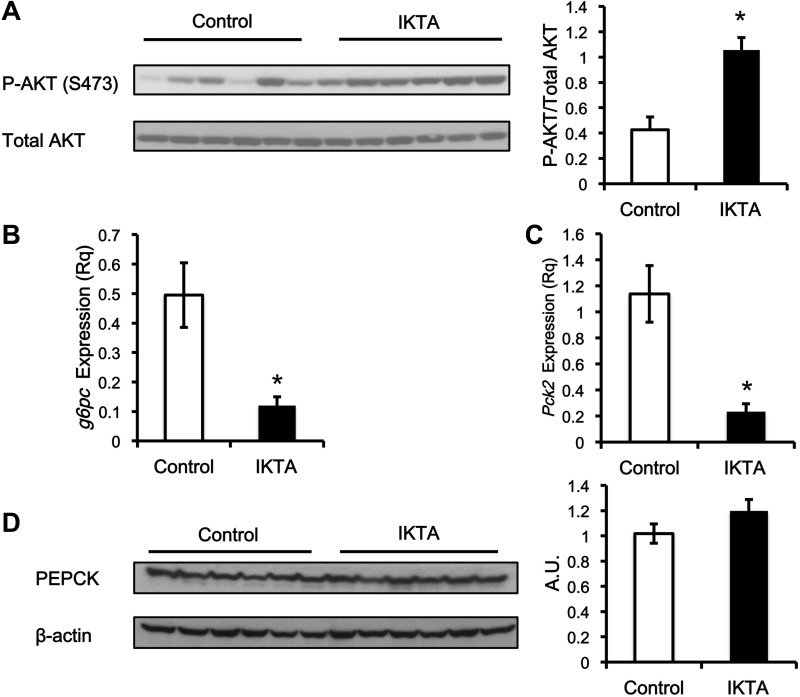
To understand the mechanism that accounts for the failure of insulin to suppress hepatic glucose production in the IKTA mice, we assessed markers of the insulin signaling pathway. The phosphorylation of Akt at Ser-473 was higher (∼2.5-fold) in the IKTA mice (Fig. 6A). Gene expression of downstream targets of insulin signaling, Pck1 (PEPCK) and G6pc (G6Pase), were also assessed (Fig. 6, B and C). Despite severe hepatic insulin resistance, the IKTA mice had a lower mRNA expression of Pck1 and G6pc. In addition, PEPCK protein content was not different between the control and IKTA mice (Fig. 6D). Glycogen synthase and phosphorylase activity were measured to determine whether activity of these enzymes was different between groups. Insulin stimulates the activity of glycogen synthase and inhibits the activity glycogen phosphorylase. The total activity of glycogen phosphorylase was not increased (P = 0.07) in the IKTA mice (Fig. 7A), and the activity ratio of glycogen phosphorylase (0.83 ± 0.03 vs. 0.85 ± 0.02; control vs. IKTA) was not different between groups. Total activity (Fig. 7B) and the activity ratio (0.17 ± 0.02 vs. 0.12 ± 0.02; control vs. IKTA) of glycogen synthase were similar between IKTA and control mice.
Fig. 6.
Insulin resistance in the liver is not explained by traditional insulin-induced signaling pathways. The insulin signaling pathway appears intact in the mice that have lung inflammation. Akt phosphorylation, pck2 expression, and g6pc expression were measured at the end of the clamp. A: Western blot for Akt phosphorylation. B: RNA expression levels of g6pc. C: RNA expression levels of pck2. D: representative Western blot for PEPCK protein. The amount of phosphorylated Akt is calculated as the average of phosphorylated protein divided by total Akt protein (AU). The protein expression of PEPCK is normalized against β-actin (AU). The RNA expression of g6pc and pck2 is normalized against the expression of gapdh (Rq). Data are expressed as means ± SE. *Significant difference, P < 0.05.
