Activating transcription factor 3 (ATF3) is an inducible transcriptional regulator of the inflammatory response. We used a loss of function approach to test whether ATF3 exerted maladaptive effects during heart failure in mice. ATF3 deletion significantly improved survival without grossly affecting histopathology during heart failure.
Keywords: hypertrophy, remodeling, ventricular dysfunction, fibrosis, inflammation
Abstract
Numerous fibrotic and inflammatory changes occur in the failing heart. Recent evidence indicates that certain transcription factors, such as activating transcription factor 3 (ATF3), are activated during heart failure. Because ATF3 may be upregulated in the failing heart and affect inflammation, we focused on the potential role of ATF3 on postinfarct heart failure. We subjected anesthetized, wild-type mice to nonreperfused myocardial infarction and observed a significant induction in ATF3 expression and nuclear translocation. To test whether the induction of ATF3 affected the severity of heart failure, we subjected wild-type and ATF3-null mice to nonreperfused infarct-induced heart failure. There were no differences in cardiac function between the two genotypes, except at the 2-wk time point; however, ATF3-null mice survived the heart failure protocol at a significantly higher rate than the wild-type mice. Similar to the slight favorable improvements in chamber dimensions at 2 wk, we also observed greater cardiomyocyte hypertrophy and more fibrosis in the noninfarcted regions of the ATF3-null hearts compared with the wild-type. Nevertheless, there were no significant group differences at 4 wk. Furthermore, we found no significant differences in markers of inflammation between the wild-type and ATF3-null hearts. Our data suggest that ATF3 suppresses fibrosis early but not late during infarct-induced heart failure. Although ATF3 deficiency was associated with more fibrosis, this did not occur at the expense of survival, which was higher in the ATF3-null mice. Overall, ATF3 may serve a largely maladaptive role during heart failure.
NEW & NOTEWORTHY
Activating transcription factor 3 (ATF3) is an inducible transcriptional regulator of the inflammatory response. We used a loss of function approach to test whether ATF3 exerted maladaptive effects during heart failure in mice. ATF3 deletion significantly improved survival without grossly affecting histopathology during heart failure.
heart failure (HF) is a complex, multifactorial disorder that affects over five million people in the United States. Heart failure leads to many significant structural and functional changes, including cardiomyocyte hypertrophy, ventricular remodeling, impaired calcium handling, and oxidative stress. Yet, recent advances in treating heart failure have been incremental and effectively palliative. Thus developing a more coherent understanding of the molecular underpinnings of heart failure represents a critical and unmet need. The role of inflammation, in particular, has received renewed attention in recent years. In fact, several transcription factors that may regulate the inflammatory response have been implicated in heart failure. One such transcription factor, activating transcription factor 3 (ATF3), is the focus of the present study.
ATF3 is a member of the cAMP response element-binding protein (CREB)/ATF family of basic region-leucine zipper (bZip) transcription factors (28, 34). ATF3 can act as either a transcriptional activator or repressor, and is involved in the rapid regulation of many genes (26). Under normal conditions, ATF3 is not detectable in the heart, but is induced rapidly following myocardial ischemia (4, 28). We recently demonstrated a role for ATF3 during ischemic preconditioning (2). Previous reports suggest that ATF3 induction is the result of cellular injury rather than inflammation (35); however, there are conflicting findings regarding whether this induction is beneficial or detrimental in the heart. Indeed, cardiac ATF3 overexpression may be sufficient to provoke conduction abnormalities and contractile dysfunction (28). Furthermore, ATF3 overexpression in otherwise healthy adult mice produces cardiac fibrosis, cardiac hypertrophy, and cardiac dysfunction (26); adult ATF3 overexpressing mice are also more susceptible to pressure overload-induced cardiac dysfunction, and complementary loss of function studies demonstrated that ATF3 deficiency reduces cardiomyocyte hypertrophy and protects the heart against pressure overload (26). Conversely, others have shown that ATF3 deficiency enhances cardiomyocyte hypertrophy in vitro, and ATF3 deficiency exacerbates cardiac fibrosis in a pressure overload model (40). Thus the role of ATF3 in the failing heart remains unclear and requires further examination.
To this end, the present study reports on the role of endogenous ATF3 during infarct-induced heart failure. We hypothesized that ATF3-deficient mice would have attenuated fibrotic and inflammatory responses thereby limiting pathophysiological changes in heart failure.
METHODS
Mice.
Male wild-type (WT) and ATF3-null mice were bred at the University of Louisville and maintained on a C57BL/6 background. ATF3-null mice (9) were obtained from Dr. Tsonwin Hai from the Department of Molecular and Cellular Biochemistry and Center for Molecular Neurobiology, The Ohio State University, Columbus, OH. The University of Louisville Institutional Animal Care and Use Committee approved all procedures.
Myocardial infarction.
Adult 12- to 20-wk-old male mice were subjected to nonreperfused, in vivo coronary artery ligation to induce heart failure, as described previously(1, 7, 18, 19, 31, 37) and in accordance with the University of Louisville Animal Care and Use Committee. Mice were anesthetized by using a combination of ketamine hydrochloride (50 mg/kg) and sodium pentobarbital (50 mg/kg), administered intraperitoneally. Mice were then intubated with PE-60 tubing and mechanically ventilated with 100% oxygen supplemented via the ventilator side port. Body temperature was maintained at 36.5–37.5°C by using a rectal thermometer interfaced with a servo-controlled heat lamp. Mice were subjected to a thoracotomy by using sterile technique, and the left coronary arteries were visualized with the aid of a dissecting microscope and permanently occluded with 7-0 silk sutures. After ligation, the chest and skin were closed by using 4-0 silk and polyester sutures, respectively. Mice were given 5 mg/kg of ketoprofen, subcutaneously, for analgesia. Upon recovery of spontaneous respiration, the intubation tube was removed and mice were allowed to recover in a temperature-controlled area supplemented with 100% oxygen. Mice were given additional doses of ketoprofen (5 mg/kg) by 24 h and 48 h postoperatively.
Infarct size analysis.
