Here we provide the first direct demonstration that the arteriogenic mediator placental growth factor is regulated by fluid shear stress (a physiological stimulus for collateral artery remodeling) in both vascular cell cocultures and isolated perfused arterioles. We further identify NADPH oxidase 4 and H2O2 as downstream mediators of this response.
Keywords: arteriogenesis, collateral circulation, hemodynamics, endothelium, vascular endothelial growth factors
Abstract
Placental growth factor (PLGF), a potent stimulator of arteriogenesis, is upregulated during outward arterial remodeling. Increased fluid shear stress (FSS) is a key physiological stimulus for arteriogenesis. However, the role of FSS in regulating PLGF expression is unknown. To test the hypothesis that FSS regulates PLGF expression in vascular cells and to identify the signaling pathways involved, human coronary artery endothelial cells (HCAEC) and human coronary artery smooth muscle cells were cultured on either side of porous Transwell inserts. HCAEC were then exposed to pulsatile FSS of 0.07 Pa (“normal,” mimicking flow through quiescent collaterals), 1.24 Pa (“high,” mimicking increased flow in remodeling collaterals), or 0.00 Pa (“static”) for 2 h. High FSS increased secreted PLGF protein ∼1.4-fold compared with static control (n = 5, P < 0.01), while normal FSS had no significant effect on PLGF. Similarly, high flow stimulated PLGF mRNA expression nearly twofold in isolated mouse mesenteric arterioles. PLGF knockdown using siRNA revealed that HCAEC were the primary source of PLGF in cocultures (n = 5, P < 0.01). Both H2O2 and nitric oxide production were increased by FSS compared with static control (n = 5, P < 0.05). NG-nitro-l-arginine methyl ester (100 μM) had no significant effect on the FSS-induced increase in PLGF. In contrast, both catalase (500 U/ml) and diphenyleneiodonium (5 μM) attenuated the effects of FSS on PLGF protein in cocultures. Diphenyleneiodonium also blocked the effect of high flow to upregulate PLGF mRNA in isolated arterioles. Further studies identified NADPH oxidase 4 as a source of reactive oxygen species for this pathway. We conclude that FSS regulates PLGF expression via NADPH oxidase 4 and reactive oxygen species signaling.
NEW & NOTEWORTHY
Here we provide the first direct demonstration that the arteriogenic mediator placental growth factor is regulated by fluid shear stress (a physiological stimulus for collateral artery remodeling) in both vascular cell cocultures and isolated perfused arterioles. We further identify NADPH oxidase 4 and H2O2 as downstream mediators of this response.
coronary artery disease (CAD) is the leading cause of morbidity and mortality in both Western and developing countries, and even optimistic projections predict it to remain so for the foreseeable future. CAD is characterized by stenosis of the coronary arteries due to atherosclerosis, resulting in decreased perfusion of downstream tissue. Decreased pressure downstream of atherosclerotic occlusion results in a pressure gradient that drives blood through small preexisting collateral vessels bridging the area of low pressure with areas of higher pressure. With time, these collateral vessels remodel outward in a process called arteriogenesis, increasing their conductive capacity and improving perfusion of the ischemic tissue. In CAD patients, the degree of collateral development correlates directly with positive outcome (31). Unfortunately, collateral development is highly variable in humans (51). Thus, new pharmacological interventions to induce arteriogenesis could prove life-saving for those CAD patients who have limited native ability to remodel collaterals. However, attempts to induce arteriogenesis by administration of growth factors such as vascular endothelial growth factor (VEGF)-A have yielded disappointing results, indicating the need for a better understanding of the mechanistic basis of arteriogenesis (21, 40).
Increased flow demand on collateral vessels downstream of a stenosis or occlusion results in an increase in fluid shear stress (FSS) on the arterial wall. The role of FSS as a key initiating stimulus for collateral remodeling has been extensively demonstrated (15, 37, 54, 56). FSS is inversely proportional to the cube of vessel diameter and rapidly decreases as the vessel wall expands. However, prolonged increased FSS achieved by arteriovenous shunt results in continued enlargement of collateral vessels (13, 37, 44, 45). Recently, compelling clinical evidence for the role of FSS in arteriogenesis was provided by Gloekler et al. (18). Diastolic coronary flow was pharmacologically increased in patients with CAD for 6 mo, resulting in a significant increase in coronary flow index compared with placebo control. The treatment group also demonstrated a decrease in the area of the ischemic zone, as indicated by ECG ST segment shift (18).
Placental growth factor (PLGF) is a member of the VEGF family. PLGF is a potent stimulator of collateral growth, inducing more collateral vessels (particularly 2nd- and 3rd-order side branches) and greater flow recovery in mouse and rabbit ischemic hindlimb than VEGF-A (28, 38). Evidence from PLGF−/− mice suggests that PLGF is required for normal collateral remodeling, as reestablishment of hindlimb perfusion after femoral artery ligation is severely delayed in these mice (4). On the other hand, overexpression of PLGF causes a marked increase in vessel size (34) and an increase in resistance to myocardial infarction (43, 50) in mice. Clinically, higher levels of PLGF predict improved patient outcome following acute myocardial infarction, as demonstrated by increased left ventricular ejection fraction (24).
The mechanism by which PLGF stimulates collateral remodeling is thought to rely on recruitment of monocytes to the vessel wall. These monocytes in turn orchestrate the remodeling process (35). Although some insights have been gained into the downstream effects of PLGF as mediated by monocytes, the mechanisms regulating PLGF expression in the vessel wall during collateral remodeling remain largely unknown. The importance of both PLGF and FSS in collateral remodeling led us to hypothesize that FSS may regulate the expression of PLGF in the vessel wall. This hypothesis is supported by our previous observation that PLGF mRNA expression is sharply increased in hindlimb collateral arterioles of both sedentary and exercise-trained rats immediately following acute femoral artery ligation and then decreases gradually as remodeling progresses (39). Resting blood flow was sufficient to support tissue metabolic needs in sedentary, but not exercising, rats, yet PLGF was upregulated similarly in both groups, suggesting that elevated FSS can upregulate PLGF, even in the absence of tissue hypoxia. The present study used an in vitro endothelial cell (EC)-smooth muscle cell (SMC) coculture model of the vessel wall and isolated perfused mouse mesenteric arterioles to directly characterize the effects of FSS on PLGF expression and to identify key signaling pathways mediating upregulation of PLGF by shear.
METHODS
Reagents.
All reagents were purchased from Sigma-Aldrich unless otherwise specified.
Perfused arterioles.
All animal experimental procedures were approved by the Animal Care and Use Committee at Oklahoma State University. Experiments were conducted on isolated second-order mesenteric arterioles (∼120–180 μm) of 6- to 8-wk-old C57BL/6J male mice (Jackson Laboratories). Mice were anesthetized with isoflurane delivered by a vaporizer, and the heart was excised. The entire mesentery with the superior mesenteric artery and vein was dissected and washed with 4°C PBS. Second-order mesenteric arterioles were isolated from the mesenteric tissue. The isolated artery was transferred to a vessel chamber (Living Systems Instrumentation) at 4°C. The chamber contained a pair of glass micropipettes and was filled with physiological saline solution (PSS; in mM: 142 NaCl, 4.7 KCl, 1.7 MgSO4, 0.5 EDTA, 2.79 CaCl2, 10 HEPES, and 1.18 KH2PO4, pH 7.4). After cannulation of the proximal (upstream) end of the vessel, the intraluminal pressure was gradually raised (<20 mmHg) to clear the lumen of clotted blood. Then the distal (downstream) end of the vessel was also cannulated. Time from euthanasia to complete cannulation was <1 h. The temperature of the bath was then raised to 37°C, and pressure was gradually raised to 60 mmHg (∼10 mmHg/10 min). The pressure increase was achieved by gradually raising two reservoirs connected by silicone tubing to each cannula. Perfusion buffer consisted of 1% bovine serum albumin in PSS. Once equilibrated at 60 mmHg, the longitudinal pressure gradient was increased from zero to 20 mmHg (“control”) or 50 mmHg (“proarteriogenic”) (9). This was achieved by lowering the distal reservoir and raising the proximal reservoir, allowing for the average intraluminal pressure to be maintained at 60 mmHg. The control flow rate was ∼75 μl/min, and the proarteriogenic flow rate was ∼170 μl/min. Vessels were then perfused for 2 h. Function of the vessel wall was determined at the end of perfusion by assessment of the vasoconstrictive response to epinephrine and the vasodilator response to acetylcholine, based on changes in vessel diameter measured by video micrometer. A moderate degree of flow-induced dilation was observed: ∼10% in the 20-mmHg condition and ∼25% in the 50-mmHg condition (data not shown). After flow-induced dilation was taken into account, FSS was ∼1.5-fold higher under the 50-mmHg condition than under the 20-mmHg condition. Flow-induced dilation was absent in vessels treated with the flavoenzyme inhibitor diphenyleneiodonium (DPI); thus FSS in those experiments was nearly twofold higher under the 50-mmHg condition than under the 20-mmHg condition (not shown).
