The present study describes pregnancy-associated increases in estrogen receptor (ER)-mediated endothelium-dependent relaxation in the aorta and inhibition of Ca2+ entry in the mesenteric artery. Increased vascular ERs could promote vasodilation, oppose vasoconstriction, and maintain normotension during normal pregnancy, and ER agonists should be examined for potential usefulness in reducing blood pressure in hypertensive pregnancy.
Keywords: estrogen, endothelium, nitric oxide, vascular smooth muscle, calcium
Abstract
Normal pregnancy is associated with adaptive hemodynamic, hormonal, and vascular changes, and estrogen (E2) may promote vasodilation during pregnancy; however, the specific E2 receptor (ER) subtype, post-ER signaling mechanism, and vascular bed involved are unclear. We tested whether pregnancy-associated vascular adaptations involve changes in the expression/distribution/activity of distinct ER subtypes in a blood vessel-specific manner. Blood pressure (BP) and plasma E2 were measured in virgin and pregnant (day 19) rats, and the thoracic aorta, carotid artery, mesenteric artery, and renal artery were isolated for measurements of ERα, ERβ, and G protein-coupled receptor 30 [G protein-coupled ER (GPER)] expression and tissue distribution in parallel with relaxation responses to E2 (all ERs) and the specific ER agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol (PPT; ERα), diarylpropionitrile (DPN; ERβ), and G1 (GPER). BP was slightly lower and plasma E2 was higher in pregnant versus virgin rats. Western blots revealed increased ERα and ERβ in the aorta and mesenteric artery and GPER in the aorta of pregnant versus virgin rats. Immunohistochemistry revealed that the increases in ERs were mainly in the intima and media. In phenylephrine-precontracted vessels, E2 and PPT caused relaxation that was greater in the aorta and mesenteric artery but similar in the carotid and renal artery of pregnant versus virgin rats. DPN- and G1-induced relaxation was greater in the mesenteric and renal artery than in the aorta and carotid artery, and aortic relaxation to G1 was greater in pregnant versus virgin rats. The nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester with or without the cyclooxygenase inhibitor indomethacin with or without the EDHF blocker tetraethylammonium or endothelium removal reduced E2, PPT, and G1-induced relaxation in the aorta of pregnant rats, suggesting an endothelium-dependent mechanism, but did not affect E2-, PPT-, DPN-, or G1-induced relaxation in other vessels, suggesting endothelium-independent mechanisms. E2, PPT, DPN, and G1 caused relaxation of Ca2+ entry-dependent KCl contraction, and the effect of PPT was greater in the mesenteric artery of pregnant versus virgin rats. Thus, during pregnancy, an increase in ERα expression in endothelial and vascular smooth muscle layers of the aorta and mesenteric artery is associated with increased ERα-mediated relaxation via endothelium-derived vasodilators and inhibition of Ca2+ entry into vascular smooth muscle, supporting a role of aortic and mesenteric arterial ERα in pregnancy-associated vasodilation. GPER may contribute to aortic relaxation while enhanced ERβ expression could mediate other genomic vascular effects during pregnancy.
NEW & NOTEWORTHY
The present study describes pregnancy-associated increases in estrogen receptor (ER)-mediated endothelium-dependent relaxation in the aorta and inhibition of Ca2+ entry in the mesenteric artery. Increased vascular ERs could promote vasodilation, oppose vasoconstriction, and maintain normotension during normal pregnancy, and ER agonists should be examined for potential usefulness in reducing blood pressure in hypertensive pregnancy.
during normal pregnancy, several maternal hemodynamic and cardiovascular changes occur to adapt to the metabolic demands of the growing fetus, including an increase in blood volume and cardiac output, systemic vasodilation, and decreased vascular resistance with slight decrease or no change in blood pressure (BP) (70, 84). These hemodynamic and vascular changes involve significant redistribution of blood flow to different maternal tissues and organs. An orchestrated network of vasodilator substances, including nitric oxide (NO), prostacyclin (PGI2), EDHF, kallikrein, angiotensin-(1–7), and VEGF, may participate in the local and systemic hemodynamic adaptations during pregnancy (82, 86). Estrogen (E2) levels also increase during pregnancy (23, 74). E2 induces long-term genomic vascular effects, such as stimulation of endothelial cell growth, upregulation of endothelial NO synthase and NO production, increased cyclooxygenase (COX) activity and PGI2 production (19, 53, 64, 77), inhibition of vascular smooth muscle (VSM) proliferation, and downregulation of VSM Ca2+ channels and PKC (33, 61). E2 also causes rapid nongenomic vasodilator effects via activation of endothelium-dependent NO, PGI2, and hyperpolarization pathways (24, 29) and inhibition of Ca2+-dependent mechanisms of VSM contraction (13, 31, 64). In addition to its effects on vascular cell growth and proliferation, E2 could also affect extracellular matrix deposition/degradation and, in turn, causes structural changes and remodeling of blood vessels (66).
Many of the vascular effects of E2 are mediated via estrogen receptors (ERs) (78). ERs have been identified in the female reproductive tract, mammary glands, and blood vessels of humans and experimental animals (9, 47, 68, 76, 78, 93). ERα and ERβ mediate many of the genomic effects of E2 (68, 78, 93), and surface membrane ERα and ERβ have been implicated in the rapid vasodilator effects of E2 (53). A transmembrane G protein-coupled ER (GPER; G protein-coupled receptor 30) may also bind E2 and mediate some of its nongenomic effects (20, 26, 43, 44, 55, 58, 71, 73). Because different tissues may have different blood flow requirements, the vasodilator effects of E2/ERs could vary in different vascular beds. In this respect, a vasodilator effect of E2 in one vascular bed could be balanced by minimal effect in other vascular beds with no net change in BP. We have recently shown region-specific ERα-, ERβ-, and GPER-mediated vasorelaxation in the cephalic, thoracic, and abdominal arteries of nonpregnant female rats (72). Regional differences in vasodilation, blood flow, and E2/ER activity are particularly important during pregnancy as they could have significant implications on the cardiovascular system and the health of mother and fetus. While E2 levels increase during pregnancy, whether the amount of vascular ERs increase in parallel is unclear. In addition, whether any pregnancy-associated changes in vascular ERs affect the structure or function of blood vessels is unknown. Furthermore, the role of specific ER subtypes and post-ER endothelium-dependent and endothelium-independent signaling mechanisms in mediating the vascular effects of E2 during pregnancy is unclear. The purpose of the present study was to test the hypothesis that pregnancy-associated vascular adaptations involve regional changes in ER expression, distribution, vasodilator activity, and post-ER signaling mechanisms in a blood vessel-specific manner. We used four blood vessels with specific roles and functions (namely, the thoracic aorta as a conduit vessel, the carotid artery supplying the cephalic circulation, the mesenteric artery with a specific role in the splanchnic circulation, and the renal artery with a specialized role in the kidney) from virgin and pregnant rats and subtype-specific ER agonists to determine the pregnancy-associated changes in vascular ER expression and distribution in different systemic vessels. We also tested whether pregnancy-associated alterations in vascular ER expression are manifested as changes in blood vessel structure and remodeling, ER-mediated vasodilator activity, post-ER endothelium-dependent relaxation pathways, and/or post-ER endothelium-independent inhibitory effects on VSM contraction mechanisms. The present study addressed two important questions: Does vascular ER expression/activity increase during pregnancy? and Is the pregnancy-related increase in vascular ER expression/activity universal or localized to specific vascular beds?
