A genetically modified form of the endothelial ligand angiopoietin-1 (Ang-1*), which has anti-inflammatory activity, prevented bleeding complications induced by fibroblast growth factor-2 (FGF-2) plus the heparin-like drug pentosan polysulfate without correcting the anticoagulant status of mice. These findings suggest that FGF-2 and Ang-1* can modulate the outcome of bleeding complications induced by heparin-like drugs.
Keywords: heparin-like drugs, fibroblast growth factor-2, angiopoietin-1, intestinal bleeding, vascular permeability
Abstract
Critically ill children can develop bleeding complications when treated with heparin-like drugs. These events are usually attributed to the anticoagulant activity of these drugs. However, previous studies showed that fibroblast growth factor-2 (FGF-2), a heparin-binding growth factor released in the circulation of these patients, could precipitate intestinal hemorrhages in mice treated with the heparin-like drug pentosan polysulfate (PPS). Yet very little is known about how FGF-2 induces bleeding complications in combination with heparin-like drugs. Here, we examined the mechanisms by which circulating FGF-2 induces intestinal hemorrhages in mice treated with PPS. We used a well-characterized mouse model of intestinal hemorrhages induced by FGF-2 plus PPS. Adult FVB/N mice were infected with adenovirus carrying Lac-Z or a secreted form of recombinant human FGF-2, and injected with PPS, at doses that do not induce bleeding complications per se. Mice treated with FGF-2 in combination with PPS developed an intestinal inflammatory reaction that increased the permeability and disrupted the integrity of submucosal intestinal vessels. These changes, together with the anticoagulant activity of PPS, induced lethal hemorrhages. Moreover, a genetically modified form of the endothelial ligand angiopoietin-1 (Ang-1*), which has powerful antipermeability and anti-inflammatory activity, prevented the lethal bleeding complications without correcting the anticoagulant status of these mice. These findings define new mechanisms through which FGF-2 and Ang-1* modulate the outcome of intestinal bleeding complications induced by PPS in mice and may have wider clinical implications for critically ill children treated with heparin-like drugs.
NEW & NOTEWORTHY
A genetically modified form of the endothelial ligand angiopoietin-1 (Ang-1*), which has anti-inflammatory activity, prevented bleeding complications induced by fibroblast growth factor-2 (FGF-2) plus the heparin-like drug pentosan polysulfate without correcting the anticoagulant status of mice. These findings suggest that FGF-2 and Ang-1* can modulate the outcome of bleeding complications induced by heparin-like drugs.
critically ill children are at high risk of developing bleeding complications when exposed to heparin-like drugs during extracorporeal membrane oxygenation or cardiopulmonary bypass (5). To date, the bleeding complications induced by heparin-like drugs have been attributed mainly to their anticoagulant activity. However, previous studies done in mice showed that fibroblast growth factor-2 (FGF-2), which is a heparin-binding growth factor, can induce spontaneous bleeding complications acting in synergy with heparin-like drugs (19). Surprisingly, the potential role that circulating heparin-binding growth factors play in the pathogenesis of bleeding complications induced by heparin-like drugs has not been explored in depth, and is not well understood. Therefore, since FGF-2 and other heparin-binding growth factors are released in the circulation of critically ill children (28, 37, 38), it is necessary to understand how these factors interact with heparin-like drugs to trigger bleeding complications.
In a previous study, we described a mouse model of lethal intestinal hemorrhages induced by the synergistic effects of FGF-2 and pentosan polysulfate (PPS). PPS is a low-molecular-weight (LMW) heparin-like drug that was used in Europe as an anticoagulant interchangeable with heparin (27). It is a well characterized molecule that has less potent anticoagulant activity than standard heparin (39) but has a similar ability to interact with heparin-binding growth factors (46). For this reason, PPS was used to modulate the angiogenic activity of heparin-binding growth factors in humans and mice with angiogenic tumors (29, 39, 46, 52). FGF-2 is a powerful heparin-binding angiogenic growth factor that is secreted in the circulation by nonconventional mechanisms, including endothelial injury (8, 11, 22). In our mouse model, PPS increases the recruitment of circulating FGF-2 in the intestine and induces hemorrhages that start in the intestinal submucosa and spread to the abdominal cavity (19). Moreover, lethal hemorrhages can also be seen in mice showing circulating FGF-2 levels similar to those seen in critically ill children (19). However, very little is known about the pathogenesis and treatment of these hemorrhages. Here we used PPS, which induces more localized intestinal hemorrhages when compared with heparin, to determine how FGF-2 triggers bleeding complications and identify new treatments to prevent them.
MATERIALS AND METHODS
Recombinant adenoviral (rAd) vectors.
rAd-vectors carrying either the Escherichia coli LacZ gene (rAd-Lac-Z), a 700-bp cDNA sequence encoding a secreted form of human recombinant FGF-2 (rAd-FGF-2), or a genetically modified form of angiopoietin-1 (Ang-1*) from Regeneron Pharm (Tarrytown, NY), were generated, amplified, and purified as previously described (13, 21, 44, 50). The particle/plaque forming unit (pfu) ratio of the virus stock used in these experiments was 100. The level of endotoxin contamination in viral preparations was <0.1 ng/mg protein.
Animal model.
The Children's Research Institute Animal Care and Use Committee approved the animal care and all experimental procedures. Male FVB/N mice (6–8 wk old) were purchased from Taconic (Germantown, MD) and kept in a temperature-controlled room (24°C) with a 12:12-h light-dark schedule, sterilized cages, autoclave bedding, and free access to water and food. Randomly selected groups of male mice (n = 10–21 per group) were injected retro-orbitally, under 3% isoflurane anesthesia, with 5 × 108 pfu/mouse of each rAd vector, as described before (50). All mice that survived were euthanized at the end of the study period by cervical dislocation under isoflurane anesthesia. Blood samples were also taken at the end of the study under isoflurane anesthesia. The serum levels of FGF-2 and Ang-1* were measured by ELISA as previously described (37, 44). After the adenoviral injections, a single dose of pentosan polysulfate (Fibrezym), from Bene Arzneimittel (Munich, Germany), was injected intraperitoneally (60 mg/kg body wt). This dose was selected based on the results of previous studies (19, 29, 46, 52), which showed that PPS was effective and safe in the treatment of experimental tumors, and did not cause bleeding complications or any other toxicity in mice.
Survival studies.
Three groups of male FVB/N mice were infected with either rAd-LacZ (n = 21); rAd-FGF-2 (n = 15); or Ad-Ad-Ang-1* + rAd-FGF-2 (n = 19) adenoviral vectors and injected intraperitoneally with PPS (60 mg/kg) 24 h and 7 days after the adenoviral infection. All mice were monitored closely after the PPS injections, and mice showing signs of shock, labored breathing, or other signs of eminent death were euthanized for humane reasons to assess whether internal bleeding was the main cause of their clinical symptoms.
Histological analysis.
For the light microcopy studies, 5-μm tissue sections corresponding to liver, spleen, kidney, lung, bladder, and small and large intestine were stained with hematoxylin and eosin (H & E) and Masson's trichrome. Changes in the structure of submucosal intestinal vessels and bleeding complications were assessed using a computer-assisted image analysis software from Optimas v6.2; Media Cybernetics (Silver Spring, MD), in five randomly selected microscopic fields (25×) per mice.