Cell culture.
Human coronary artery EC (HCAEC) and human coronary artery SMC (HCASMC) were purchased from Lonza. For HCASMC, donors included a 12-yr-old male, a 56-yr-old female, and a 30-yr-old male, while HCAEC donors were a 21-yr-old male and a 30-yr-old male. HCAEC were cultured in EBM-2 basal medium supplemented with EGM-2MV SingleQuot factors (HCAEC complete medium; Lonza). HCASMC were cultured in SMBM basal medium supplemented with SmGM-2 SingleQuot factors (HCASMC complete medium; Lonza). All cells were grown in a humidified incubator in 5% CO2 at 37°C. Cells were used between passages 5 and 6 for all experiments. Serum-reduced medium for experiments to be performed in room air was prepared by dilution of the appropriate complete medium in low-glucose DMEM (Hyclone, Fisher) at a ratio of 2:3, yielding a 2% serum medium, and supplementation with 15 mmol/l HEPES. Phenol red-free medium was used for experiments requiring fluorescent assays.
Coculture model.
To model the vessel wall in vitro, porous Transwell inserts (Corning Costar, 0.4-μm pore size) were used as described elsewhere (20). Inserts were inverted, and the bottom surface was coated with 0.1% gelatin in DMEM. Inserts were then placed in a humidified incubator in 5% CO2 at 37°C for 1 h. Next, HCASMC (104 cells/cm2) were seeded onto the inverted insert, and inserts were returned to the incubator overnight. On the following day, inserts were placed into six-well plates containing SmGM-2 medium and incubated for an additional 24 h. The top surface of the insert was then coated with 0.1% gelatin in DMEM and incubated for 1 h, after which HCAEC (25,000/cm2) were seeded on the top surface of the insert. EGM-2 MV was added to the insert, and the system was again incubated overnight. When possible, HCASMC and HCAEC donors were matched. Confluence of the cocultures was confirmed by Hoffman modulation contrast microscopy (Olympus IX71). Lastly, the confluent cocultures were incubated in serum-reduced medium for 24 h prior to experiments. For HCAEC monoculture experiments, no HCASMC were seeded on the bottom of the insert, but cultures were otherwise processed as described above.
Shear stress exposure.
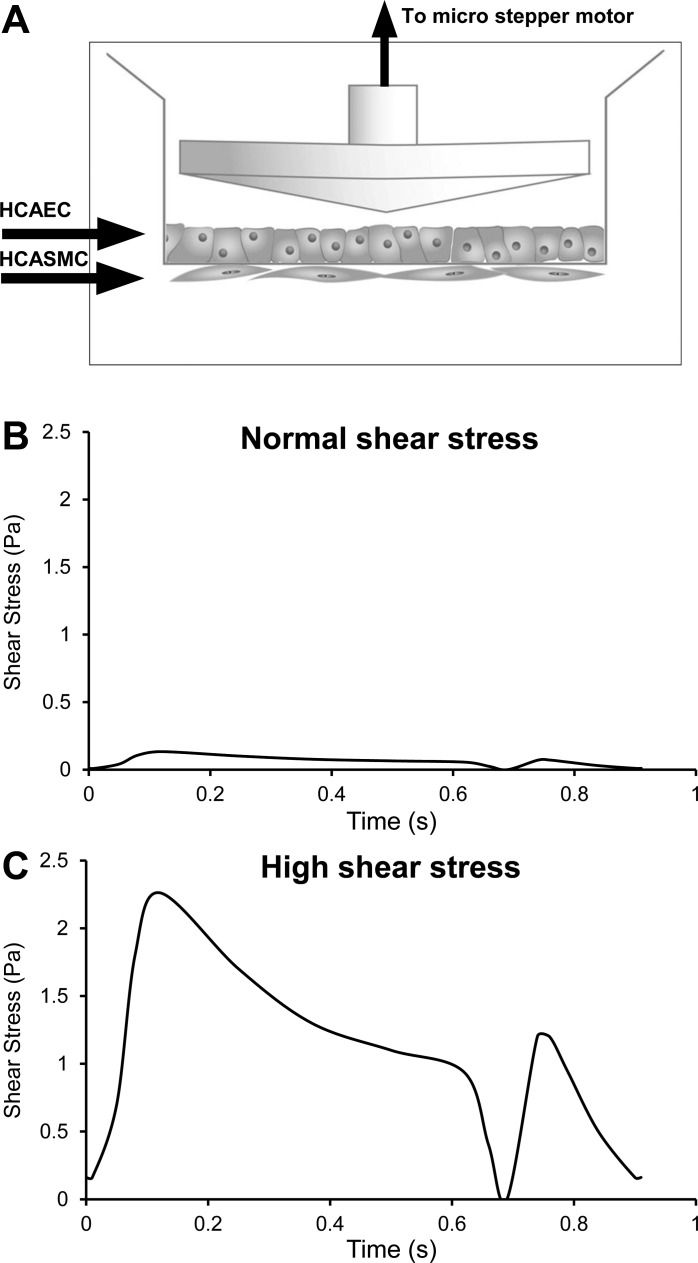
Only the HCAEC layer of the coculture was directly exposed to FSS. FSS was applied using a cone-and-plate viscometer shearing system. The system consisted of four ultra-high-molecular-weight polyethylene 0.5° cones connected to microstepper motors (Optimal Engineering Systems) linked to a controller unit (Optimal Engineering Systems) (Fig. 1A). Cones were placed into the four peripheral inserts of the six-well plate, while the central two inserts were not exposed to FSS and were used as a static control. Cocultures were then exposed to one of two pulsatile FSS waveforms, based on previously published information (29). The “normal” waveform had time-averaged FSS of 0.07 Pa (Fig. 1B) and modeled flow through a quiescent collateral vessel downstream of a patent coronary artery. The high waveform had time-averaged FSS of 1.24 Pa (Fig. 1C) and modeled flow through an actively remodeling collateral vessel downstream of a coronary artery with 60% stenosis. Shear experiments were performed on a laboratory bench top in HEPES-buffered medium with temperature maintained at 37°C. Cocultures were exposed to FSS for 2 h (except where noted). Culture medium and/or cell lysates were collected for analysis at various time points from preshear to 0–24 h postshear.
Fig. 1.
A: coculture system with the cone in place. Transwell inserts were placed inside 6-well plates. Human coronary artery endothelial cells (HCAEC) were seeded on the top side of the insert, while human coronary artery smooth muscle cells (HCASMC) were seeded on the underside. The 0.4-μm pore size prevented either cell type from migrating completely through the membrane, which was readily permeable to soluble factors and allowed some direct cell-cell contact. B and C: shear stress waveforms, shown over 1 cardiac cycle (0.9 s). B: waveform representing “normal” shear stress in a collateral arising from a coronary artery free of stenosis. C: waveform representing “high” shear stress in a collateral arising from a coronary artery with 60% stenosis.
siRNA knockdown.
HCAEC were seeded into six-well plates at a density of 210,000 cells/well. After 24 h, cells were transfected with PLGF siRNA [siPLGF; 5′-AGGUGGAAGUGGUACCCUU-3′ (sense), overhang dTdT; 5′-AAGGGUACCACUUCCACCU-3′ (antisense), overhang dCdT], predesigned Nox4 siRNA (Silencer Select; s27013), or negative control scrambled siRNA (Silencer no. 1 siRNA), all purchased from Invitrogen. Prior to addition to cells, 5 nM siRNA was precomplexed with Lipofectamine RNAiMAX transfection reagent (Invitrogen) in Opti-MEM medium (Gibco) for 20 min. Cells were exposed to transfection medium (DMEM + 10% FBS containing precomplexed siRNA) for 6 h and then trypsinized and seeded onto the upper surface of inserts precoated with 0.1% gelatin. The lower surface of the inserts had been previously seeded with wild-type HCASMC, as described above. Cocultures were incubated overnight in reduced-serum medium as described above before exposure to shear stress. In a separate group of cocultures, HCASMC were transfected similarly to HCAEC after seeding onto the lower surface of inserts. At the end of the transfection period, untreated HCAEC were seeded onto the upper surface as described above. Cell specificity and efficacy of target mRNA knockdown were determined by real-time PCR.
