This is the first study on ErbB2 overexpression in the heart that identifies cardioprotection after doxorubicin treatment. Our findings suggest that an adverse effect on redox signaling pathways necessary for maintaining mitochondrial antioxidant defenses may be an important mechanism of cardiac toxicity induced by drugs that target ErbB2 and Abl kinases.
Keywords: ErbB2, glutathione peroxidase, reactive oxygen species, nRTK, mitochondria
Abstract
Levels of the HER2/ErbB2 protein in the heart are upregulated in some women during breast cancer therapy, and these women are at high risk for developing heart dysfunction after sequential treatment with anti-ErbB2/trastuzumab or doxorubicin. Doxorubicin is known to increase oxidative stress in the heart, and thus we considered the possibility that ErbB2 protein influences the status of cardiac antioxidant defenses in cardiomyocytes. In this study, we measured reactive oxygen species (ROS) in cardiac mitochondria and whole hearts from mice with cardiac-specific overexpression of ErbB2 (ErbB2tg) and found that, compared with control mice, high levels of ErbB2 in myocardium result in lower levels of ROS in mitochondria (P = 0.0075) and whole hearts (P = 0.0381). Neonatal cardiomyocytes isolated from ErbB2tg hearts have lower ROS levels and less cellular death (P < 0.0001) following doxorubicin treatment. Analyzing antioxidant enzyme levels and activities, we found that ErbB2tg hearts have increased levels of glutathione peroxidase 1 (GPx1) protein (P < 0.0001) and GPx activity (P = 0.0031) in addition to increased levels of two known GPx activators, c-Abl (P = 0.0284) and Arg (P < 0.0001). Interestingly, although mitochondrial ROS emission is reduced in the ErbB2tg hearts, oxygen consumption rates and complex I activity are similar to control littermates. Compared with these in vivo studies, H9c2 cells transfected with ErbB2 showed less cellular toxicity and produced less ROS (P < 0.0001) after doxorubicin treatment but upregulated GR activity (P = 0.0237) instead of GPx. Our study shows that ErbB2-dependent signaling contributes to antioxidant defenses and suggests a novel mechanism by which anticancer therapies involving ErbB2 antagonists can harm myocardial structure and function.
NEW & NOTEWORTHY
This is the first study on ErbB2 overexpression in the heart that identifies cardioprotection after doxorubicin treatment. Our findings suggest that an adverse effect on redox signaling pathways necessary for maintaining mitochondrial antioxidant defenses may be an important mechanism of cardiac toxicity induced by drugs that target ErbB2 and Abl kinases.
the erbb2 receptor tyrosine kinase is a member of the epidermal growth factor receptor (EGFR) family and has an essential role in cardiac function and development (9, 39, 47, 50). ErbB2 is also important in cancer biology, and dysregulated ErbB2 signaling is implicated in various types of cancer, most notably in breast and ovarian cancer (30, 49). The clinical link between ErbB2 and heart function was discovered when 27% of ErbB2-overexpressing breast cancer patients treated with doxorubicin and anti-ErbB2 (Trastuzumab/Herceptin) subsequently developed a synergistic cardiac toxicity (57, 65). We previously reported that doxorubicin treatment induces the expression of ErbB2 in rat heart (19), but it is still unknown whether this ErbB2 upregulation might serve to counteract oxidative stress induced by doxorubicin. If so, an upregulation of ErbB2 protein in cardiomyocytes could represent a molecular target for anti-ErbB2 therapy to reverse any cardioprotective mechanisms attributed by ErbB2.
Indeed, experimental evidence does suggest that ErbB2 could have a role in regulating antioxidant defenses in cancer (55). In the heart, however, cardiomyocytes treated with an antibody against ErbB2 have increased cellular death through a reactive oxygen species (ROS)- and mitochondrial-dependent mechanism while siRNA or knockout models report that ErbB2 is essential for protecting against cellular death and oxidative stress (23, 24, 51, 56). Moreover, activation of the related ErbB4 signaling pathway by the ligand neuregulin (NRG1β) has antioxidant properties (21, 67). However, the specific role of ErbB2 as it is related to ROS-scavenging mechanisms has not been defined, and critical in vivo studies to investigate how ErbB2 affects ROS defenses and mitochondrial function in the heart have not been done, largely because ErbB2 knockout mice die prematurely, precluding the study of specific mechanisms of cardiac protection. We therefore sought to further investigate the possible protective role of cardiac ErbB2 upregulation using a recently developed transgenic murine model that selectively overexpresses ErbB2 in cardiomyocytes (62). Specifically, we used this transgenic mouse model to examine the role of ErbB2 upregulation on mitochondrial function and antioxidant defense mechanisms in the heart. Our studies demonstrate that an upregulation in ErbB2 can induce further upregulation and activation of pathways that converge on protection against oxidative stress.
MATERIALS AND METHODS
Animals
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (2011) of the National Institutes of Health (NIH). The protocol was approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions (Animal Welfare Assurance No. A-3273-01). The wild-type (WT) and transgenic (TG; ErbB2tg) mice used in this study were developed as described previously (62).
Cell Culture and ErbB2 Overexpression in H9c2 Cell Line
The H9c2 rat cardiomyoblast cell line was obtained from ATTC (Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotic at 37°C and 5% CO2. The cells were subcultured at a ratio of 1:4 every 3–4 days. H9c2 cells were transfected with rat-neu/ErbB2 or pcDNA 3.1(+) control plasmid DNA using the Fugene HD (Promega, Madison, WI) reagent. Two-day-old litters were genotyped and excised hearts were pooled into WT or ErbB2tg groups, and ventricular cardiomyocytes were isolated (70) and cultured in MEM supplemented with 20% FBS and 1% penicillin-streptomycin antibiotic. Cultures were treated between 5–6 days when cells were 50% confluent.
Immunoblotting
Frozen left ventricle from WT and ErbB2tg was rapidly homogenized in lysis buffer (25 mM Na2HPO4, 25 mM NaH2PO4, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1% Triton X-100, and 100-fold dilution of protease and phosphatase inhibitors). The lysis buffer was similarly used to lyse the transfected H9c2 cell pellets. Standard gel electrophoresis and immunoblotting were performed (62) on heart tissue and H9c2 cell lysates. The primary antibodies used were directed against glutathione peroxidase 1 (GPx1), glutathione reductase (GR; Abcam, Cambridge, MA), Akt, cytoplasmic Abelson (c-Abl), phospho-tyrosine (Cell Signaling, Danvers, MA), ErbB2, EGFR, thioredoxin reductase 2 (TrxR2), SOD2, Prohibitin, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), Abelson-related gene (Arg; US Biological, Salem, MA), and catalase (EMD Millipore, Billerica, MA). SOD1 antibody was generated against C. elegans SOD1 (40). Densitometry was performed using ImageJ (NIH) software. Levels of Akt (62) and GAPDH proteins were measured to normalize protein quantities across tissue and cell lysate samples, respectively.