Immunohistochemistry.
Paraffin-embedded tissues were cut into 5-μm sections and processed for immunohistochemistry using the Histostain SP kit from Zymed (South San Francisco, CA) and the heat induced epitope retrieval method as previously described (19, 38). The following primary antibodies were used: von Willebrand Factor (vWF) antibody from Dako (Carpinteria, CA); rat anti-mouse F4/80 (AbD Serotec, Raleigh, NC; 1:20 dilution), and rabbit anti-collagen IV (Merck Millipore, Darmstadt, Germany; 1:50 dilution). All sections were blocked with 10% goat serum from Sigma-Aldrich (St. Louis, MO) for 30 min and incubated with primary antibodies overnight at 40C. Secondary antibodies were either biotinylated anti-rat from Dako (1:200 dilution) or biotinylated anti-rabbit (1:8,000 dilution) from Invitrogen (Carlsbad, CA). Subsequently, the sections were incubated with streptavidin-peroxidase and developed using AEC staining kit (both from Invitrogen). Hematoxylin (Invitrogen) was used for counterstaining the sections. The quantification of submucosal collagen was assessed in five randomly selected microscopic fields (25×) per mouse, using the computer-assisted image analysis described above.
Vascular permeability.
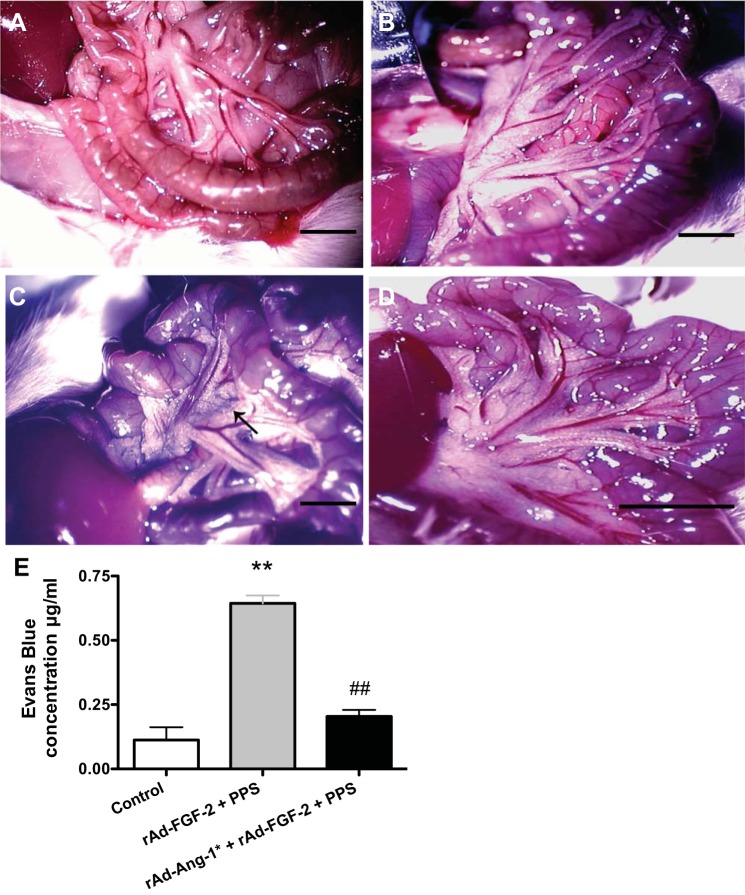
Mice were infected with rAd-LacZ or rAd-FGF2 vectors (5 × 108 pfu/mouse) alone or in combination of rAd-Ang-1* (5 × 108 pfu/mouse), and injected with PPS (60 mg/kg body wt ip) as described above. Vascular permeability was assessed using Evans Blue (EB) as previously described (36, 47). Briefly, all mice were injected intravenously with 100 μl of the EB dye (5 mg/ml in PBS) from Sigma-Aldrich (St. Louis, MO). Fifteen minutes after the EB injection, a laparotomy was performed to assess the leakage of EB in the mesenteric vessels and abdominal cavity, as shown in Fig. 3, and mice were observed for a period up to 2 h. At intervals of 15–30 min, the concentration of the EB dye in the peritoneal fluid was analyzed at 620 nm using a Beckman Du 640 spectrophotometer.
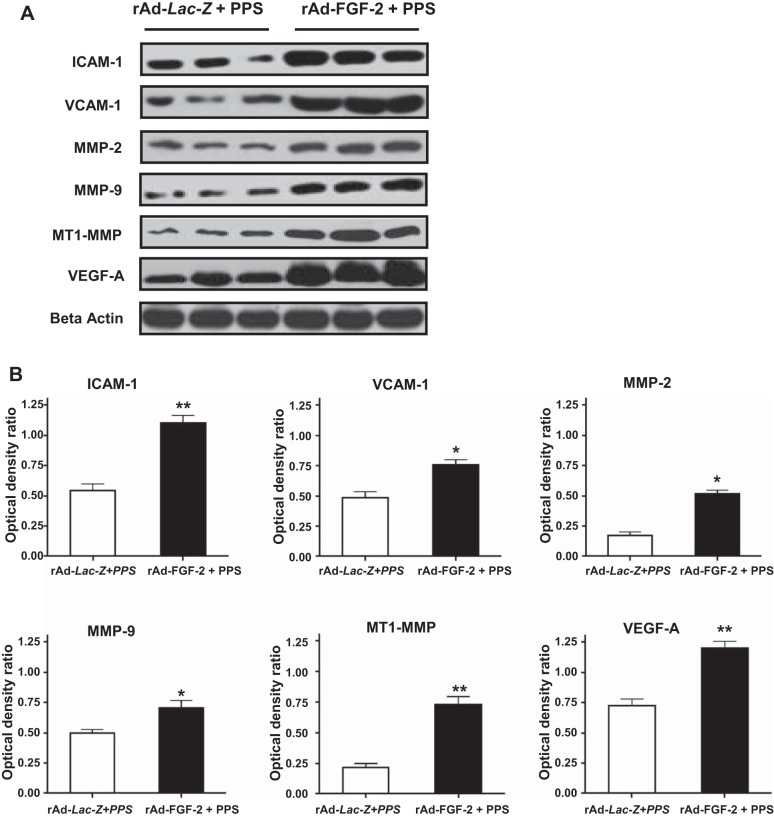
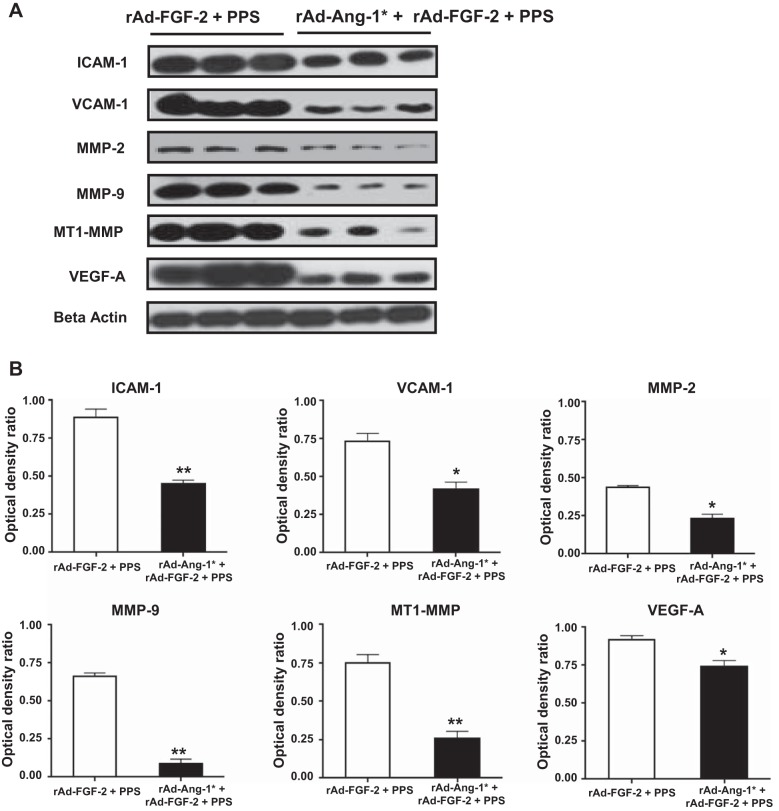
Fig. 3.