PLGF ELISA.
Media samples were collected from sheared cells and their corresponding static controls, treated with protease inhibitor cocktail (1 mM PMSF, 1 mM Na3VO4, 1 μg/ml leupeptin, 1 mM benzamindine-HCl, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A), and stored at −80°C until further processing. PLGF was measured using the DuoSet ELISA development kit (R & D Systems) according to the manufacturer's protocol. All samples were assayed in duplicate. Data were normalized to total protein concentration, as determined by bicinchoninic acid assay (Pierce). All assay plates were read on a BioTek Synergy HT plate reader.
Real-time PCR.
After exposure to shear stress and media collection, cells were rinsed gently with PBS (HyClone) and trypsinized (TrypLE Express, Gibco). Collected cells were resuspended in 1% β-mercaptoethanol in RLT lysis buffer (Qiagen) and frozen at −80°C for later processing. Cannulated vessels were placed in RLT lysis buffer immediately after perfusion and sonicated on ice using a sonic dismembrator (model D100, Fisher). Total RNA was isolated using RNeasy mini columns (Qiagen) following the manufacturer's directions. Total RNA quantity and quality were determined spectrophotometrically using a Take3 Micro-Volume plate in a Synergy HT plate reader (BioTek). Reverse transcription was carried out using the QuantiTect reverse transcription kit (Qiagen) following the manufacturer's instructions. Real-time PCR was performed on an ABI 7500 Fast instrument (Applied Biosystems) using PerfeCTa SYBR Green FastMix, Low ROX (Quanta Biosystems). Relative abundance of target mRNA was determined using the comparative cycle threshold (Ct) method and the following primer pairs: 5′-CCTACGTGGAGCTGACGTTCT-3′ (forward) and 5′-TCCTTTCCGGCTTCATCTTCT-3′ (reverse) for human PLGF, 5′-TTGGAGCAGGAATTGGGGTC-3′ (forward) and 5′-AATGCTGCATGACCAACCTTT-3′ (reverse) for human Nox1, 5′-GGGAACTGGGCTGTGAATGA-3′ (forward) and 5′-CCAGTGCTGACCCAAGAAGT-3′ (reverse) for human Nox2, 5′-GGGGTTAAACACCTCTGCCT-3′ (forward) and 5′-ACACAATCCTAGCCCCAACA-3′ (reverse) for human Nox4, and 5′-CTGCTGGGAACAACTCAACAGA-3′ (forward) and 5-′GCGACCCCACACTTCGTT-3′ (reverse) for mouse PLGF. Gene expression was normalized to β-actin as amplified with the following primers: 5′-TGCCGACAGGATGCAGAAG-3′ (forward) and 5′-CTCAGGAGGAGCAATGATCTTGAT-3′ (reverse) for human β-actin and 5′-AGTTCGCCATGGATGACGAT-3′ (forward) and 5′-TGCCGGAGCCGTTGTC-3′ (reverse) for mouse β-actin. The relative stability of β-actin after treatment was determined using the comparative Ct method with GAPDH as the housekeeping gene: 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′(reverse) for human GAPDH. No significant change in β-actin was detected.
H2O2 assay.
The effect of shear stress on the H2O2 concentration in coculture medium was assessed using the Amplex Red H2O2 assay kit (Invitrogen). Samples were collected in dark tubes, protected from light, and immediately frozen at −80°C until analysis. Further processing of samples was carried out on ice in reduced-light conditions according to the manufacturer's directions.
Nitrate/nitrite assay.
Total nitrate and nitrite concentration was determined in samples of culture medium from sheared and static control cocultures as an index of total nitric oxide (NO) production using a nitrate/nitrite fluorometric assay kit (Cayman Chemical).
Statistical analyses.
All data are presented as means ± SE. Experiments were replicated at least five times. Data were analyzed by two-way repeated-measures ANOVA or one-way ANOVA, as appropriate. Both were followed by Tukey's range test. The level of significance was set at P < 0.05.
RESULTS
Increased FSS upregulates PLGF expression.
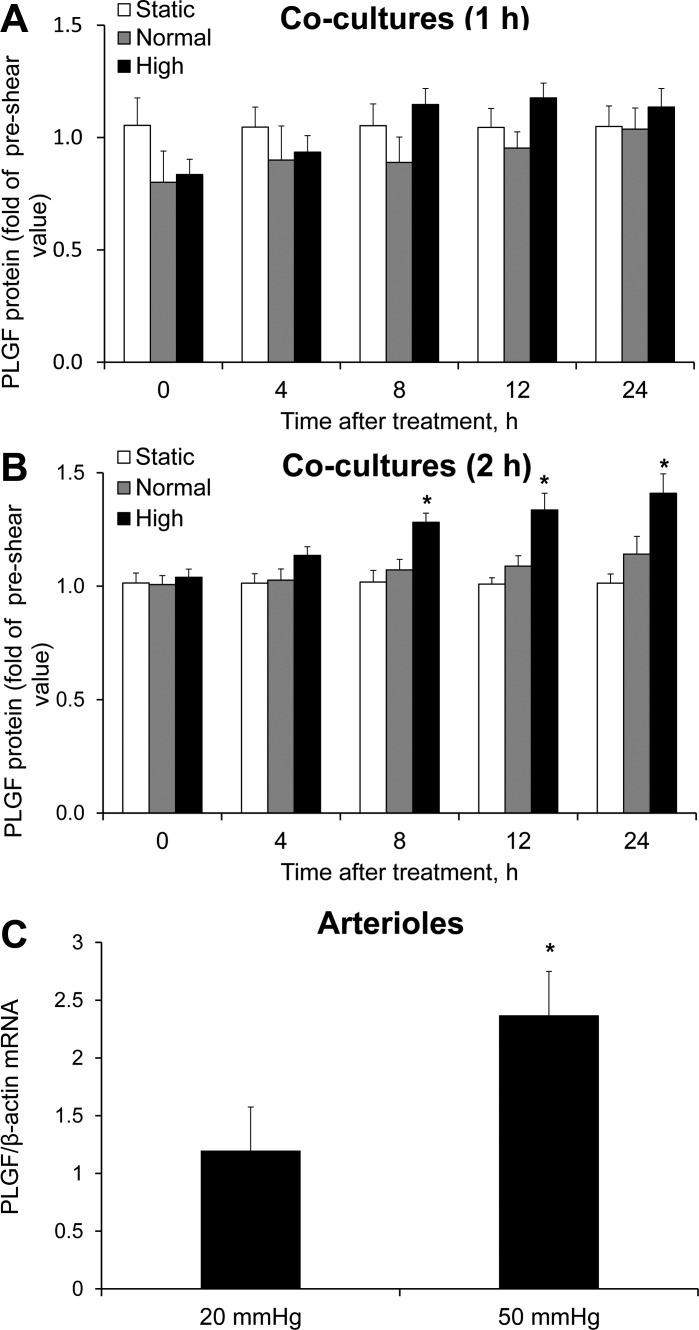
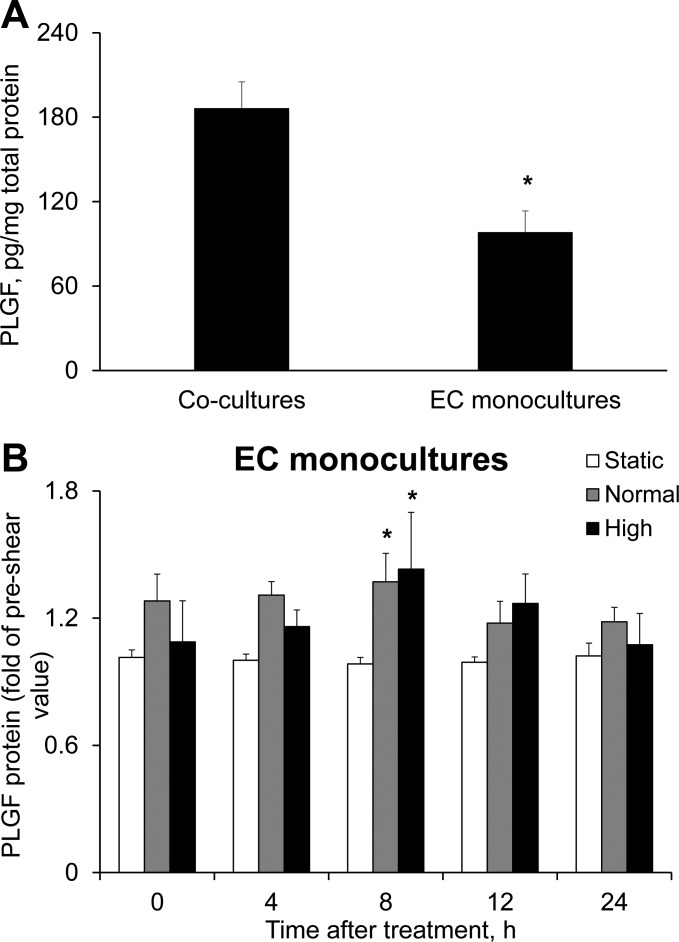
To characterize the effects of FSS on PLGF expression, cocultures were exposed to normal or high FSS for 1, 2, or 4 h. Medium was sampled immediately before and 0, 4, 8, 12, and 24 h after FSS exposure. Exposure to either FSS waveform for 1 h had no significant effect on PLGF protein levels in the medium (compared with static control) at any time point up to 24 h (Fig. 2A). However, 2 h of high FSS significantly increased PLGF protein levels in medium starting 8 h after shear exposure compared with static control conditions. PLGF protein levels continued to increase up to 24 h after shear exposure (Fig. 2B) and returned to static control levels by 36 h after shear exposure (data not shown). In contrast to the effect of high FSS, 2 h of treatment with normal FSS had no significant effect on PLGF protein (Fig. 2B). Exposure to 4 h of FSS coincided with increased cell detachment, as subjectively determined by contrast microscopy (data not shown). Therefore, a 2-h exposure was selected for further experiments.