Immunoprecipitation
Lysates from heart tissue and H9c2 cells were prepared for immunoprecipitation as previously described (62). Briefly, lysates were incubated with antibodies directed against ErbB2 (Santa Cruz Biotechnology), c-Abl (Santa Cruz Biotechnology), or Arg (US Biological) at 4°C overnight. Depending on the antibody, the lysates were then incubated with either Protein A or G beads (GE Healthcare Life Sciences, Pittsburgh, PA) at 4°C overnight; the supernatant was discarded and urea buffer with beta-mercaptoethanol was added to the beads. The supernatant was loaded on a SDS gel and the immunoblotting protocol was followed as described above.
Mitochondria Isolation from Hearts
Mitochondria were isolated from hearts as described previously (20). Briefly, hearts were excised from WT and ErbB2tg mice, minced, and placed in a 30-ml homogenizer containing 3 ml of mitochondrial isolation buffer (0.7 mg/ml per mouse heart; 10 mM HEPES, 0.25 M sucrose, 1 mM EGTA, Nagarse, and Protease Type XXIV from Bacillus licheniformis; Sigma-Aldrich, St. Louis, MO). The tissue was homogenized, followed by a series of differential centrifugations at 4°C (20). The final mitochondrial pellet was resuspended in the mitochondrial resuspension buffer (10 mM HEPES, 0.25 M sucrose, and 0.1% BSA) with protease inhibitors (Roche Applied Science, Indianapolis, IN) and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich) added at a 100-fold dilution.
ROS Measurements-Amplex Red
The Amplex Red assay (Invitrogen, Carlsbad, CA) was used to measure hydrogen peroxide (H2O2) content from the isolated heart mitochondria. The mitochondria were energized with succinate (1, 63) in both unstimulated (−ADP) and stimulated (+ADP) states.
Electron paramagnetic resonance analysis.
For preparation of tissue for electron paramagnetic resonance spectroscopy (EPR), frozen ventricles were homogenized in lysis buffer [12.5 mM Na2HPO4, 12.5 mM NaH2PO4, 75 mM NaCl, 10% glycerol, 0.5 mM EDTA, 1:100 of phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich), and 1 tablet of complete mini EDTA-free protease inhibitor (Roche Applied Science, Indianapolis, IN)] using Precellys Homogeneizer (Precellys, Montigny-le-Bretonneux, France). Nonsoluble fractions were removed by centrifuge at 15,000 g. Protein concentrations were determined by Pierce BCA protein assay kit (Life Technologies) and normalized to 1 μg/μl. Stock solutions of 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (10 mM CPH; Enzo Life Sciences, Farmingdale, NY) were made in nitrogen purged 0.9% (wt/vol) NaCl, 25 g/l Chelex 100 (Bio-Rad), and 0.1 mM DTPA. Seventy microliters of protein extracts were combined with the CPH probe (1 mM) (15). Samples were assayed in 50-μl glass capillary tubes at room temperature using the Bruker X-band EPR spectrometer (Bruker, Billerica, MA). Spectrometer settings were as follows: sweep width, 100 G; microwave frequency, 9.75 GHz; modulation amplitude, 1 G; microwave power, 3.2 mW; conversion time, 5.12 ms; time constant, 5.12 ms; receiver gain, 2 × 104; and number of scans, 4. EPR intensities were converted to the density of spin probe and calculated ROS production rate (pmol·μg−1·min−1).
ROS measurements-DCF-DA.
The formation of ROS in transfected H9c2 cells or mouse neonatal ventricular cardiomyocytes treated with vehicle/doxorubicin (Bedford Laboratories, Bedford, OH) was measured using 50 μM of 2,7-dichlorodihydrofluorescein diacetate (DCF-DA; Sigma-Aldrich) at an excitation of 485 and 522 nm emission.
MTT and LDH Assays
Cell viability of the vehicle- or doxorubicin-treated, transfected H9c2 cells was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay to assess mitochondrial dehydrogenase function. The cells were incubated with 5 mg/ml MTT reagent in PBS at 37°C for 4 h. The insoluble purple formazan was dissolved in 100 μl of dimethyl sulfoxide and the absorbance was read at 570 nm on a microplate reader. For cell viability studies in isolated neonatal mouse cardiomyocytes, the release of cytosolic lactate dehydrogenase (LDH) was measured based on the protocol found in Ref. 6 using reagents obtained from the CytoTox-ONE Homogenous Membrane Integrity Assay kit (Promega).
Mitochondrial Respiration Studies
Respiration was measured using freshly isolated heart mitochondria (200 μg of mitochondrial protein) that were added to a 25°C chamber containing mitochondrial respiration buffer (120 mM KCl, 1 mM EGTA, 5 mM MOPS, 5 mM K2HPO4, and 0.2% BSA). Oxygen consumption was monitored with a Clark-type O2 electrode (Instech, Plymouth Meeting, PA) connected to an O2 monitor (model 5300; YSI, Yellow Springs, OH) (10, 31). Glutamate/malate (10 mM/2 mM) was added to the chamber followed by ADP (0.5 mM) for measurement of state 3 and 4 respiratory rates.
Complex I Activity Assay
Complex I activity was measured based on the 2,6-dichlorindophenol (DCIP) method (32, 42). Mitochondria isolated from WT and ErbB2tg hearts were added to a reaction mixture (20 μl of 10 μg/ml of mitochondrial protein resuspended in 180 μl of 25 mM KPi pH 7.4, 3 mg/ml BSA, 60 μM decylubiquinone, 160 μM DCIP, 80 μM NADH, 2 μM antimycin, and 2 mM KCN) in a 96-well plate. The assay rate was kinetically monitored at 595 nm for 3 min. Rotenone (4 μM) was added to the reaction mixture to assess rotenone-insensitive complex I activity.
Activity Assays for GPx, GR, and Quantification of Glutathione
GPx activity in whole heart homogenates and isolated heart mitochondria was measured with a NADPH-linked enzymatic assay to monitor the decrease in NADPH absorbance at 340 nm. Reagents were obtained from the GPx activity assay kit ADI-900-158 (Enzo Life Sciences, Plymouth Meeting, PA). GR activity in whole heart homogenates was measured by the rate of NADPH oxidation at an absorbance of 340 nm. Reagents were obtained from a GR activity assay kit (Cayman Chemical, Ann Arbor, MI). Glutathione was quantified as described in Ref. 41 using 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and GR. NADPH was added to the samples and the absorbance was recorded at 412 nm for 3 min.
TrxR2 Activity Assay
Mitochondria were isolated from WT and ErbB2tg mouse hearts and the mitochondrial membranes were removed by a series of freeze-thaw cycles between dry ice and ethanol at 37°C. The supernatant was collected and prepared in a master mix (5 mM DTNB, 8% ethanol, and 300 μM NADPH) and PE buffer (100 mM potassium phosphate and 2 mM EDTA, pH 7.0). TrxR2 activity was measured by the reduction of 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB) by TrxR2 and NADPH at an absorbance of 412 nm (60).
SOD Enzymatic Activity
SOD enzymatic activity was assayed according to the protocols described previously in (17, 40). Analysis of SOD activity was performed by nondenaturing gel electrophoresis with nitro blue tetrazolium staining (17).