METHODS
Animals.
Time-pregnant (day 11 of gestation) and age-matched virgin female Sprague-Dawley rats (12 wk of age) were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed in the animal facility for at least 1 wk and had access to ad libitum standard rat chow and tap water on a 12:12-h light-dark cycle. All experimental procedures followed the National Institutes of Health (nih) Guide for the Care and Use of Laboratory Animals and the guidelines of the Harvard Medical Area Standing Committee on Animals.
BP.
On day 19 of pregnancy or the equivalent in virgin rats, rats were anaesthetized with isoflurane, and a PE-50 catheter was inserted in the carotid artery and exteriorized at the back of the neck. Rats were allowed to recover from anesthesia for at least 1 h. The carotid arterial catheter was connected to a pressure transducer attached to an amplifier and BP recorder (Living System Instrumentation, Burlington, VT). BP in conscious rats was recorded every 20 min for 1 h, and the average BP was measured (50).
Plasma E2.
Blood samples from virgin and pregnant rats were collected in EDTA, and plasma estradiol (E2) was measured by ELISA (Rat E2 ELISA Kit, MBS702969, MyBioSource). Virgin rats were studied during estrus to control for reproductive and endocrine confounders. The stage of the estrous cycle was determined by taking a vaginal lavage with a pasteur pipette (52). An estrus lavage primarily contained anucleated cornified squamous cells (89) and was confirmed before all experiments.
Tissue preparation.
Pregnant and virgin rats were euthanized by CO2 inhalation. The thoracic aorta, right and left carotid, mesentry and mesenteric arterial arcade, and kidneys and renal arteries were excised and placed in Krebs solution. We chose the thoracic aorta, carotid artery, and mesenteric artery as representatives of the thoracic, cephalic, and abdominal circulation and the renal arteries for their role in the regulation of renal function, plasma volume, and BP. Uteri of virgin and pregnant rats were also examined as known targets and positive controls for ERs. The virgin uterus was cleaned of the surrounding fat and weighed. The pregnant uterus was cut open, the placenta and pups were separated, and the litter size, individual pup weight, and uterine weight were recorded. The kidneys were also cleaned and weighed. With the aid of a dissection microscope, the aorta, carotid artery, mesenteric artery, and renal artery were carefully cleaned of fat and connective tissue and cut into 3-mm-wide rings for vascular function experiments. For endothelium-intact arteries, extreme care was taken to minimize injury to the endothelium. For endothelium-denuded segments, the endothelium was removed by scraping the vessel interior five times around the tip of forceps (for the aorta) or around thin tungsten wire (for the carotid, mesenteric, and renal artery). Different segments from each vascular bed were used for different experimental protocols and pharmacological manipulations. The remainder of the vascular tissue was stored at −80°C for Western blots, histology, and immunohistochemistry.
Western blots.
The aorta, carotid artery, mesenteric artery, and renal artery as well as the uterus were homogenized in homogenization buffer containing 20 mM MOPS, 4% SDS, 10% glycerol, 2.3 mg DTT, 1.2 mM EDTA, 0.02% BSA, 5.5 μM leupeptin, 5.5 μM pepstatin, 2.15 μM aprotinin, and 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride using a 2-ml tight-fitting homogenizer (Kontes Glass, Vineland, NJ). The homogenate was centrifuged at 10,000 g for 10 min, the supernatant was collected, and protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA). Protein extracts (20 μg) were combined with an equal volume of 2× Laemmli loading buffer, boiled for 5 min, and size fractionated by electrophoresis on 8% SDS-polyacrylamide gels. Proteins were transferred from the gel to a nitrocellulose membrane by electroblotting. Membranes were incubated in 5% nonfat dry milk in Tris-buffered saline-Tween for 1 h and then with polyclonal rabbit ERα antibody (sc-542, 1:1,000), ERβ antibody (sc-8974, 1:1,000), and GPER antibody (sc-134576, 1:1,000, Santa Cruz Biotechnology, Dallas, TX) for 24 h at 4°C. Membranes were washed three times for 15 min in Tris-buffered saline-Tween and then incubated with horseradish peroxidase-conjugated secondary antibody (1:1,000) for 2 h, and immunoreactive bands were detected using enhanced chemiluminescence Western blot detection reagent (GE Healthcare Bio-Sciences, Piscataway, NJ). Blots were subsequently reprobed for β-actin (1:5,000). Reactive bands were analyzed by optical densitometry and ImageJ software (NIH, Bethesda, MD). Densitometry values represented the pixel intensity normalized to β-actin to correct for loading.
Histology and quantitative morphometry.
Segments of the arteries and uteri of virgin and pregnant rats were cryopreserved in Tissue Tek 4583 optimal cutting temperature compound (Fisher Scientific) and stored at −80°C. Cross-sectional 6-μm-thick cryosections from the arteries and uterus were placed on glass slides and prepared for staining with hematoxylin and eosin. Stained sections were coded and labeled in a blinded fashion. Images were acquired on a Nikon microscope using the same magnification, light intensity, and camera gain and analyzed using ImageJ software. Images of arteries were acquired using bright-field and ×4 and ×40 objectives. Because the rat uterus is large, 20 picture frames of sequential parts of the uterine tissue section were acquired using the ×4 objective, and the composite image of the whole uterine section was reconstituted as previously described (39). Images of tissue sections were analyzed using ImageJ. Outlines of the tissue exterior and interior were used to define the whole tissue area and lumen area, respectively, and the wall area was calculated as whole tissue area minus lumen area. The number of pixels in each area was then translated into millimeters squared using a calibration bar. Using higher-magnification images of the aorta, carotid artery, renal artery, mesenteric artery (×400), and uterus (×40) and ImageJ software, a line was drawn across the tissue wall, and the number of pixels in the line scan was counted and translated into total wall thickness (in mm) using a calibration bar. The relative thickness of the vascular tissue layers (intima, media, and adventitia) and uterine tissue layers (endometrium, myometrium, and perimetrium) was also measured and presented in millimeters or as a percentage of total wall thickness.
Immunohistochemistry.