FGF-2 + PPS increases the expression of inflammatory molecules in the intestine of mice. A: representative Western blots done with samples derived from the small intestine of mice infected with rAd-Lac-Z or rAd-FGF-2 and treated with PPS (60 mg/kg). B: the densitometric analysis corresponding to the Western blots. Results are expressed in arbitrary optical density units as a ratio of the beta-actin expression (means ± SE). Samples from the small intestine of 6 different mice were included in each group (*P < 0.05, **P < 0.001 compared with control mice treated with rAd-Lac-Z + PPS).
Bleeding time and coagulation studies.
The bleeding time was measured using the murine tail-bleeding method as previously described (12). For the clotting studies, whole blood was collected from anesthetized mice after a cardiac puncture (9 parts of blood was mixed with 1 part of a 3.8% sodium citrate solution). Both the prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured using the AMAX-200 coagulation analyzer from Trinity Biotech (Wicklow, Ireland) as per manufacturer's instructions. Mean platelet counts (k/ml) were determined in triplicate by manual counting.
Western blot analysis.
Western blots were done in samples collected from the small intestine of controls and experimental mice. These samples were homogenized using the RIPA lysis buffer, which contains protease inhibitors and the phosphatase inhibitor cocktail 2 from Sigma-Aldrich. The lysates were centrifuged at 1,000 g for 10 min at 4°C, and the supernatants were collected to measure the protein concentration using the BCA Protein assay kit from Pierce Biotechnology (Rockford, IL). Subsequently, samples containing equal amounts of protein (20–50 μg) were loaded onto 4–16% Bis-Tris gel from Invitrogen and then transferred to nitrocellulose membrane from Bio-Rad (Hercules, CA). The membranes were probed with the following primary antibodies: anti-MMP-2 and anti-MMP-9 rabbit polyclonal antibodies both from Calbiochem (Spring Valley, CA); anti-MT-1 MMP, rabbit polyclonal antibody from Abcam (Cambridge, MA); anti-mouse ICAM-1 (CD-54) from BioLegend (San Diego, CA); anti-VCAM-1 rabbit polyclonal from Santa Cruz Biotechnology (Santa Cruz CA); anti-mouse VEGF-A from R&D System (Minneapolis, MN), and the beta-Actin (AC-15) mouse monoclonal antibody from Sigma-Aldrich. All primary antibodies were diluted 1:1,000 and incubated overnight at 4°C. Then membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) depending on the primary antibody used: goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP from Bio-Rad. Antibody detection was performed using the super signal West Pico Chemiluminescent Substrate from Thermo Scientific (Rockford, IL) following the manufacturer's instruction. The membrane was exposed to Kodak film (X-OMAT) from Kodak Scientific Imaging and developed using automated developer. Densitometric analysis was conducted using Adobe Photoshop 6.0. The results are representative of three independent experiments.
Statistical analysis.
Differences between two groups were compared using the Students' t-test and two-tailed P values. When more than two means were compared, we used one-way analysis of variance (ANOVA) followed by multiple comparisons using the Student-Newman-Keuls test. The Kruskal-Wallis nonparametric test was used to analyze the aPTT and bleeding results. The power calculations made to select the number of mice required for each experiment were based on the results of our previous study (19). Survival curves were analyzed using the Kaplan-Meier survival analysis, using the log rank test with the Graph Pad Prism 6 software (San Diego, CA). P values < 0.05 were considered significant.
RESULTS
FGF-2 + PPS induces intestinal vascular inflammatory changes and recruitment of macrophages in mice.
Previous studies reported that FGF-2 alone can induce inflammatory changes in mice (1, 30); however, these changes in the absence of PPS did not induce bleeding complications (19). In addition, other studies showed that PPS alone did not induce spontaneous bleeding complications or inflammatory changes in mice (19, 52). Thus, considering that the anticoagulant activity of PPS is essential to induce bleeding complications in mice treated with FGF-2 (19), we hypothesized that FGF-2-induced inflammatory changes play a critical role in the pathogenesis of the bleeding complications induced by FGF-2 + PPS. To test this hypothesis we first assessed the inflammatory changes induced by rAd-FGF-2 + PPS in the intestine of mice. Since adenoviral vectors per se can induce systemic inflammatory changes, mice treated with rAd-Lac-Z + PPS were used as controls. Briefly, two groups of mice (n = 10 per group) were infected with adenoviruses (108 pfu/mouse) carrying either the coding sequence of the LacZ gene (controls) or a secreted form of human recombinant FGF-2, and injected with PPS (60 mg/kg ip), 24 h and 7 days after the adenoviral infection. The dose and timing of the PPS injections were selected based on the results of previous studies (19, 29, 30, 44–46, 50, 52), which show that mice treated in this manner did not develop bleeding complications. In addition, intestinal sections derived from normal untreated control mice of similar age and sex (n = 5) were used to assess the changes in the normal intestine. A detailed analysis of the intestinal sections collected in mice treated with FGF-2 + PPS revealed prominent changes in the structure of submucosal intestinal vessels during the early stages of the bleeding complications compared with control mice treated with rAd-Lac-Z + PPS and untreated control mice (Fig. 1). More specifically, submucosal intestinal vessels of mice treated with FGF-2 + PPS were distorted and dilated, facilitating the leakage of inflammatory and red blood cells in the intestinal submucosa (Fig. 1). Because no significant histological differences were found between the submucosal intestinal vessels of untreated control mice and mice treated with rAd-Lac-Z + PPS (Fig. 1), in all subsequent studies we used the latter group of mice as controls.