Treatment of H9c2 Cells with Doxorubicin
Dosages for doxorubicin were determined according to published studies of doxorubicin-treated H9c2 cells (43). For ErbB2 overexpression studies, doxorubicin was added to the H9c2 cells at 24 h posttransfection. Assays for ROS production and cellular viability were performed 6–24 h after drug treatment.
Statistical Methods
GraphPad Prism software (GraphPad, La Jolla, CA) was used to perform statistical analysis. After determining means and standard deviations, the Student unpaired t-test was used to determine significance of differences between two groups. Two-way ANOVA and Tukey's post hoc tests were performed for multiple comparisons. P < 0.05 was accepted as a significant difference.
RESULTS
GPx Expression and Activity Are Increased in Hearts Overexpressing ErbB2
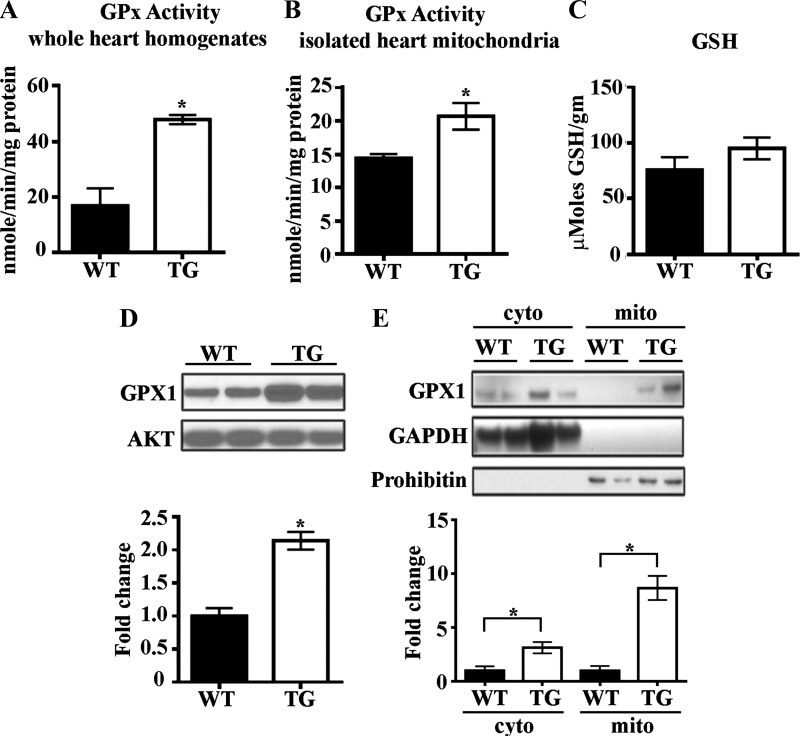
Recognizing that total cellular levels of ROS represent the balance of production and scavenging (1, 2), we first focused on how ErbB2 overexpression affects mechanisms of ROS removal by analyzing the activities of multiple antioxidant enzymes. We found that total GPx activity is increased in ErbB2tg whole hearts and isolated mitochondria compared with levels in WT controls (Fig. 1, A and B). We did not detect any differences in GSH levels when comparing WT and ErbB2tg hearts (Fig. 1C). Consistent with GPx increased activity, we also noted that ErbB2tg hearts express significantly higher levels of GPx1 protein compared with WT hearts (Fig. 1D), with cell fractionation experiments showing that cytosolic fractions have roughly twofold higher GPx1 protein but the majority of the protein is from the mitochondrial fractions with eightfold higher levels of GPx1 protein expression in ErbB2tg than in WT hearts (Fig. 1E).
Fig. 1.
Levels of glutathione peroxidase 1 (GPx1) protein and GPx activity are increased by cardiac ErbB2 overexpression. A and B: whole heart homogenates (n = 4 per group) and mitochondria (n = 8 per group) isolated from the ErbB2tg hearts have higher GPx activity than wild-type (WT) hearts. C: glutathione (GSH) levels are not different between WT and transgenic (TG) animals (n = 6 per group). D: representative immunoblot shows higher expression of GPx1 in whole heart homogenates of transgenic hearts compared with WT hearts (n = 12 per group). E: GPx1 is elevated in cytosolic and mitochondrial fractions from ErbB2tg hearts as shown in representative immunblots (n = 6 per group). Values represent means ± SD. Akt is the loading control protein, and values are fold increase over controls. *P < 0.05.
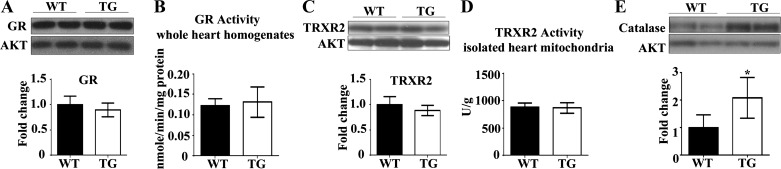
Since neuregulin-1β treatment upregulates GR mRNA in adult rat ventricular cardiomyocytes (67), we also measured GR protein expression and activity from myocardium under ErbB2 overexpression. However, we found no difference (Fig. 2, A and B) in GR protein expression or activity between WT and ErbB2tg mouse myocardium.
Fig. 2.
Glutathione reductase (GR) and thioredoxin reductase 2 (TRXR2) are unchanged by overexpression of ErbB2 in the heart. Catalase protein levels are increased in the ErbB2tg hearts. A and B: GR protein levels and activity (n = 7 per group) are not different between the WT and TG hearts. C and D: no difference in TRXR2 protein levels (n = 5 per group) or activity (n = 6 per group) was found between both groups of animals. E: catalase protein levels are increased in the TG hearts (n = 6 per group). Values represent the means ± SD. Representative immunoblots are shown. Akt is the loading control protein and values are fold increase over controls. *P < 0.05.
Next, we assessed whether the antioxidant thioredoxin pathway is regulated by ErbB2 overexpression. TrxR2 protein levels and activity were measured in whole heart homogenates and mitochondria, with no significant differences found between hearts from WT and ErbB2tg mice (Fig. 2, C and D). However, we determined that ErbB2 overexpression in the heart upregulates the antioxidant catalase (Fig. 2E), an important scavenger of H2O2.
ErbB2tg Heart Mitochondria Have Lower Levels of H2O2 and Similar Mitochondrial Function as WT Heart Mitochondria
Since catalase and GPx were both increased in the hearts of ErbB2tg mice, we next sought to measure H2O2. ErbB2 can signal to protective pathways through homodimerization without ligand activation (4, 72), yet it is not known if ErbB2 upregulation and signaling is protective against oxidative stress, similar to neuregulin-1β signaling. For example, in vitro studies have shown that ErbB4 pathway activation in rat cardiomyocytes treated with neuregulin-1β (a ligand for ErbB4) causes an upregulation of specific antioxidant enzymes, such as GR, thioredoxin, thioredoxin reductase 1, and glutathione S-transferase P1 as well as protection against oxidative stress (21, 67). We asked whether ErbB2 upregulation in cardiomyocytes could induce a similar protective antioxidant condition.