Fig. 2.
Effect of fluid shear stress (FSS) exposure on placental growth factor (PLGF) protein expression in cocultures and arterioles. A: cocultures were exposed to static control conditions (no FSS), normal FSS (0.07 Pa), or high FSS (1.24 Pa). PLGF protein in medium was assessed prior to and up to 24 h after shear exposure. A: neither waveform had a significant effect on PLGF protein levels after 1 h of exposure (n = 5). B: 2 h of exposure to high, but not normal, FSS induced a significant increase in PLGF protein compared with static control (n = 5). *P < 0.001. C: mesenteric arterioles (∼150 μm ID) were cannulated and perfused with 1% BSA-PSS for 2 h. Perfusion with a pressure gradient of 50 mmHg significantly increased PLGF mRNA compared with perfusion with a pressure gradient of 20 mmHg (n = 5). *P < 0.05.
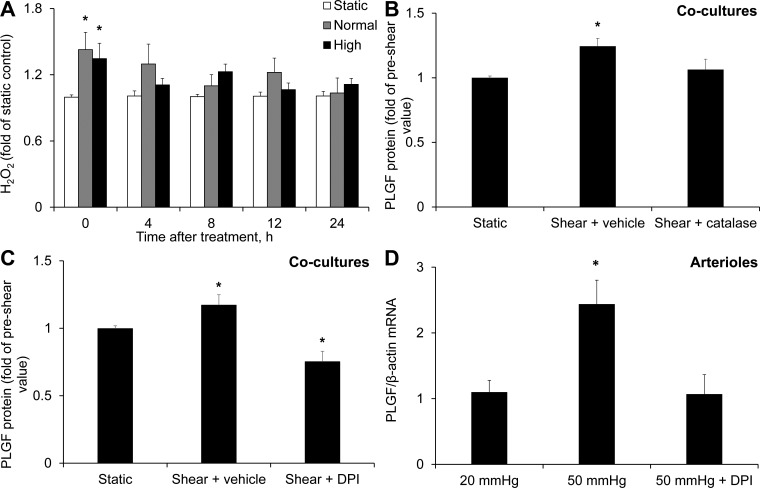
To confirm that PLGF is regulated by FSS in intact vessels, we perfused mouse second-order mesenteric arterioles using a 50-mmHg (high flow) or a 20-mmHg (normal flow) longitudinal pressure gradient. PLGF mRNA was significantly increased after 2 h of perfusion at 50 mmHg compared with control arterioles perfused at 20 mmHg (2.37 ± 0.36 fold of control; Fig. 2C).
SMC increase EC responsiveness to FSS.
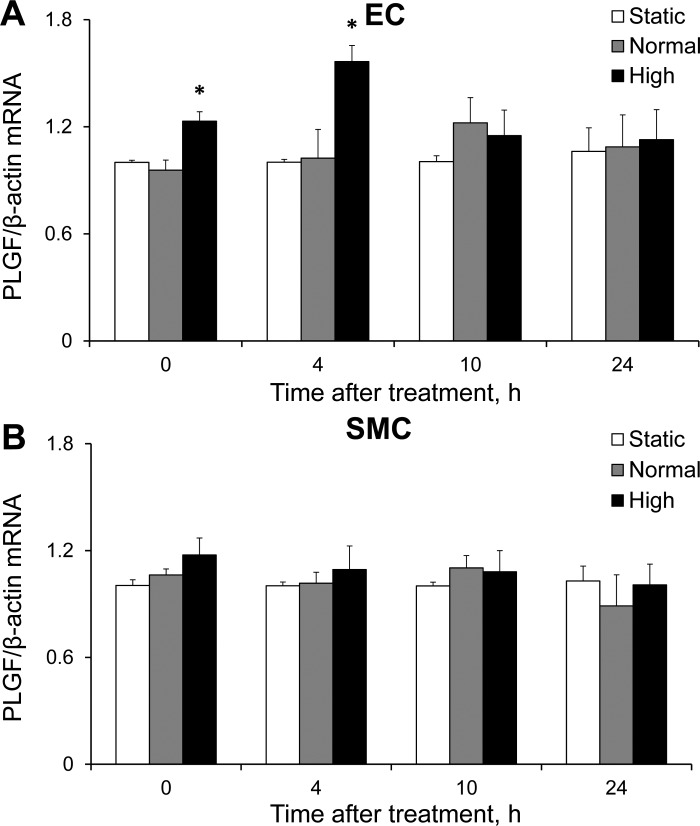
We next sought to determine which cell type in the coculture was responsible for the increased PLGF protein expression in response to FSS by analyzing PLGF mRNA in HCAEC and HCASMC at 0, 4, 10, and 24 h after FSS exposure. High, but not normal, FSS produced an immediate and significant increase in PLGF mRNA expression in HCAEC, which was maintained until 4 h after shear exposure (Fig. 3A). Neither level of FSS had a significant effect on PLGF mRNA in HCASMC (Fig. 3B).
Fig. 3.
Effect of FSS on PLGF mRNA expression in HCAEC (EC) and HCASMC (SMC). A: HCAEC demonstrated an immediate increase in PLGF gene expression in response to high FSS, which was maintained for up to 4 h after shear exposure (n = 5). *P < 0.001. Normal FSS had no significant effect on PLGF mRNA in HCAEC (n = 5). B: HCASMC showed no significant change in PLGF gene expression, regardless of the level of shear exposure received by the coculture (n = 5). HCASMC were not directly exposed to FSS.
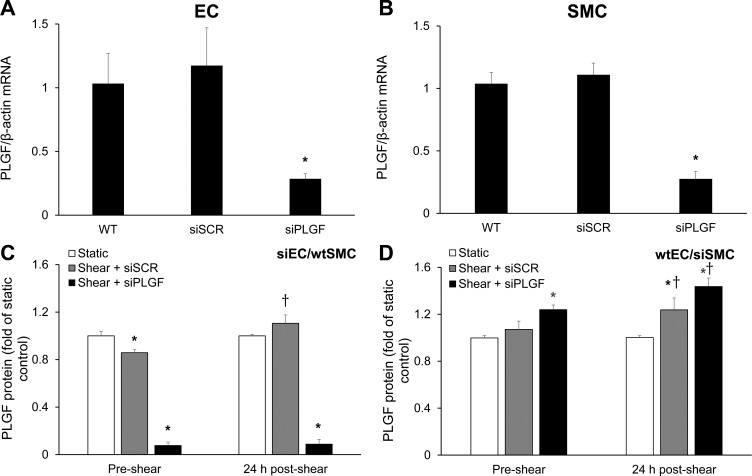
To further characterize the role of HCAEC in PLGF production by cocultures, PLGF was knocked down in HCAEC or HCASMC using siRNA. PLGF knockdown was confirmed by RT-PCR (Fig. 4, A and B). PLGF knockdown in HCAEC did not affect PLGF mRNA in cocultured HCASMC, or vice versa (not shown). Treatment of HCAEC with siPLGF drastically and significantly decreased total PLGF levels in cocultures and abolished the effect of FSS to increase PLGF protein at 24 h after shear exposure (Fig. 4C). Surprisingly, siPLGF treatment of HCASMC resulted in a significant increase in PLGF protein concentration in coculture medium compared with medium from cocultures containing untreated or scrambled siRNA-treated HCASMC (Fig. 4D). Treatment of HCASMC with siPLGF did not prevent the FSS-induced increase in PLGF protein, in contrast to siPLGF treatment of HCAEC.