Given that increased glycogenolysis was not detected, we examined the factors important in regulating gluconeogenesis. While the expression of genes that control gluconeogenic capacity was not increased in the IKTA mice, circulating concentrations of the gluconeogenic substrate lactate was increased (Fig. 7C). Since increases in the amount of lactate in the plasma can increase gluconeogenesis, this could contribute to the persistent glucose production in the presence of insulin by the liver in the IKTA mice (13, 36, 48).
DISCUSSION
Lung diseases are associated with an increased risk for development of Type 2 diabetes. As they also increase known risk factors (e.g., obesity), the direct link between lung inflammation and glucose dyshomeostasis is unclear (6, 40, 44). This study highlights a direct link between airway inflammation and whole body insulin action. The aim of this study was to understand whether localized airway inflammation, similar to what occurs during lung disease, has a negative impact on the insulin regulation of glucose metabolism. Previous work has correlated lung diseases with impaired insulin action, but this link has not been directly tested. Additionally, these studies do not address whether the inflammation that occurs with many lung diseases impacts insulin action. We assessed the impact of airway-specific inflammation on insulin action using a mouse model in which we could chronically and selectively induce inflammation in the airway epithelium. Our findings show that targeted induction of low-grade inflammation in the lung airway epithelium that does not result in systemic hypoxemia triggers systemic insulin resistance and inflammation in liver and peripheral tissues.
The model system that was used in these studies triggers lung airway inflammation specifically in the epithelium (10, 43). The activation of NF-κB in the lung airway not only triggers inflammation and the accumulation of immune cells in the lung, but also causes an increase in circulating plasma proinflammatory cytokines IL-6, IL-5, and TNF-α (Fig. 1). While prior studies used a dose of Dox that induced enough airway injury to induce hypoxemia (10, 46), in the present study, the dose and duration of doxycycline were optimized to avoid the confounding effects of hypoxemia.
Impairment in hepatic insulin action is the primary mechanism, whereby airway inflammation impairs whole body insulin action in IKTA mice. The glucose requirements during the clamps for mice with inflammation in the airway epithelium were decreased by 23% in the IKTA mice relative to the control mice, despite higher insulin concentrations in the IKTA mice. On the basis of the calculated tracer-determined whole body glucose flux, the lower glucose requirements were primarily due to a failure of insulin to suppress hepatic glucose production (Fig. 2C). In control mice, insulin completely suppressed hepatic glucose production. In contrast, hepatic glucose production in the IKTA mice was not suppressed despite higher insulin concentrations.
The inability of insulin to inhibit the production of glucose by the liver was associated with hepatic inflammation and systemic inflammation. Like other tissues, the liver had an increase in the expression of proinflammatory makers (Il6, Tnf, and Nos2), which could induce hepatic insulin resistance (21). In addition, circulating concentrations of TNF-α, IL-5, and IL-6 were increased, which could also contribute to the observed hepatic insulin resistance. As has been reported with lung injury, IL-5 is increased and may explain the accumulation of polymorphonuclear neutrophils in the lung (15). The role of IL-5 in insulin action is unclear; a recent report suggests that the lack of IL-5 exacerbates insulin resistance when animals are on a high-fat diet (32). In individuals with airway disease, systemic IL-6 is increased and may, along with increased TNF-α, contribute to an increased diabetes risk (16, 17, 21). In the current study, we used mice on a C57BL/6 background to evaluate insulin action as opposed to Balb/c, as they are prone to dietary and inflammation-induced insulin resistance and have a robust cytokine response (2, 33, 35). However, it is possible that the absolute increase in plasma cytokines in response to airway injury may differ from that on a Balb/c background (46). The increase in inflammation in the liver could explain the insulin resistance. Insulin resistance is observed during systemic inflammatory events like obesity and endotoxemia, which also trigger an inflammatory response in the liver (7, 8, 18, 19, 24, 30). There are reports showing a crosstalk between the liver and the lung, although most of the prior work is focused on the impact of liver injury on lung inflammation (23). The present data suggest that the crosstalk is reciprocal; inflammation in the lung induces hepatic inflammation. Besides inducing inflammation in the liver, airway inflammation increased CHOP expression. CHOP, which is commonly increased with ER stress, may contribute to the hepatic insulin resistance (27). However, BiP, another ER stress marker, was not increased. Therefore, activation of ER stress alone would not be enough to explain the hepatic insulin resistance.