To evaluate for potential differences in the extent of initial injury, mice from both experimental and control groups were subjected to nonreperfused coronary artery ligation as described above. After 72 h of coronary occlusion, the mice were anesthetized with 5% isoflurane. Once the mice were fully nonresponsive, hearts were excised and perfused with 60 mM of potassium chloride. The hearts were then embedded in 4% agarose gel and sectioned by using a tissue chopper (McIlwain). The 1-mm heart sections were then stained with 2,3,5-triphenyl-tetrazolium chloride (TTC; Sigma-Aldrich) for 5 min at 37°C. After staining, the sections were removed from the TTC solution and photographed with a Nikon D90 digital camera, and the pictures were analyzed with the 64-bit version of ImageJ (1.47c) as described previously(5, 6, 10–18, 20–23, 27, 29, 30, 32, 33, 37, 39).
Echocardiography.
Transthoracic echocardiography of the left ventricle was performed as previously described(3, 5, 7, 17–20, 36, 37) with a Vevo 770 echocardiography system. Body temperature was maintained at 36.5–37.5°C by using a rectal thermometer interfaced with a servo-controlled heat lamp. Mice were anesthetized with 2% isoflurane then maintained under anesthesia with ∼1.5% isoflurane. By using the Vevo rail system, the mouse was placed chest up on an examination board interfaced with the Vevo 770. The board was outfitted with EKG electrodes for all limbs. Next, depilatory cream was applied to the mouse's chest and wiped clean to remove all hair in the area of interest. The 707-B (30 MHz) scan head was used to obtain 2D images (100 fps) of the parasternal long axis. M-modes were taken from the same images. The probe was then rotated to acquire a short axis view of the heart. Beginning at the level of the papillary muscles and moving apically, serial 2D images were taken every millimeter. All measurements were taken by utilizing the Vevo 770's rail system to maintain probe placement and allow for minute adjustments of position. Left ventricular diameters during diastole and systole (LVIDd and LVIDs) were determined from long axis M-modes along with heart rate (HR). Left ventricular fractional shortening (%FS) was calculated as [(LVIDd-LVIDs)/LVIDd]×100%. Diastolic and systolic volumes were acquired by applying Simpson's rule of discs to the serially acquired short axis images. Stroke volume (SV) was calculated as diastolic volume − systolic volume. Ejection Fraction was calculated as (SV/diastolic volume)×100%. Cardiac output was determined by SV×HR. Relative wall thickness was calculated as (diastolic posterior wall thickness + diastolic anterior wall thickness)/LVIDd. Velocity of circumferential shortening corrected for heart rate (Vcfc) was calculated as [(FS/ET)/√RR].
Euthanasia of mice and tissue samples.
At the conclusion of each myocardial infarction protocol, 160 mg/kg sodium pentobarbital was administered to each mouse for euthanasia. Body weight, tibia length, and heart weights were recorded. A midmyocardial section of the left ventricle was cut by using a Zivic Instruments coronal mouse heart slicer (HSMS005-1) and retained for histopathology and immunofluorescent studies. The remaining left ventricular tissue was separated into noninfarcted and infarcted portions and snap frozen in liquid nitrogen for protein and mRNA expression analyses.
Protein preparation and Western blot analysis.
Cytosolic and nuclear proteins were prepared from frozen myocardial samples according to routine methods. Myocardial tissue samples were homogenized in buffer A [10 mmol/l HEPES (pH 7.9), 1.5 mmol/l MgCl2, 10 mmol/l KCl, 1 mmol/l DTT, 25 μg/ml leupeptin and 1 mmol/l PMSF]. Lysates were incubated on ice for 10 min and centrifuged at 1,850 × g for 10 min in a 4°C temperature-controlled room. The supernatant was collected as the cytosolic fraction and stored on ice. The pellets were dissolved in buffer B (buffer A + 0.1% Triton X-100), incubated on ice for 10 min, and centrifuged again at 1,850 × g for 10 min in a 4°C temperature-controlled room. The pellet was washed with buffer A and then resuspended in buffer C [20 mmol/l HEPES (pH 7.9), 25% glycerol, 0.42 mol/l NaCl, 1.5 mmol/l MgCl2, 0.2 mmol/l EDTA, 0.5 mmol/l DTT and 1 mmol/l PMSF] for 30 min on ice. The lysates were then centrifuged at 25,000 × g for 30 min in a refrigerated ultracentrifuge at 4°C. The supernatant was collected as the nuclear fraction and stored on ice until protein content determination. Protein concentration was measured by using a Pierce 660 protein assay kit. Lysates were stored at −80°C until gel electrophoresis. Western blot analysis was performed with standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting. To ensure equal protein loading in all lanes of the gel, the total amounts of proteins transferred from each lane to the polyvinylidene difluoride membranes during blotting were stained with Ponceau S. The specific signals of the detected proteins with immunoblotting were quantitated with densitometry and further normalized to the corresponding α-tubulin Western blot by densitometric analysis. The following primary antibodies were used for Western blot analyses: ATF3 and specificity protein-1 polyclonal antibodies (Santa Cruz Biotechnologies), GRP78 antibodies (Cell Signaling), and α-tubulin (Sigma).
Quantitative (q)RT-PCR.
RNA was extracted from tissues by using the RNeasy mini kit (Qiagen), and RNA concentration was measured using the Nanodrop 1000A Spectrometer. cDNA was prepared and real-time PCR amplification was performed with SYBR-Green qPCR Master Mix (Qiagen) by using a 7900HT Fast Real-Time PCR system (Applied Biosystems). Primers for ATF3, hypoxanthine guanine phosphoribosyl transferase (HPRT), Il-1β, IL-6, and TNF-α were obtained from SABiosciences. The primers for α-myosin heavy chain (MHC), β-MHC, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), connective tissue growth factor, and transforming growth factor-β1 were obtained from Integrated DNA technologies by using the sequences listed in Table 1. Relative gene expression was determined by the 2−ΔΔCT method by internal normalization to HPRT. All samples were analyzed in triplicate. Melt curves for each sample were verified visually for sharp peaks. Representative samples for each group in each primer set were subjected to agarose gel electrophoresis for confirmation of 1 amplicon at the anticipated number of base pairs.
Table 1.