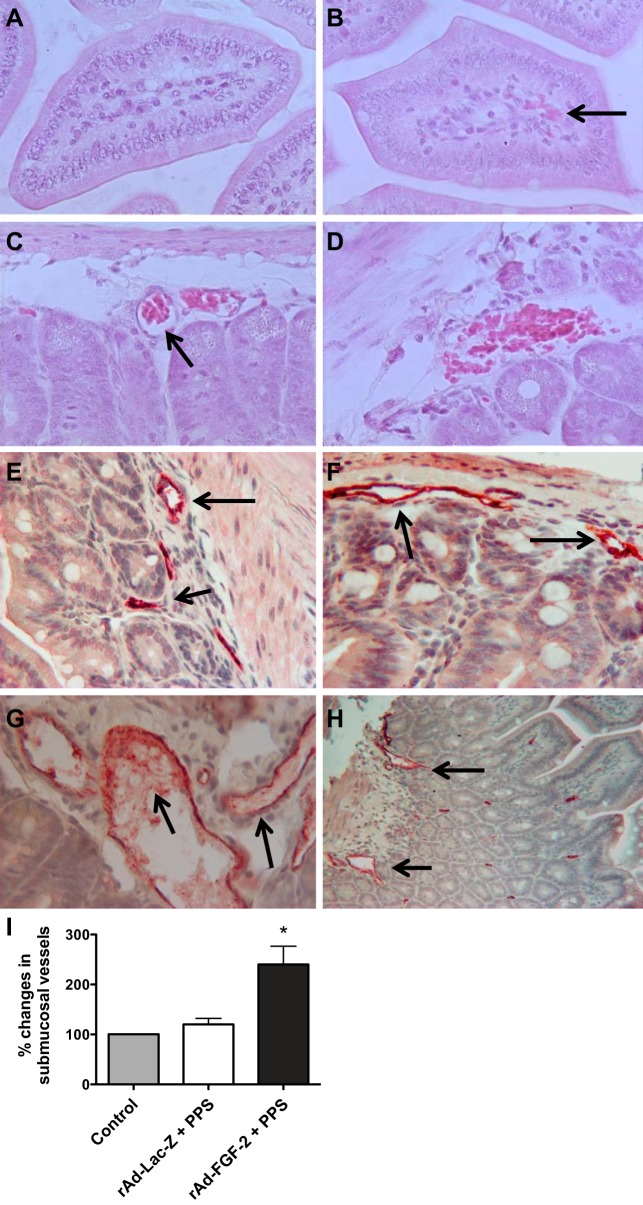
Fig. 1.
Fibroblast growth factor-2 (FGF-2) + pentosan polysulfate (PPS) induces structural changes in submucosal intestinal vessels, facilitating the leakage of red blood cells (RBC). A and B: representative hematoxylin and eosin (H & E)-stained sections collected from mice treated with rAd-Lac-Z + PPS (A) or rAd-FGF-2 + PPS (B). The black arrow points to a focal area with vascular leakage. C and D: representative intestinal sections of mice treated with rAd-FGF-2 + PPS. RBCs are shown in dilated capillaries (C), and leaking into the intestinal submucosa (D). E–H: a representative immunohistochemistry staining for the endothelial antigen vWF (red color) in the intestinal submucosal vessels of control untreated mice (E), control mice treated with rAd-Lac-Z + PPS (F), and mice treated with rAd-FGF-2 + PPS (G and H). The black arrows point to the intestinal submucosal vessels. I: graph represents the quantification of dilated and disrupted submucosal intestinal vessels expressed as % changes relative to control untreated mice. *Kruskal-Wallis test: P < 0.0041; n = 5 mice per group; Dunn's multiple comparison test: controls vs. rAd-Lac-Z + PPS: P > 0.05 (ns); rAd-Lac-Z + PPS vs. rAd-FGF-2 + PPS: P < 0.05. Original magnification: A and B, 400×; C–G, 250×; and H, 100×.
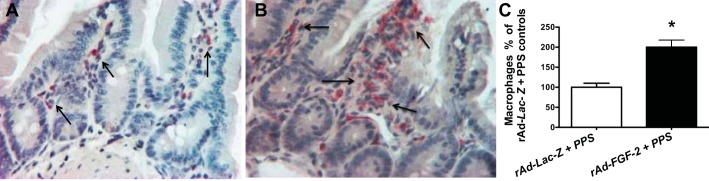
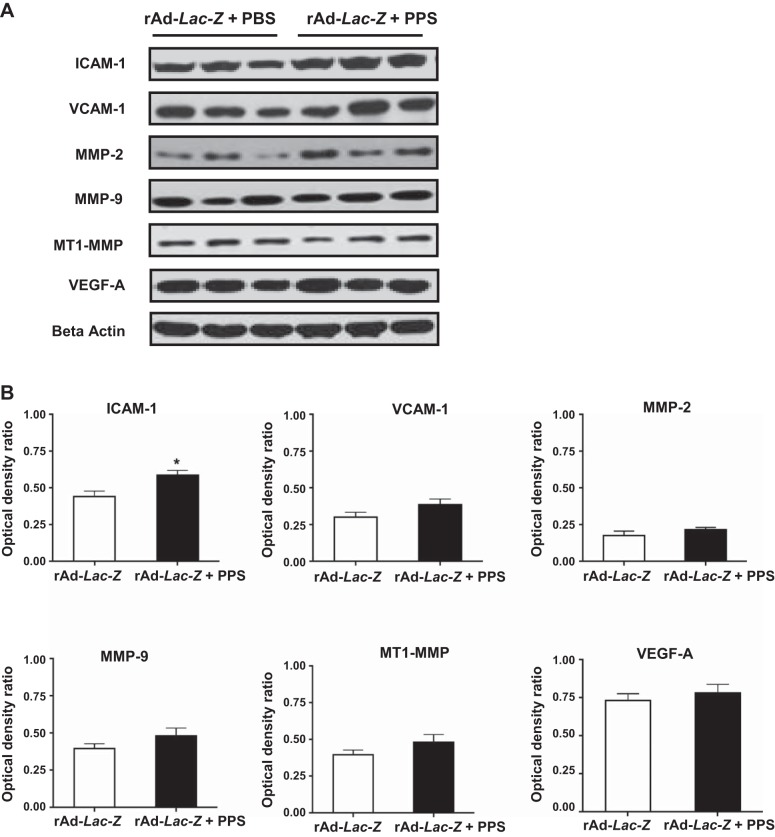
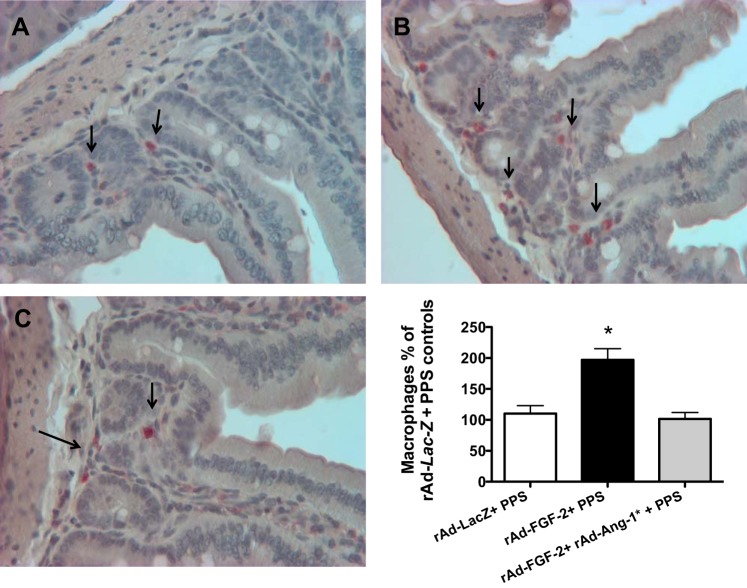
In agreement with the findings described above, the vascular intestinal changes induced by rAd-FGF-2 + PPS were accompanied by the recruitment of macrophages (Fig. 2). Furthermore, the expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM), and vascular endothelial cell growth factor-A (VEGF-A) was increased in the intestine of mice treated with FGF-2 + PPS (Fig. 3). These changes were associated with an upregulated expression of matrix metalloproteinases (MMPs) MMP-2/MMP-9, and the membrane type-1-MMP (MT1-MMP), which are all involved in tissue vascular remodeling and inflammation (Fig. 3). In contrast, no relevant differences were noted in the expression of these inflammatory molecules between mice infected with rAd-Lac-Z viral vectors and treated either with PPS or phosphate-buffered saline (PBS), with the exception of ICAM-1 (Fig. 4). These findings suggest that FGF-2 plays a critical role in the pathogenesis of the inflammatory changes that trigger the bleeding complications.
