This manuscript provides new information to suggest that while cardiac output seems to account for the majority of the exercise pressor reflex in young individuals, vascular conductance becomes more prominent in mediating this reflex in the old. This age-related change may, in part, explain the limited exercise-induced peripheral vasodilation often associated with aging.
Keywords: afferent feedback, dynamic exercise, exercise pressor reflex
Abstract
We investigated the influence of aging on the group III/IV muscle afferents in the exercise pressor reflex-mediated cardiovascular response to rhythmic exercise. Nine old (OLD; 68 ± 2 yr) and nine young (YNG; 24 ± 2 yr) males performed single-leg knee extensor exercise (15 W, 30 W, 80% max) under control conditions and with lumbar intrathecal fentanyl impairing feedback from group III/IV leg muscle afferents. Mean arterial pressure (MAP), cardiac output, leg blood flow (QL), systemic (SVC) and leg vascular conductance (LVC) were continuously determined. With no hemodynamic effect at rest, fentanyl blockade during exercise attenuated both cardiac output and QL ∼17% in YNG, while the decrease in cardiac output in OLD (∼5%) was significantly smaller with no impact on QL (P = 0.8). Therefore, in the face of similar significant ∼7% reduction in MAP during exercise with fentanyl blockade in both groups, LVC significantly increased ∼11% in OLD, but decreased ∼8% in YNG. The opposing direction of change was reflected in SVC with a significant ∼5% increase in OLD and a ∼12% decrease in YNG. Thus while cardiac output seems to account for the majority of group III/IV-mediated MAP responses in YNG, the impact of neural feedback on the heart may decrease with age and alterations in SVC become more prominent in mediating the similar exercise pressor reflex in OLD. Interestingly, in terms of peripheral hemodynamics, while group III/IV-mediated feedback plays a clear role in increasing LVC during exercise in the YNG, these afferents seem to actually reduce LVC in OLD. These peripheral findings may help explain the limited exercise-induced peripheral vasodilation often associated with aging.
NEW & NOTEWORTHY
This manuscript provides new information to suggest that while cardiac output seems to account for the majority of the exercise pressor reflex in young individuals, vascular conductance becomes more prominent in mediating this reflex in the old. This age-related change may, in part, explain the limited exercise-induced peripheral vasodilation often associated with aging.
during physical activity, mechanical and chemical stimuli within the contracting muscle increase the discharge frequency of group III and IV muscle afferents which project, via the dorsal horn of the spinal cord, to the ventral lateral medulla and the nucleus tractus solitarii. This stimulus drives much of the cardiovascular response to exercise, a regulatory mechanism termed the “exercise pressor reflex” (9, 26, 35, 38, 39). The pharmacologic stimulation of spinal μ-opioid receptors temporarily inhibits group III/IV muscle afferent feedback to the cardiovascular control centers in the brainstem (25, 36) without affecting the force-generating capacity of skeletal muscle (2, 22). Taking advantage of this phenomenon, previous studies have addressed the role of the exercise pressor reflex in determining the overall circulatory response to exercise in animals (21, 36, 44) and humans (1, 3). With this approach, conservative estimates suggest that muscle reflexes account for up to 20% of the central and peripheral hemodynamic responses exhibited by young healthy humans during exercise.
Normal healthy aging is associated with various structural and functional changes in the cardiovascular system. Specifically, changes in myocardial contractile properties, impairments in the autonomic regulation of the heart via β-adrenergic desensitization, vascular dysfunction, and changes in the baroreflex control of blood pressure have all been documented to occur with advancing age (14, 15, 28, 37). Although many of these changes are a direct consequence of aging, others may actually be appropriate secondary adaptations that ensure the correct autonomicly mediated cardiovascular response to these original physiological changes in older individuals. For example, it is well accepted that while young individuals maintain circulatory homeostasis during head-up tilt primarily via a cardiac response, the elderly are more reliant on alterations in peripheral vascular resistance (28). However, little is known about the influence of age on the role of the group III/IV muscle afferents in the exercise pressor reflex.
As the exercise pressor reflex plays a key role in regulating circulation during exercise in young individuals (53), potential age-related alterations in this mechanism may play an important role in the altered cardiovascular response to physical activity associated with the elderly (29). Indeed, based on a limited number of experiments utilizing handgrip exercise and postexercise circulatory occlusion (PECO), the exercise pressor reflex has been suggested to be attenuated in older individuals (24, 34). However, potential age-related differences in metabolite accumulation during PECO and the associated differences in the magnitude of the afferent stimulus in the young compared with the old (57, 58) may have masked the real effect of aging on the muscle reflex control of the cardiovascular response to exercise.
Consequently, to better understand the impact of age on the role of the group III/IV muscle afferents in the exercise pressor reflex-mediated cardiovascular response in humans, we utilized lumbar intrathecal fentanyl to temporarily attenuate lower limb muscle afferent feedback during dynamic knee extensor exercise in young and old individuals. We hypothesized that 1) in terms of the blood pressure response to exercise, the contribution of group III/IV-mediated muscle reflexes remains unaltered with advancing age; 2) the main mechanism responsible for the exercise pressor reflex-induced increase in blood pressure shifts from being predominantly driven by an increase in cardiac output in the young to being determined more by a decrease in vascular conductance in the old; and 3) in terms of peripheral hemodynamics, the exercise pressor reflex facilitates leg blood flow (QL) in the young, but restricts QL in the elderly.
METHODS
Participants
Eight recreationally active young (age: 24 ± 4 years; body wt: 77 ± 13 kg; height: 1.70 ± 0.02 m) and old (age: 69 ± 2 years; body wt: 79 ± 4 kg; height: 1.80 ± 0.02 m) men participated in this study. All participants were normotensive, nonsmokers, and none were taking any medications. Written consent was obtained from each participant. All experimental procedures were approved by the University of Utah and the Salt Lake City Veteran Affairs Medical Center Institutional Review Boards and conformed to the Declaration of Helsinki.
Experimental Protocol
Participants were familiarized with the experimental procedures during an initial visit. In a follow-up session, participants performed an incremental single-leg knee extensor test (0 ± 5 W/min) to exhaustion to determine their maximum attainable workload (Wpeak). Between 48 to 72 h following this incremental exercise test, participants returned to the laboratory where their right femoral artery and vein were catheterized (18 gauge central line catheters, Arrow International, Reading) by using the Seldinger technique. Following 10 min of rest, CO2 sensitivity was evaluated by determining the ventilatory response to three levels of inspiratory CO2 (FiCO2) while the participant was comfortably seated. This test was conducted to assess the potential migration of intrathecal fentanyl to the level of the brainstem, which, if occurring, could bind to the medullary opioid receptors and directly affect neurons that are involved in cardiovascular and ventilatory responses (31). Although unlikely, this would undermine the main premise behind the current experimental approach, as previously described (1, 3).