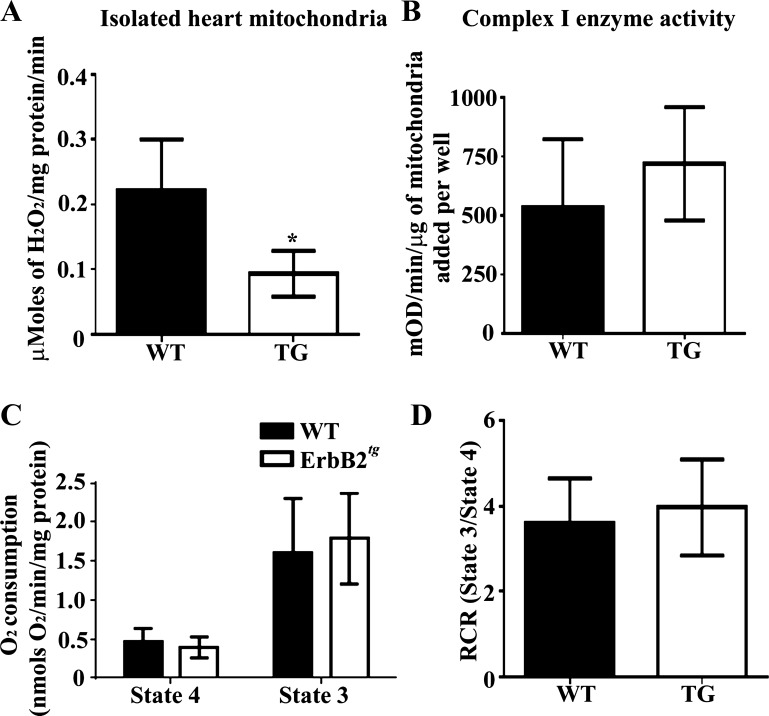
Since mitochondria represent an important source of ROS (35, 46), we measured H2O2 emission during reverse electron transport (RET) with succinate using the Amplex Red assay. We show that isolated mitochondria from ErbB2tg hearts release significantly less H2O2 under RET than WT heart mitochondria (Fig. 3A). The reduced H2O2 is consistent with the findings that mitochondrial GPx is significantly increased in ErbB2tg hearts, since GPx metabolizes and reduces levels of H2O2. Since ROS are a normal product of the mitochondrial electron transport chain, next, we assessed complex I activity and did not find any difference between WT or ErbB2tg heart mitochondria (Fig. 3B). We further assessed mitochondrial function by measuring the mitochondrial oxygen consumption rate with substrates of complex I (glutamate/malate). The data indicate that the amount of oxygen consumed under states 4 and 3 respiration was also similar between WT and ErbB2tg mitochondria; furthermore, cardiac mitochondria from both groups of animals have similar respiratory control ratio values (Fig. 3, C and D).
Fig. 3.
ErbB2tg heart mitochondria release less H2O2 compared with WT hearts, and functional studies with complex I substrates indicate that the respiratory function of isolated mitochondria from ErbB2tg hearts is not different compared with controls. A: isolated mitochondria from ErbB2tg hearts have lower mitochondrial H2O2 emission than the WT hearts as determined by the Amplex Red assay (n = 6 per group). B: results from isolated heart mitochondria show that complex I enzyme activity (n = 6 per group); state 4 and state 3 respiratory rates (C; n = 8 per group) and the respiratory control ratio (RCR; state 3/state 4) are similar between both groups of animals (D; n = 8 per group). Values represent the means ± SD. *P < 0.05.
ErbB2tg Hearts Produce Less ROS with No Effect on SOD Protein or Activity
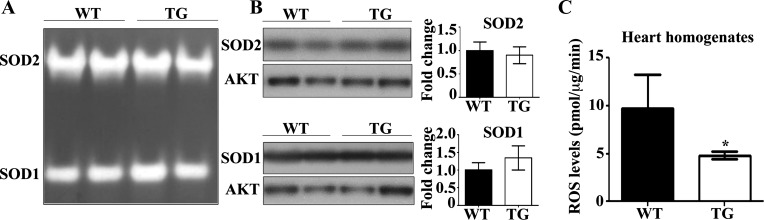
We found that the ErbB2tg mitochondria emit or release less H2O2 measured by the Amplex Red method and one likely factor is the elevation in total GPx activity, an enzyme that reduces H2O2. Furthermore, we wanted to explore other possible factors that could influence the ROS scavenging capacity of the ErbB2tg mitochondria. For example, SOD1 and SOD2 levels could be altered and have an effect on mitochondrial H2O2 basal levels since SOD metabolizes superoxide to H2O2. We found that SOD levels and activity were similar between ErbB2tg and WT hearts (Fig. 4, A and B). Thus a decrease in SOD is not the explanation for reduced basal mitochondrial H2O2 emission in the ErbB2tg mice. Taken together, these data further indicate that the heightened GPx protein levels in the mitochondrial fraction from the ErbB2tg mice are mainly responsible for decreasing basal H2O2 release from mitochondria.
Fig. 4.
ErbB2 overexpression decreases reactive oxygen species (ROS) levels and does not alter SOD activity or protein levels. A and B: SOD activity was assessed by native gel electrophoresis and nitro blue tetrazolium staining. Activities and protein levels of SOD1 and SOD2 are not different between both groups of animals (n = 4 per group). Akt is the loading control protein, and values are fold increase over controls. Representative immunoblots are shown. C: results from an EPR analysis using the 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (CPH) probe indicate that the ErbB2tg hearts have lower levels of ROS compared with WT hearts (n = 4 per group). Values represent the means ± SD. *P < 0.05.
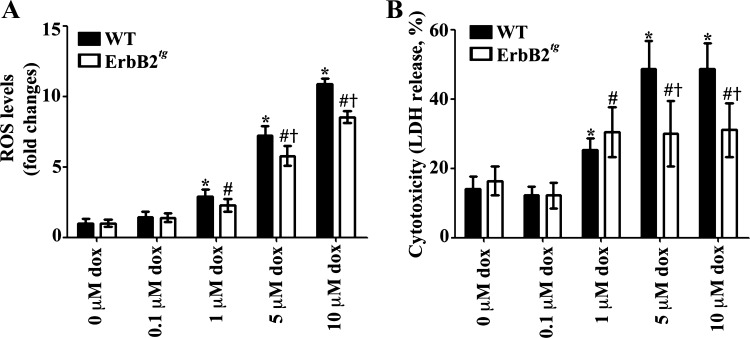
To further analyze whether ErbB2tg mice hearts exhibit less oxidative stress, we measured basal oxidative stress of whole hearts using the EPR method and the CPH cyclic hydroxylamine spin probe. This probe will react with reactive species other than superoxide such as peroxynitrite (14, 15, 37). We found that ErbB2tg hearts have a lower baseline oxidative stress, which is in agreement with our aforementioned findings (Fig. 4C). Furthermore, in studies using isolated neonatal mouse cardiomyocytes from WT and ErbB2tg hearts, we determined that ErbB2 overexpression is protective against doxorubicin-induced ROS and cellular death by the DCF-DA and LDH assay, respectively (Fig. 5, A and B).
Fig. 5.