List of primers used for quantitative PCR
| Gene Product | Forward Primer | Reverse Primer |
|---|---|---|
| α-MHC | 5′-AGCTGACAGGGGCCATCAT-3′ | 5′-ACATACTCGTTCCCCACCTTC-3′ |
| β-MHC | 5′-AGACTGTCAACACTAAGAGGGT-3′ | 5′-CCAATGGCGGCAATAACAGC-3′ |
| ANP | 5′-GGAGCCTACGAAGATCCAGC-3′ | 5′-TCCAATCCTGTCAATCCTACCC-3′ |
| BNP | 5′-GAGGTCACTCCTATCCTCTGG-3′ | 5′-GCATCTTGAATTGCTCTGGAGA-3′ |
| CTGF | 5′-GACCCAACTATGATGCGAGCC-3′ | 5′-TCCCACAGGTCTTAGAACAGG-3′ |
| TGF-β1 | 5′-CCGCAACAACGCCATCTATG-3′ | 5′-CCCGAATGTCTGACGTATTGAAG-3′ |
α-MHC, alpha-myosin heavy chain; β-MHC, beta-myosin heavy chain; ANP: atrial natriuretic peptide; BNP, brain natriuretic peptide; CTGF, connective tissue growth factor; TGF-β1, transforming growth factor beta 1.
Tissue fixation and histochemical staining.
Myocardial tissue samples were fixed in 10% neutral-buffered formalin overnight and stored in 70% ethanol until tissue processing. Tissue was processed and paraffin embedded prior to sectioning at 4-μm thickness. Tissue sections were mounted on slides, deparaffinized in xylene, and rehydrated in decreasing concentrations of ethanol. Fast green/Sirius red and hematoxylin and eosin staining protocols were performed by using standard histochemical techniques. Stained tissue sections were coated with mounting medium and coverslips added to protect the tissue. All images were acquired from the noninfarcted myocardium. Relative areas of collagen, deposited in the noninfarcted region, were measured by using NIS Elements software and expressed as fractional percentage of normal myocardium.
Tissue immunofluorescence.
Fixed tissue samples were deparaffinized as described above and incubated with 5 μg/ml of wheat germ agglutinin (WGA) conjugated to Texas Red (Molecular Probes). Slides were mounted with VECTASHIELD mounting medium with 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories), and fluorescence was observed by using an EVOS fluorescence microscope (Advanced Microscopy Group) or a Nikon A1 confocal microscope. Cross-sectional areas of round cells with a centrally located nucleus were assessed by using Nikon NIS elements software. All images were acquired from the noninfarcted myocardium.
Statistical analysis.
Data are reported as means ± SD. Statistical analyses were performed by using GraphPad Prism 5.0. Results were analyzed either by unpaired Student's t-test for two groups or by a one-way ANOVA for three or more groups, followed by Bonferroni correction to test for significant differences between groups. For the survival data, Kaplan-Meier survival curves were compared for significant differences with the Log-rank (Mantel-Cox) test. Groups were considered statistically significant when P < 0.05.
RESULTS
ATF3 is activated by myocardial infarction.
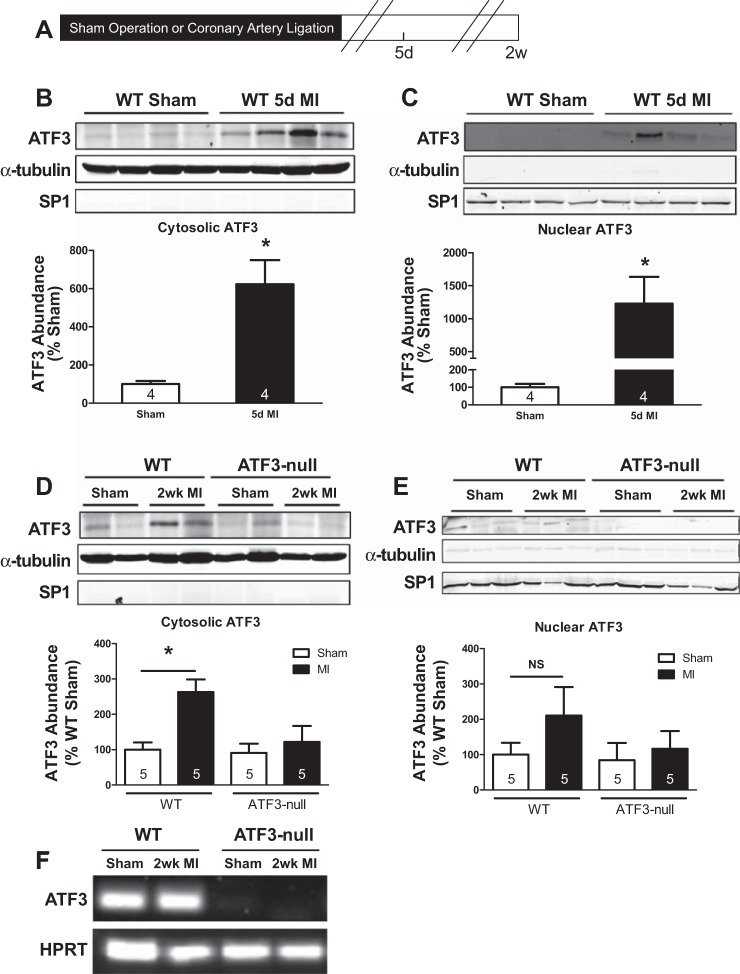
To assess whether myocardial infarction (MI) activates ATF3, WT mice were subjected to sham operation or MI and euthanized 5 days after surgery (Fig. 1A). Immunoblotting of tissue lysates from the noninfarcted myocardium, or the corresponding regions from the sham operated mice, showed a sixfold increase in cytosolic ATF3 abundance 5 days after MI (Fig. 1B). A 12-fold increase in nuclear abundance of ATF3 was observed in hearts subjected to MI (Fig. 1C) indicating that, in addition to increased cytosolic abundance of ATF3, MI stimulated activation and nuclear translocation of ATF3. To further evaluate ATF3 induction by MI, WT and ATF3-null mice were subjected to sham operation or MI and euthanized 2 wk after surgery. As indicated in Fig. 1D, cytosolic abundance of ATF3 was significantly elevated in the WT noninfarcted myocardium 2 wk after MI compared with the sham; ATF3 was not detected in ATF3-null hearts after MI, confirming the protein detected on Western blot is ATF3. Nuclear abundance of ATF3 was also evaluated in WT and ATF3-null mice, and no significant increase was detected 2 wk after MI (Fig. 1E). Genetic deletion of ATF3 was confirmed by PCR (Fig. 1F). Thus ATF3 was induced early (5 days) after MI, suggesting ATF3 may be important in regulating early post-MI transcriptional events.