Following a short break, measurements of QL, cardiovascular/pulmonary variables, and arterial/venous blood samples were taken under control conditions (Ctrl) at rest and then during exercise. Specifically, participants performed three, 3-min, constant-load right leg knee extensor exercise bouts (60 revolutions/min) consisting of two absolute (15 and 30 W) and one relative exercise intensity (80% of Wmax; young: 45 ± 6 W; old: 35 ± 5 W). Both absolute and relative workloads were included because while QL is determined predominantly by absolute workload (12), MAP is primarily dictated by relative exercise intensity (49). Pulmonary variables, cardiac output, and QL were recorded continuously while arterial/venous blood samples were taken during the final minute of each workload.
Following a 2-h rest period, 1 ml of opioid analgesic fentanyl (0.025 mg/ml), recognized to have no effect on the force-generating capacity of the quadriceps, was delivered through vertebral interspace L3–L4 (1). To minimize the potential risk of cephalad movement within the cerebrospinal fluid, participants remained in the upright seated position throughout the study. Approximately 20 min post fentanyl administration, all measurements, including the CO2 sensitivity test, and procedures were repeated. To ensure optimal pharmacological blockade, the time to completion of all experiments was kept to less than 60 min post fentanyl injection.
Measurements
Femoral artery blood flow.
Blood velocity (Vmean; cm/s) and vessel diameter were measured simultaneously in the common femoral artery, distal to the inguinal ligament and proximal to the bifurcation of the deep and superficial femoral arteries, by using a Logic 7 ultrasound Doppler (General Electric Medical Systems, Milwaukee, WI). QL (liters/min) was calculated as = Vmean π (vessel diameter/2)2 × 60. All blood velocity measurements were performed with the probe positioned to maintain an insonation angle of 60° or less.
Pulmonary and cardiovascular responses.
Ventilation and pulmonary gas exchange were measured continuously by using an open circuit system (True Max 2400, Parvo Medics, Sandy, UT). HR was measured from the R-R interval by using a three lead electrocardiogram (Biopac systems, Goleta, CA). SV was estimated beat by beat from pressure waveforms assessed by finger photoplethysmography (7) by using the modelflow method (Beatscope version 1.1, Finapress Medical Systems, Amsterdam, The Netherlands), and cardiac output was calculated as the product of SV and HR. In addition to the beat-to-beat noninvasive assessment of arterial blood pressure by photoplethysmography, arterial and venous blood pressure measurements were collected continuously from within the femoral artery and vein by using pressure transducers (Transpac IV, Hospira, Lake Forest, IL) placed at the level of the arterial and venous catheters. Mean arterial pressure (MAP) was calculated as arterial diastolic pressure + 1/3 (arterial systolic pressure − arterial diastolic pressure), and mean venous pressure (MVP) was calculated as the average of venous systolic and diastolic pressure. Perfusion pressure was calculated as MAP − MVP. LVC was calculated as QL/(MAP − MVP). SVC was calculated as cardiac output/MAP (from photoplethysmography).
Blood derived variables.
Femoral arterial and venous blood samples were collected anaerobically and assessed in a co-oximeter and blood gas analyzer (GEM 4000, Instrumentation Laboratory, Bedford, MA). Blood gases were not corrected for blood temperature. Arterial (CaO2) and venous (CvO2) oxygen content were calculated as 1.39 (Hb) × [(oxyhaemoglobin saturation/100) + 0.003 × Po2]. Percentage O2 extraction was calculated as [(CaO2 − CvO2)/CaO2] × 100. Leg O2 delivery was calculated as the product of QL and CaO2, and leg oxygen consumption (V̇o2) as the product of CaO2 − CvO2 difference and QL.
Steady-state CO2 response test.
Measurements were carried out by using a steady-state open circuit technique (6). After baseline responses to eupnoeic air were recorded (5 min), ventilatory responses to two different concentrations of CO2 (3 and 6% CO2 with 70% O2 and balance N2) were measured in all participants. The participants breathed each gas mixture for 4 min, and the tests were separated by 5 min of exposure to room air to allow the ventilatory variables to return to baseline levels between conditions. Arterial blood samples were collected during the final 30 s of each condition and analyzed for Pco2. In addition, breathing frequency and tidal volume were assessed and averaged over the final minute in each condition.
Statistical analysis.
All statistical analyses were performed with SigmaPlot (version 12.0). Two-way ANOVA with repeated measures on the factors absolute workload and condition were performed separately for the young and the old to evaluate the effect of fentanyl blockade on central and peripheral hemodynamics. Two-way ANOVA on factors absolute workload and age were performed on Ctrl data to evaluate the effect of age on central and peripheral hemodynamics. Tukey's post hoc tests were used to identify means that were significantly different, with P ≤ 0.05. Finally, paired t-tests were used to test for differences between Ctrl and fentanyl blockade at 80% of Wmax. All results are expressed as means ± SE.
RESULTS
Resting Ventilatory Responses to CO2
Eupneic air breathing was not altered following fentanyl injection as evidenced by the similar breathing pattern and PaCO2 values exhibited by all participants in both the Ctrl and the fentanyl conditions (P > 0.2; Table 1). Additionally, exposure to the two levels of increased FiCO2 resulted in similar PaCO2 and hypercapnic ventilatory responses in both the Ctrl and fentanyl conditions in the young and the old (P > 0.5).
Table 1.
Ventilatory response to CO2 at rest
| FiCO2, % | Arterial Pco2, mmHg |
fR, breaths min−1 |
VT, liters |
|||
|---|---|---|---|---|---|---|
| Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | |
| 0.03 | ||||||
| Old | 28.1 ± 1.9 | 28.4 ± 1.9 | 13.3 ± 1.2 | 15.0 ± 1.3 | 0.9 ± 0.1 | 1.0 ± 0.2 |
| Young | 36.0 ± 1.7 | 35.7 ± 1.7 | 11.8 ± 1.3 | 13.0 ± 1.3 | 1.0 ± 0.3 | 0.9 ± 0.2 |
| 3 | ||||||
| Old | 33.9 ± 2.1 | 32.1 ± 1.8 | 12.8 ± 1.3 | 14.9 ± 1.8 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Young | 39.4 ± 1.6 | 39.1 ± 1.6 | 12.0 ± 1.0 | 13.9 ± 1.2 | 1.2 ± 0.2 | 1.0 ± 0.2 |
| 6 | ||||||
| Old | 37.9 ± 2.2 | 38.0 ± 1.3 | 14.6 ± 1.8 | 15.6 ± 1.6 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| Young | 45.0 ± 1.1 | 44.4 ± 1.1 | 15.7 ± 1.0 | 15.5 ± 1.4 | 1.4 ± 0.1 | 1.5 ± 0.1 |
Arterial Pco2, partial pressure of carbon dioxide in arterial blood; fR, breathing frequency; VT, tidal volume; FeCO2, inspired CO2 fraction; Ctrl, control; Fent, fentanyl.