Fig. 4.
Contribution of HCAEC and HCASMC to basal and FSS-stimulated PLGF expression in cocultures. PLGF expression was knocked down using siRNA in HCAEC or HCASMC. PLGF knockdown was confirmed by real-time PCR in HCAEC (A; n = 5) and HCASMC (B; n = 5). *P < 0.01. Medium was sampled immediately prior to exposure to 2 h of high FSS and 24 h thereafter. WT, wild-type; siSCR, negative control scrambled siRNA; siPLGF, PLGF siRNA. C: in cocultures containing siPLGF-treated HCAEC (siEC) but wild-type HCASMC (wtSMC), PLGF was significantly decreased compared with levels in untreated cocultures (n = 5) and failed to increase in response to FSS. *P < 0.001. Cocultures with negative control scrambled siRNA-treated HCAEC showed a slight, but significant, decrease in preshear PLGF levels compared with untreated cells (n = 5); however, exposure to high FSS significantly increased PLGF as expected (n = 5). *P < 0.001; †P < 0.05. D: in cocultures containing wild-type HCAEC and siPLGF-treated HCASMC (siSMC), there was a significant increase in total PLGF protein in the medium compared with untreated cocultures (n = 5). *P < 0.001. PLGF was further increased by exposure to high FSS in both negative control scrambled siRNA- and siPLGF-treated groups compared with static untreated controls (n = 5) and the corresponding preshear values for each treatment condition (n = 5). *P < 0.001; †P < 0.05.
To assess the effect of communication between HCAEC and HCASMC on PLGF production by EC, we next compared HCAEC monocultures with HCAEC-HCASMC cocultures. Baseline PLGF protein levels were significantly lower in medium of HCAEC monocultures than HCAEC-HCASMC cocultures (Fig. 5A). In addition, the sensitivity to FSS intensity (normal vs. high) appeared to be lost in HCAEC monocultures, since exposure of HCAEC monocultures to both normal and high FSS resulted in a significant and similar increase in PLGF protein. Furthermore, the effect of FSS in monocultures was transient and was significant only at 8 h after shear exposure (Fig. 5B), yielding a time course of PLGF expression that was dissimilar to our observations in cocultures. These data suggest that although HCAEC are clearly the primary source of PLGF in the coculture model, HCASMC nevertheless play a role in modulating both shear-induced and baseline expression of PLGF.
Fig. 5.
Effect of FSS exposure on PLGF levels in HCAEC monocultures. A: baseline (unstimulated) level of PLGF protein was significantly lower in medium of HCAEC monocultures than in medium of HCAEC-HCASMC cocultures (n = 5). *P < 0.001. B: HCAEC monocultures were exposed to static conditions, normal FSS, or high FSS for 2 h as described for cocultures. Culture medium was sampled before and after shear exposure. High FSS and normal FSS induced a significant increase in PLGF protein, but only at 8 h after exposure (n = 5). *P < 0.05. Thus the time course and intensity dependence of the effect were altered in the absence of HCASMC.
Expression of PLGF induced by FSS is dependent on H2O2.
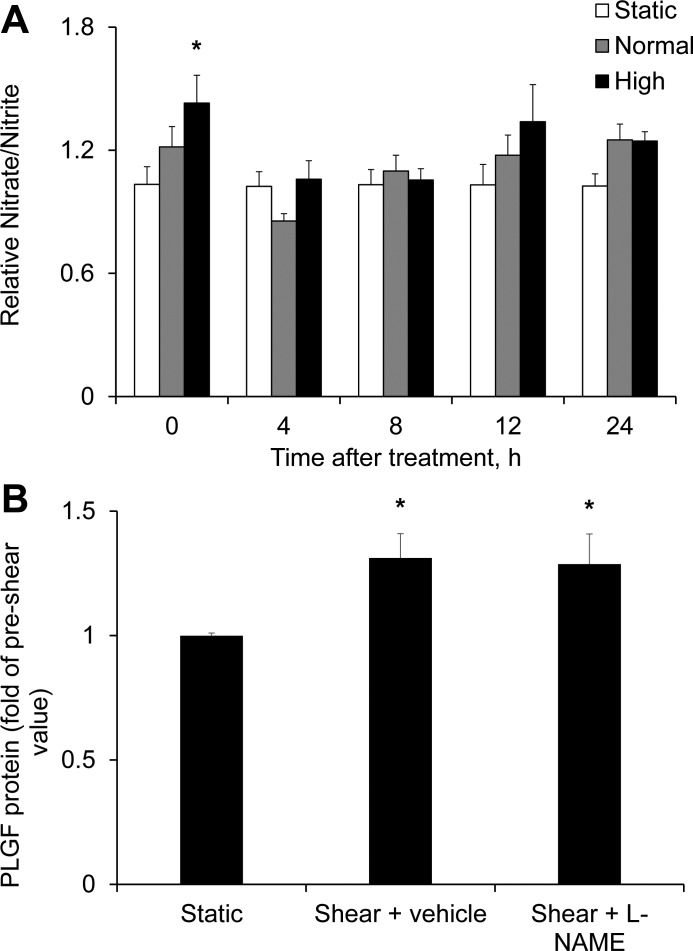
We next investigated possible signaling mechanisms mediating upregulation of PLGF by FSS. The soluble mediator NO was evaluated first on the basis of its well-known role in mediating FSS-dependent responses in EC. Total nitrate/nitrite concentrations in coculture media were determined as a measure of NO production. High, but not normal, FSS significantly increased total nitrite/nitrate (1.43 ± 0.14 fold of static control) immediately after exposure (Fig. 6A). To assess the role of NO in the regulation of PLGF expression, cocultures were treated with the NO synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 μM) 30 min prior to FSS exposure. Medium was sampled for PLGF protein levels immediately prior to FSS exposure and 24 h after shear exposure (the time point at which the maximal effect of FSS on PLGF protein levels in untreated cells was observed; Fig. 2B). Inhibition of NOS by l-NAME had no significant effect on the FSS-induced increase in PLGF protein (Fig. 6B).
Fig. 6.
Role of nitric oxide (NO) in the FSS-induced increase in PLGF protein. A: high, but not normal, FSS significantly increased nitrate/nitrite levels immediately after shear exposure compared with static control (n = 5). *P < 0.01. B: the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 μM) had no effect on the shear-induced increase in PLGF protein (n = 5). *P < 0.05.
We next tested the effect of FSS on H2O2. Both FSS waveforms caused a significant increase in H2O2 immediately after exposure: 1.43 ± 0.16 and 1.35 ± 0.14 fold of static control for normal and high FSS, respectively (Fig. 7A). Treatment with the H2O2 scavenger catalase (500 U/ml) 30 min prior to FSS exposure abolished the effect of high FSS on PLGF protein (Fig. 7B), suggesting that H2O2 is a key mediator of this response. The effects of catalase were mimicked by 5 μM DPI, the flavoenzyme inhibitor (Fig. 7C). Treatment with 5 μM DPI also prevented the increase in PLGF mRNA in arterioles perfused at 50 mmHg compared with 20 mmHg (Fig. 7D).
Fig. 7.
Role of H2O2 in the FSS-induced increase in PLGF protein. A: high and normal FSS significantly increased H2O2 in cocultures immediately after shear exposure compared with static control (n = 5). *P < 0.05. B: catalase (500 U/ml) blocked the effect of FSS to upregulate PLGF (n = 5). *P < 0.01. C: diphenyleneiodonium (DPI, 5 μM) also prevented the increase in PLGF protein in response to FSS and further decreased PLGF protein below static control levels (n = 5). *P < 0.01. D: DPI (5 μM) also inhibited the flow-induced increase in PLGF mRNA in cannulated arterioles [20 mmHg (n = 6), 50 mmHg (n = 5), and DPI + 50 mmHg (n = 4)]. *P < 0.01.
Nox4 is necessary for the FSS-induced increase in PLGF and H2O2 in vascular cocultures.