To determine the tissue distribution of ERα, ERβ, and GPER, cryosections of the aorta, carotid artery, mesenteric artery, and renal artery as well as the uterus were thawed and fixed in ice-cold acetone for 30 min. Endogenous peroxidase was quenched in 1.5% H2O2 solution for 30 min, and nonspecific binding was blocked in 10% horse serum for 30 min. Tissue sections were incubated with polyclonal ERα (1:100), ERβ (1:100), and GPER (1:100) antibody (Santa Cruz Biotechnology). After being rinsed with PBS, tissue sections were incubated with biotinylated anti-rabbit secondary antibody, rinsed with PBS, and then incubated with avidin-labeled peroxidase (VectaStain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Positive labeling was visualized using diaminobenzadine and appeared as brown spots. Negative control slides were run simultaneously with no primary antibody. Specimens were counterstained with hematoxylin for 40 s, rinsed with PBS, topped with cytoseal 60, and covered with slide coverslips. Images were acquired on a Nikon microscope using the same microscope magnification, light intensity, and camera gain. Images of the aorta, carotid artery, mesenteric artery, and renal artery were acquired using bright-field and ×40 objectives, and images of the virgin uterus were acquired with a ×4 objective. Because the rat pregnant uterus is large, 20 picture frames of sequential parts of the uterine wall were acquired using the ×4 objective, and the composite image of the whole uterine section was reconstituted as previously described (39). Images were analyzed using ImageJ, the total number of pixels in the tissue section image was defined, and the number of brown spots (pixels) was counted and then presented as a percentage of total pixels. In addition, the number of pixels in the specific tissue layer (intima, media, and adventitia for arteries and endometrium, myometrium, and perimetrium for the uterus) was defined and transformed into the area (in μm2) using a calibration bar. The number of brown spots (pixels) representing ERα, ERβ, and GPER in each tissue layer was then counted and presented as the number of pixels per micrometer squared.
Isometric contraction/relaxation.
Vascular rings were suspended between two wire hooks; one hook was fixed at the bottom of a tissue bath, and the other hook was connected to a Grass force transducer (FT03, Astro-Med, West Warwick, RI). Arterial rings were stretched under 2 g (aorta), 1 g (carotid artery), or 0.5 g (mesenteric and renal artery) of basal tension and allowed to equilibrate for 45 min in Krebs solution bubbled with 95% O2-5% CO2 at 37°C. These basal tensions produced maximal KCl contraction, and further increases in basal tension did not cause further contraction to KCl (72). Changes in contraction/relaxation were recorded on a Grass polygraph (model 7D, Astro-Med).
Control contraction to 96 mM KCl was first elicited. Once KCl reached a plateau, the tissue was washed in Krebs solution three times for 10 min. To investigate endothelial function, vascular rings were precontracted with phenylephrine (Phe; 6 × 10−7) and then treated with ACh (10−9–10−5 M), and the percent relaxation was measured. To test the role of ERs, vascular segments precontracted with Phe were treated with increasing concentrations (10−12–10−5 M) of E2 (activator of all ERs), 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol (PPT; ERα agonist) (79, 81), diarylpropionitrile (DPN; selective ERβ agonist) (27), or (±)-1-[(3aR*,4S*,9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G1; GPER agonist) (21, 37), and the percent vascular relaxation was measured. For concentration-response curves, each ER agonist concentration was added, the vascular response was observed until it reached steady state or for 10 min, and the next concentration was then added. We previously tested the specificity of E2, PPT, DPN, and G1 and found that treatment of the vessels with the vehicle ethanol (0.1%, for E2) or DMSO (0.1%, for PPT, DPN, and G1) did not significantly change Phe-induced contraction (72). In addition, the effects of PPT, DPN, and G1 on Phe-induced contraction were prevented in vessels pretreated with the ERα antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole (MPP), the ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), and the GPER antagonist (3aS*,4R*,9bR*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-3H-cyclopenta[c]quinoline (G15), respectively, supporting specificity of the effects of PPT, DPN, and G1 (51, 72).
To test the role of endothelium-dependent NO, PGI2, and the hyperpolarization pathway, vascular relaxation was measured in arterial segments treated with the NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M), COX inhibitor indomethacin (10−5 M), and tetraethylammonium (TEA; 30 mM), a blocker of K+ channels and the hyperpolarization pathway. To test for endothelium-independent effects on VSM, the effects of ER agonists were measured in endothelium-denuded arteries precontracted with Phe. To test for ER-mediated effects on Ca2+ entry into VSM, the effects of ER agonists were tested on endothelium-denuded arteries precontracted with 96 mM KCl, which causes membrane depolarization and activates Ca2+ entry through Ca2+ channels into VSM (61).
Solutions and drugs.
Krebs solution contained (in mM) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2 at pH 7.4. High-KCl (96 mM) or TEA solution (30 mM) was prepared as described for Krebs solution but with equimolar substitution of NaCl with KCl or TEA, respectively. Stock solutions of Phe (10−1 M), ACh (10−1 M), and l-NAME (10−1 M, Sigma) were prepared in deinoized water. E2 (10−2 M, Sigma) was prepared in 100% ethanol. PPT, DPN, and G1 (10−1 M, Tocris, Ellisville, MO) and indomethacin (10−1 M, Sigma) were prepared in DMSO. The final concentration of ethanol or DMSO in the experimental solution was <0.1%. PBS was used to rinse the slides in the immunohistochemistry experiments and contained (in mM) 137 NaCl, 2.7 KCl, 8 Na2HPO4, and 2 KH2PO4 at pH 7.4. All other chemicals were of reagent grade or better.
Statistical analysis.
Data from different rats are presented as means ± SE, and n indicates the number of rats. For vascular reactivity experiments, individual concentration-relaxation curves were constructed, sigmoidal curves were fitted to the data using the least-squares method, and the pD2 values (−log EC50, drug concentration evoking half-maximal response) were measured using Prism (version 6.01, GraphPad Software, San Diego, CA). Data were first analyzed using two-way ANOVA with multiple classification criteria [rat type (pregnant vs. virgin), condition of the endothelium (denuded vs. intact), treatment (treated with l-NAME or TEA vs. nontreated)] (3, 59). When a statistical difference was observed, the data were further analyzed using a Student-Newman-Keuls post hoc test for multiple comparisons and an unpaired Student's t-test for comparison of two means. Differences were considered significant if P < 0.05.
RESULTS
BP, plasma E2, body weight, and tissue weight.
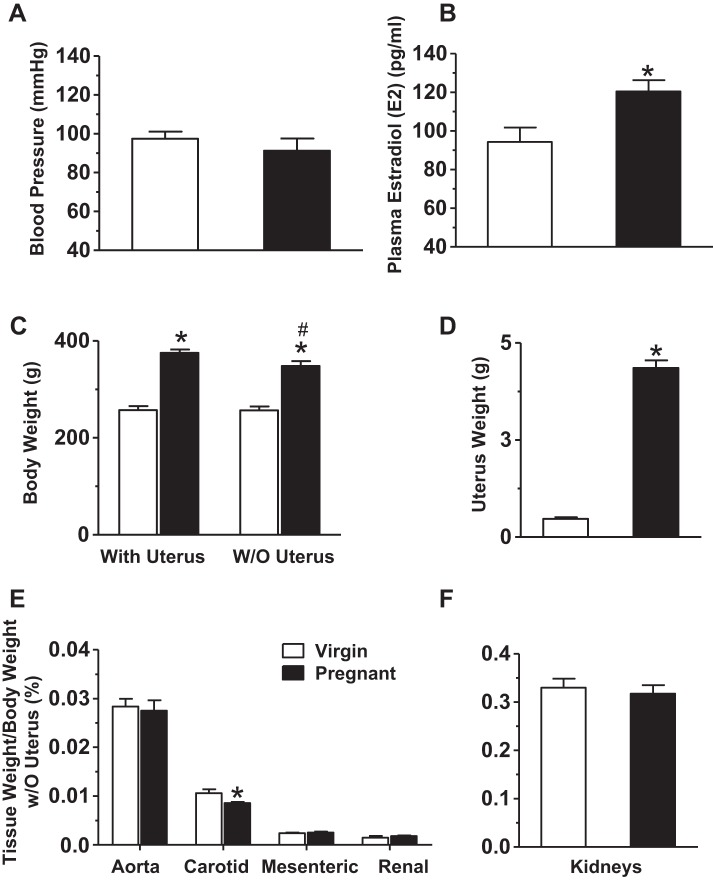
Mean arterial pressure was slightly but not significantly reduced (Fig. 1A) and plasma E2 levels were significantly higher (Fig. 1B) in pregnant versus virgin rats. The body weight of intact animals was greater in pregnant versus virgin rats (Fig. 1C). When rats were euthanized and the uterus, pups, and placentae removed, the body weight without the uterus was still greater in pregnant versus virgin rats (Fig. 1C). The uterus weight without pups and placentae was also dramatically greater in pregnant versus virgin rats (Fig. 1D). The vascular tissue weight as a percentage of body weight was not different in the aorta, mesenteric artery, and renal artery but was reduced in the carotid artery of pregnant versus virgin rats (Fig. 1E). Kidney weight was not statistically different in pregnant versus virgin rats (Fig. 1F).