Central Hemodynamic Responses
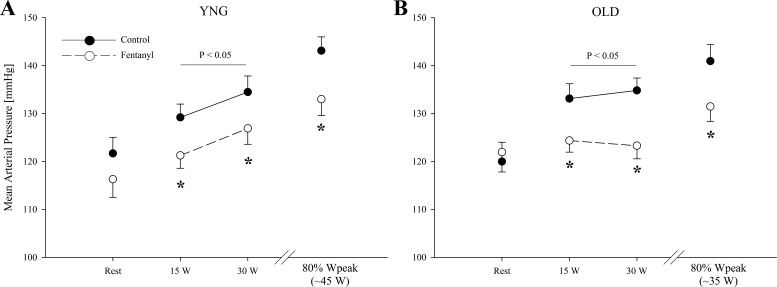
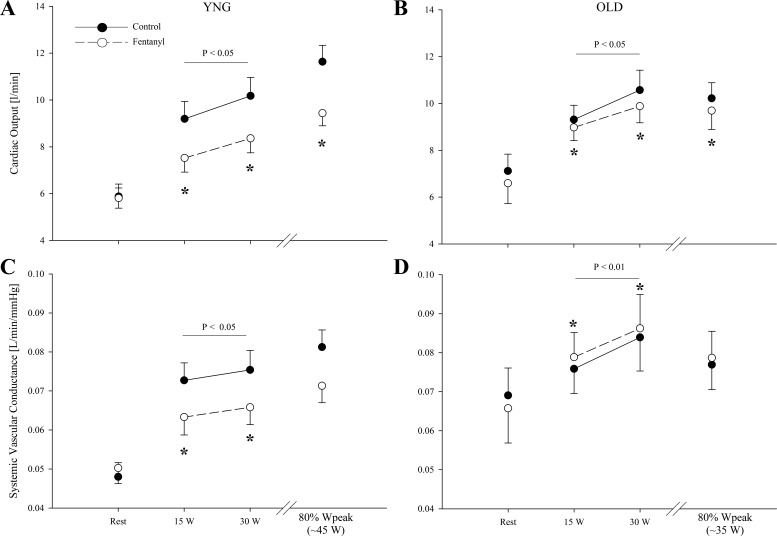
At rest, MAP and MVP were similar between the Ctrl and fentanyl conditions for both groups (P > 0.2; Fig. 1). During control exercise at the same absolute intensities, both MAP and MVP were not different between groups (P = 0.2). In addition, MAP and MVP were also not different between the young and the old during the same relative intensity (P > 0.3). MAP and perfusion pressure was 5–10% lower during the absolute and relative workloads with fentanyl blockade in both the young (P < 0.05) and the old (P < 0.05). The blockade-induced decrease in MAP during exercise was similar in both groups (P = 0.2). Fentanyl blockade had no effect on MVP in either group (P > 0.3). At rest, SVC was similar between conditions in both the young and old (P > 0.4; Fig. 2). During exercise, SVC was not different between groups in the Ctrl condition at the absolute workloads (P = 0.4). At 80% of Wpeak, SVC was also not different between the young and old (P = 0.6). Furthermore, while SVC significantly decreased (∼12%) with fentanyl blockade during the absolute and the relative workloads in the young, fentanyl blockade increased SVC by ∼4% during the absolute workloads (P < 0.01) in the old. The blockade-induced changes in SVC during exercise were different between groups (P < 0.01).
Fig. 1.
Mean arterial pressure at rest and during the final minute of each work rate during control (●) and fentanyl (○) exercise in young (YNG) and older (OLD) participants. Variables were not different between YNG vs. OLD during control exercise. *P < 0.05 vs. control.
Fig. 2.
Cardiac output and systemic vascular conductance at rest and during the final minute of each work rate during control (●) and fentanyl (○) exercise in YNG and OLD. Variables were not different between YNG vs. OLD during control exercise. *P < 0.05 vs. control.
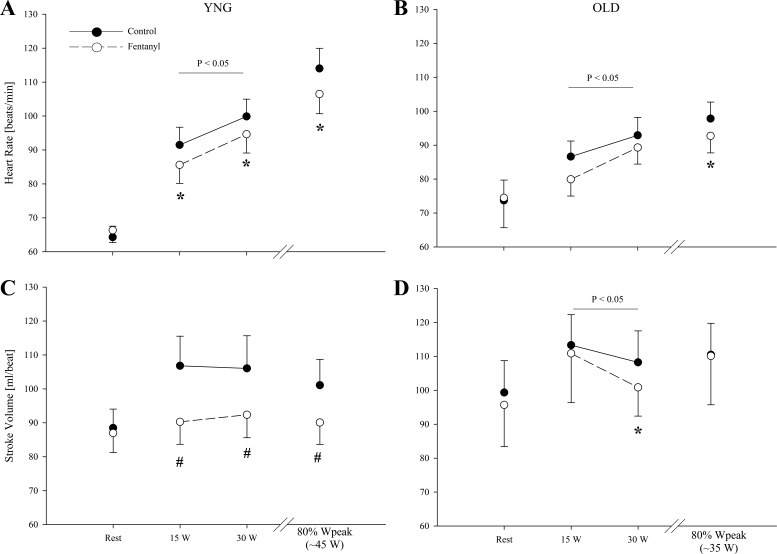
At rest, cardiac output, SV, and HR were similar in the young and old (P > 0.1; Figs. 2 and 3). Furthermore, fentanyl blockade had no effect on resting cardiac output, SV, and HR in either group (P > 0.2). During exercise in the Ctrl condition, cardiac output and SV at the absolute work rates were similar in the young and old (P > 0.2). HR was also not different across the two absolute workloads in the young and old under Ctrl conditions (P = 0.3). During exercise at 80% of Wpeak, there were no differences in cardiac output and SV between the young and old (P > 0.2); however, HR was lower in the old (P = 0.05). After fentanyl blockade in young, cardiac output and HR were ∼17 and ∼6% lower (P < 0.05), respectively. After fentanyl blockade in the old, cardiac output was only ∼5% and HR ∼6% lower (P < 0.05). While there was no main effect of age on the blockade-induced decrease in HR (P = 0.9), the fentanyl-induced changes in cardiac output during exercise were different between groups (P < 0.01). In addition, there was a reduction in HR of 7 ± 1% (P < 0.05) during 80% Wpeak in the young and 5 ± 2% (P < 0.05) during 80% of Wpeak in the old. Under Ctrl conditions in the young, SV increased from rest to 15 W (P < 0.05), but remained unchanged with further increases in workload (P = 0.8). In contrast, SV did not change from rest to exercise during the fentanyl blockade in the young (P = 0.8). In the old, SV was ∼3% lower with fentanyl blockade during absolute work rates (P < 0.05). Furthermore, while SV increased from rest to 15 W during Ctrl exercise in the old (P < 0.05), it remained unchanged with further increases in workload (P = 0.3), and this pattern was unaltered with fentanyl blockade.
Fig. 3.
Heart rate and stroke volume at rest and during the final minute of each work rate during control (●) and Fentanyl (○) exercise in YNG and OLD. *P < 0.05 vs. control; #not different from rest.