Fig. 1.
Activating transcription factor 3 (ATF3) is induced following myocardial infarction (MI) in wild-type (WT) but not ATF3-null mice. A: mice were euthanized 5 days and 2 wk after sham operation and MI. B and D: immunoblotting reveals increased cytosolic ATF3 expression in WT mice 5 days and 2 wk following MI, which is not present in ATF3-null mice. C and E: nuclear ATF3 is significantly elevated 5 days after MI in WT but not 2 wk after MI in either WT or ATF3-null mice. F: genetic deletion of ATF3 was confirmed by PCR. *P < 0.05. SP1, stimulating protein-1; HPRT, hypoxanthine guanine phosphoribosyl transferase; NS, not significant.
Deletion of ATF3 improves survival after MI.
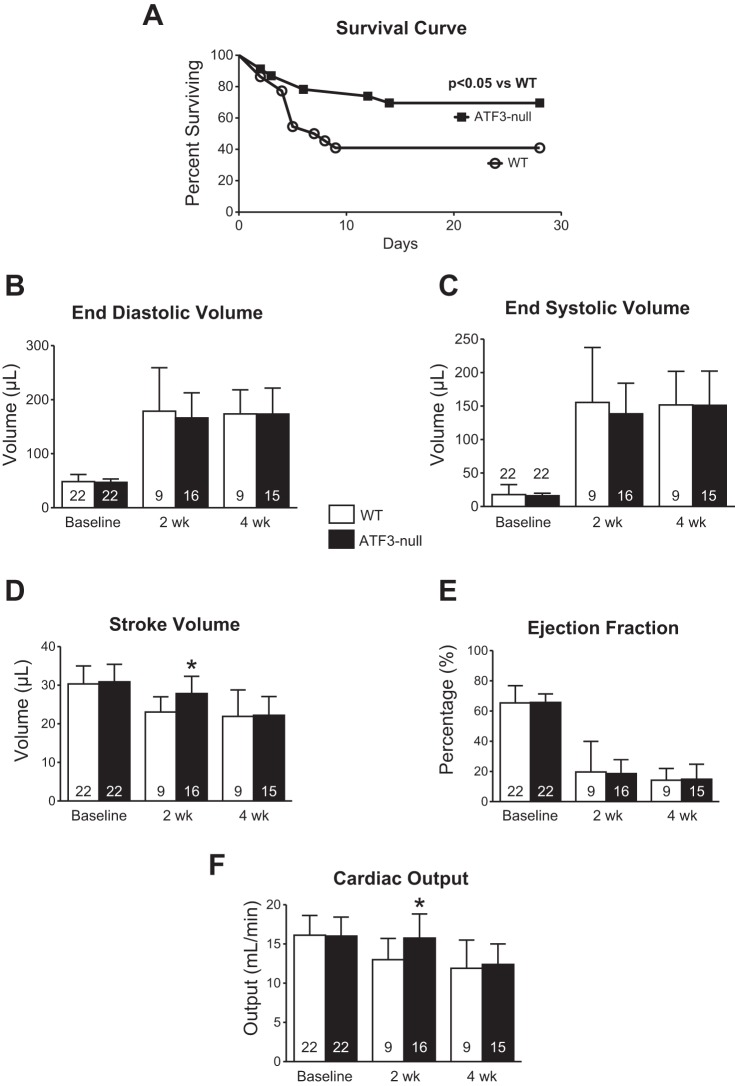
To investigate whether ATF3 affects survival and cardiac function after MI, WT and ATF3-null mice were subjected to left coronary artery ligation and followed for 4 wk. Kaplan-Meier survival curves (Fig. 2A) indicate significantly more ATF3-null mice (70% of the initial n = 23) surviving after MI compared with WT (41% of the initial n = 22). It could be argued that such a difference is due to smaller infarcts; however, this is unlikely because we used a nonreperfused model of MI and we measured infarct size, which demonstrated no significant difference between WT (n = 9) and ATF3-null (n = 7) mice subjected to infarction (43+/−11% vs. 44+/−12%, respectively; expressed as percent of total left ventricle). Echocardiography was used to evaluate cardiac function in WT and ATF3-null mice at baseline and at 2 wk and 4 wk after coronary artery ligation. There were no differences in heart rate (not shown), end diastolic volume (Fig. 2B), end systolic volume (Fig. 2C), or ejection fraction (Fig. 2E). We observed a significantly greater stroke volume in ATF3-null mice at 2 wk compared with WT (Fig. 2D). As a result, cardiac output was greater 2 wk after infarction in ATF3-null mice compared with WT (Fig. 2F). No group differences were observed in any of the echocardiography-derived parameters at 4 wk. At 4 wk, there were no significant differences in body weights, tibia lengths, or heart rate between WT and ATF3-null mice (data not shown). Given the significant improvement in survival, we next queried whether changes were evident at the myocardial level.
Fig. 2.
ATF3-null mice exhibited improved survival 4 wk following MI compared with WT mice, despite no significant difference in cardiac function. A: Kaplan-Meier survival curve of significantly improved survival 4 wk after MI in ATF3-null mice vs. WT (70 vs. 41%, respectively). B and C: ventricular volumes were not different in WT and ATF3-null mice following MI. D: a transient, nominal increase in stroke volume was observed 2 wk following MI, which did not persist through the 4-wk time point. E: ejection fraction was unchanged in ATF3-null mice compared with WT following MI. F: a transient increase in cardiac output was seen 2 wk following MI, which did not persist through the 4-wk time point. *P < 0.05.