Fig. 1.
Blood pressure (BP), plasma estrogen/estradiol (E2), body weight, and tissue weight in virgin versus pregnant (Preg) rats. BP was recorded (A) and plasma E2 levels were measured by ELISA (B) in virgin and pregnant (day 19) rats. Body weight with and without (w/o) the uterus (C) and uterus weight (D) were compared. Arterial tissue weight (E) and kidney weight (F) were measured and presented as a percentage of body weight without the uterus. Bar graphs represent means ± SE; n = 5–6 rats/group. *P < 0.05, pregnant vs. virgin rats; #P < 0.05, without the uterus vs. with the uterus.
ERα expression and tissue distribution.
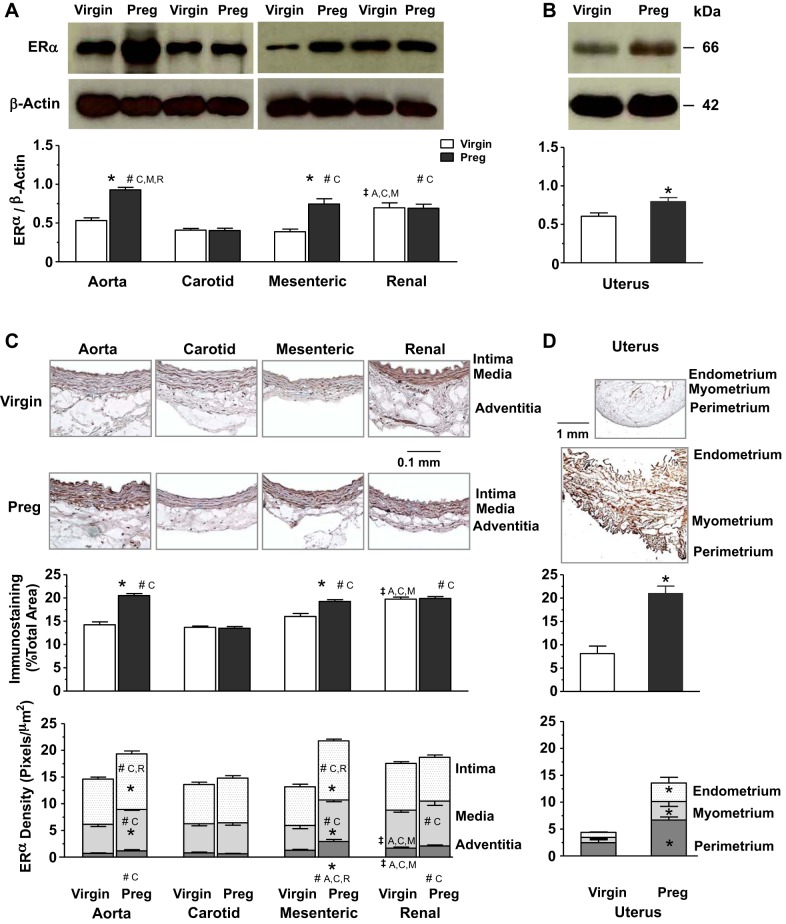
Western blots for ERα revealed a band at 66 kDa (Fig. 2A). In vessels of virgin rats, the ERα-immunoreactive band relative to actin was greater in the renal artery than in the aorta, carotid artery, and mesenteric artery. In vessels of pregnant rats, ERα was most prominent in the aorta and showed greater levels in the mesenteric and renal artery than in the carotid artery. ERα levels were also higher in the aorta and mesenteric artery of pregnant versus virgin rats (Fig. 2A). Parallel Western blots on the uterus revealed a greater amount of ERα in pregnant versus virgin rats (Fig. 2B). Immunohistochemistry revealed that in vessels of virgin rats, ERα immunostaining was greater in the renal artery, particularly in the media and adventitia, than in the aorta, carotid artery, and mesenteric artery (Fig. 2C). In vessels of pregnant rats, total ERα was higher in the aorta, mesenteric artery, and renal artery than in the carotid artery, and the relative distribution of ERα in the intima, media and adventitia of the aorta and mesenteric artery and media and adventitia of the renal artery was greater than in the carotid artery. Total ERα and its relative distribution in the intima, media, and adventitia was also greater in the aorta and mesenteric artery of pregnant versus virgin rats (Fig. 2C). Parallel immunohistochemistry experiments revealed a greater total amount and relative distribution of ERα in the endometrium, myometrium, and perimetrium in uterine tissue sections of pregnant versus virgin rats (Fig. 2D).
Fig. 2.
Protein amount and tissue distribution of estrogen receptor (ER)α in the aorta (A), carotid artery (C), mesenteric artery (M), and renal artery (R) as well as the uterus of virgin versus pregnant rats. Tissue homogenates of blood vessels (A) and the uterus (B) were prepared for Western blots using ERα antibody (1:1,000). ERα and G protein-coupled ER (GPER; shown in Fig. 4) were run on the same gel and therefore have the same actin control. Vascular tissue sections (C) and uterine sections (D) were stained with ERα antibody using immunohistochemistry, and the total amount and relative distribution of ERα brown immunostaining in different layers of the tissue wall were measured using ImageJ. Bar graphs represent means ± SE; n = 4–6 rats/group. *P < 0.05, pregnant vs. virgin rats; ‡significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of virgin rats; #significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of pregnant rats.
ERβ expression and tissue distribution.