Peripheral Hemodynamic Responses
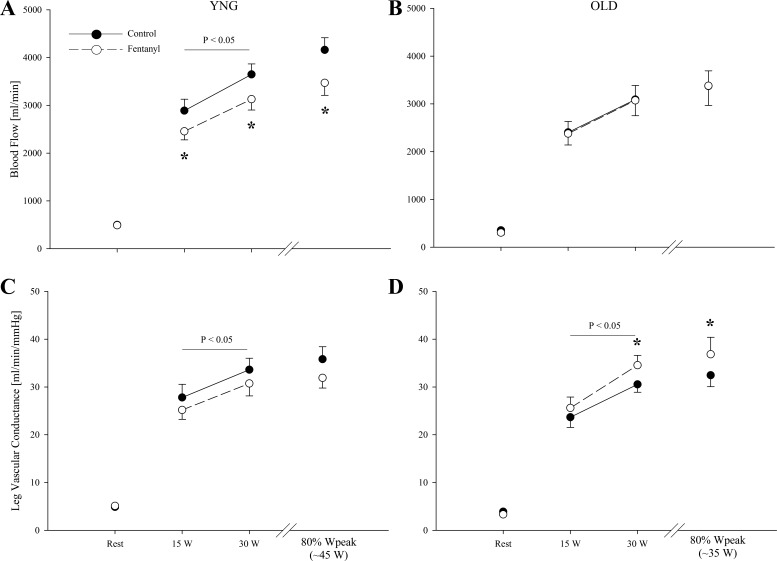
At rest, QL and LVC were similar between conditions in both the young and old (P > 0.2; Fig. 4). During Ctrl exercise, both QL and LVC were ∼16 and ∼12% lower in the old compared with the young (P < 0.05). Fentanyl blockade reduced QL during the absolute workloads in the young by 13 to 14% (P < 0.05) but had no effect on QL in the old (P = 0.8). At 80% of Wpeak, fentanyl blockade reduced QL by ∼16% (P < 0.05) in the young but had no effect in the old (P = 0.9). The blockade-induced change in QL during exercise was different between groups (P < 0.05). Fentanyl had a main effect on LVC during the absolute workloads in both groups (P < 0.05). In the young, LVC during the absolute workloads with fentanyl blockade was 6 to 9% lower compared with Ctrl (P < 0.05). In contrast, in the old, LVC increased by ∼11% during the absolute workloads with fentanyl blockade compared with Ctrl exercise (P < 0.05). The blockade-induced changes in LVC during exercise were different between groups (P < 0.01). At 80% of Wpeak, fentanyl blockade had no effect on LVC in the young (P = 0.1) but increased LVC in the old (P = 0.05).
Fig. 4.
Femoral blood flow and leg vascular conductance at rest and during the final minute of each work rate during control (●) and Fentanyl (○) exercise in YNG and OLD. *P < 0.05 vs. control.
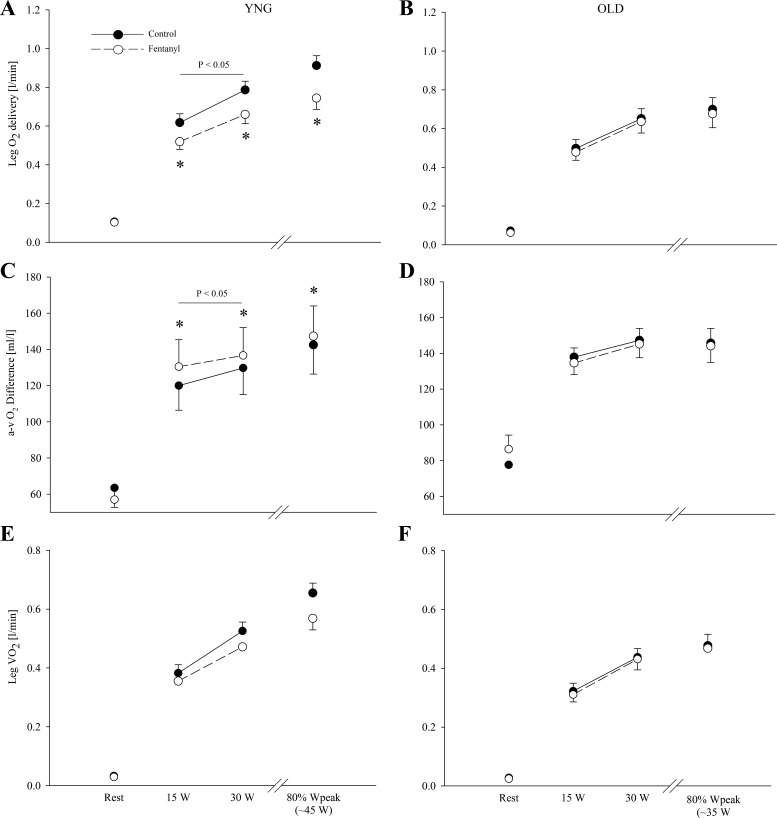
Leg O2 Supply and O2 Utilization
At rest, leg O2 delivery (young: ∼0.11 liters/min; old: ∼0.07 liters/min), arteriovenous O2 difference (young: ∼63 ml/dl; old: ∼78 ml/dl), and leg V̇o2 (young: ∼0.03 liters/min; old: ∼0.03 liters/min) were similar in the two conditions and in both groups (P > 0.2; Table 2 and Fig. 5). Within the Ctrl condition at the absolute workloads, arteriovenous O2 difference was not different between the young and old (P = 0.2); however, both leg O2 delivery and leg V̇o2 was lower in the old compared with the young (P < 0.05). At 80% of Wpeak during Ctrl exercise, leg O2 delivery and leg V̇o2 was lower in the old compared with the young (P < 0.05). Likely as a consequence of reduced O2 delivery with fentanyl blockade in the young (P < 0.05), arteriovenous O2 difference was higher with fentanyl blockade (P < 0.01) which resulted in an unchanged leg V̇o2 (P = 0.1). In contrast, leg O2 delivery (P = 0.5), arteriovenous O2 difference (P = 0.7), and leg V̇o2 (P = 0.6) were unaltered with fentanyl blockade in the old.
Table 2.