Isolated neonatal cardiomyocytes from ErbB2tg hearts have lower ROS levels and less cellular death from doxorubicin (dox). A: WT and ErbB2tg neonatal cardiomyocytes were incubated with vehicle or dox for 24 h. ROS production was determined using the DCF-DA fluorescent assay for general oxidative stress (n = 8 per treatment group). B: cell viability was determined by the LDH assay (n = 8 per treatment group). Values represent the means ± SD. Two-way ANOVA < 0.05; *P < 0.05 vs. vector with 0 μM dox; #P < 0.05 vs. ErbB2 with 0 μM dox; †P < 0.05 vs. vector with dox dose.
ErbB2 Overexpression Upregulates Nonreceptor Tyrosine Kinases c-Abl and Arg, Two Kinases Linked to GPx and Catalase Activation
Since ErbB2 overexpression decreases mitochondrial H2O2 and related scavenging enzymes are elevated in the heart of ErbB2 overexpressors, we next investigated a potential mechanism of ErbB2's role in activation of H2O2-metabolizing enzymes. The nRTKs c-Abl and Arg have been recently linked to GPx activity regulation. For example, in vitro studies in mouse embryo fibroblasts (WT and c-abl−/−arg−/−), 293 and SH-SY5Y cells showed that GPx activity is regulated by c-Abl and Arg kinases (5). Furthermore, the Abl kinase inhibitor imatinib mesylate is known to decrease GPx activity in vivo in the rat heart (53). Additionally, Abl kinases have been shown to regulate catalase activity (6).
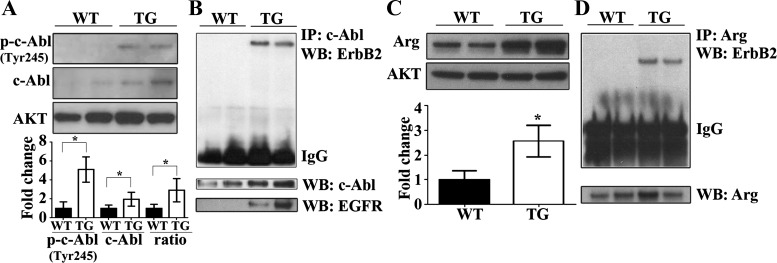
We hypothesized that ErbB2 overexpression in the heart, with increased GPx activity, and less basal mitochondrial H2O2 levels, would have elevated and activated c-Abl and Arg that would then regulate antioxidant enzyme activity. The underlying rationale of our hypothesis is provided by cancer studies showing coimmunoprecipitation of ErbB2 with c-Abl and Arg peptides (58, 59) and is important in Abl kinase activation. To our knowledge, the overarching role of ErbB2 in the regulation of nRTKs, with the subsequent activation of GPx, has not been investigated. Interestingly, representative immunoblots and densitometric analysis of phosphorylated (activated) (Y245) c-Abl and total c-Abl demonstrated that, compared with WT, the hearts from ErbB2tg mice exhibit increased levels of these proteins (Fig. 6A). Phosphorylation at the tyrosine 245 moiety located in the kinase domain of c-Abl contributes to c-Abl kinase activation (25), and ErbB2tg hearts have higher levels at baseline indicating high activity (Fig. 6A). Furthermore, our immunoprecipitation data reveal that c-Abl binds to ErbB2 in the transgenic hearts (Fig. 6B) further connecting ErbB2 to the Abl kinases in vivo.
Fig. 6.
c-Abl and Arg proteins that are known to regulate GPx activity are upregulated and coimmunoprecipitate with ErbB2 in the heart. A: representative immunoblots show higher expression of phosphorylated c-Abl at Tyr245 [p-c-Abl (Tyr245)] and total c-Abl protein in ErbB2tg hearts (n = 7 per group). B: c-Abl was immunoprecipitated (IP) from whole heart homogenates and coimmunoprecipitated with ErbB2 (n = 6 per group) and epidermal growth factor receptor (EGFR; n = 2 per group) as shown in representative immunoblots. WB, Western blot. C: representative immunoblot of Arg shows higher expression of Arg in the ErbB2tg hearts compared with the WT group (n = 7 per group). D: Arg was immunoprecipitated from whole heart homogenates and coimmunoprecipitated with ErbB2 (n = 6 per group). Values represent the means ± SD. Akt is the loading control protein and values are fold increase over controls. *P < 0.05.
Our previous studies on ErbB2 overexpression in the heart showed a concurrent upregulation of EGFR (62), as this phenomena has been observed in cancer cells with ErbB2 upregulation (22, 28). Furthermore, in addition to ErbB2, EGFR is also known to bind and activate Abl kinases in breast cancer cells; consequently, the activities of EGFR cannot be excluded here (59). Parallel in vitro studies have shown that activated Abl kinases phosphorylate EGFR and prevent ligand-dependent internalization of the EGFR receptor (66), a potential mechanism of EGFR upregulation under ErbB2 overexpression. Our immunoblotting results show that c-Abl also binds to EGFR, as well as ErbB2 (Fig. 6B). Additionally, the protein level of another nRTK Arg is also elevated in ErbB2tg mice hearts (Fig. 6C) and we demonstrate that Arg binds to the ErbB2 protein in the hearts overexpressing ErbB2 (Fig. 6D).
H9c2 cells Overexpressing ErbB2 Are Protected Against Doxorubicin-Induced Oxidative Stress and Cellular Death
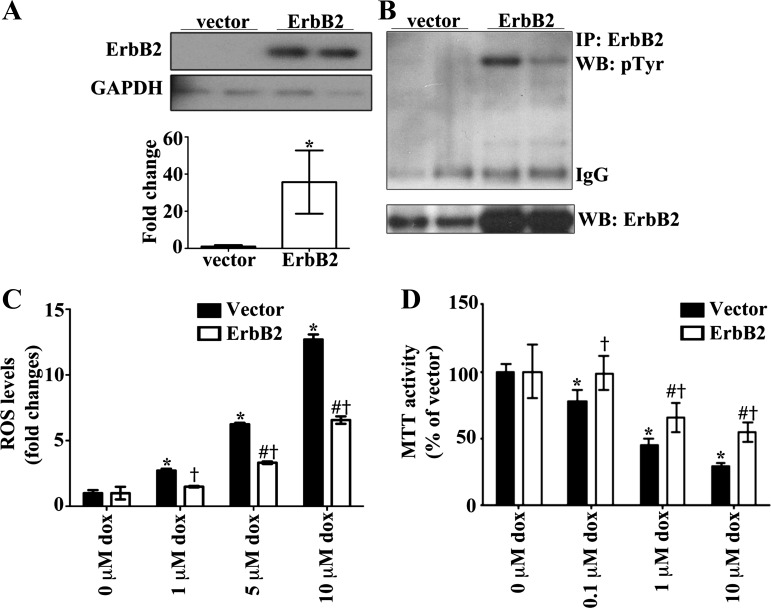
Since our results show that ErbB2 overexpression in the heart regulates GPx and Abl kinases, with ROS emission reduced, we sought to determine whether ErbB2 overexpression is protective against oxidative stress and cytotoxicity induced by doxorubicin in H9c2 cells, a cardiomyoblast cell line that is often used as an alternative in vitro model to cardiomyocytes and in doxorubicin toxicity studies (45, 54, 71). Representative immunoblots of lysates from H9c2 cells with transient overexpression of ErbB2 compared with the vector control (Fig. 7A) show that ErbB2 is upregulated and phosphorylated, indicative of activation (Fig. 7B).