The insulin resistance that occurs due to inflammation typically results in suppression of the Akt signaling pathway (14, 18, 20, 49). It is not clear in vivo whether impaired insulin signaling in the liver via Akt is the contributor to insulin resistance. In support of the idea that Akt signaling does not always explain the insulin resistance phenotype, Akt phosphorylation and signaling in the liver was intact in IKTA mice. If hepatic insulin signaling were impaired due to systemic inflammation, there should be a blunted response to insulin on downstream gene targets, as well as a blunted phosphorylation of Akt (25). However, the RNA expression of Pck2 and G6pc was not increased; in fact, it was significantly lower in IKTA mice. Although mRNA expression of Pck2 was lower in the IKTA mice, protein levels of PEPCK were unchanged. In addition to gene targets, insulin also acts via the Akt signaling pathway to suppress the breakdown of glycogen by suppressing the activity of glycogen phosphorylase (25, 38). However, the activity of glycogen phosphorylase was not increased and trended lower in the mice with inflammation in their lung airway. Thus, insulin resistance in the liver cannot be explained by a failure of insulin to activate the traditional insulin-signaling pathway.
The increased production of glucose in the presence of insulin by the liver in IKTA mice could result from increased gluconeogenesis. As mentioned, the activities of glycogen synthase and phosphorylase between mice with and without inflammation of the lung airway cannot explain the failure of insulin to suppress hepatic glucose production. The protein expression of PEPCK was unchanged; however, the availability of gluconeogenic substrates, e.g., lactate, was increased and could drive gluconeogenic flux (13, 36). This elevated lactate could enter the liver and drive an increase in glucose production through gluconeogenesis in spite of intact insulin signaling. It is unlikely that the rise in lactate alone can explain the impaired suppression of hepatic glucose production. However, it may synergize with low-grade hepatic inflammation.
Airway inflammation impaired glucose uptake into various peripheral tissues. Glucose uptake was significantly decreased in the gastrocnemius muscle and trended toward a decrease in the vastus lateralis and soleus muscle. White adipose tissue glucose uptake was unaffected. The expression of Il6 and Nos2 in the gastrocnemius and vastus muscles was increased, likely reflecting increases in muscle inflammation. These increases combined with the increase in circulating IL-6 and TNF-α, probably contribute to the decrease in muscle insulin action. Interestingly, we also observed a decrease in glucose uptake and increased expression of proinflammatory markers (Tnf, Nos2, and Il6) in the brain. Various reports indicate that decreases in blood flow and endothelial dysfunction can negatively impact glucose uptake into muscle tissue. It is possible that this could contribute to the decrease in the glucose uptake into the brain (4, 5, 20, 28, 39). It is also possible that inflammation in the lung airway epithelium could impact glucose homeostasis due to a negative effect on muscle blood flow and microvascular recruitment without necessarily impacting insulin signaling (11).
Perspectives and Significance
This study indicates that there is a direct link between low-grade inflammation in the lung airway epithelium and systemic insulin resistance. Individuals with lung inflammation (COPD, asthma, and pneumonia) are at greater risk of developing hyperglycemia or diabetes. Although they have lung inflammation, they also have other associated issues that can cause insulin resistance (e.g., hypoxia, obesity, or steroid treatment). Thus, it was not known whether primary airway inflammation without the accompanying comorbidities could cause systemic inflammation and insulin resistance. In our studies, we demonstrate that primary airway inflammation does cause systemic inflammation and insulin resistance. Moreover, the liver was a major site of the insulin resistance. Lung airway induced hepatic inflammation, suggesting crosstalk between the liver and the lung. Thus, airway inflammation, even without the accompanying hypoxemia or obesity that can develop in various lung diseases, may contribute to the increased prevalence of insulin resistance and hyperglycemia in these individuals (3, 6, 9, 17, 29, 42, 47). Thus, new therapies that can limit airway inflammation to treat lung injury may have additional benefits beyond improving lung function. They may lower the risk of systemic inflammation and insulin resistance.