Fig. 2.
FGF-2 + PPS induces the recruitment of macrophages in the intestinal submucosa. A and B: representative immunohistochemistry staining of macrophages in control mice treated with rAd-Lac-Z +PPS (A) or rAd-FGF-2 + PPS (B). Macrophages are shown in red color stained with a rat anti-mouse antibody against the F4/80 antigen. All sections were counterstained with hematoxylin. C: the graph represents the quantification of macrophages expressed as % changes relative to control mice treated with rAd-Lac-Z + PPS. *P < 0.001, n = 4 per group, means ± SE. Original magnification (A and B), 250×.
Fig. 4.
PPS did not affect the expression of metalloproteinases or VEGF-A in the intestine of mice infected with rAd-LacZ. A: representative pictures of the Western blots done with samples derived from the small intestinal of mice infected with rAd-LacZ vectors and treated with PPS (60 mg/kg) or phosphate-buffered saline (PBS). Only the intestinal expression of ICAM-1 was significantly increased in mice treated with rAd-LacZ + PPS relative to the control mice infected with rAd-LacZ (*P < 0.05; n = 6 samples per group). B: the densitometric analysis corresponding to the Western blots. Results are expressed in arbitrary optical density units as ratio of the beta-actin expression (means ± SE). Samples from 6 different mice were included in each group.
Angiopoietin-1* (Ang-1*) decreases the vascular permeability and inflammatory changes in the intestine of mice treated with FGF-2 + PPS, and prevents lethal intestinal hemorrhages in mice.
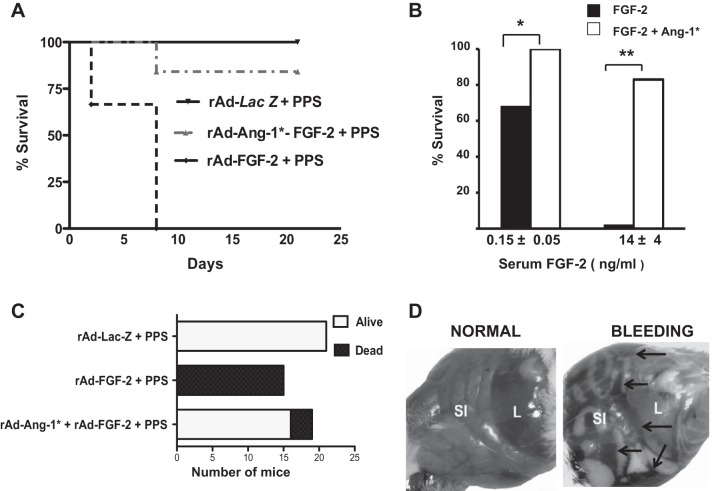
Considering that FGF-2 + PPS induced the expression of VEGF-A in the intestine of mice, we examined the possibility that Ang-1* could prevent the vascular permeability and inflammatory changes induced by FGF-2 + PPS (44, 45, 48). Ang-1* is a recombinant Ang-1 from Regeneron Pharmaceuticals in which the first 73 amino acids of human angiopoietin-2 are fused to the portion of human Ang-1 beginning at the 77th amino acid residue, and with a replacement of Cys265 to Ser (44). These changes increase the stability of Ang-1 in the circulation, as shown in previous studies in which rAd-Ang-1* blocked the permeability changes induced by VEGF-A and other inflammatory agents in mice (44). Therefore, we performed vascular permeability studies in mice injected intravenously with the Evans Blue (EB) dye, to determine how rAd-Ang-1* affected the permeability of the mesenteric vessels of mice treated with rAd-FGF-2 + FGF-2. As shown in Fig. 5, Ang-1* decreased the vascular permeability changes in the mesenteric vessels of mice treated with rAd-FGF-2 + PPS. Based on these findings, we explored the possibility that Ang-1* could prevent the development of lethal hemorrhages induced by FGF-2 + PPS in mice. To test this hypothesis, one group of FVB/N mice (n = 15) was infected with rAd-FGF-2 and injected with a single lethal dose of PPS (60 mg/kg) 24 h and 7 days after the infection, while a second group of mice (n = 19) was infected simultaneously with both adenoviral vectors (rAd-FGF-2 + rAd-Ang-1*) and treated in a similar manner. Additional control mice (n = 21) were infected with rAd-Lac-Z and injected with PPS in a similar manner. All mice (100%; 15 of 15) injected with rAd-FGF-2 + PPS died within 24 h of the second PPS injection. In contrast, all control mice survived (Fig. 6). Moreover, ∼84% (16 of 19) of the mice infected simultaneously with rAd-FGF-2 + rAd-Ang-1* and injected with PPS survived (Fig. 6, A–C), and these results were more dramatic in mice with the lowest levels of circulating FGF-2 (Fig. 6B). Finally, since intestinal endothelial cells cannot be infected through a systemic intravenous injection of rAd-Ang-1*, and all mice injected with rAd-Ang-1* show high serum levels of Ang-1* (8 ± 2 ng/ml, mean ± SD), the beneficial effects of Ang-1* were attributed to its circulating levels.
Fig. 5.
Angiopoietin-1 (Ang-1*) decreases the vascular permeability in the mesenteric vessels of mice treated with rAd-FGF-2 + PPS. FVB/N mice were injected with rAd-FGF-2 alone or in combination with rAd-Ang-1* (5 × 108 pfu/mouse for each adenoviral vector). Seven days later, all mice were injected with PPS (60 mg/kg), anesthetized, and injected retro-orbitally with 100 μl of Evans Blue (EB) dye (5 mg/ml in PBS). The EB injection alone produced a homogeneous bluish staining of the gastrointestinal tract in control mice (B) compared with control mice not injected with EB (A). C: a representative sample of mice treated with PPS plus rAd-FGF-2 demonstrating more significant leakage of EB compared with mice treated with PPS + rAd-FGF-2 + rAd-Ang-1* (D). E: the leakage of the EB dye was quantified in fluid collected from the peritoneal cavity, using arbitrary optical density units, and the results are expressed as means ± SE (n = 3 mice per group; one way ANOVA **P < 0.001 compared with control mice; ##P < 0.001 compared with mice treated with rAd-FGF-2 + PPS). Bars = 0.5 cm.