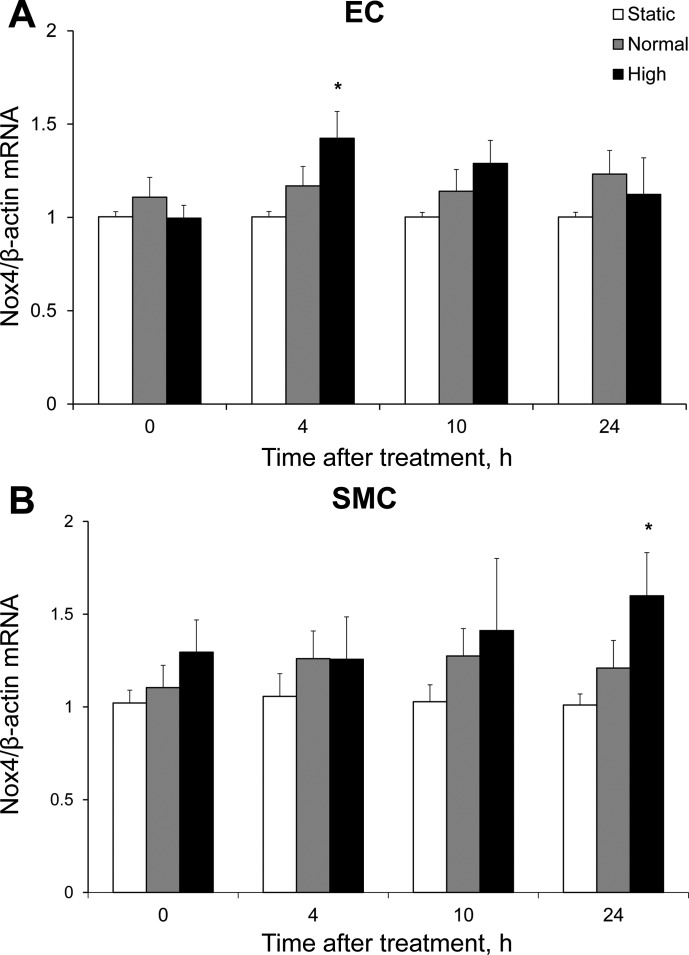
We next investigated the role of the reactive oxygen species (ROS)-producing Nox as a possible source of H2O2 for this pathway. Real-time PCR was performed on mRNA isolated from static cocultures to determine the relative abundance of mRNA for Nox1, Nox2, and Nox4, three isoforms previously implicated in the outward remodeling of coronary vessels (Table 1). In HCASMC and HCAEC, the predominant isoform was Nox4. We next assessed Nox4 mRNA levels in cocultures at 0, 4, 10, and 24 h after FSS exposure. In HCAEC, Nox4 mRNA was significantly increased 4 h after exposure to high FSS (1.42 ± 0.14 fold of static control) and returned to control levels by 10 h (Fig. 8A). High FSS significantly increased HCASMC Nox4 mRNA 24 h after exposure (1.60 ± 0.23 fold of control; Fig. 8B). Normal FSS had no significant effect on Nox4 mRNA in either cell type.
Table 1.
Relative Nox isoform/β-actin mRNA
| Nox1 | Nox2 | Nox4 | |
|---|---|---|---|
| HCAEC | 1.00 ± 0.09 | 0.30 ± 0.05 | 1,164.14 ± 60.43*† |
| HCASMC | 1.17 ± 0.11 | 0.97 ± 0.14 | 15.92 ± 2.57*† |
Values are means ± SE (n = 5). Real-time PCR revealed NADPH oxidase (Nox) 4 to be the predominant isoform of Nox expressed in cocultures. HCAEC, human coronary artery endothelial cells; HCASMC, human coronary artery smooth muscle cells.
P < 0.01 vs. Nox1;
P < 0.01 vs. Nox2.
Fig. 8.
Effect of FSS on NADPH oxidase 4 (Nox4) gene expression in HCAEC and HCASMC. A: in HCAEC, high FSS significantly increased Nox4 mRNA compared with static control at 4 h after exposure, while normal FSS had no significant effect on Nox4 mRNA (n = 5). *P < 0.01. B: high FSS increased Nox4 mRNA only at 24 h after exposure in HCASMC (n = 5). *P < 0.05.
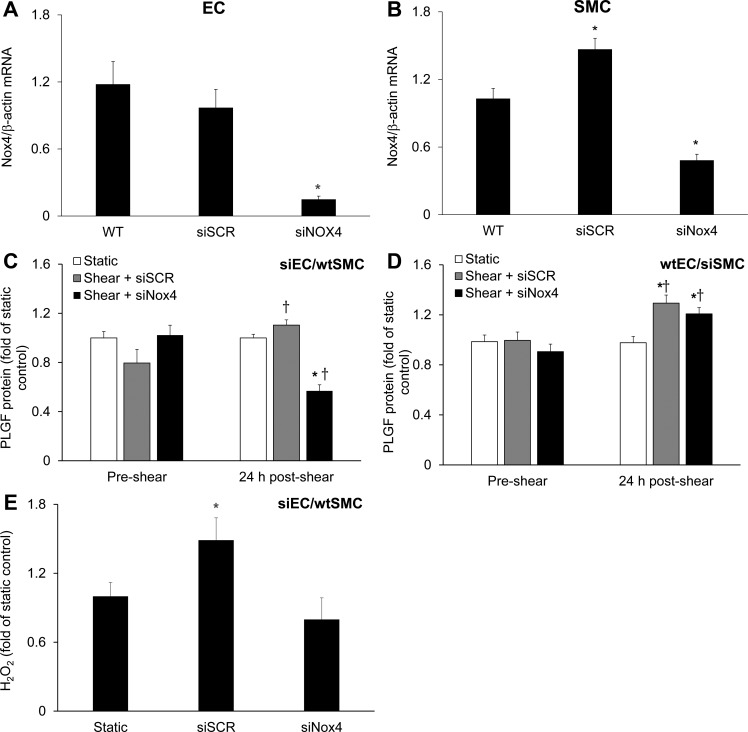
Lastly, to determine whether Nox4 plays a role in the FSS-induced increase in PLGF, we used siRNA to knock down Nox4 in HCAEC or HCASMC. Knockdown was verified by RT-PCR (Fig. 9, A and B). Nox4 knockdown in HCAEC did not affect Nox4 mRNA in cocultured HCASMC, or vice versa (data not shown). Knockdown of Nox4 in HCAEC abolished the effect of high FSS to increase PLGF protein levels (Fig. 9C). In contrast, knockdown of Nox4 in HCASMC had no significant effect on the response of PLGF to high FSS (Fig. 9D). Knockdown of Nox4 in HCAEC also prevented the increase in H2O2 observed immediately after high FSS in untreated cells (Fig. 9E).
Fig. 9.
Role of endothelial Nox4 in the FSS-induced increase in PLGF protein. A and B: real-time PCR confirmed knockdown of Nox4 in HCAEC (n = 5) and HCASMC (n = 5). siNox4, Nox4 siRNA. *P < 0.01. C: in cocultures containing siNox4-treated HCAEC but wild-type HCASMC, the FSS-induced increase in PLGF protein was inhibited and PLGF was decreased to below static control levels (n = 5). *P < 0.01. PLGF remained responsive to shear in cocultures with negative control scrambled siRNA-treated HCAEC (n = 5). †P < 0.05. D: response of PLGF to FSS was unaffected in cocultures containing wild-type HCAEC and siNox4-treated HCASMC (n = 5). *P < 0.01 vs. static. †P < 0.05 vs. preshear. E: knockdown of Nox4 in HCAEC prevented the effect of high FSS to increase H2O2, identifying endothelial Nox4 as a key source of FSS-induced H2O2 production in the coculture system (n = 5). *P < 0.05.
DISCUSSION
Our findings demonstrate that PLGF is regulated by FSS in a vascular cell coculture model and in intact vessels and that this response is sensitive to the magnitude and duration of FSS. We identified EC as the primary cell type producing PLGF in the coculture model; however, we also found that endothelial PLGF production is positively influenced by the presence of SMC. PLGF mRNA was increased in EC immediately after exposure to shear stress, whereas the increase in PLGF protein levels became detectable 8 h after exposure to shear stress. This time course is similar to the time course we previously reported for PLGF upregulation by H2O2 in SMC (48). We also previously showed that PLGF protein levels can be regulated independently of PLGF mRNA levels (47). Thus the differing time course for upregulation of PLGF mRNA and protein is not unexpected. Investigation of the mechanisms involved in FSS-induced PLGF expression revealed that this pathway is dependent on H2O2 and identified Nox4 as a likely source of H2O2 in this context. Further definition of the molecular mechanism by which FSS regulates PLGF may identify new therapeutic targets to promote or inhibit PLGF expression and arteriogenesis, depending on the context (e.g., ischemic cardiovascular disease vs. cancer).