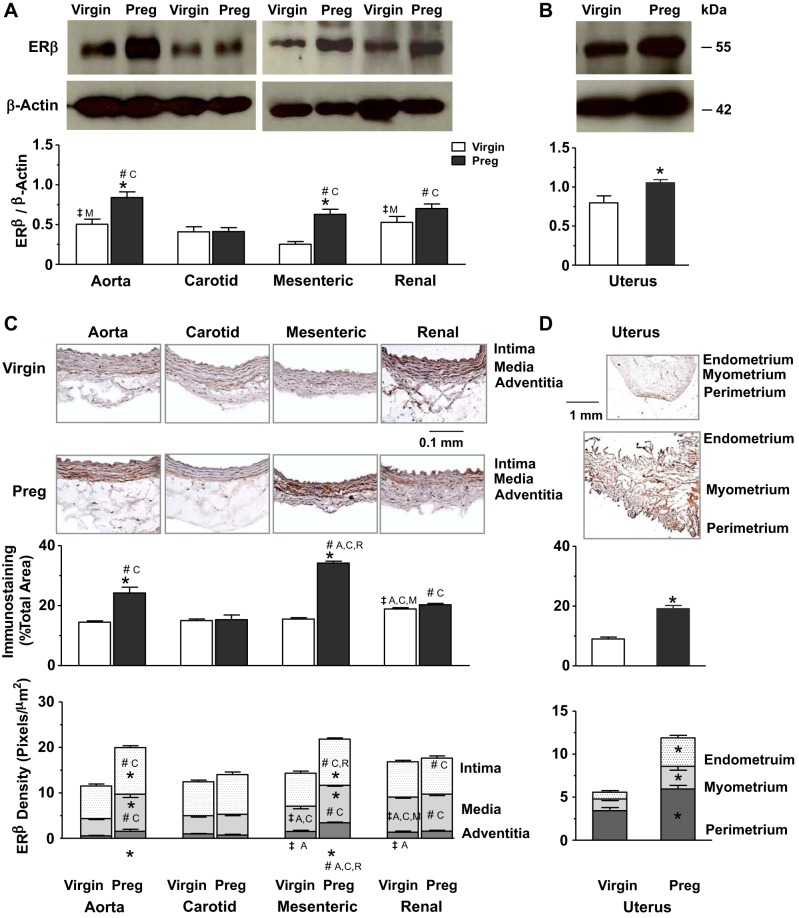
Western blots for ERβ in blood vessels revealed a band at 55 kDa (Fig. 3A). In vessels of virgin rats, ERβ relative to actin was greater in the aorta and renal artery than in the mesenteric artery. In vessels of pregnant rats, ERβ was most prominent in the aorta, with greater amounts in the aorta, mesenteric artery, and renal artery than in the carotid artery. ERβ was also higher in the aorta and mesenteric artery of pregnant versus virgin rats (Fig. 3A). Parallel Western blots on the uterus showed higher levels of ERβ in pregnant versus virgin rats (Fig. 3B). Immunohistochemistry revealed that in vessels of virgin rats, ERβ was most detectable in the renal artery, with higher levels in the media and adventitia, compared with other vessels. In vessels of pregnant rats, total ERβ and its relative distribution in the intima and media were more apparent in the aorta, mesenteric artery, and, to some extent, the renal artery than in the carotid artery. ERβ was also higher in the adventitia of the mesenteric artery versus the aorta, carotid artery, and renal artery of pregnant rats. Total ERβ and its relative distribution in the intima, media, and adventitia were also greater in the aorta and mesenteric artery of pregnant versus virgin rats (Fig. 3C). Parallel immunohistochemistry revealed a greater total amount and relative distribution of ERβ in the endometrium, myometrium, and perimetrium in the uterus of pregnant versus virgin rats (Fig. 3D).
Fig. 3.
Protein amount and tissue distribution of ERβ in the aorta, carotid artery, mesenteric artery, and renal artery as well as the uterus of virgin versus pregnant rats. Tissue homogenates of blood vessels (A) and the uterus (B) were prepared for Western blots using ERβ antibody (1:1,000). Vascular tissue sections (C) and uterine sections (D) were stained with ERβ antibody using immunohistochemistry, and the total amount and relative distribution of ERβ brown immunostaining in different layers of the tissue wall were measured using ImageJ. Bar graphs represent means ± SE; n = 4–6 rats/group. *P < 0.05, pregnant vs. virgin rats; ‡significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of virgin rats; #significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of pregnant rats.
GPER expression and tissue distribution.
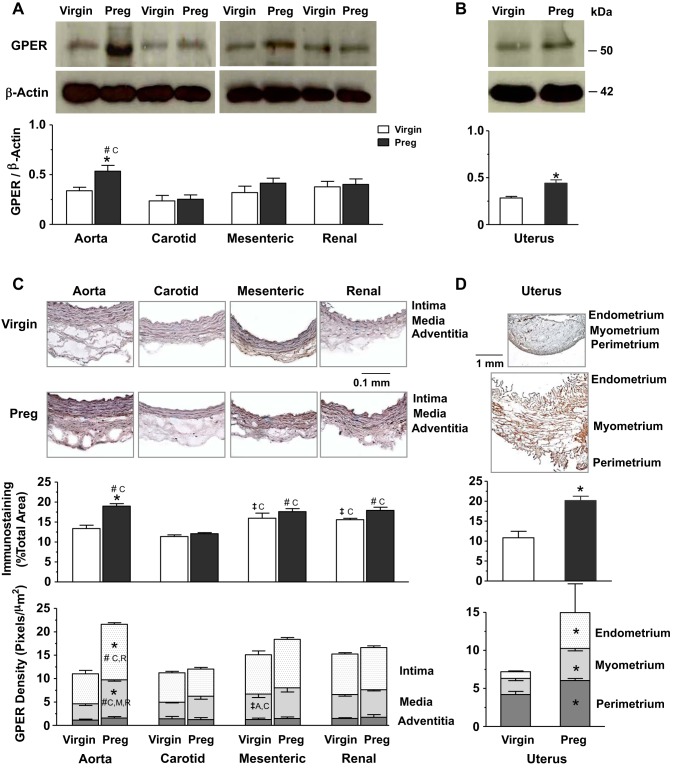
Western blots for GPER revealed a band at ∼50 kDa that was not different among vessels of virgin rats and was only significantly greater in the aorta versus other vessels of pregnant rats or in the aorta of pregnant versus virgin rats (Fig. 4A). GPER levels were also greater in the uterus of pregnant versus virgin rats (Fig. 4B). Immunohistochemistry revealed more GPER particularly in the media of mesenteric and renal artery than in the carotid artery of virgin rats. In vessels of pregnant rats, total GPER was more apparent in the aorta, mesenteric artery, and renal than in the carotid artery, and its relative distribution in the intima and media was greater in the aorta than in the other vessels. Total GPER and its relative distribution in the intima and media were higher in the aorta of pregnant versus virgin rats (Fig. 4C). In addition, the GPER total amount and relative distribution were greater in the endometrium, myometrium, and perimetrium in uterine sections of pregnant versus virgin rats (Fig. 4D).
Fig. 4.
Protein amount and tissue distribution of GPER in the aorta, carotid artery, mesenteric artery, and renal artery as well as the uterus of virgin versus pregnant rats. Tissue homogenates of blood vessels (A) and the uterus (B) were prepared for Western blots using GPER antibody (1:1,000). GPER and ERα (shown in Fig. 2) were run on the same gel and therefore have the same actin control. Vascular tissue sections (C) and uterine sections (D) were stained with GPER antibody using immunohistochemistry, and the total amount and relative distribution of GPER brown immunostaining in different layers of the tissue wall were measured using ImageJ. Bar graphs represent means ± SE; n = 4–6 rats/group. *P < 0.05, preg vs. virgin rats; ‡significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of virgin rats; #significantly different (P < 0.05) from corresponding measurements in the aorta, carotid artery, mesenteric artery, and renal artery of pregnant rats.