Fig. 6.
Ang-1* increases the survival of mice treated with rAd-FGF-2 + PPS. A: a Kaplan-Meier survival curve. A single dose of PPS (60 mg/kg) was injected 24 h and 7 days after the infection with the corresponding adenoviral (rAd) vectors (n = 15–21 mice per group). Log-rank test comparisons of survival curves: P < 0.001 in mice treated with rAd-Lac-Z + PPS vs. all the other groups. B: the survival of mice with different FGF-2 plasma levels *P < 0.05; **P < 0.0001. C summarizes the number of mice alive or dead at the end of the experiment (rAd-FGF + PPS vs. rAd-Ang-1* +rAd-FGF-2 + PPS; Chi-square test, P < 0.0001; rAd-Lac-Z + PPS vs. rAd-Ang-1* rAd-FGF-2 + PPS, P > 0.05). D: representative pictures of the lethal abdominal bleeding in mice. SI, small intestine; L, liver. Black arrows point to the hemorrhages.
Ang-1* decreases the inflammatory changes induced by FGF-2 + PPS.
To confirm that the circulating levels of Ang-1* were capable of decreasing the inflammatory changes in the intestine of mice treated with FGF-2 + PPS, we assessed the expression of VCAM-1, ICAM-1, MMP-2, MMP-9, MT1-MMP, and VEGF-A. By Western blots, we found that Ang-1* induced a significant reduction in the protein expression of all these inflammatory molecules (Fig. 7). In addition, Ang-1* was also effective decreasing the recruitment of macrophages in the intestine (Fig. 8).
Fig. 7.
Ang-1* decreases the expression of inflammatory molecules in the intestine of mice treated with rAd-FGF-2 + PPS. A: representative Western blots in samples derived from the small intestine of mice treated with rAd-FGF-2 + PPS or rAd-FGF-2 + rAd-Ang1* + PPS (60 mg/kg). B: the densitometric analysis corresponding to the Western blots. Results are expressed in arbitrary optical density units as a ratio of the beta-actin expression (means ± SE). Samples from the small intestine of 6 different mice were included in each group (*P < 0.05, **P < 0.001 compared with mice treated with rAd-FGF-2 + PPS).
Fig. 8.
Ang-1* decreases the intestinal recruitment of macrophages induced by FGF-2 + PPS. A–C: a representative immunohistochemistry staining of macrophages in control mice treated with either rAd-Lac-Z +PPS (A); mice treated with rAd-FGF-2 + PPS (B); and mice treated with rAd-Ang-1* + rAd-FGF-2 + PPS (C). Macrophages are shown in red color stained with a rat anti-mouse antibody against the F4/80 antigen. All sections were counterstained with hematoxylin. The graph represents the quantification of macrophages expressed as % changes relative to control mice treated with rAd-Lac-Z + PPS. *Kruskal-Wallis test, P < 0.0082, n = 4 per group; Dunn's Multiple Comparison Test: rAd-Lac-Z + PPS vs. rAd-Ang-1* + rAd-FGF-2 + PPS, P > 0.05; and rAd-Lac-Z + PPS vs. rAd-FGF-2 PPS, P < 0.05. Original magnification (A–C), 200×.
Ang-1* did not correct the anticoagulation status of mice treated with FGF-2 + PPS.
To determine whether Ang-1* prevented the development of lethal intestinal hemorrhages by normalizing the anticoagulant activity of PPS, we measured the activated partial thromboplastin time (aPTT) and the bleeding time, since they both are affected by PPS (41). As expected, within the first hours of the PPS injection, all mice treated with PPS showed prolonged aPTT (>300 s) and bleeding times (>250 s), independently of the presence or absence of rAd-Ang-1* and other vectors (Table 1). In contrast, all mice infected with the rAd-vectors alone, but not injected with PPS, showed a normal coagulation profile. PPS did not induce significant changes in platelet counts or prothrombin time (Table 1).
Table 1.
Coagulation and hematological analysis in blood samples collected from anesthetized mice 7 days after the adenoviral infection, and 1.5 h after the injection of a single dose of PPS (60 mg/kg)
| Group | PT, s | aPTT, s | Bleeding time, s | Platelets count, × 109/liter |
|---|---|---|---|---|
| Control | 9 ± 0.9 | 25 ± 3.3 | 123 ± 21 | 640 ± 197 |
| Control + PPS | 10 ± 0.7 | >300*a | >240*b | 659 ± 146 |
| Ad-Lac-Z | 10 ± 0.5 | 20 ± 2.1 | 143 ± 17 | 740 ± 199 |
| Ad-FGF-2 | 9 ± 0.7 | 19 ± 0.4 | 144 ± 47 | 623 ± 193 |
| Ad-Lac-Z + PPS | 12 ± 2.1 | >300*a | >240*b | 764 ± 87 |
| Ad-FGF-2 + PPS | 10 ± 1.1 | >300*a | >240*b | 648 ± 138 |
| Ad-FGF-2 + Ad-Ang-1* + PPS | 11 ± 1.2 | >300*a | >240*b | 647 ± 163 |
All values are expressed as the means ± SD.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured in blood collected through a heart puncture (5-14 mice per group). Platelet counts and bleeding times were measured in blood collected from the tail as described in methods. PPS, pentosan polysulfate; FGF-2, fibroblast growth factor-2; rAd, recombinant adenovirus; ND, not done.
aPTT > 300 s cannot be accurately analyzed with our assay.
Tail bleeding times were terminated after 240 s.
Kruskal-Wallis test P value = <0.0001 (n = 5–14 per group).
DISCUSSION
In this study, we demonstrated that FGF-2 release into the circulation of mice treated with the heparin-like drug PPS induces inflammatory and vascular permeability changes in the intestine of mice. These changes disrupt the structure of intestinal vessels, and in combination with the anticoagulant activity of PPS, precipitate lethal intestinal hemorrhages. Furthermore, we found that a recombinant modified form of angiopietin-1 (Ang-1*), an endothelial ligand with powerful antipermeability and anti-inflammatory activity (44, 45, 48), prevented the lethal bleeding complications by decreasing the intestinal inflammatory and vascular permeability changes, without normalizing the anticoagulant status of these mice. These findings suggest that heparin-binding growth factors and heparin-like drugs, acting in a synergistic manner, can precipitate severe bleeding complications through the induction of inflammatory and anticoagulant changes, and identify Ang-1* as a potential new treatment to prevent these disorders.
FGF-2 is a heparin binding angiogenic growth factor that lacks a leader sequence for secretion and is released by several alternative pathways, including endothelial injury (8, 11, 22). The latter mechanism of FGF-2 release explains the elevated plasma levels of FGF-2 seen in critically ill children with endothelial injury due to hypoxia, sepsis, and cardiac diseases (8, 28, 37, 38). FGF-2 released into the circulation is rapidly taken up by heparan sulfate proteoglycans (HSPG) located on the surface of vascular endothelial cells (20, 49). In this fashion, HSPG modulate the angiogenic activity of FGF-2 by affecting its binding to high-affinity receptors (32, 42). The interactions between FGF-2 and HSPG also reflect its tight binding to immobilized heparin, a property that was used in the initial isolation of FGF-2 (8, 11, 51). For all these reasons, heparin-like molecules have been used to modulate the biological activity of FGF-2 in patients with angiogenic diseases (6, 8, 17). In fact, PPS was used as a systemic treatment in patients with AIDS-associated Kaposi's sarcoma, because it is a well characterized LMW heparin-like drug that modulates the activity of heparin-binding growth factors and has less potent anticoagulant activity than standard heparin (35, 46, 52). Nonetheless, its use as an anti-angiogenic drug was also limited due to the bleeding complications seen in these patients (29, 35).