Although the cone-and-plate viscometer used to generate shear stress in the coculture system allows accurate control of the angular velocity of the cone (and, thus, FSS) in our in vitro model, exposure times >2 h were prohibited by detachment of the EC. The possibility that cessation of elevated FSS at the end of the exposure period affects PLGF expression (in addition to, or instead of, initiation of elevated FSS) cannot be completely excluded. However, the dependence of the PLGF protein response on the duration and magnitude of FSS exposure and the immediate effect of FSS on PLGF mRNA in both cocultures and perfused vessels suggest that upregulation of PLGF is primarily due to initiation of elevated FSS.
In the physiological context of arteriogenesis in vivo, the vessel wall is exposed to multiple stimuli in addition to FSS, including stretch (cyclic, tangential, and circumferential) and hypoxia. Our coculture model allows us to study the effects of FSS without confounding effects from these other stimuli, which is one advantage of the system. However, it is likely that PLGF is also affected by these other cues during arteriogenesis in vivo. Indeed, stretch has been shown to induce PLGF expression in human bronchial airway epithelial cells. Interestingly, in contrast to our findings with FSS, the response of PLGF to stretch was shown to be NO-dependent (32). Similarly, our group previously demonstrated upregulation of PLGF protein in HCAEC by hypoxia. Thus the potential contribution of stretch and hypoxia to the overall regulation of PLGF expression in vascular cells during arteriogenesis remains to be investigated. The potential interactions between the signaling pathways mediating the effects of FSS, stretch, and hypoxia on PLGF are another important area for further study. It is also of interest to determine whether PLGF is differentially regulated in tissues where arteriogenesis occurs within or near hypoxic regions (e.g., the coronary circulation) compared with tissues in which the site of collateral enlargement is relatively distant from the site of hypoxia (e.g., the peripheral circulation).
In intact vessels, EC and SMC interact physically and also communicate through secreted mediators to maintain normal vessel function. The coculture model used in this study provides a close approximation of this situation by allowing for both physical and biochemical interaction. Our data show that PLGF is primarily produced by HCAEC in our model, consistent with our previously published findings (59). Indeed, knockdown of PLGF in HCAEC decreased baseline media PLGF concentrations to <10% of the level in untreated cocultures. Nevertheless, HCASMC appear to play a major role in modulating PLGF levels in the coculture system. HCAEC-HCASMC cocultures secreted almost twofold more PLGF than HCAEC monocultures. This effect cannot be explained by a simple additive effect of HCASMC PLGF production on total PLGF levels, since we previously showed that HCAEC produce approximately two orders of magnitude more PLGF than HCASMC (59). Furthermore, knockdown of PLGF in HCASMC cocultures yielded the seemingly contradictory result of increased PLGF media levels. One possible explanation for these observations is that HCASMC-produced PLGF may exert negative feedback on HCAEC PLGF production, whereas other factors generated by HCASMC have a stimulatory effect. Consistent with our findings for PLGF, several other growth factors, including VEGF-A, platelet-derived growth factor-AA, platelet-derived growth factor-BB, monocyte chemotactic protein-1, and transforming growth factor-β1, have been reported to increase in EC-SMC cocultures compared with EC monocultures (5, 22). Furthermore, coculture of EC with SMC leads to an increase in SMC differentiation (16), EC elongation, and EC permeability (6), demonstrating the importance of EC-SMC interaction on signaling and function in both cell types. In keeping with these results in static cocultures and monocultures, we found the effect of FSS on PLGF to be transient and diminished in HCAEC monocultures compared with cocultures. These data suggest that HCAEC-HCASMC interaction and signaling are essential for both baseline PLGF expression and its upregulation by the physiological stimulus of increased FSS. Dysfunction in either of these vascular cell types may therefore lead to abnormal PLGF production and signaling, which may have important consequences for the vasculature in pathophysiological settings such as atherosclerosis.
It is well established that, in the vasculature, FSS increases the production of NO by endothelial NO synthase (eNOS). Both eNOS and NO production are increased during arteriogenesis, and blocking NO production with l-NAME or eNOS knockout inhibits collateral growth in several models of arteriogenesis (27, 33, 36, 60). We verified that the high-FSS condition used in this study was sufficient to increase NO production, as expected. However, inhibition of NO production with l-NAME had no effect on the FSS-induced expression of PLGF in our in vitro model system. As noted above, eNOS knockout mice exhibit diminished arteriogenesis after femoral artery occlusion (33). However, collateral growth in these mice has been demonstrated to “catch up” with that in wild-type mice later in the arteriogenic process (2), suggesting that non-NO-dependent processes also play a role.
The role of ROS in arteriogenesis has been clearly demonstrated in numerous studies evaluating the effects of antioxidants on collateral growth. Oxidative stress exerts a strong inhibitory effect on collateral remodeling. For instance, induction of oxidative stress by knockout of endothelial-specific superoxide dismutase (SOD, which dismutates O2·−, the superoxide radical anion, to H2O2) impairs flow recovery in mouse ischemic hindlimb (25). Interestingly, suppression of ROS generation is also inhibitory. In the dog the increase in coronary blood flow induced by repeated coronary occlusion is inhibited by the antioxidant N-acetylcysteine (19). Similarly, in mice the antioxidants N-acetylcysteine, ebselen, and tempol either delay or inhibit blood flow recovery in the hindlimb following femoral artery ligation (12, 25, 52). These seemingly contradictory observations have been made even within studies. In the rat heart, oxidative stress produced by treatment with the SOD inhibitor diethyldithiocarbamate inhibits the increase in coronary collateral blood flow following repeated coronary occlusion, but a similar degree of inhibition is also observed when ROS generation is suppressed with either DPI or the Nox inhibitor apocynin (42). These contradictory results suggest that arteriogenesis requires an optimal ROS concentration. An alternative interpretation is that H2O2 may be an important regulator of arteriogenesis. Nox inhibition results in a decrease in both H2O2 and O2·−, whereas inhibition of SOD would also result in a decrease in H2O2 but an increase in O2·−. These data may therefore suggest a potential positive role for H2O2 in regulating arteriogenesis.
In the vasculature, the predominant sources of ROS are Nox, xanthine oxidases, uncoupled eNOS, and the mitochondrial electron transport chain. Of these, Nox are the only family of enzymes that produce ROS as their primary function, rather than as a by-product (3). Nox-derived ROS activates several proarteriogenic signaling pathways, including the Akt, MAPK, Src, and PKC pathways (17, 57). Consistent with these findings, various Nox isoforms are also implicated in collateral remodeling in vivo (7, 10, 46, 52). Furthermore, FSS increases both Nox expression and Nox-derived ROS generation (1, 8, 11), and Nox isoforms colocalize with the mechanosensing machinery of the cell (23, 58).
We report that the flavoenzyme inhibitor DPI blocks the effects of FSS on PLGF expression in vascular cell cocultures and intact perfused vessels. In our in vitro model, we found Nox4 to be the predominant Nox isoform, consistent with previous reports for EC (14) and SMC (53). Nox4, unlike other Nox isoforms, is constitutively active and only requires association with p22phox for its activity; furthermore, it uniquely produces mostly H2O2, rather than O2·− (30). Interestingly, Nox4 activity does not result in peroxynitrite production and NO inactivation (49), both of which are linked to the activation of other Nox isoforms in the vasculature (26, 55). Rather, endothelial-specific Nox4 overexpression in mice results in increased H2O2 production and vasodilation, along with lower systemic blood pressure (41). Nox4-overexpressing mice also exhibit accelerated flow recovery following induction of hindlimb ischemia (7). Furthermore, similar to PLGF knockout mice, mice lacking Nox4 fail to recover hindlimb perfusion after femoral artery ligation (46).
In this study we found that both endothelial and smooth muscle Nox4 mRNA were increased by high FSS in our coculture system, although the time course differed between cell types. H2O2 levels were increased immediately after exposure to shear, whereas Nox4 mRNA was not upregulated until 4 h after exposure to shear. However, knockdown of Nox4 in HCAEC prevented the effects of shear stress to increase H2O2 and PLGF. We therefore hypothesize that the increase in H2O2 immediately after exposure to shear was due to increased Nox4 activity, rather than increased Nox4 protein levels. In contrast to its effects in HCAEC, Nox4 knockdown in HCASMC did not alter the response of PLGF to shear stress. These findings suggest that endothelial Nox4 plays an important role in the regulation of PLGF by FSS and ROS. We speculate that endothelial Nox4-generated H2O2 did not increase PLGF expression by HCASMC in the coculture system, since it was localized to the EC layer.
Nox4 is predominantly localized intracellularly, in association with the perinuclear endoplasmic reticulum (58), the nucleus, and focal adhesion points (23). One possible mechanism for FSS induction of Nox4 activity is by transfer of the mechanical forces of FSS on the plasma membrane to integrins and focal adhesion points via the cytoskeleton. Another possible mechanism for FSS-mediated Nox4 activation is a direct effect of FSS on the glycosylated extracellular domain of plasma membrane-associated Nox4.