GRANTS
This work was supported by National Institutes of Health Grants DK-043748 and DK-078188 (to principle investigator: O. P. McGuinness) and the Department of Veteran's Affairs (1l01BX-002378) (to T. S. Blackwell). T. J. Cyphert and T. M. Barnes were supported by T32-DK-007563 and L. M. House, was supported by T32-DK-007061. Special thanks are given to the Mouse Metabolic Phenotyping Center (DK-59637) and Hormone Assay and Analytical Services Core (DK-059637 and DK-020593).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.J.C., R.T.M., L.M.H., T.M.B., Y.F.O., and R.Z. performed experiments; T.J.C., R.T.M., L.M.H., R.Z., and O.P.M. analyzed data; T.J.C. and O.P.M. interpreted results of experiments; T.J.C. and R.Z. prepared figures; T.J.C. drafted manuscript; T.J.C., R.T.M., L.M.H., T.M.B., Y.F.O., F.E.Y., T.S.B., and O.P.M. edited and revised manuscript; R.T.M., W.J.B., R.P.H., F.E.Y., T.S.B., and O.P.M. conception and design of research; O.P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
O. P. McGuinness had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Ayala JE, Bracy DP, Malabanan C, James FD, Ansari T, Fueger PT, McGuinness OP, Wasserman DH. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp 57: 3188, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baker EH, Janaway CH, Philips BJ, Brennan AL, Baines DL, Wood DM, Jones PW. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 61: 284–289, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD, Clark MG. Role of blood flow in the regulation of muscle glucose uptake. Annu Rev Nutrit 17: 487–499, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol Endocrinol Metab 266: E248–E253, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bolton CE, Evans M, Ionescu AA, Edwards SM, Morris RH, Dunseath G, Luzio SD, Owens DR, Shale DJ. Insulin resistance and inflammation. A further systemic complication of COPD. COPD 4: 121–126, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos MR, Szerszen A, Saifan C, Zigelboym I, Khoueiry G, Abi Rafeh N, Wetz RV, Kleiner M, Aoun N, Weiserbs KF, Maniatis T, Rothman J. Fasting hyperglycemia upon hospital admission is associated with higher pneumonia complication rates among the elderly. Int Arch Med 3: 16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Ds Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-B pathway. J Immunol 178: 6504–6513, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Djuric SW, Grihalde N, Lin CW. Glucagon receptor antagonists for the treatment of type II diabetes: current prospects. Curr Opin Investig Drugs 3: 1617–1623, 2002. [PubMed] [Google Scholar]
- 13.Felipe A, Remesar X, Pastor-Anglada M. l-lactate uptake by rat liver: effect of food deprivation and substrate availability. Biochem J 273: 195–198, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamment M, Hajduch E, Ferre P, Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23: 381–390, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 183: 195–201, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res 6: 316–324, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation 17: 192–205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res 107: 579–591, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52: 2784–2789, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Koskela HO, Salonen PH, Romppanen J, Niskanen L. Long-term mortality after community-acquired pneumonia-impacts of diabetes and newly discovered hyperglycaemia: a prospective, observational cohort study. Br Med J 4: e005715, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster LH, Christman JW, Blackwell TR, Koay MA, Blackwell TS. Suppression of lung inflammation in rats by prevention of NF-κB activation in the liver. Inflammation 25: 25–31, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lang CH, Bagby GJ, Spitzer JJ. Carbohydrate dynamics in the hypermetabolic septic rat. Metabolism 33: 959–963, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 14: 9–19, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd B, Burrin J, Smythe P, Alberti KG. Enzymic fluorometric continuous-flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutyrate. Clin Chem 24: 1724–1729, 1978. [PubMed] [Google Scholar]