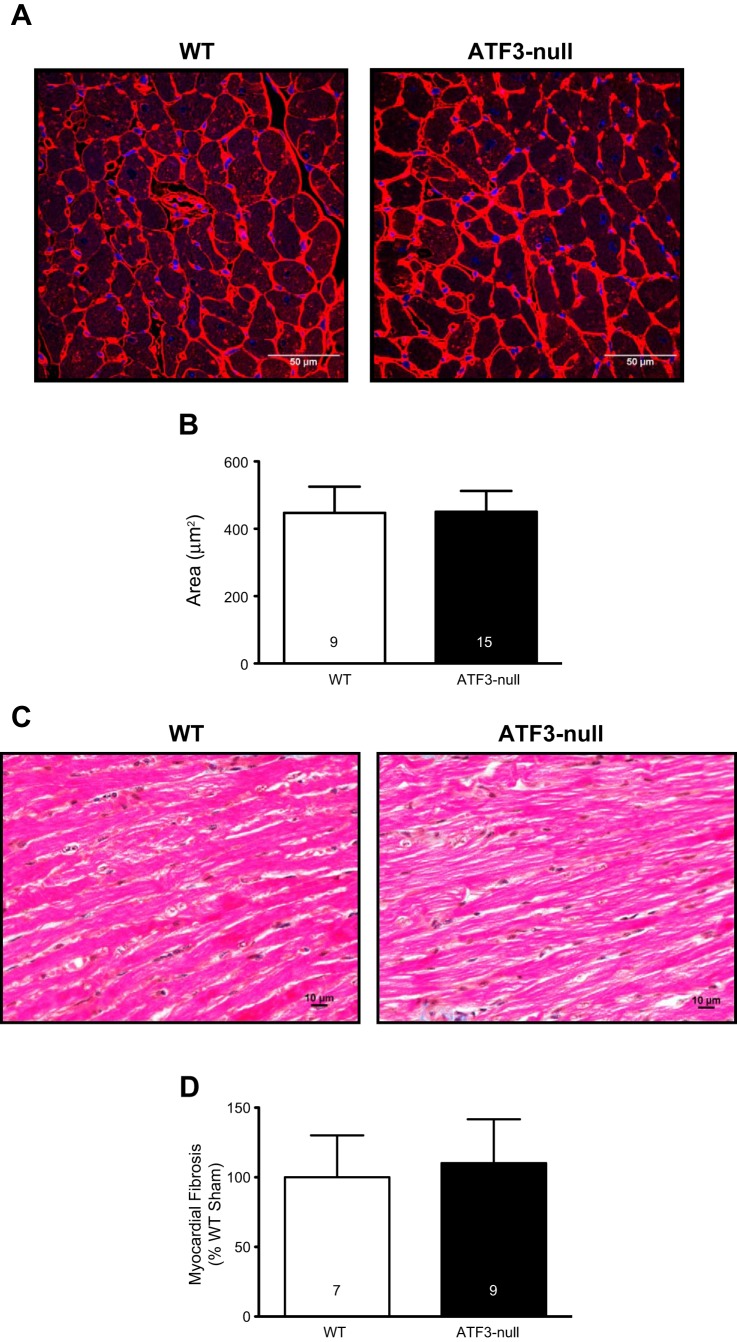
ATF3 deletion does not exacerbate cardiomyocyte hypertrophy.
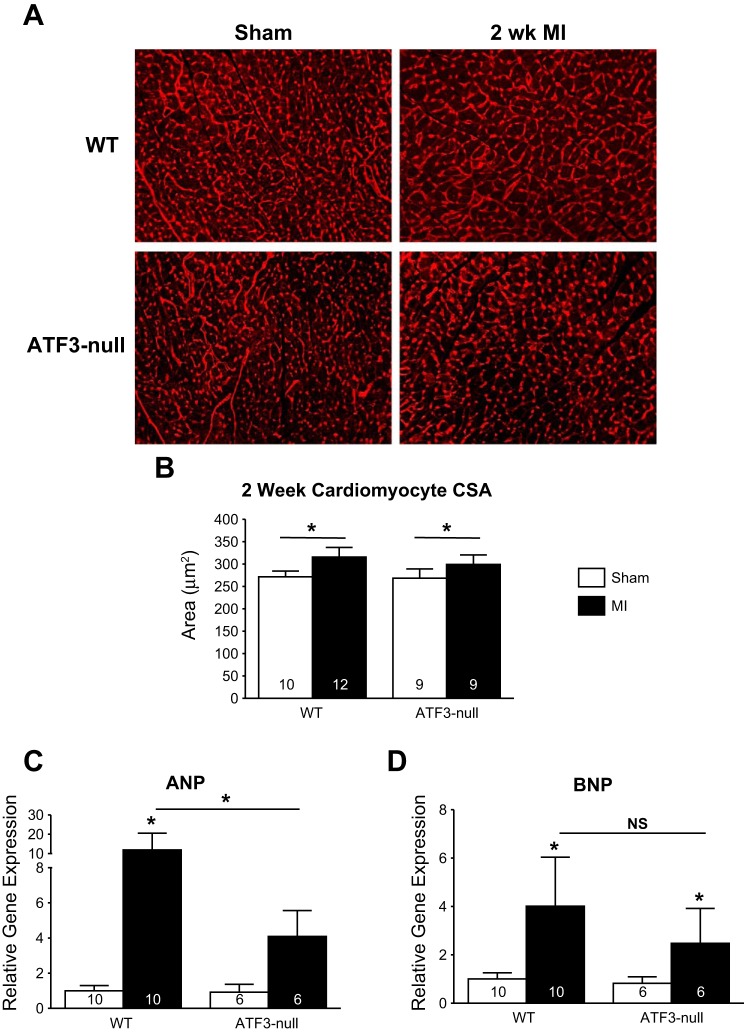
Cardiomyocyte hypertrophy represents an important aspect of cardiac remodeling. To ascertain whether differences in hypertrophy might explain the improvement in survival in the ATF3-null group, cardiomyocyte cross-sectional area was assessed at 2 wk after MI (Fig. 3A). Both WT and ATF3-null mice showed significantly increased cross-sectional area compared with their respective sham groups; however, there were no differences in hypertrophy between WT and ATF3-null hearts (Fig. 3B). To further examine potential alterations in the hypertrophic response, we also performed qPCR analyses for ANP and BNP (hallmarks of hypertrophy) mRNA levels. These data (Fig. 3, C and D) indicate a significant induction of ANP and BNP expression in WT and ATF3-null hearts at 2 wk. While the induction of ANP was slightly lower in ATF3-null hearts, there was no significant difference in BNP induction. Thus significant differences in the hypertrophic response are an unlikely explanation for differences in survival. This led us to evaluate potential differences in the fibrotic response.