Tissue histology and morphometry.
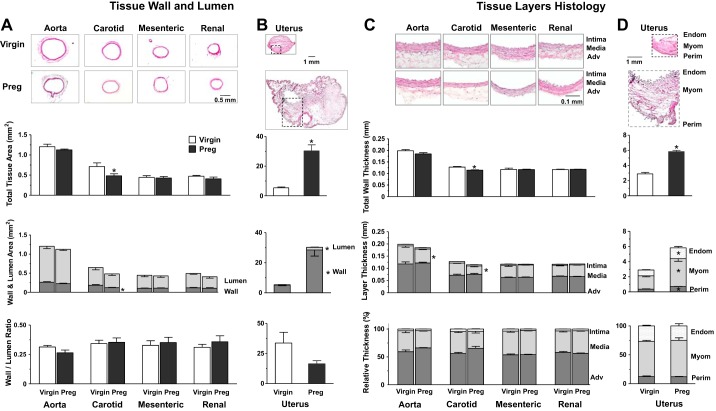
We assessed if pregnancy-induced changes in vascular ERs are associated with changes in vessel structure. The external vessel diameter in pregnant rats (aorta: 1,206 ± 57 μm, carotid artery: 836 ± 88 μm, mesenteric artery: 737 ± 38 μm, and renal artery: 721 ± 39 μm) was not significantly different from that in virgin rats (aorta: 1,357 ± 65 μm, carotid artery: 961 ± 70 μm, mesenteric artery: 802 ± 49 μm, and renal artery: 781 ± 36 μm). Histological analysis of aortic sections showed no significant changes in total tissue area, wall and lumen area, wall-to-lumen ratio (Fig. 5A), and total wall thickness (Fig. 5C) but a significant decrease in aortic media thickness (Fig. 5C) in pregnant versus virgin rats. Carotid artery sections showed decreases in total and wall area (Fig. 5A), total wall thickness, and media thickness (Fig. 5C) in pregnant versus virgin rats. No significant differences in tissue wall, lumen thickness, and relative thickness of the intima, media, and adventitia were observed in the mesenteric and renal artery of pregnant versus virgin rats (Fig. 5, A and C). Parallel measurements in uterine sections revealed increases in total tissue area, wall and lumen area (Fig. 5B), total wall thickness, and endometrium and myometrium layer thickness (Fig. 5D) in pregnant versus virgin rats.
Fig. 5.
Histology and morphometric analysis of the aorta, carotid artery, mesenteric artery, and renal artery (A and C) as well as the uterus (B and D) of virgin versus pregnant rats. Cryosections were prepared for hematoxylin and eosin staining, and tissue images were analyzed using ImageJ. Total tissue area, lumen area, whole wall area, and wall-to-lumen ratio were measured. Total wall thickness and the relative thickness of the different vascular layers (intima, media, and adventitia) and uterine layers (endometrium, myometrium, and perimetrium) were also measured. Bar graphs represent means ± SE; n = 4–6 rats/group. *P < 0.05, pregnant vs. virgin rats.
E2-, PPT-, DPN-, and G1-induced relaxation of Phe-induced contraction.
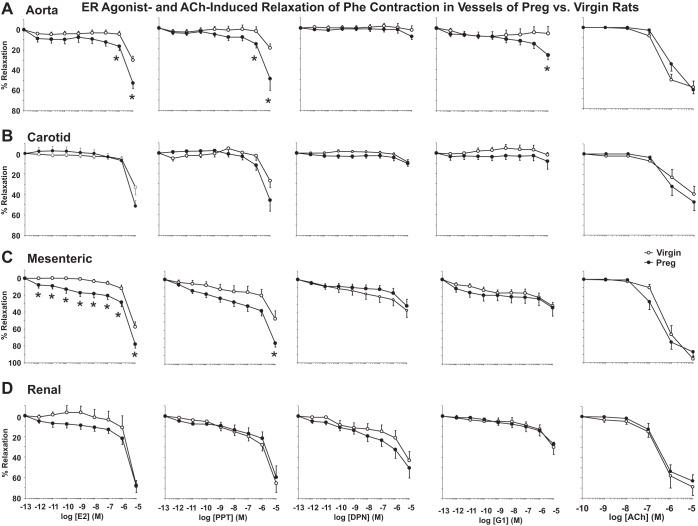
We assessed if pregnancy-induced changes in vascular ERs could affect vascular function. In blood vessels isolated from virgin rats and precontracted with Phe, all ER agonists caused greater maximum relaxation with no difference in EC50 in the mesenteric and renal artery versus the aorta and carotid artery (Fig. 6 and Table 1). In vessels of pregnant rats, maximum relaxation to E2 and the ERα agonist PPT was greater in the mesenteric artery and maximum relaxation to the ERβ agonist DPN was greater in the mesenteric and renal artery versus the aorta and carotid artery. In vessels of pregnant rats, PPT was more potent in causing relaxation in the mesenteric artery and G1 was more potent in the aorta and mesenteric artery than in the carotid artery (Table 1). The relaxation to E2 and PPT was greater in the aorta (Fig. 6A) and mesenteric artery (Fig. 6C) but not different in the carotid artery (Fig. 6B) or renal artery (Fig. 6D) of pregnant versus virgin rats (Table 1). DPN (ERβ) and G1 (GPER) relaxation was generally less than that induced by E2 or PPT in all vessels tested (Fig. 6 and Table 1). DPN maximum relaxation and EC50 were not different in all vessels of pregnant versus virgin rats. G1-induced maximum relaxation was only greater in the aorta of pregnant versus virgin rats (Fig. 6A and Table 1). In comparison, ACh, a known stimulator of endothelium-dependent relaxation, caused relaxation that was not different in the aorta, carotid artery, mesenteric artery, or renal artery of pregnant versus virgin rats (Fig. 6, A–D).
Fig. 6.
Differential effects of E2, 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol (PPT), diarylpropionitrile (DPN), G1, and ACh on relaxation of the phenylephrine (Phe)-precontracted aorta (A), carotid artery (B), mesenteric artery (C), and renal artery (D) of virgin versus pregnant rats. Endothelium-intact segments of the thoracic aorta, carotid artery, mesenteric artery, and renal artery were precontracted with Phe. Increasing concentrations (10−12–10−5 M) of E2 (activator of all ERs), PPT (ERα agonist), DPN (ERβ agonist), or G1 (GPER agonist) were added, and the percent relaxation of Phe-induced contraction was measured. In parallel experiments, the effects of increasing concentrations (10−9–10−5 M) of ACh on the relaxation of Phe-precontracted vessels were measured. Data represent means ± SE; n = 8–10 rats/group. *P < 0.05, pregnant vs. virgin rats.
Table 1.