Femoral arterial-venous O2 transport and gas exchange variables obtained at rest and during final minute of exercise
| Rest |
15 W |
30 W |
80% Wpeak |
ANOVA Ctrl Only Across Age | ANOVA Blockade | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | |||
| Hb, g/dl | ||||||||||
| Old | 15.2 ± 0.5 | 15.2 ± 0.5 | 15.4 ± 0.6 | 15.1 ± 0.5 | 15.8 ± 0.5 | 15.5 ± 0.4 | 15.4 ± 0.6 | 15.1 ± 0.5 | P = 0.5 | P = 0.4 |
| Young | 15.7 ± 0.2 | 15.5 ± 0.3 | 15.8 ± 0.3 | 15.6 ± 0.2 | 15.9 ± 0.3 | 15.7 ± 0.3 | 16.2 ± 0.2 | 15.8 ± 0.4 | P = 0.05 | |
| CaO2, ml/dl | ||||||||||
| Old | 20.2 ± 0.6 | 20.2 ± 0.6 | 20.6 ± 0.8 | 20.1 ± 0.7 | 21.2 ± 0.7 | 20.7 ± 0.5 | 20.7 ± 0.8 | 20.2 ± 0.6 | P = 0.2 | P = 0.3 |
| Young | 21.3 ± 0.3 | 21.1 ± 0.4 | 21.5 ± 0.4 | 21.1 ± 0.4 | 21.6 ± 0.4 | 21.2 ± 0.4 | 22.0 ± 0.3 | 21.4 ± 0.6 | P < 0.05 | |
| CvO2, ml/dl | ||||||||||
| Old | 12.6 ± 0.8 | 11.8 ± 0.6 | 7.1 ± 0.3 | 6.9 ± 0.3 | 6.8 ± 0.3 | 6.5 ± 0.2 | 6.5 ± 0.5 | 6.1 ± 0.3 | P = 0.03 | P = 0.3 |
| Young | 14.6 ± 0.9 | 15.0 ± 0.6 | 8.2 ± 0.3 | 6.7 ± 0.4* | 7.2 ± 0.3 | 6.0 ± 0.4* | 6.2 ± 0.3 | 5.0 ± 0.4* | P < 0.001 | |
| SaO2, % | ||||||||||
| Old | 94.8 ± 0.3 | 94.9 ± 0.4 | 95.4 ± 0.3 | 94.7 ± 0.4 | 95.1 ± 0.2 | 94.9 ± 0.3 | 95.5 ± 0.2 | 95.1 ± 0.3 | P < 0.001 | P = 0.09 |
| Young | 96.5 ± 0.3 | 96.7 ± 0.3 | 96.5 ± 0.3 | 96.1 ± 0.4 | 96.4 ± 0.3 | 95.9 ± 0.3 | 96.5 ± 0.2 | 96.2 ± 0.2* | P = 0.10 | |
| SvO2, % | ||||||||||
| Old | 58.8 ± 2.5 | 55.7 ± 2.8 | 33.3 ± 1.5 | 32.9 ± 1.4 | 30.7 ± 1.4 | 30.1 ± 1.4 | 30.1 ± 1.9 | 28.9 ± 1.6 | P = 0.07 | P = 0.4 |
| Young | 64.4 ± 4.0 | 68.8 ± 2.4 | 36.9 ± 1.1 | 30.4 ± 1.6* | 31.9 ± 1.3 | 27.0 ± 1.3* | 27.1 ± 1.3 | 22.3 ± 1.3* | P < 0.01 | |
| PaO2, mmHg | ||||||||||
| Old | 78.6 ± 2.5 | 83.2 ± 3.3 | 89.2 ± 4.4 | 84.6 ± 2.9 | 87.9 ± 2.8 | 87.6 ± 3.5 | 91.3 ± 1.7 | 88.0 ± 2.6 | P = 0.8 | P = 0.2 |
| Young | 84.7 ± 2.5 | 87.6 ± 3.6 | 88.6 ± 3.2 | 84.8 ± 4.0* | 87.0 ± 1.7 | 83.2 ± 2.3* | 89.9 ± 2.2 | 86.7 ± 1.5* | P < 0.01 | |
| PaCO2, mmHg | ||||||||||
| Old | 30.4 ± 2.2 | 29.0 ± 1.5 | 26.6 ± 2.1 | 31.1 ± 1.5 | 28.5 ± 1.5 | 28.2 ± 1.7 | 28.0 ± 0.9 | 28.3 ± 1.5 | P < 0.001 | P = 0.2 |
| Young | 35.6 ± 1.6 | 34.8 ± 2.0 | 34.7 ± 1.9 | 36.0 ± 1.9* | 35.2 ± 1.4 | 36.0 ± 1.4* | 34.1 ± 1.6 | 35.7 ± 1.6* | P < 0.05 | |
| PvO2, mmHg | ||||||||||
| Old | 34.3 ± 1.4 | 34.2 ± 1.9 | 25.7 ± 1.3 | 24.9 ± 0.9 | 26.0 ± 1.3 | 24.4 ± 1.0 | 25.7 ± 1.2 | 24.7 ± 1.0 | P = 0.1 | P = 0.2 |
| Young | 40.7 ± 3.1 | 41.1 ± 1.9 | 25.0 ± 0.8 | 22.3 ± 0.8* | 23.2 ± 0.7 | 21.2 ± 0.9* | 21.3 ± 0.8 | 19.8 ± 1.0* | P < 0.01 | |
| PvCO2, mmHg | ||||||||||
| Old | 37.9 ± 1.3 | 40.6 ± 1.8 | 53.0 ± 1.9 | 55.0 ± 1.8 | 57.1 ± 2.5 | 57.6 ± 1.9 | 61.3 ± 2.4 | 63.6 ± 2.2 | P = 0.5 | P = 0.4 |
| Young | 42.8 ± 1.6 | 42.7 ± 1.9 | 55.3 ± 2.3 | 54.0 ± 2.9 | 58.0 ± 2.0 | 59.3 ± 2.2 | 65.1 ± 2.4 | 69.6 ± 2.9 | P = 0.5 | |
| O2 extraction (%) | ||||||||||
| Old | 38.2 ± 2.7 | 41.6 ± 2.9 | 65.2 ± 1.5 | 65.4 ± 1.4* | 67.8 ± 1.5 | 67.6 ± 1.4* | 68.6 ± 2.0 | 69.7 ± 1.6* | P = 0.1 | P = 0.6 |
| Young | 31.5 ± 3.9 | 29.2 ± 2.2 | 61.9 ± 1.0 | 68.5 ± 1.5* | 66.9 ± 1.3 | 71.9 ± 1.3* | 72.0 ± 1.3 | 76.8 ± 1.3* | P < 0.001 | |
| Arterial pH | ||||||||||
| Old | 7.43 ± 0.02 | 7.42 ± 0.02 | 7.41 ± 0.02 | 7.37 ± 0.01* | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.38 ± 0.01 | 7.37 ± 0.01 | P = 0.2 | P = 0.09 |
| Young | 7.43 ± 0.01 | 7.43 ± 0.02 | 7.42 ± 0.02 | 7.42 ± 0.03 | 7.41 ± 0.01 | 7.39 ± 0.02 | 7.40 ± 0.01 | 7.38 ± 0.01* | P = 0.6 | |
| Venous pH | ||||||||||
| Old | 7.36 ± 0.01 | 7.35 ± 0.01 | 7.27 ± 0.01 | 7.26 ± 0.02 | 7.23 ± 0.01 | 7.22 ± 0.02 | 7.21 ± 0.01 | 7.19 ± 0.01* | P < 0.001 | P = 0.1 |
| Young | 7.39 ± 0.01 | 7.38 ± 0.02 | 7.31 ± 0.02 | 7.32 ± 0.02 | 7.29 ± 0.01 | 7.28 ± 0.01 | 7.25 ± 0.02 | 7.22 ± 0.01* | P = 0.8 | |
| Arterial Lactate, mmol/liter | ||||||||||
| Old | 0.78 ± 0.15 | 0.66 ± 0.09 | 1.37 ± 0.20 | 1.20 ± 0.14* | 2.10 ± 0.25 | 1.93 ± 0.22 | 2.47 ± 0.30 | 2.34 ± 020 | P = 0.5 | P = 0.07 |
| Young | 0.57 ± 0.04 | 0.69 ± 0.14 | 1.31 ± 0.18 | 1.29 ± 0.21 | 1.78 ± 0.33 | 1.79 ± 0.38 | 2.68 ± 0.50 | 2.86 ± 0.52 | P = 0.8 | |
| Venous Lactate, mmol/liter | ||||||||||
| Old | 0.81 ± 0.12 | 0.76 ± 0.09 | 2.14 ± 0.32 | 1.92 ± 0.23 | 3.31 ± 0.36 | 3.00 ± 0.31 | 3.99 ± 0.44 | 3.86 ± 0.27 | P = 0.4 | P = 0.1 |
| Young | 0.72 ± 0.05 | 0.83 ± 0.14 | 2.09 ± 0.42 | 1.89 ± 0.40 | 2.54 ± 0.58 | 2.54 ± 0.63 | 4.21 ± 0.90 | 4.43 ± 0.87 | P = 0.5 | |
| Lactate efflux, mmol/min | ||||||||||
| Old | 0.01 ± 0.02 | 0.02 ± 0.01 | 1.87 ± 0.46 | 1.74 ± 0.40 | 3.63 ± 0.61 | 3.35 ± 0.66 | 4.99 ± 0.68 | 4.90 ± 0.62 | P = 0.8 | P = 0.5 |
| Young | 0.08 ± 0.01 | 0.07 ± 0.02 | 2.34 ± 0.79 | 1.57 ± 0.60* | 2.76 ± 0.86 | 2.24 ± 0.78 | 6.06 ± 1.55 | 5.42 ± 1.32 | P < 0.05 | |
Hb, hemoglobin; CaO2, arterial oxygen content; CvO2, venous oxygen content; SaO2, arterial oxygen saturation; SvO2, venous oxygen saturation; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; PvO2, partial pressure of oxygen in venous blood; PvCO2, partial pressure of carbon dioxide in venous blood.
P < 0.05, fentanyl exercise value is different from that at control exercise.
Fig. 5.
Leg O2 supply and demand at rest and during the final minute of each work rate during control (●) and fentanyl (○) exercise in YNG and OLD. *P < 0.05 vs. control.
DISCUSSION