Although FGF-2 can trigger hemorrhages in mice treated either with PPS or standard heparin, PPS increases the recruitment of circulating FGF-2 in the intestine (19) and produces a more localized intestinal bleeding pattern that can be followed in a more predictable and sequential manner. For this reason we used PPS instead of heparin in our mouse model. It is worth noting that intestinal cells cannot be infected through single intravenous injections of adenoviral vectors (50), and therefore both FGF-2 and Ang-1* present in the circulation can affect intestinal vessels, as described in the present and other studies (44, 45). In addition, endothelial cells from intestinal submucosal vessels express high-affinity FGF receptors and are very sensitive to the proangiogenic activity of FGF-2 (34). Therefore, the intestine is a major target of circulating FGF-2 (34). On the basis of this principle, FGF-2 has been used to prevent apoptosis of intestinal endothelial cells (14, 34), or to accelerate the healing of gastrointestinal ulcers in different experimental rodent models (25). Interestingly, both events are regulated by HSPG and heparin-like drugs (3, 4, 24, 25). It could be argued that PPS should antagonize the action of FGF-2 by blocking its interactions with the high-affinity receptors. Nonetheless our data show that PPS was unable to block the intestinal inflammatory changes induced by FGF-2. Therefore, since the inhibitory effects of PPS can be fully reversed by adding more FGF-2 (46), it appears that submucosal intestinal vessels are very sensitive to FGF-2. Alternatively, it is tempting to speculate that PPS may interact with FGF-2 by mimicking the action of FGF-binding proteins, which mobilize FGF-2 and increase its signaling activity (31, 51). In this regard, previous studies reported that two different FGF-binding proteins can induce lethal hemorrhages in chicken embryos (31, 51).
Capillary permeability and bleeding are two different pathological processes. FGF-2 alone does not induce significant permeability changes in cultured endothelial cells (7, 9). Therefore, a novel finding of our study is that FGF-2, acting in a synergistic manner with PPS in vivo, increased the permeability of intestinal vessels. In support of our findings, previous studies reported that FGF-2 can induce capillary permeability changes in tumor endothelial cells implanted under the skin of mice (16), or in hyperglycemic rats (43). Moreover, we found that FGF-2 induced the expression of VEGF-A and increased the recruitment of macrophages in the intestine, and both events can explain the enhanced vascular permeability changes. In addition, previous studies point to other significant interactions between FGF-2 and VEGF (15, 40) and demonstrate that FGF-2 can modulate the activity of VEGF-A and the expression of the VEGF receptor-2 (VEGFR-2) in other cell types (26, 33). Alternatively, we found that FGF-2 + PPS also increased the expression of the matrix metalloproteinases MMP-2, MMP-9, and membrane type 1-MMP (MT-1-MMP). These proteolytic enzymes can degrade type IV collagen (2) and disrupt the structure of the basement membranes of intestinal submucosal vessels. Moreover, MMP-9 can cleave matrix-bound isoforms of VEGF-A, releasing soluble fragments that stimulate the phosphorylation of the VEGFR2 on endothelial cells, and inducing capillary dilation (23). Taken together, these studies may explain the remarkable dilatation of the submucosal intestinal vessels seen in mice treated with FGF-2 + PPS. More studies are needed, however, to elucidate the basic signaling mechanisms through which FGF-2 + PPS affect the structure and integrity of intestinal vessels.
Studies done in rodents with thrombocytopenia, tumors, or treated with anticoagulants have shown that inflammation can precipitate spontaneous hemorrhages (10, 16, 18). In fact, FGF-2 induces a proinflammatory signature in cultured endothelial cells, and conditioned media collected from microvascular endothelial cells exposed to FGF-2 also enhances the recruitment of monocytes (1). Likewise, we found a significant recruitment of macrophages in the intestinal submucosa of the FGF-2 + PPS-treated mice, and these changes were partially reverted by Ang-1*. Macrophages release several inflammatory cytokines and proteolytic enzymes (e.g., VEGF-A, MMP-9) that can upregulate the expression of VCAM-1 and ICAM-1. Indeed, all these factors, acting in synergy modulate the permeability and migration of leukocytes through endothelial cells. Previous studies carried out in the chick embryo chorioallantoic membrane or the matrigel angiogenesis assay (1) found a significant recruitment of monocytes and macrophages in focal areas of FGF-2-driven neovascularization. Taken together, these studies support the notion that the recruitment of macrophages may play a role in the pathogenesis of the intestinal hemorrhages induced by FGF-2 + PPS.
A remarkable finding of our study is that Ang-1* prevented the development of lethal intestinal bleeding in mice without correcting their anticoagulant status. Ang-1* is a potent endothelium-specific proangiogenic ligand, and a potent inhibitor of vascular permeability, acting through the Tie2 receptor (48). The Ang-1/Tie2 receptor complex is known to contribute to vascular remodeling, attenuation of apoptosis, and inflammation (44, 45, 48). More specifically, Ang-1 promotes the attachment of the endothelium to surrounding matrix and cells and tightens the cell junctions of endothelial cells (44). Thus these findings provide additional support to the notion that changes in capillary permeability and inflammation play key roles in the pathogenesis of bleeding disorders induced by FGF-2 and heparin-like drugs. Previous studies showed that mice can tolerate circulating levels of Ang-1* that are more than ten times higher than the serum levels of Ang-1* detected in our study, for at least 25 days without developing clinical symptoms, alterations in fluid balance, or changes in kidney function (44). Thus, although any potential short-term adverse effects of circulating Ang-1* remain to be determined in children treated with heparin-like drugs, it is tempting to speculate that these findings may have wider clinical implications to prevent hemorrhages in critically ill children treated with heparin during extracorporeal membrane oxygenation or cardiopulmonary bypass surgery. However, further studies are warranted to define how FGF-2 interacts with other heparin-like drugs and to validate this notion in children with angiogenic diseases treated with heparin-like drugs.
GRANTS
This study was supported by National Institutes of Health Grants R01-HL-55605 and R01-HL-102497.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J., J.R.D., P.T., E.W., and P.E.R. performed experiments; M.J., J.R.D., P.T., E.W., and P.E.R. analyzed data; M.J., E.W., and P.E.R. interpreted results of experiments; M.J., J.R.D., P.T., and P.E.R. prepared figures; M.J., J.R.D., and P.E.R. drafted manuscript; M.J., E.W., and P.E.R. edited and revised manuscript; M.J., J.R.D., P.T., E.W., and P.E.R. approved final version of manuscript; P.E.R. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. J. Rudge from Regeneron Pharmaceuticals for providing the Ang-1* adenoviral vectors and performing the Ang-1* assays.