Fig. 3.
Cardiomyocyte hypertrophy is evident 2 wk following MI, but not significantly altered in ATF3-null mice. A: representative images showing wheat germ agglutinin (WGA) staining in WT and ATF3-null mice. B: quantification of cardiomyocyte cross-sectional area (CSA) following MI in WT and ATF3-null mice indicating no difference in MI-induced hypertrophy in ATF3-null mice compared with WT. C: atrial natriuretic peptide (ANP) expression in ATF3-null mice is significantly lower than in WT mice subjected to MI. D: brain natriuretic peptide (BNP) is significantly elevated following MI in both WT and ATF3-null mice. *P < 0.05.
Deletion of ATF3 transiently enhances the fibrotic response to MI.
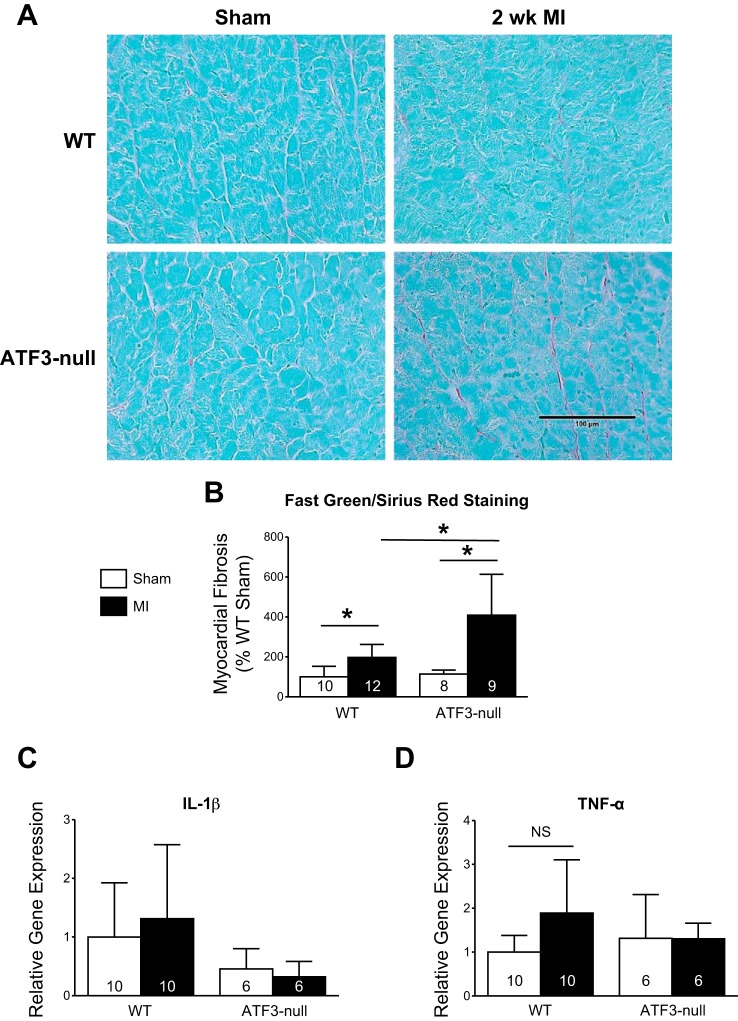
Both genotypes were subjected to sham or MI and harvested 2 wk later to assess the fibrotic response. Representative fast green/sirius red stained left ventricular sections are shown in Fig. 4A. Myocardial fibrosis was quantified as sirius red staining as a percentage of fast green staining, and normalized relative to WT sham (Fig. 4B). The data indicate ATF3-null hearts subjected to MI had significantly more fibrosis in the noninfarcted myocardium compared with WT MI at 2 wk following MI. To ascertain whether such changes were associated with an alteration in the inflammatory response, IL-1β (Fig. 4C) and TNFα (Fig. 4D) mRNA levels were measured by qPCR; however, no significant differences were observed. Although we observed changes in the fibrotic response at 2 wk, it was not clear whether relatively gross histopathologic changes were evident at 4 wk. Hence, mice were subjected to MI and followed for 4 wk. We found no evidence for group differences in cardiomyocyte hypertrophy (via WGA; Fig. 5, A and B) or fibrosis, according to board-certified veterinary pathologist's blinded assessment of the slides (Fig. 5, C and D). Incidentally, the pathologist (Dr. Annamalai) identified evidence of global nuclear atypia in the hearts of ATF3-null mice 4 wk after MI compared with the local atypia seen in WT mice. Thus it appears that the deleterious effect of ATF3 induction occurs early in the course of infarct-induced heart failure.
Fig. 4.
ATF3-null mice exhibit increased fibrosis 2 wk after MI based on fast green/sirius red (FGSR) staining, despite no change in expression of genes implicated in the inflammatory response. A: representative images of FGSR staining 2 wk following MI. B: FGSR quantification indicating increased fibrosis 2 wk after MI in ATF3-null mice compared with WT. C and D: TNF-α and IL-1β are not significantly altered in either the WT or the ATF3-null mice 2 wk after MI. *P < 0.05.
Fig. 5.
ATF3-null mice did not exhibit altered cardiac hypertrophy or fibrosis 4 wk after MI. A: representative WGA staining 4 wk after MI in WT and ATF3-null mice. B: cardiomyocyte cross-sectional area is not different between WT and ATF3-null mice following MI. C: representative images of hematoxylin and eosin stained hearts from WT and ATF3-null mice 4 wk after MI. D: there is no significant change in the amount of fibrosis observed in WT and ATF3-null mice 4 wk after MI.
DISCUSSION
As our understanding of the molecular regulation of inflammation has become more nuanced, there has been a resurgence in our collective interest in the role of inflammation in heart failure. Here, we focused on ATF3, a putative early response gene in inflammation, to ascertain its role in the failing heart. To this end, we used a loss of function approach to determine the role of endogenous ATF3 during heart failure. We hypothesized that post-MI induction of ATF3 exacerbates heart failure through the promotion of inflammation. Our present data indicate that loss of ATF3 improves postinfarct survival, but without a lasting impact on post-MI inflammation and fibrosis.