Fig. 7.
ErbB2 overexpression in H9c2 cells protects against dox-induced oxidative stress and cell death. A: H9c2 cells were transfected with pcDNA (3.1)+ vector or ErbB2 plasmid. B: representative immunoblot shows that transfection with ErbB2 plasmid increases the levels of total and phosphorylated ErbB2 proteins (n = 6 per group). C: after 48 h of transfection the cells were incubated with vehicle or dox for 6 h. ROS production was determined using the DCF-DA fluorescent assay for general oxidative stress (n = 6 per treatment group). D: cell viability was determined by the MTT assay (n = 6 per treatment group). Values represent the means ± SD. GAPDH is the loading control protein and values are fold increase over controls. *P < 0.05; two-way ANOVA < 0.05; *P < 0.05 vs. vector with 0 μM dox; #P < 0.05 vs. ErbB2 with 0 μM dox; †P < 0.05 vs. vector with dox dose.
A widely known consequence for inhibiting ErbB2 is cellular death (23, 24, 51, 56). While many studies have shown the deleterious effects from blocking ErbB2, no studies have been performed in an ErbB2 overexpression model to identify cardioprotective effects. Our results indicate that ErbB2 when overexpressed is protective against oxidative stress elicited by doxorubicin treatment as measured using the DCF-DA fluorescent probe (Fig. 7C). Additionally, there is less cytotoxicity in cells treated with doxorubicin when they overexpress ErbB2, as measured by a MTT assay (Fig. 7D), which parallels the reduction in ROS levels.
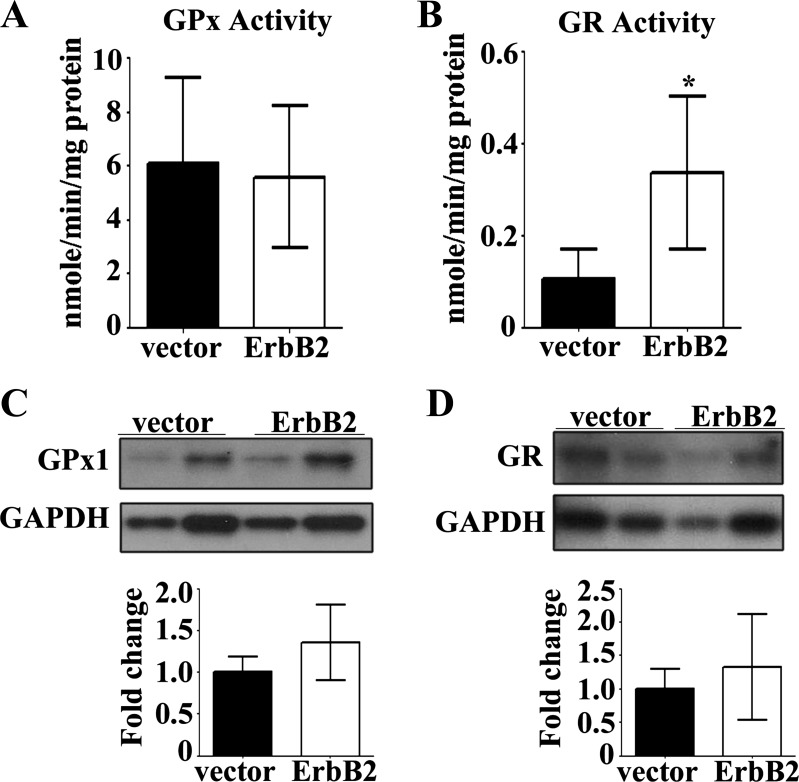
We next explored whether the in vivo role of ErbB2 in the heart, regarding upregulation of antioxidant enzymes, could be recapitulated in an in vitro system of H9c2 cells. Surprisingly, GPx enzyme activity was not elevated in H9c2 cells (Fig. 8A), as was observed in the heart of ErbB2tg mice (Fig. 1A). Since neuregulin-1β treatment of cardiomyocytes in cell culture resulted in increased GR gene expression (67), we next measured GR activity in ErbB2 transfected cells and found that GR was indeed elevated (Fig. 8B), suggesting that GR contributes to the cellular protection and lower ROS emission seen with ErbB2 transfection after doxorubicin treatment. Protein levels of GPx1 and GR proteins, however, are unchanged by overexpression of ErbB2 in H9c2 cells (Fig. 8, C and D).
Fig. 8.
GR activity is increased in ErbB2 overexpressing H9c2 cells. GPx activity (A; n = 6 per group) and GR activity were measured in H9c2 cells transfected with vector or ErbB2 plasmid (B; n = 5 per group). Transfection with ErbB2 plasmid did not affect levels of GPx1 protein (C; n = 5 per group) or GR protein (D; n = 6 per group) as shown in representative immunoblots. Values represent the means ± SD. GAPDH is the loading control protein and values are fold increase over controls. *P < 0.05.
DISCUSSION
The antioxidant role of ErbB2 upregulation in the heart has not been studied in any in vitro or in vivo cardiac system. Here we show that ErbB2 overexpression significantly decreased the levels of ROS while increasing the protein level of GPx1 as well as its activity in the heart. Accordingly, using EPR methods in whole heart and Amplex Red detection in isolated heart mitochondria we found significantly lower levels of ROS in the ErbB2 overexpressors. The ErbB2-mediated regulatory mechanism of GPx activity may be related to the action of c-Abl and Arg nonreceptor tyrosine kinases. These Abl kinases and active forms were elevated under ErbB2 expression in the heart and are also known to activate both GPx and catalase enzymes in cancer cells (5, 6). We also found that catalase is upregulated when ErbB2 is overexpressed.
The H2O2-scavenging enzyme GPx1 is primarily located in the mitochondria, where H2O2 was significantly decreased under ErbB2 overexpression. When mitochondrial function was evaluated we found that it was not adversely affected by ErbB2 overexpression in cardiomyocytes in vivo. While studies in neonatal rat cardiomyocytes have shown that ErbB2 inhibition leads to cellular death and ROS generation through mitochondrial-dependent pathways (23, 24), we observed that with ErbB2 overexpression complex I activity and O2 consumption rate from complex I substrates were comparable between WT and ErbB2tg mice mitochondria. The ability to reduce mitochondrial ROS release by increased intramitochondrial scavenging would be expected to protect the heart under conditions of enhanced ROS production, such as doxorubicin toxicity.
GPx and catalase enzymes are both regulated by Abl kinases. In the current study, both ErbB2 and EGFR immunoprecipitated with c-Abl and Arg kinases, and this protein:protein interaction has been shown to activate Abl kinases in cancer cells (58, 59). Additionally, c-Abl and Arg are known to bind to EGFR and are activated by EGF in breast cancer cell lines (59). Our findings that ErbB2 overexpression induces upregulation of c-Abl and Arg and that ErbB2 immunoprecipitates with both c-Abl and Arg constitute a first demonstration in the heart. Interestingly, ErbB2 overexpression in the heart and in cancer cells is associated with a corresponding upregulation of EGFR.