Utilizing intrathecal fentanyl to temporarily attenuate muscle afferent feedback during dynamic single-leg knee extensor exercise, we investigated the effect of aging on the role of group III/IV leg afferents in the exercise pressor reflex-mediated cardiovascular response. With no cardiovascular effects at rest, fentanyl blockade attenuated both cardiac output and QL during exercise in the young, whereas in the old, the drug-induced decrease in cardiac output was substantially smaller with no impact on QL. Furthermore, LVC and SVC increased following fentanyl blockade in the old but decreased in the young. Despite these significant age-related differences, the group III/IV-mediated contribution to the overall blood pressure response to exercise remained similar with age. However, the afferent feedback-related mechanisms contributing to the MAP response to exercise appear to be impacted by age. Specifically, while cardiac output seems to account for the majority of the group III/IV-mediated MAP response in the young, the effect of neural feedback on the heart decreases with age and alterations in SVC become more prominent in terms of mediating the exercise pressor reflex in the old. Interestingly, in terms of peripheral hemodynamics, while group III/IV-mediated feedback plays a clear role in increasing LVC during exercise in the young, these afferents seem to actually reduce LVC in the elderly. Although the current study cannot address the possible existence of sex-related differences, this finding could potentially help to explain the limited exercise-induced peripheral vasodilation often associated with aging (12, 47).
Aging and the Exercise Pressor Reflex
The effects of aging on exercise-induced increases in blood pressure remain equivocal, with some studies revealing an exaggerated response in the elderly (17, 18), whereas others have found no difference across age groups (24, 30, 32, 56). Since the MAP response to both absolute and relative exercise intensities was similar in both groups (Fig. 1), the current study supports the conclusion that the exercise-induced increase in blood pressure is not affected by aging.
Earlier studies investigating the influence of aging on the role of muscle reflexes in determining exercise-induced MAP responses have relied on PECO techniques (24, 34). This approach traps exercise-induced metabolites within a muscle by the inflation of a blood pressure cuff proximal to the muscle that was contracting to maintain, and even raise, neural feedback for as long as the muscle is held ischemic. During PECO following rhythmic handgrip exercise in old and young individuals, MAP has been reported to be lower in the old, which led to the conclusion that aging attenuates the exercise pressor reflex (34). However, recognizing the well-documented age-related shift in muscle fiber type toward the fatigue resistant type I phenotype (33, 50), which is associated with a slower metabolite accumulation and consequently a reduced stimulus for metabosensitive muscle afferents (57, 58), this conclusion may have been in error. Specifically, the observed difference in blood pressure responses during PECO in old compared with young individuals might actually be a consequence of reduced metaboreceptor stimulation in the old and entirely independent of any functional changes in the muscle reflex arc. The present investigation circumvented this and various other issues that have been associated with PECO (4), and clearly did not reveal an effect of aging on the contribution of the group III/IV muscle afferents to the blood pressure response during exercise (Fig. 1).
Aging and Exercise-Induced Central Hemodynamics
Although resting cardiac output is generally similar in young and old individuals (51), cardiac output achieved during maximal exercise is markedly decreased with age (20). However, findings from studies addressing the effect of aging on cardiac output during a given submaximal work rate are, in contrast, surprisingly inconsistent and may be related to the nature of the exercise, with some studies reporting no difference during cycling exercise (nonweight bearing) (46, 51), whereas others document an age-related decrease during treadmill exercise (weight bearing) (42, 55). In the present study, the increase in cardiac output from rest to single-leg knee extensor exercise (nonweight bearing) was similar in young and old (Fig. 2), agreeing with earlier work also based on nonweight-bearing cycle exercise (46, 51).
Input from thin fiber muscle afferents exert inotropic and chronotropic effects on the heart to facilitate an increase in cardiac output and MAP, and enhance blood flow to the working muscle of the exercising animal (41) and human (3, 8). Indeed, the ∼17% reduction in cardiac output with the afferent blockade in the young participants (Fig. 2) confirms these previous findings (3) and further emphasizes the critical role of neural feedback in regulating central hemodynamic responses during exercise. Interestingly, compared with the large effect in young individuals, fentanyl blockade had a substantially smaller, but still significant, effect on cardiac output in the old (∼5% decrease), a discrepancy suggestive of an age-related decline in the role of the group III/IV-mediated muscle reflexes in facilitating central hemodynamics during exercise. The small effect of the afferent blockade on cardiac output in the old is likely attributable to the age-related impairment in the autonomic regulation of the heart, a phenomenon which is largely accounted for by β-adrenergic desensitization (14).