ATF3 belongs to the cAMP responsive element-binding (CREB) protein family of transcription factors(8). There are at least two isoforms of ATF3: the longer isoform represses transcription of promoters with ATF3 binding elements; the shorter isoform (deltaZip2) does not bind DNA and does not contain the leucine zipper motif. The shorter isoform may stimulate transcription by decoying inhibitory elements away from ATF3 binding sites in promoters. In general, ATF3 acts as an early response gene supporting inflammation. Although several potential gene targets have been identified, the focus of the present study was providing clarification on the role of ATF3 in heart failure.
Here, we observed a significant increase in ATF3 protein levels following myocardial infarction; using ATF3 deficient mice allowed us to test whether ATF3 expression was involved in post-MI remodeling. Infarction caused significant mortality in the WT mice, which was reduced in ATF3 deficient mice; however, there were limited changes in cardiac function during the 4-wk observation period. Although stroke volume and cardiac output were statistically higher in the ATF3 deficient mice (compared with WT), the magnitude of the difference was quite small, and ejection fraction never differed significantly. Because of the acute difference in survival, we focused on the 2-wk time point.
In terms of a mechanistic explanation, it is possible that ATF3 deletion may bolster the early fibrotic/reparative response. As expected, MI produced a significant increase in myocardial fibrosis (remote from the infarct) in the WT hearts. Interestingly, ATF3 deficient hearts exhibited significantly more fibrosis than the WT hearts at 2 wk post-MI. Although ATF3 may negatively regulate MMP2 expression(38), this is likely not an important aspect of its regulation in the present context because the group with the greatest survival (i.e., ATF3 deficient mice) had the greatest extent of fibrosis. Regardless, the difference in fibrosis at 2 wk was not maintained through 4 wk. We speculate that the endogenous limitation of early post-MI fibrosis by ATF3 may partially account for its maladaptive effects. Although this may be counterintuitive to some, it is important to realize many investigators identify myocardial rupture as a significant contributor to death in the mouse model of nonreperfused MI. Therefore, an acutely fibrotic ventricle may improve survival by avoiding ventricular rupture. Because we did not perform autopsies in mice that died in the animal facility, this is only one potential explanation for what might be happening in the early phases of post-MI remodeling.
In addition to its potential role as a regulator of fibrotic mediators, ATF3 plays a robust role in inflammation. Several groups have documented a role for ATF3 in promoting(24) or antagonizing(25) inflammation. We examined mRNA levels of the foundational cytokines (TNFα and IL-1β) and found no difference between WT and ATF3 deficient hearts. According to the pathologist (blinded to group assignment), there were also no obvious differences in the extents of cellular infiltrates in WT and ATF3 deficient hearts. Thus we do not see an obvious role for ATF3 in the post-MI inflammatory response, though it is possible that there may be a limited and/or phasic role for ATF3 in inflammation.
The significant improvement in survival was one of the most striking findings of the study. Despite such improvement in survival, there were no lasting improvements in ventricular function. It is possible that the lack of sustained improvement in cardiac function was a casualty of the improved survival in the ATF3 deficient mice. We speculate that, in the WT failing group, the mice with the most severe heart failure died (earlier), which precluded their inclusion in subsequent functional analyses (where, arguably, they would have worse cardiac function). In contrast, fewer ATF3-null mice died; thus more mice with the most severe heart failure lived. These differences were not, of course, attributable to infarct size because there was no difference in acute infarct size. So, even though improvement in survival is a striking finding, it may have occurred at the expense of a group-level improvement in cardiac function. Until additional studies identify the exact cause, this explanation remains purely speculative.
As with all studies, basic science and clinical, limitations exist. Many clinical studies are plagued by lack of blinding in the performance of surgical protocols and subsequent functional analyses; however, this was not the case in the present study, which was performed in a blinded fashion. Nevertheless, other limitations related to the mice existed. For example, these were relatively healthy mice, they had no risk factors, and they were relatively young. Clearly, this is not reflective of the majority of the patient population. Similarly, all of the mice were males, which again, is not reflective of human patient populations. Another often overlooked limitation is the absence of pharmacologic intervention, much less polypharmacy; human heart failure patients are aggressively medically managed with, at minimum, β-blockers and angiotensin-converting enzyme inhibitors. Yet, the results from this study provide critical conceptual insight into the role of ATF3 in the failing heart.
In summary, our findings suggest that endogenous ATF3 transiently suppresses fibrosis early but not late during heart failure. Although the survival difference manifested early in the course of the study, it is not clear whether such a difference is directly caused by post-MI changes in fibrosis-associated ATF3 deletion. Future studies are required to address the reason for the improvement in survival, which may be related to currently unappreciated events soon after MI.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-083320, R01 HL-094419, P20 RR-024489, and P01 HL-078825 (to S. Jones and A. Bhatnagar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.C.B., A.B., and S.P.J. conception and design of research; A.C.B., A.M.D., R.E.B., and K.R.B. performed experiments; A.C.B., A.M.D., R.E.B., and K.R.B. analyzed data; A.C.B., A.B., and S.P.J. interpreted results of experiments; A.C.B. and A.M.D. prepared figures; A.C.B., A.M.D., and S.P.J. drafted manuscript; A.C.B., A.M.D., R.E.B., K.R.B., A.B., and S.P.J. approved final version of manuscript; A.B. and S.P.J. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Imaging and Physiology Core of the Diabetes and Obesity Center. Lakshmanan Annamalai, DVM, PhD, provided expert insight related to histopathology. Ms. Susan Dougherty performed most of the qPCR. Ms. Emily Steinmetz and Mr. Don Mosley were responsible for mouse husbandry.