- 27.Maris M, Overbergh L, Gysemans C, Waget A, Cardozo AK, Verdrengh E, Cunha JP, Gotoh T, Cnop M, Eizirik DL, Burcelin R, Mathieu C. Deletion of C/EBP homologous protein (Chop) in C57BL/6 mice dissociates obesity from insulin resistance. Diabetologia 55: 1167–1178, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Mauvais-Jarvis F. Novel link between inflammation, endothelial dysfunction, and muscle insulin resistance. Diabetes 62: 688–690, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care 28: 810–815, 2005. [DOI] [PubMed] [Google Scholar]
- 30.McGuinness OP. Defective glucose homeostasis during infection. Annu Rev Nutrit 25: 9–35, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res 27: e27, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210: 535–549, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56: 1129–1139, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee K, Sowards KJ, Brooks SE, Norris PR, Boord JB, May AK. Insulin resistance increases before ventilator-associated pneumonia in euglycemic trauma patients. Surg Infect (Larchmt) 15: 713–720, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Mulligan KX, Morris RT, Otero YF, Wasserman DH, McGuinness OP. Disassociation of muscle insulin signaling and insulin-stimulated glucose uptake during endotoxemia. PloS One 7: e30160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newgard CB, Hirsch LJ, Foster DW, McGarry JD. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem 258: 8046–8052, 1983. [PubMed] [Google Scholar]
- 37.Niswender KD, Shiota M, Postic C, Cherrington AD, Magnuson MA. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J Biol Chem 272: 22,570–22,575, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Ortmeyer HK, Bodkin NL, Hansen BC. Insulin regulates liver glycogen synthase and glycogen phosphorylase activity reciprocally in rhesus monkeys. Am J Physiol Endocrinol Metab 272: E133–E138, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Premilovac D, Bradley EA, Ng HL, Richards SM, Rattigan S, Keske MA. Muscle insulin resistance resulting from impaired microvascular insulin sensitivity in Sprague Dawley rats. Cardiovasc Res 98: 28–36, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, Speizer FE, Barr RG, Camargo CA Jr. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 27: 2478–2484, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 8: 1281–1290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rueda AM, Ormond M, Gore M, Matloobi M, Giordano TP, Musher DM. Hyperglycemia in diabetics and non-diabetics: effect on the risk for and severity of pneumococcal pneumonia. J Infect 60: 99–105, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Sadikot RT, Han W, Everhart MB, Zoia O, Peebles RS, Jansen ED, Yull FE, Christman JW, Blackwell TS. Selective I κB kinase expression in airway epithelium generates neutrophilic lung inflammation. J Immunol 170: 1091–1098, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sevenoaks MJ, Stockley RA. Chronic obstructive pulmonary disease, inflammation and comorbidity—a common inflammatory phenotype? Respir Res 7: 70, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B, Blackwell TS. Epithelial NF-κB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci USA 104: 18,514–18,519, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Hove CL, Maes T, Cataldo DD, Gueders MM, Palmans E, Joos GF, Tournoy KG. Comparison of acute inflammatory and chronic structural asthma-like responses between C57BL/6 and BALB/c mice. Int Arch Allergy Immunol 149: 195–207, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Ye X, Steinberg H, Liu SF. Selective blockade of endothelial NF-κB pathway differentially affects systemic inflammation and multiple organ dysfunction and injury in septic mice. J Pathol 220: 490–498, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Young AA, Wang MW, Cooper GJ. Amylin injection causes elevated plasma lactate and glucose in the rat. FEBS Lett 291: 101–104, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-α signaling through IKK2. J Biol Chem 283: 35,375-35,382, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]