The link between Abl kinases and GPx has already been reported in the heart (53). We suggest that ErbB2 regulation of GPx activity may be related to the action of c-Abl and Arg nonreceptor tyrosine kinases, as these kinases and active forms were elevated under ErbB2 expression and are also known to activate both GPx and catalase enzymes (5, 6). The importance of c-Abl and Arg in cardiac growth and development (8, 52) is highlighted in studies showing that drugs such as imatinib mesylate (Gleevec) that target and inhibit c-Abl/Arg also cause cardiac dysfunction with subsequent reduction of cardiac GPx activity (27, 29, 33, 53). Our findings are in agreement with these previous studies with additional evidence showing that ErbB2 protein upregulates c-Abl/Arg and GPx expression and activity. Furthermore, these data are in agreement with the idea that the Abl signaling pathway is another potential target to induce cardiac toxicity when inhibited by anti-ErbB2 cancer therapy.
ErbB2 overexpression significantly decreases doxorubicin toxicity in H9c2 cells by decreasing ROS levels and preserving mitochondrial dehydrogenase function. Similarly, neonatal cardiomyocytes isolated from ErbB2tg hearts have lower ROS levels and less cellular death following treatment with doxorubicin. This may be clinically relevant as doxorubicin likely upregulates ErbB2 in human heart, acting as an antioxidant defense mechanism. Oxidative stress is one important mechanism in doxorubicin-induced cardiac dysfunction (7, 48) and this drug is known to generate ROS by redox cycling at complex I in the respiratory chain (7, 11, 16). Clinical studies using radiolabeled anti-ErbB2 and SPECT imaging showed that more anti-ErbB2 binds in the heart after doxorubicin treatment (3). These clinical studies are in agreement with our earlier findings that doxorubicin increases ErbB2 in the rat heart (19). In the current study, our findings that GPx and catalase activities are elevated additionally support the antioxidant role of ErbB2.
Together, our data suggest a new role for ErbB2 in the regulation of antioxidant systems such as GPx and catalase and the associated decrease in ROS levels in cardiomyocytes. Furthermore, GPx is upregulated in cardiac hypertrophy and downregulated in heart failure (13, 26, 34) and levels of ErbB2 in the human heart likely reflect the same expression profile as GPx. This transgenic model of cardiac ErbB2 overexpression exhibits hypertrophy that does not progress to overt heart failure with wall thinning. Oxidative stress has been reported to favor the progression from compensated hypertrophy to overt heart failure (12, 64). Consequently, we suggest that the cardioprotective role of ErbB2 in the hypertrophic state is promoted by its ability to reduce oxidative stress.
In vivo vs. in vitro overexpression of ErbB2 has different outcomes in regard to regulation of ROS-scavenging enzymes. In vitro studies of ErbB2 overexpression in H9c2 cells revealed that ErbB2 upregulates GR activity, yet in vivo studies showed that GPx enzymatic activity was upregulated in transgenic hearts overexpressing ErbB2. We suspect the redox differences of the heart compared with cell culture conditions could be a factor determining which enzyme is upregulated. Others have shown that GR mRNA was also upmodulated in cultured rat adult cardiomyocytes treated with neuregulin-1β, the ligand for ErbB3 and ErbB4 (67). Possibly, cultured cells have a greater dependence on GR activity. As a caveat, in our studies, H9c2 cells were incubated in DMEM that does not contain glutathione. Other differences between in vivo and in vitro models of ErbB2 overexpression could be important in the regulation of GR vs. GPx including cell cycle differences, the contribution from multiple cell types in vitro (i.e., adult vs. neonatal cardiomyocytes) and in vivo (e.g., cardiomyoctyes, endothelial cells, fibroblasts) (68), and the extent of hypertrophic signaling or the timing effects such as acute upregulation in cell culture but chronic upregulation in vivo in the heart.
We cannot rule out the possibility that the hypertrophic phenotype is necessary for GPx activity in the ErbB2tg mouse heart, since GPx activity is increased in models of chronic, stable hypertrophy without heart failure. Yet, both the in vivo and in vitro ErbB2 overexpression models studied here had a similar outcome, in that ErbB2 overexpression provides an enzymatic means to reduce oxidative stress in agreement with the mechanism of cell protection observed in vitro after doxorubicin treatment.
In the chronic elevation of the receptor tyrosine kinase ErbB2, a negative feedback mechanism may be present to reduce H2O2, to subsequently reduce chronic signaling. This could occur since multiple receptor tyrosine kinases have been linked to H2O2 production for amplification of the signal. For example, the phosphatase and tensin homolog on chromosome 10 (PTEN) was shown to be inactivated when oxidized by H2O2 but not superoxide (38). Many studies have focused on H2O2 in signaling and it is relatively stable in cells key in this role (18, 61). H2O2 has been called an insulinomimetic in RTK insulin receptor signaling (44). Furthermore, there is evidence that H2O2 can increase phosphorylation of ErbB4, independent of neuregulin (36). Thus we suggest a feedback system is in place where the RTKs EGFR and ErbB2 are in a complex with the Abl kinases, known to increase GPx levels and activity, and reduce H2O2. Since higher levels of H2O2 would further amplify ErbB2 signaling, a feedback mechanism to reduce H2O2 is activated to modulate pathway activity for cell homeostasis.
Conclusions and Translational Implications
The findings herein contribute to our understanding of toxicological mechanisms that can increase the vulnerability of cardiac mitochondria and heart function after anti-ErbB2 and doxorubicin treatments. It is possible that drug therapies that specifically target ErbB2 are effectively inhibiting antioxidant systems that are necessary for maintaining normal heart function, especially conditions of hypertrophy or oxidative stress triggered by doxorubicin. Our findings suggest that adverse effect on crucial redox signaling pathways involved in maintaining antioxidant defenses may be an important mechanism of cardiac toxicity induced by drugs that target ErbB2 and Abl kinases.
GRANTS
This work is supported by the National Heart, Lung, and Blood Institute Grant R01-HL-088649 (to K. Gabrielson) and the National Institute of Environmental Health Sciences Training Grant ES07141 (to F. Belmonte).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.L.G., F.B., and S.D. conception and design of research; K.L.G., F.B., S.D., P.S.-S., V.S., B.S., X.G., N.P., M.A.A., M.N., P.K., and C.S. interpreted results of experiments; K.L.G. and F.B. drafted manuscript; K.L.G., F.B., S.D., V.S., B.S., N.P., M.A.A., and C.S. edited and revised manuscript; K.L.G., F.B., S.D., N.P., M.A.A., P.K., and C.S. approved final version of manuscript; F.B., S.D., P.S.-S., V.S., B.S., X.G., and M.N. performed experiments; F.B., S.D., P.S.-S., V.S., B.S., X.G., and M.N. analyzed data; F.B. and V.S. prepared figures.
ACKNOWLEDGMENTS
We are grateful to Dr. Valeria Culotta for providing reagents and Dr. Nadja de Souza Pinto for critical conversations and review of the figures.