REFERENCES
- 1.Andres G, Leali D, Mitola S, Coltrini D, Camozzi M, Corsini M, Belleri M, Hirsch E, Schwendener RA, Christofori G, Alcami A, Presta M. A pro-inflammatory signature mediates FGF2-induced angiogenesis. J Cell Mol Med 13: 2083–2108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res 86: 226–235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79: 1005–1013, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A, David G, Vlodavsky I, Yayon A. Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem 269: 114–121, 1994. [PubMed] [Google Scholar]
- 5.Chauhan S, Malik M, Malik V, Chauhan Y, Kiran U, Bisoi AK. Extra corporeal membrane oxygenation after pediatric cardiac surgery: a 10 year experience. Ann Card Anaesth 14: 19–24, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet 354: 62–65, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333: 1757–1763, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol 162: 1913–1926, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O'Donnell E, Zhao BQ,Cifuni SM, Wagner DD. Inflammation induces hemorrhage in thrombocytopenia. Blood 111: 4958–4964, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev 8: 95–114, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM; Animal Models Subcommittee of the Scientific and Standardization Committee of The Isth. Towards a standardization of the murine tail bleeding model. J Thromb Haemost 8: 2820–2822, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AR, Dejneka NS, D'Amato RJ, Yang Z, Syed N, Maguire AM, Bennett J. Strain-dependent anterior segment neovascularization following intravitreal gene transfer of basic fibroblast growth factor (bFGF). J Gene Med 3: 252–259, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK 3rd, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med 186: 1831–1841, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata Y, Rook SL, Aiello LP. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes 48: 1145–1155, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA 95: 4607–4612, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest 108: 349–355, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaques LB. Spontaneous hemorrhage with anticoagulants. Circulation 25: 130–139, 1962. [DOI] [PubMed] [Google Scholar]
- 19.Jerebtsova M, Wong E, Przygodzki R, Tang P, Ray PE. A novel role of fibroblast growth factor-2 and pentosan polysulfate in the pathogenesis of intestinal bleeding in mice. Am J Physiol Heart Circ Physiol 292: H743–H750, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Klagsbrun M, Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell 67: 229–231, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Kozarsky K, Grossman M, Wilson JM. Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat Cell Mol Genet 19: 449–458, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Ku PT, D'Amore PA. Regulation of basic fibroblast growth factor (bFGF) gene and protein expression following its release from sublethally injured endothelial cells. J Cell Biochem 58: 328–343, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169: 681–691, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine A, Kenet G, Bruck R, Avni Y, Avinoach I, Aeed H, Matas Z, David M, Yayon A. Effect of heparin on tissue binding activity of fibroblast growth factor and heparin-binding epidermal growth factor in experimental colitis in rats. Pediatr Res 51: 635–640, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang HY, Cho CH. Association of heparin with basic fibroblast growth factor, epidermal growth factor, and constitutive nitric oxide synthase on healing of gastric ulcer in rats. J Pharmacol Exp Ther 290: 789–796, 1999. [PubMed] [Google Scholar]
- 26.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res 17: 6130–6139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lush RM, Figg WD, Pluda JM, Bitton R, Headlee D, Kohler D, Reed E, Sartor O, Cooper MR. A phase I study of pentosan polysulfate sodium in patients with advanced malignancies. Ann Oncol 7: 939–944, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Mankhambo LA, Banda DL, Group IPDS, Jeffers G, White SA, Balmer P, Nkhoma S, Phiri H, Molyneux EM, Hart CA, Molyneux ME, Heyderman RS, Carrol ED. The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care 14: R91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall JL, Wellstein A, Rae J, DeLap RJ, Phipps K, Hanfelt J, Yunmbam MK, Sun JX, Duchin KL, Hawkins MJ. Phase I trial of orally administered pentosan polysulfate in patients with advanced cancer. Clin Cancer Res 3: 2347–2354, 1997. [PubMed] [Google Scholar]
- 30.Mattison PC, Soler-Garcia AA, Das JR, Jerebtsova M, Perazzo S, Tang P, Ray PE. Role of circulating fibroblast growth factor-2 in lipopolysaccharide-induced acute kidney injury in mice. Pediatr Nephrol 27: 469–483, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonnell K, Bowden ET, Cabal-Manzano R, Hoxter B, Riegel AT, Wellstein A. Vascular leakage in chick embryos after expression of a secreted binding protein for fibroblast growth factors. Lab Invest 85: 747–755, 2005. [DOI] [PubMed] [Google Scholar]
- 32.McKeehan WL, Wu X, Kan M. Requirement for anticoagulant heparan sulfate in the fibroblast growth factor receptor complex. J Biol Chem 274: 21511–21514, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest 121: 2668–2678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293: 293–297, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Pluda JM, Shay LE, Foli A, Tannenbaum S, Cohen PJ, Goldspiel BR, Adamo D, Cooper MR, Broder S, Yarchoan R. Administration of pentosan polysulfate to patients with human immunodeficiency virus-associated Kaposi's sarcoma. J Natl Cancer Inst 85: 1585–1592, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp e50062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray P, Acheson D, Chitrakar R, Cnaan A, Gibbs K, Hirschman GH, Christen E, Trachtman H. Basic fibroblast growth factor among children with diarrhea-associated hemolytic uremic syndrome. J Am Soc Nephrol 13: 699–707, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Ray PE, Liu XH, Xu L, Rakusan T. Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol 13: 586–593, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Scully MF, Weerasinghe KM, Ellis V, Djazaeri B, Kakkar VV. Anticoagulant and antiheparin activities of a pentosan polysulphate. Thromb Res 31: 87–97, 1983. [DOI] [PubMed] [Google Scholar]
- 40.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 141: 1659–1673, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soria C, Soria J, Ryckewaert JJ, Holmer E, Caen JP. Anticoagulant activities of a pentosane polysulphate: comparison with standard heparin and a fraction of low molecular weight heparin. Thromb Res 19: 455–463, 1980. [DOI] [PubMed] [Google Scholar]
- 42.Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 79: 1015–1024, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Stephan CC, Chang KC, LeJeune W, Erichsen D, Bjercke RJ, Rege A, Biediger RJ, Kogan TP, Brock TA, Williamson JR, Tilton RG. Role for heparin-binding growth factors in glucose-induced vascular dysfunction. Diabetes 47: 1771–1778, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Wellstein A, Zugmaier G, Califano JA, 3rd Kern F, Paik S, Lippman ME. Tumor growth dependent on Kaposi's sarcoma-derived fibroblast growth factor inhibited by pentosan polysulfate. J Natl Cancer Inst 83: 716–720, 1991. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 42: 789–794, 2001. [PubMed] [Google Scholar]
- 48.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 407: 242–248, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64: 841–848, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Ye X, Jerebtsova M, Ray PE. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum Gene Ther 11: 621–627, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Chen Y, Swift MR, Tassi E, Stylianou DC, Gibby KA, Riegel AT, Wellstein A. Effect of FGF-binding protein 3 on vascular permeability. J Biol Chem 283: 28329–28337, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zugmaier G, Lippman ME, Wellstein A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J Natl Cancer Inst 84: 1716–1724, 1992. [DOI] [PubMed] [Google Scholar]