Our finding that FSS-stimulated ROS production is a key signal for PLGF expression raises questions about the growing use of antioxidants as a prophylactic measure in healthy individuals. While antioxidants may be beneficial in individuals with oxidative stress (by returning ROS to physiological levels), our data and data of others supporting the “redox window” hypothesis of arteriogenesis suggest that overuse of antioxidants could potentially inhibit beneficial collateral remodeling. It is therefore of interest to determine the effects of impaired oxidative balance on shear-induced PLGF expression.
In this study we report that proarteriogenic FSS increases PLGF protein levels by ∼40% compared with static control. Although the magnitude of this effect is moderate, studies in humans suggest that a PLGF increase in this range is likely to be physiologically significant. Patients categorized as having “rich” coronary collaterals (Rentrop classification 2 and 3) were found to have 40% higher plasma PLGF levels than patients with “poor” coronary collaterals (Rentrop classification 0 and 1) (61). These clinical observations suggest that the increase in our human in vitro model could have a significant effect on human coronary collateral circulation in vivo. Furthermore, the increase in PLGF mRNA in isolated intact vessels in response to high flow was more robust than the effect in the coculture model. When considered together with our data showing that EC-SMC communication plays an important role in modulating the response of PLGF to shear, this observation suggests that the response of PLGF to increased FSS in vivo is likely to be more pronounced than the response in the coculture model.
In summary, we provide the first demonstration that the important arteriogenic growth factor PLGF is upregulated in both cocultured vascular cells and isolated vessels by the key arteriogenic stimulus of elevated FSS. We further show that this response is mediated by a novel signaling pathway involving H2O2 and Nox4 and that it is influenced by EC-SMC interaction. These findings have important clinical implications, as a better understanding of the molecular mechanisms regulating arteriogenesis may suggest new therapies for ischemic cardiovascular disease.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant R01 HL-084494 (to P. G. Lloyd).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.A.R. and P.G.L. developed the concept and designed the research; N.A.R. performed the experiments; N.A.R. analyzed the data; N.A.R. and P.G.L. interpreted the results of the experiments; N.A.R. prepared the figures; N.A.R. drafted the manuscript; N.A.R. and P.G.L. edited and revised the manuscript; N.A.R. and P.G.L. approved the final version of the manuscript.
REFERENCES
- 1.Bretón-Romero R, González de Orduña C, Romero N, Sánchez-Gómez FJ, de Álvaro C, Porras A, Rodríguez-Pascual F, Laranjinha J, Radi R, Lamas S. Critical role of hydrogen peroxide signaling in the sequential activation of p38 MAPK and eNOS in laminar shear stress. Free Radic Biol Med 52: 1093–1100, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Brevetti LS, Chang DS, Tang GL, Sarkar R, Messina LM. Overexpression of endothelial nitric oxide synthase increases skeletal muscle blood flow and oxygenation in severe rat hind limb ischemia. J Vasc Surg 38: 820–826, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech 37: 531–539, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101: 2667–2674, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res 103: 477–484, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Distasi MR, Unthank JL, Miller SJ. Nox2 and p47phox modulate compensatory growth of primary collateral arteries. Am J Physiol Heart Circ Physiol 306: H1435–H1443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576: 557–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169: 719–728, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res 99: 656–662, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Evangelista AM, Thompson MD, Bolotina VM, Tong X, Cohen RA. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med 53: 2327–2334, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fath SW, Burkhart HM, Miller SC, Dalsing MC, Unthank JL. Wall remodeling after wall shear rate normalization in rat mesenteric arterial collaterals. J Vasc Res 35: 257–264, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res 67: 169–178, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloekler S, Traupe T, Stoller M, Schild D, Steck H, Khattab A, Vogel R, Seiler C. The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart 100: 160–166, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, Chilian WM, Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol 285: H1582–H1589, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol 293: C1824–C1833, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107: 1359–1365, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Heydarkhan-Hagvall S, Helenius G, Johansson BR, Li JY, Mattsson E, Risberg B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem 89: 1250–1259, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Iwama H, Uemura S, Naya N, Imagawa Ki Takemoto Y, Asai O, Onoue K, Okayama S, Somekawa S, Kida Y, Takeda Y, Nakatani K, Takaoka M, Kawata H, Horii M, Nakajima T, Doi N, Saito Y. Cardiac expression of placental growth factor predicts the improvement of chronic phase left ventricular function in patients with acute myocardial infarction. J Am Coll Cardiol 47: 1559–1567, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res 101: 409–419, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Koller A, Toth P, Ungvari Z, Henrion D. Functional adaptation and remodeling of arteries to hemodynamic forces: role of reactive oxygen species and the vascular renin-angiotensin system. In: Systems Biology of Free Radicals and Antioxidants, edited by Laher I. Berlin: Springer, 2014, p. 1213–1237. [Google Scholar]
- 27.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2528–H2538, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Mack PJ, Zhang Y, Chung S, Vickerman V, Kamm RD, García-Cardeña G. Biomechanical regulation of endothelium-dependent events critical for adaptive remodeling. J Biol Chem 284: 8412–8420, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 116: 975–983, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed KA, Nasreen N, Tepper RS, Antony VB. Cyclic stretch induces PlGF expression in bronchial epithelial cells via nitric oxide release. Am J Physiol Lung Cell Mol Physiol 292: L559–L566, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci 115: 2559–2567, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Pardali E, Waltenberger J. Monocyte function and trafficking in cardiovascular disease. Thromb Haemost 108: 804–811, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Park B, Hoffman A, Yang Y, Yan J, Tie G, Bagshahi H, Nowicki PT, Messina LM. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J Vasc Surg 51: 165–173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol 24: 1664–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res 92: 378–385, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 108: 1933–1938, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Roncal C, Buysschaert I, Chorianopoulos E, Georgiadou M, Meilhac O, Demol M, Michel JB, Vinckier S, Moons L, Carmeliet P. Beneficial effects of prolonged systemic administration of PlGF on late outcome of post-ischaemic myocardial performance. J Pathol 216: 236–244, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Schierling W, Troidl K, Mueller C, Troidl C, Wustrack H, Bachmann G, Kasprzak PM, Schaper W, Schmitz-Rixen T. Increased intravascular flow rate triggers cerebral arteriogenesis. J Cereb Blood Flow Metab 29: 726–737, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Schierling W, Troidl K, Troidl C, Schmitz-Rixen T, Schaper W, Eitenmuller IK. The role of angiogenic growth factors in arteriogenesis. J Vasc Res 46: 365–374, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Shaw JH, Lloyd PG. Post-transcriptional regulation of placenta growth factor mRNA by hydrogen peroxide. Microvasc Res 84: 155–160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw JH, Xiang L, Shah A, Yin W, Lloyd PG. Placenta growth factor expression is regulated by hydrogen peroxide in vascular smooth muscle cells. Am J Physiol Cell Physiol 300: C349–C355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda Y, Uemura S, Iwama H, Imagawa K, Nishida T, Onoue K, Takemoto Y, Soeda T, Okayama S, Somekawa S, Ishigami K, Takaoka M, Kawata H, Kubo A, Horii M, Nakajima T, Saito Y. Treatment with recombinant placental growth factor (PlGF) enhances both angiogenesis and arteriogenesis and improves survival after myocardial infarction. Circ J 73: 1674–1682, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Teunissen PF, Horrevoets AJ, van Royen N. The coronary collateral circulation: genetic and environmental determinants in experimental models and humans. J Mol Cell Cardiol 52: 897–904, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Tulis DA, Unthank JL, Prewitt RL. Flow-induced arterial remodeling in rat mesenteric vasculature. Am J Physiol Heart Circ Physiol 274: H874–H882, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol Heart Circ Physiol 271: H914–H923, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264: 85–97, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Xiang L, Varshney R, Rashdan NA, Shaw JH, Lloyd PG. Placenta growth factor and vascular endothelial growth factor A have differential, cell-type specific patterns of expression in vascular cells. Microcirculation 21: 368–379, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang HT, Yan Z, Abraham JA, Terjung RL. VEGF121- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol Heart Circ Physiol 280: H1097–H1104, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Wang P, Huang YB, Li J, Zhu J, Luo X, Shi HM, Li Y. Plasma cathepsin L and its related pro/antiangiogenic factors play useful roles in predicting rich coronary collaterals in patients with coronary heart disease. J Int Med Res 38: 1389–1403, 2010. [DOI] [PubMed] [Google Scholar]