REFERENCES
- 1.Aon MA, Cortassa S, O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta 1797: 865–877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr TM, Behe M, Wormann B. Trastuzumab and breast cancer. N Engl J Med 345: 995–996, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18: 847–855, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278: 39609–39614, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cao C, Leng Y, Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem 278: 29667–29675, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 34: 106–135, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Chislock EM, Ring C, Pendergast AM. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci USA 110: 12432–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459–465, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res 103: 983–991, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies a KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261: 3060–3067, 1986. [PubMed] [Google Scholar]
- 12.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol 28: 506–514, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol Heart Circ Physiol 266: H1280–H1285, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Dikalov S, Skatchkov M, Fink B, Bassenge E. Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide 1: 423–431, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Dikalov SI, Kirilyuk IA, Voinov M, Grigorév IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 45: 417–430, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doroshow JH, Davies a KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261: 3068–3074, 1986. [PubMed] [Google Scholar]
- 17.Flohé L, Otting F. Superoxide dismutase assays. Methods Enzymol 105: 93–104, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Frijhoff J, Dagnell M, Godfrey R, Ostman A. Regulation of protein tyrosine phosphatase oxidation in cell adhesion and migration. Antioxid Redox Signal 20: 1994–2010, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Gabrielson K, Bedja D, Pin S, Tsao A, Gama L, Yuan B, Muratore N. Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Res 67: 1436–1441, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gabrielson KL, Hogue BA, Bohr VA, Cardounel AJ, Nakajima W, Kofler J, Zweier JL, Rodriguez ER, Martin LJ, de Souza-Pinto NC, Bressler J. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am J Pathol 159: 1507–1520, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraud MN, Fluck M, Zuppinger C, Suter TM. Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1beta. J Appl Physiol 99: 313–322, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Angulo AM, Hennessy BT, Meric-Bernstam F, Sahin A, Liu W, Ju Z, Carey MS, Myhre S, Speers C, Deng L, Broaddus R, Lluch A, Aparicio S, Brown P, Pusztai L, Symmans WF, Alsner J, Overgaard J, Borresen-Dale AL, Hortobagyi GN, Coombes KR, Mills GB. Functional proteomics can define prognosis and predict pathologic complete response in patients with breast cancer. Clin Proteomics 8: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem 284: 2080–2087, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol 44: 2231–2238, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 13: 559–571, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta M, Singal PK. Higher antioxidative capacity during a chronic stable heart hypertrophy. Circ Res 64: 398–406, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Hasinoff BB, Patel D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol Appl Pharmacol 249: 132–139, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Henjes F, Bender C, von der Heyde S, Braun L, Mannsperger HA, Schmidt C, Wiemann S, Hasmann M, Aulmann S, Beissbarth T, Korf U. Strong EGFR signaling in cell line models of ERBB2-amplified breast cancer attenuates response towards ERBB2-targeting drugs. Oncogenesis 1: e16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu W, Lu S, McAlpine I, Jamieson JD, Lee DU, Marroquin LD, Heyen JR, Jessen BA. Mechanistic investigation of imatinib-induced cardiac toxicity and the involvement of c-Abl kinase. Toxicol Sci 129: 188–199, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res 95: 734–741, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Janssen AJ, Trijbels FJ, Sengers RC, Smeitink JA, van den Heuvel LP, Wintjes LT, Stoltenborg-Hogenkamp BJ, Rodenburg RJ. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem 53: 729–734, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12: 908–916, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Kirshenbaum LA, Hill M, Singal PK. Endogenous antioxidants in isolated hypertrophied cardiac myocytes and hypoxia-reoxygenation injury. J Mol Cell Cardiol 27: 263–272, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem 279: 51141–7, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70: 343–354, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA 101: 16419–16424, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378: 394–398, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Leitch JM, Jensen LT, Bouldin SD, Outten CE, Hart PJ, Culotta VC. Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS. J Biol Chem 284: 21863–21871, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes 51: 174–181, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Long J, Ma J, Luo C, Mo X, Sun L, Zang W, Liu J. Comparison of two methods for assaying complex I activity in mitochondria isolated from rat liver, brain and heart. Life Sci 85: 276–280, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res 97: 77–87, 2013. [DOI] [PubMed] [Google Scholar]
- 44.May JM, de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem 254: 2214–2220, 1979. [PubMed] [Google Scholar]
- 45.Merten KE, Jiang Y, Feng W, Kang YJ. Calcineurin activation is not necessary for doxorubicin-induced hypertrophy in H9c2 embryonic rat cardiac cells: involvement of the phosphoinositide 3-kinase-Akt pathway. J Pharmacol Exp Ther 319: 934–940, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res 59: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52: 1213–1225, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hübner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA 99: 8880–8885, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugatsch T, Abedat S, Lotan C, Beeri R. Anti-erbB2 treatment induces cardiotoxicity by interfering with cell survival pathways. Breast Cancer Res 8: R35, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu Z, Cang Y, Goff SP. c-Abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci USA 107: 1136–1141, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad SY, Alkharfy KM, Arafah MM. Cardiotoxic effects of arsenic trioxide/imatinib mesilate combination in rats. J Pharm Pharmacol 58: 567–573, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Sardão VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Morphological alterations induced by doxorubicin on H9c2 myoblasts: nuclear, mitochondrial, and cytoskeletal targets. Cell Biol Toxicol 25: 227–243, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461: 109–113, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh KK, Shukla PC, Quan A, Lovren F, Pan Y, Wolfstadt JI, Gupta M, Al-Omran M, Leong-Poi H, Teoh H, Verma S. Herceptin, a recombinant humanized anti-ERBB2 monoclonal antibody, induces cardiomyocyte death. Biochem Biophys Res Commun 411: 421–426, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasan D, Kaetzel DM, Plattner R. Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal 21: 1143–1150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res 66: 5648–5655, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D, Watson WH, Aon MA, Paolocci N. Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J Biol Chem 286: 33669–33677, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270. [DOI] [PubMed] [Google Scholar]
- 62.Sysa-Shah P, Xu Y, Guo X, Belmonte F, Kang B, Bedja D, Pin S, Tsuchiya N, Gabrielson K. Cardiac-specific over-expression of epidermal growth factor receptor 2 (ErbB2) induces pro-survival pathways and hypertrophic cardiomyopathy in mice. PLoS One 7: e42805, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46: 1283–1297, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241–248, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Tan-chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23: 7811–7819, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Tanos B, Pendergast AM. Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J Biol Chem 281: 32714–32723, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol 41: 845–854, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Vuorenpää H, Ikonen L, Kujala K, Huttala O, Sarkanen JR, Ylikomi T, Aalto-Setälä K, Heinonen T. Novel in vitro cardiovascular constructs composed of vascular-like networks and cardiomyocytes. In Vitro Cell Dev Biol Anim 50: 275–286, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Wang GW, Kang YJ. Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther 288: 938–944, 1999. [PubMed] [Google Scholar]
- 70.Wang GW, Schuschke DA, Kang YJ. Metallothionein-overexpressing neonatal mouse cardiomyocytes are resistant to H2O2 toxicity. Am J Physiol Heart Circ Physiol 276: H167–H175, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. Vitr Cell Dev Biol Anim 47: 125–131, 2011. [DOI] [PubMed] [Google Scholar]
- 72.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001. [DOI] [PubMed] [Google Scholar]