Maximum response and EC50 for ER agonist-induced relaxation of the thoracic aorta, carotid artery, mesenteric artery, and renal artery isolated from virgin and pregnant rats and precontracted with phenylephrine or KCl
| Virgin Rats |
Pregnant Rats |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aorta | Carotid artery | Mesenteric artery | Renal artery | Aorta | Carotid artery | Mesenteric artery | Renal artery | |
| Relaxation of phenylephrine-induced contraction maximum, % | ||||||||
| E2 | 29.99 ± 3.85 | 33.20 ± 7.38 | 57.40 ± 5.86†A,C | 67.47 ± 4.66†A,C | 52.76 ± 5.45* | 51.49 ± 5.50 | 77.87 ± 4.65*‡A,C | 68.13 ± 6.19 |
| PPT | 19.33 ± 3.76 | 26.94 ± 6.44 | 46.48 ± 9.47†A | 65.16 ± 8.81†A,C | 49.27 ± 11.35* | 45.89 ± 10.77 | 75.36 ± 4.54*‡A,C | 59.45 ± 11.15 |
| DPN | 2.04 ± 4.14 | 8.83 ± 2.56 | 37.24 ± 8.17†A,C | 43.12 ± 8.57†A,C | 8.28 ± 2.95 | 10.20 ± 2.78 | 31.89 ± 8.03‡A,C | 50.46 ± 9.87‡A,C |
| G1 | 5.34 ± 6.70 | 1.87 ± 2.50 | 31.19 ± 4.07†A,C | 30.20 ± 5.03†A,C | 26.17 ± 4.35* | 8.10 ± 7.74 | 33.21 ± 9.97 | 27.22 ± 10.06 |
| pD2 (−log M) | ||||||||
| E2 | 5.74 ± 0.11 | 5.63 ± 0.07 | 5.72 ± 0.06 | 5.83 ± 0.12 | 6.24 ± 0.34 | 5.71 ± 0.04 | 6.16 ± 0.26 | 5.92 ± 0.13 |
| PPT | 6.94 ± 0.81 | 6.59 ± 0.69 | 6.52 ± 0.56 | 6.19 ± 0.18 | 5.92 ± 0.13 | 5.81 ± 0.10 | 7.21 ± 0.45‡A,C | 6.37 ± 0.28 |
| DPN | 6.43 ± 0.78 | 5.72 ± 0.10 | 7.57 ± 0.79 | 6.43 ± 0.34 | 6.30 ± 0.50 | 6.90 ± 0.74 | 6.60 ± 0.64 | 6.70 ± 0.34 |
| G1 | 7.73 ± 1.07 | 6.18 ± 0.68 | 8.25 ± 0.76 | 6.82 ± 0.55 | 7.43 ± 0.66 | 5.81 ± 0.17‡A | 8.86 ± 1.03‡C | 6.23 ± 0.28 |
| Relaxation of KCl-induced contraction maximum, % | ||||||||
| E2 | 18.13 ± 4.27 | 47.77 ± 10.03†A | 60.51 ± 6.42†A | 79.12 ± 8.20†A,C | 16.43 ± 3.45 | 49.40 ± 7.27‡A | 68.36 ± 3.56‡A,C | 70.19 ± 6.47‡A |
| PPT | 12.53 ± 6.87 | 31.11 ± 12.66 | 52.86 ± 8.11†A | 82.04 ± 5.91†A,C,M | 7.49 ± 4.03 | 25.58 ± 6.89‡A | 69.73 ± 5.45‡A,C | 62.65 ± 14.07‡A,C |
| DPN | 5.98 ± 5.73 | 11.92 ± 4.69 | 36.25 ± 4.47†A,C | 36.76 ± 17.30 | 16.43 ± 3.45 | 13.21 ± 4.12 | 39.60 ± 8.63‡A,C | 37.17 ± 7.97‡A,C |
| G1 | 9.06 ± 7.03 | 19.41 ± 9.49 | 44.02 ± 10.41†A | 47.87 ± 9.62†A | 0.56 ± 1.56 | 12.60 ± 3.54‡A | 56.78 ± 9.20‡A,C | 54.49 ± 8.94‡A,C |
| pD2 (−log M) | ||||||||
| E2 | 5.51 ± 0.01 | 6.41 ± 0.50 | 5.82 ± 0.07†A | 5.76 ± 0.14 | 5.50 ± 0.01 | 5.74 ± 0.04‡A | 5.73 ± 0.06‡A | 6.01 ± 0.36 |
| PPT | 5.99 ± 0.25 | 7.27 ± 0.76 | 7.6 ± 0.43†A | 8.91 ± 0.68†A | 5.85 ± 0.25 | 6.47 ± 0.53 | 8.63 ± 0.57‡A,C | 8.75 ± 0.83‡A,C |
| DPN | 5.83 ± 0.27 | 5.78 ± 0.10 | 7.45 ± 0.52†A,C | 7.26 ± 0.67 | 6.11 ± 0.61 | 6.37 ± 0.40 | 7.03 ± 0.55 | 6.24 ± 0.22 |
| G1 | 6.10 ± 0.53 | 6.17 ± 0.30 | 7.63 ± 0.49†C | 7.71 ± 0.60†C | 5.77 ± 0.14 | 6.98 ± 0.76 | 7.45 ± 0.47‡A | 6.57 ± 0.44 |
Data represent means ± SE; n = 7–11 rats/group.
ER, estrogen receptor; E2, estradiol; PPT, 4,4′,4"-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol; DPN, diarylpropionitrile.
P < 0.05, pregnant vs. virgin rats;
significantly different (P < 0.05) from corresponding measurements in the aorta (A), carotid artery (C), and mesenteric artery (M) of virgin rats;
significantly different (P < 0.05) from corresponding measurements in the aorta and carotid artery of pregnant rats.
Contribution of endothelial NOS, COX, and EDHF to ER-mediated relaxation.
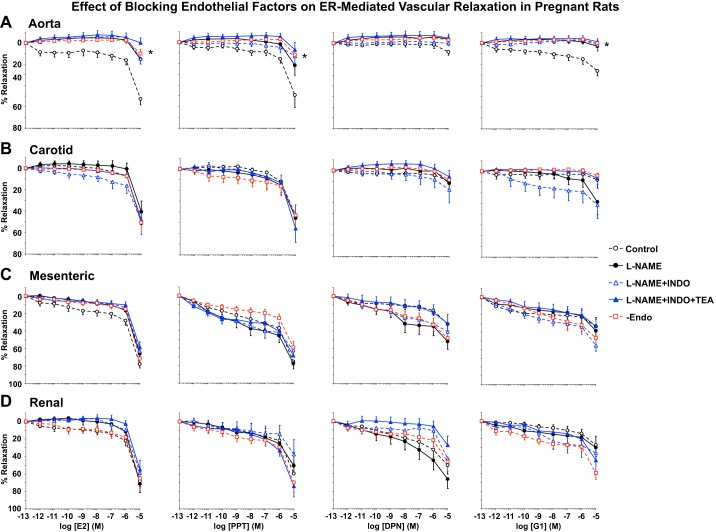
We have previously reported differences in the sensitivity of the ACh relaxation response to the NOS blocker l-NAME, COX inhibitor indomethacin, and the hyperpolarization pathway in different vascular beds of virgin rats (72) and in mesenteric vessels of virgin versus pregnant rats (49). In the present study, we assessed the role of endothelial vasodilators in ER agonist-induced relaxation of blood vessels of pregnant rats. Pretreatment with the NOS inhibitor l-NAME (3 × 10−4 M) for 15 min reduced E2-, PPT-, DPN-, and G1-induced relaxation of Phe-induced contraction in the aorta (Fig. 7A) but not in the carotid, mesenteric, or renal artery (Fig. 7, B–D, and Table 2). Additional treatment with the COX inhibitor indomethacin (10−5 M), EDHF inhibitor TEA (30 mM), or endothelium removal did not cause further inhibition of ER agonist-induced relaxation in any of the arteries tested (Fig. 7, A–D, and Table 2). Interestingly, in l-NAME + indomethacin + TEA-treated and endothelium-denuded vessels, maximum relaxation to most ER agonists remained greater in the mesenteric and renal artery than in the aorta and carotid artery, and E2- and PPT-induced relaxation was greater in the carotid artery than in the aorta (Table 2). Pretreatment with blockers of endothelium-derived vasodilators or endothelium removal did not affect ER agonist EC50 in any of the vessels tested (Table 2).