Aging and Exercise-Induced Peripheral Hemodynamics
Although not always evident (45), the majority of evidence suggests that there is an age-related reduction in skeletal muscle blood flow during exercise (32, 48, 49). This attenuation in exercise-induced hyperemia has been attributed to various age-related alterations in the vascular mechanisms that regulate muscle perfusion (5, 40). The lower QL in the old compared with the young participants observed in our experiments supports these earlier observations (Fig. 4).
The findings of the current study suggest that aging causes an alteration in the muscle reflex control of peripheral blood flow during exercise. Specifically, in the young, group III/IV muscle afferent blockade attenuated QL as a consequence of a significant reduction in both perfusion pressure and LVC. In the old participants, afferent blockade caused a similar significant decrease in perfusion pressure, but, in contrast, LVC increased with the overall effect of no change in QL. This suggests that during exercise with an intact afferent feedback mechanism, group III/IV muscle afferents (and the associated limited LVC response to exercise) might hinder QL in the elderly. This observation could help to explain the lower blood flow observed in the older individuals in this study (Fig. 4) and earlier findings also documenting compromised QL during exercise in the elderly (32, 48, 49).
The Potential Role of the Baroreflex in the Observed Fentanyl-Induced Hemodynamic Changes
While blockade of group III/IV muscle afferents dampens hemodynamic responses to exercise, baroreflex-mediated compensatory cardiovascular adjustments attempt to counterbalance the associated blunted pressor response to return MAP to its set point pressure (13). Consequently, although the role of the baroreflex was not measured in the current study, the observed circulatory responses still need to be considered as the net outcome of these two opposing factors which are also likely to be affected by age. Specifically, young individuals have been reported to rely on both decreases in SVC [i.e., increases in sympathetic nerve activity (SNA)] and, to a lesser extent, increases in cardiac output to return MAP to its set point pressure during a hypotensive stimulus (10, 27). However, it is important to consider that muscle afferent feedback also contributes to the resetting of the carotid baroreflex to operate at a higher arterial pressure (54). Spinal blockade, in turn, impairs this resetting of the baroreflex, resulting in a lower set point pressure operating during exercise with fentanyl blockade (54). Therefore, the observed responses in the young likely underestimate the real hemodynamic effects of blocking the muscle afferents (3).
With advancing age, presumably secondary to the age-related impairment in arterial baroreceptor control of the heart, older individuals are typically characterized by a substantially greater reliance on a decrease in SVC, rather than an increase in cardiac output, to restore MAP during hypotension (10, 15). Furthermore, the sensitivity of arterial baroreceptors to detect a deviance in MAP from the set point pressure is markedly blunted with age (16). It is therefore likely that the blockade-induced decrease in MAP generated a rather small, or, perhaps, nonexistent baroreceptor error signal in the old. In addition, older individuals are characterized by higher resting SNA to muscle and a substantially lower rise to a given physiological stress (52). Taken together, these likely age-related changes in the baroreceptors and SNA responses suggest that while baroreflex-mediated cardiovascular adjustments might have masked an even greater effect of muscle afferent blockade on the hemodynamic responses during exercise in the young, these compensatory mechanisms were presumably either not, or to a much smaller degree, engaged in the old. Therefore, these considerations further support the concept that there is an age-related alteration of the contribution of the group III/IV muscle afferents to the hemodynamic response to exercise.
Group III/IV Afferents and Oxygen Transport and Utilization during Exercise with Age
Interestingly, the fentanyl blockade of group III/IV muscle afferent feedback reduced CaO2 by decreasing arterial Po2 and HbO2 saturation in the young but not the old participants (Table 2). This was likely the consequence of the afferent blockade-induced hypoventilation that also resulted in a lower V̇e (but not V̇e/V̇co2) during exercise in the young but not the old (Table 3). Although the blockade-induced hypoventilation in the young was not as pronounced during the current knee extensor exercise modality as previously documented during cycling exercise (1), in combination the attenuated CaO2 and QL led to a reduction in O2 delivery which was compensated for by an increase in O2 extraction, with a net effect of no change in leg V̇o2. In terms of peripheral hemodynamics, it is important to note that the documented afferent blockade-induced fall in QL in the young participants would likely have been somewhat greater if it were not for this reduction in CaO2, which would independently increase QL (19). In contrast, fentanyl blockade during exercise in the elderly had no effect on ventilation or CaO2, and therefore leg V̇o2 remained unchanged (Table 2 and 3 and Fig. 5), confirming the similar findings from a recent cycling study which included old participants as controls for patients with heart failure (43).
Table 3.
Ventilatory responses at rest and during final minute of exercise
| Rest |
15 W |
30 W |
80% Wpeak |
ANOVA Ctrl Only Across Age | ANOVA Blockade | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | |||
| V̇e, liters/min | ||||||||||
| Old | 9.3 ± 1.5 | 9.4 ± 1.2 | 21.8 ± 4.0 | 17.7 ± 1.1 | 22.4 ± 2.0 | 23.1 ± 1.6 | 28.3 ± 4.3 | 27.5 ± 3.3 | P = 0.3 | P = 0.3 |
| Young | 8.6 ± 0.7 | 9.9 ± 1.5 | 22.8 ± 2.4 | 22.0 ± 2.6 | 26.9 ± 1.2 | 25.4 ± 1.8* | 37.5 ± 3.2 | 34.4 ± 2.2* | P < 0.05 | |
| fR, breaths/min | ||||||||||
| Old | 12.8 ± 1.5 | 14.9 ± 1.4 | 24.0 ± 1.5 | 22.6 ± 1.8 | 24.3 ± 2.1 | 24.4 ± 1.9 | 25.1 ± 2.5 | 24.8 ± 2.1 | P = 0.6 | P = 0.5 |
| Young | 12.7 ± 1.5 | 13.9 ± 1.4 | 21.3 ± 1.4 | 21.4 ± 1.7 | 24.6 ± 1.4 | 22.9 ± 1.9 | 28.2 ± 1.9 | 26.5 ± 2.2 | P = 0.2 | |
| VT, liters | ||||||||||
| Old | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | P = 0.9 | P = 0.2 |
| Young | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | P = 0.7 | |
| V̇o2, liters/min | ||||||||||
| Old | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | P < 0.01 | P = 0.4 |
| Young | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | P = 0.8 | |
| V̇co2, liters/min | ||||||||||
| Old | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.7 ± 0.1 | 0.7 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.1 | P = 0.1 | P = 0.3 |
| Young | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.8 ± 0.0 | 0.7 ± 0.1* | 1.1 ± 0.1 | 1.1 ± 0.1 | P < 0.05 | |
| RER | ||||||||||
| Old | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | P = 0.5 | P = 0.3 |
| Young | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0* | 0.9 ± 0.0 | 0.8 ± 0.0* | 1.0 ± 0.0 | 0.9 ± 0.0* | P < 0.01 | |
| V̇e/V̇o2, liters/min | ||||||||||
| Old | 43.6 ± 5.0 | 44.3 ± 3.4 | 41.0 ± 8.3 | 36.5 ± 1.9 | 35.1 ± 1.8 | 36.7 ± 3.4 | 36.5 ± 2.0 | 37.5 ± 3.0 | P < 0.05 | P = 0.4 |
| Young | 31.6 ± 1.1 | 36.2 ± 2.8 | 29.1 ± 2.2 | 28.3 ± 2.2 | 28.1 ± 0.8 | 27.6 ± 1.4 | 31.3 ± 2.1 | 29.6 ± 1.4 | P = 0.3 | |
| V̇e/V̇co2, liters/min | ||||||||||
| Old | 50.2 ± 2.3 | 51.2 ± 2.0 | 43.4 ± 2.7 | 44.4 ± 1.4 | 40.9 ± 1.7 | 41.7 ± 2.1 | 40.1 ± 1.7 | 39.8 ± 2.2 | P = 0.5 | P = 0.7 |
| Young | 39.4 ± 1.2 | 40.8 ± 1.0 | 34.7 ± 1.7 | 36.8 ± 2.5 | 32.9 ± 1.1 | 33.5 ± 1.8 | 32.8 ± 1.6 | 32.2 ± 1.3 | P = 0.1 | |
V̇e, ventilation; V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e/V̇o2, ventilatory equivalent ratio for oxygen; V̇e/V̇co2, ventilatory equivalent ratio for carbon dioxide.