REFERENCES
- 1.Brainard RE, Watson LJ, DeMartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One 8: e83174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks AC, Guo Y, Singh M, McCracken J, Xuan YT, Srivastava S, Bolli R, Bhatnagar A. Endoplasmic reticulum stress-dependent activation of ATF3 mediates the late phase of ischemic preconditioning. J Mol Cell Cardiol 76: 138–147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang WL, Jones SP, Lefer DJ, Welbourne T, Sun G, Yin L, Suzuki H, Huang J, Granger DN, van der Heyde HC. CD8(+)-T-cell depletion ameliorates circulatory shock in Plasmodium berghei-infected mice. Infect Immun 69: 7341–7348, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol 16: 1157–1168, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condorelli G, Roncarati R, Ross J Jr, Pisani A, Stassi G, Todaro M, Trocha S, Drusco A, Gu Y, Russo MA, Frati G, Jones SP, Lefer DJ, Napoli C, Croce CM. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad U S A 98: 9977–9982, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girod WG, Jones SP, Sieber N, Aw TY, Lefer DJ. Effects of hypercholesterolemia on myocardial ischemia-reperfusion injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 19: 2776–2781, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Greer JJ, Kakkar AK, Elrod JW, Watson LJ, Jones SP, Lefer DJ. Low-dose simvastatin improves survival and ventricular function via eNOS in congestive heart failure. Am J Physiol Heart Circ Physiol 291: H2743–H2751, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M, Grey ST, Ron D, Hai T. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol 24: 5721–5732, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res 87: 812–817, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmeyer MR, Scalia R, Ross CR, Jones SP, Lefer DJ. PR-39, a potent neutrophil inhibitor, attenuates myocardial ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 279: H2824–H2828, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Jones SP, Gibson MF, Rimmer DM 3rd Gibson TM, Sharp BR, Lefer DJ. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J Am Coll Cardiol 40: 1172–1178, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Jones SP, Girod WG, Granger DN, Palazzo AJ, Lefer DJ. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. Am J Physiol Heart Circ Physiol 277: H763–H769, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Jones SP, Girod WG, Huang PL, Lefer DJ. Myocardial reperfusion injury in neuronal nitric oxide synthase deficient mice. Coron Artery Dis 11: 593–597, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Jones SP, Girod WG, Marotti KR, Aw TY, Lefer DJ. Acute exposure to a high cholesterol diet attenuates myocardial ischemia-reperfusion injury in cholesteryl ester transfer protein mice. Coron Artery Dis 12: 37–44, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd'Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol Heart Circ Physiol 276: H1567–H1573, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, Van Haperen R, De Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 286: H276–H282, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci U S A 100: 4891–4896, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SP, Greer JJ, Ware PD, Yang J, Walsh K, Lefer DJ. Deficiency of iNOS does not attenuate severe congestive heart failure in mice. Am J Physiol Heart Circ Physiol 288: H365–H370, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 284: H277–H282, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Jones SP, Trocha SD, Lefer DJ. Cardioprotective actions of endogenous IL-10 are independent of iNOS. Am J Physiol Heart Circ Physiol 281: H48–H52, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Jones SP, Trocha SD, Lefer DJ. Pretreatment with simvastatin attenuates myocardial dysfunction after ischemia and chronic reperfusion. Arterioscler Thromb Vasc Biol 21: 2059–2064, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kaszubska W, Hooft van Huijsduijnen R, Ghersa P, DeRaemy-Schenk AM, Chen BP, Hai T, DeLamarter JF, Whelan J. Cyclic AMP-independent ATF family members interact with NF-kappa B and function in the activation of the E-selectin promoter in response to cytokines. Mol Cell Biol 13: 7180–7190, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawauchi J, Zhang C, Nobori K, Hashimoto Y, Adachi MT, Noda A, Sunamori M, Kitajima S. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J Biol Chem 277: 39025–39034, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Koren L, Elhanani O, Kehat I, Hai T, Aronheim A. Adult cardiac expression of the activating transcription factor 3, ATF3, promotes ventricular hypertrophy. PLoS One 8: e68396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefer DJ, Scalia R, Jones SP, Sharp BR, Hoffmeyer MR, Farvid AR, Gibson MF, Lefer AM. HMG-CoA reductase inhibition protects the diabetic myocardium from ischemia-reperfusion injury. FASEB J 15: 1454–1456, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y, Chaves A, Chen J, Kelley R, Jones K, Weed HG, Gardner KL, Gangi L, Yamaguchi M, Klomkleaw W, Nakayama T, Hamlin RL, Carnes C, Altschuld R, Bauer J, Hai T. Transgenic mice with cardiac-specific expression of activating transcription factor 3, a stress-inducible gene, have conduction abnormalities and contractile dysfunction. Am J Pathol 159: 639–650, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palazzo AJ, Jones SP, Anderson DC, Granger DN, Lefer DJ. Coronary endothelial P-selectin in pathogenesis of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 275: H1865–H1872, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Palazzo AJ, Jones SP, Girod WG, Anderson DC, Granger DN, Lefer DJ. Myocardial ischemia-reperfusion injury in CD18- and ICAM-1-deficient mice. Am J Physiol Heart Circ Physiol 275: H2300–H2307, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7: 634–642, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM 3rd Trocha SD, Huang PL, Smith MB, Lefer AM, Lefer DJ. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation 103: 2598–2603, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 282: H2422–H2426, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Starks D, Arriba LN, Enerson CL, Brainard J, Nagore N, Chiesa-Vottero A, Uribe JV, Belinson J. Mexican Cervical Cancer Screening Study II: 6-month and 2-year follow-up of HR-HPV women treated with cryotherapy in a low-resource setting. J Low Genit Tract Dis 18: 333–337, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci 15: 170–182, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Wang Q, Watson LJ, Jones SP, Epstein PN. Cardiac overexpression of 8-oxoguanine DNA glycosylase 1 protects mitochondrial DNA and reduces cardiac fibrosis following transaortic constriction. Am J Physiol Heart Circ Physiol 301: H2073–H2080, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A 107: 17797–17802, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan C, Wang H, Boyd DD. ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter. J Biol Chem 277: 10804–10812, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Jones SP, Suhara T, Greer JJ, Ware PD, Nguyen NP, Perlman H, Nelson DP, Lefer DJ, Walsh K. Endothelial cell overexpression of fas ligand attenuates ischemia-reperfusion injury in the heart. J Biol Chem 278: 15185–15191, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Shen DF, Bian ZY, Zong J, Deng W, Zhang Y, Guo YY, Li H, Tang QZ. Activating transcription factor 3 deficiency promotes cardiac hypertrophy, dysfunction, and fibrosis induced by pressure overload. PLoS One 6: e26744, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]