P < 0.05, fentanyl exercise value is different from that at control exercise.
A relevant consideration for the interpretation of our findings is the potential effect of aging on the efficacy of fentanyl to attenuate μ-opioid receptor-mediated afferent feedback. Although there is no data in humans supporting this hypothesis, a recent animal study documented an age-related decrease in the affinity of spinal μ-receptors to bind to the very specific opioid agonist DAMGO (23). However, there is no clinical necessity to increase opioid dosages in older patients, and the similar impact of spinal blockade on MAP during exercise in both age groups (Fig. 1) do not support the existence of this age-related effect in humans, at least not with fentanyl.
It is important to realize that the heightened reliance on vascular resistance to adequately control blood pressure during exercise in the elderly is not only accounted for by increases in the afferent feedback-mediated sympathetic vasoconstriction, but other age-related modifications of various vasoconstrictor pathways and vascular tone may also play a role. For example, age-related decreases in functional sympatholysis (11) and changes in various neurohumoral mechanisms controlling vascular smooth muscle and the endothelium of the skeletal vasculature (59) may also affect, and maybe even support, the regulation of blood pressure during exercise, particularly in the face of a decreased contribution from the heart (42, 55).
Conclusion
The contribution of group III/IV muscle afferents in regulating MAP during exercise remains unaltered by the aging process. However, whereas young individuals predominantly rely on alterations in cardiac output to change MAP, the role of the heart decreases with age and peripheral vasomotor activation becomes a more prominent mechanism in determining the exercise pressor reflex in the elderly. Additionally, although group III/IV muscle afferents play a clear role in increasing LVC during exercise in the young, it seems that these afferents may actually compromise exercise-induced peripheral hemodynamics in the old.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-103786, HL-116579, and HL-091830; American Heart Association Grant 14POST17770016; and Veteran Affairs Merit Grant E6910R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.K.S. and M.A. conception and design of research; S.K.S., J.C.W., M.V., M.J.R., B.S.G., A.D.B., R.S.R., and M.A. performed experiments; S.K.S. analyzed data and prepared figures and tables; S.K.S. and M.A. interpreted results of experiments; S.K.S. prepared the first draft of manuscript; all authors edited, revised, and approved the final version of the manuscript.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188: 19–23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70: 554–565, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkenbosch A, Bovill JG, Dahan A, DeGoede J, Olievier IC. The ventilatory CO2 sensitivities from Read's rebreathing method and the steady-state method are not equal in man. J Physiol 411: 367–377, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol 199: 367–383, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. Beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol 4: 1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol 95: 2591–2597, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JP, Kim A, Hartwich D, Fadel PJ. New insights into the effects of age and sex on arterial baroreflex function at rest and during dynamic exercise in humans. Auton Neurosci 172: 13–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher JP, Kim A, Young CN, Fadel PJ. Carotid baroreflex control of arterial blood pressure at rest and during dynamic exercise in aging humans. Am J Physiol Regul Integr Comp Physiol 299: R1241–R1247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H777–H783, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol 57: 1374–1379, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68: 2466–2472, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Hilty L, Lutz K, Maurer K, Rodenkirch T, Spengler CM, Boutellier U, Jancke L, Amann M. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp Physiol 96: 505–517, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Hoskins DL, Gordon TL, Crisp T. The effects of aging on mu and delta opioid receptors in the spinal cord of Fischer-344 rats. Brain Res 791: 299–302, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Houssiere A, Najem B, Pathak A, Xhaet O, Naeije R, Van De Borne P. Chemoreflex and metaboreflex responses to static hypoxic exercise in aging humans. Med Sci Sports Exerc 38: 305–312, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kalliomaki J, Luo XL, Yu YB, Schouenborg J. Intrathecally applied morphine inhibits nociceptive C fiber input to the primary somatosensory cortex (SI) of the rat. Pain 77: 323–329, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB and Shepherd JT. New York: Oxford Univ. Press, 1996, p. 381–447. [Google Scholar]
- 27.Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454–H2465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laitinen T, Niskanen L, Geelen G, Lansimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol 96: 2333–2340, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Lakatta EG. Cardiovascular aging in health. Clin Geriatr Med 16: 419–444, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Lalande S, Sawicki CP, Baker JR, Shoemaker JK. Effect of age on the hemodynamic and sympathetic responses at the onset of isometric handgrip exercise. J Appl Physiol 116: 222–227, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50, Spec No: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Markel TA, Daley JC 3rd, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation 107: 675–678, 2003. [DOI] [PubMed] [Google Scholar]
- 35.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meintjes AF, Nobrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 105: 1652–1660, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol Heart Circ Physiol 233: H374–H378, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa T, Spina RJ, Martin WH 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 99: 414–426, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res 381: 385–389, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-V̇o2 relationships during submaximal cycle ergometry. J Appl Physiol 84: 599–605, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev 42: 45–52, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation 69: 203–213, 1984. [DOI] [PubMed] [Google Scholar]
- 52.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp Physiol 97: 59–69, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551: 1013–1021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas SG, Paterson DH, Cunningham DA, McLellan DG, Kostuk WJ. Cardiac output and left ventricular function in response to exercise in older men. Can J Physiol Pharmacol 71: 136–144, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, Mitchell JH. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbits. J Appl Physiol 79: 1744–1752, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Wilson LB, Mitchell JH. Exercise pressor reflex: studies on the effect of skeletal muscle fiber type and spinal cord transmission. Adv Exp Med Biol 381: 187–197, 1995. [PubMed] [Google Scholar]
- 59.Wray DW, Richardson RS. ‘Fine-tuning’ blood flow to the exercising muscle with advancing age: an update. Exp Physiol 100: 589–602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]