Fig. 7.
Effect of blockade of nitric oxide, prostglandins, and EDHF or endothelium removal (Endo) on the vascular relaxation effects of E2, PPT, DPN, and G1 in the aorta (A), carotid artery (B), mesenteric artery (C), and renal artery (D) of pregnant rats. Vascular segments from pregnant rats were either nontreated or pretreated with the nitric oxide inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M), cyclooxygenase inhibitor indomethacin (Indo; 10−5 M), and the hyperpolarization blocker tetraethylammonium (TEA; 30 mM) for 15 min or endothelium denuded. Vascular segments were then precontracted with submaximal concentrations of Phe. Increasing concentrations (10−12–10−5 M) of E2, PPT, DPN, and G1 were then added, and the relaxation response was measured. Data represent means ± SE; n = 7–11 rats/group. *Significantly different (P < 0.05) from corresponding measurement in control nontreated intact vessels.
Table 2.
Effect of blockade of nitric oxide (l-NAME), PGI2(indomethacin), and hyperpolarization (TEA) or endothelium removal on the maximum response and EC50 for ER agonist-induced relaxation in the thoracic aorta, carotid artery, mesenteric artery, and renal artery isolated from pregnant rats and precontracted with phenylephrine
| Maximum Relaxation, % |
pD2 (−log M) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aorta | Carotid artery | Mesenteric artery | Renal artery | Aorta | Carotid artery | Mesenteric artery | Renal artery | |
| E2 | 52.76 ± 5.45 | 51.49 ± 5.50 | 77.87 ± 4.65†A,C | 68.13 ± 6.19 | 6.24 ± 0.34 | 5.71 ± 0.04 | 6.16 ± 0.26 | 5.92 ± 0.13 |
| +l-NAME | 15.32 ± 4.44* | 40.59 ± 9.98†A | 66.13 ± 7.17†A,C | 71.32 ± 10.17†A | 5.73 ± 0.11 | 5.61 ± 0.04 | 5.97 ± 0.23 | 5.73 ± 0.07 |
| +l-NAME + Indo | 14.99 ± 5.12* | 49.89 ± 11.82†A | 63.33 ± 6.00†A | 58.78 ± 16.23†A | 5.53 ± 0.02 | 5.95 ± 0.18 | 5.75 ± 0.07 | 5.79 ± 0.16 |
| +l-NAME + Indo + TEA | 0.06 ± 4.72* | 48.34 ± 10.29†A | 58.45 ± 5.90*†A | 54.81 ± 10.47†A | ND | 5.65 ± 0.06 | 5.69 ± 0.07 | 5.61 ± 0.04 |
| +Endothelium removal | 10.12 ± 3.98* | 50.80 ± 9.58†A | 71.73 ± 6.70†A | 65.06 ± 9.61†A | 5.47 ± 0.06 | 5.66 ± 0.03 | 5.71 ± 0.06 | 5.82 ± 0.12 |
| PPT | 49.27 ± 11.35 | 45.89 ± 10.77 | 75.36 ± 4.54†A,C | 59.45 ± 11.15 | 5.92 ± 0.13 | 5.81 ± 0.10 | 7.21 ± 0.45 | 6.37 ± 0.28 |
| +l-NAME | 21.49 ± 9.36 | 45.73 ± 10.87 | 77.45 ± 6.40†A,C | 50.59 ± 15.26 | 5.99 ± 0.30 | 6.23 ± 0.34 | 7.56 ± 0.54 | 6.80 ± 0.43 |
| +L-NAME + Indo | 12.95 ± 8.03* | 42.91 ± 9.53†A | 62.55 ± 7.61†A | 37.52 ± 16.81 | 6.28 ± 0.49 | 6.23 ± 0.26 | 9.04 ± 0.71* | 7.04 ± 0.56 |
| +l-NAME + Indo + TEA | 6.96 ± 6.74* | 55.44 ± 13.09†A | 68.54 ± 7.51†A | 74.05 ± 12.19†A | 6.11 ± 0.49 | 6.08 ± 0.31 | 7.92 ± 0.86 | 6.59 ± 0.35 |
| +Endothelial removal | 11.76 ± 5.29* | 43.30 ± 12.46†A | 59.40 ± 6.79†A | 69.68 ± 11.00†A | 5.55 ± 0.03* | 6.98 ± 0.67 | 6.96 ± 0.66 | 7.82 ± 0.83 |
| DPN | 8.28 ± 2.95 | 10.20 ± 2.78 | 31.89 ± 8.03†A,C | 50.46 ± 9.87†A,C | 6.30 ± 0.50 | 6.90 ± 0.74 | 6.60 ± 0.64 | 6.70 ± 0.34 |
| +l-NAME | 0.00 ± 0.00* | 11.85 ± 7.49 | 51.83 ± 8.93†A,C | 66.06 ± 10.76†A,C | ND | 5.88 ± 0.20 | 7.78 ± 0.59 | 7.21 ± 0.57 |
| +l-NAME + Indo | 0.05 ± 2.44 | 18.00 ± 12.24 | 40.64 ± 8.87†A | 42.42 ± 14.71†A | ND | 6.59 ± 0.39 | 8.50 ± 0.79 | 6.47 ± 0.54 |
| +l-NAME + Indo + TEA | 0.00 ± 0.00* | 5.83 ± 5.85 | 31.31 ± 10.48†A | 27.29 ± 10.56†A | ND | 5.87 ± 0.11 | 7.01 ± 0.45 | 6.09 ± 0.22 |
| +Endothelial removal | 0.00 ± 0.00* | 8.95 ± 5.02 | 48.35 ± 8.97†A,C | 46.49 ± 10.82†A,C | ND | 5.79 ± 0.09 | 7.37 ± 0.46 | 6.61 ± 0.50 |
| G1 | 26.17 ± 4.35 | 8.10 ± 7.74 | 33.21 ± 9.97 | 27.22 ± 10.06 | 7.43 ± 0.66 | 5.81 ± 0.17 | 8.86 ± 1.03 | 6.23 ± 0.28 |
| +l-NAME | 3.08 ± 4.37* | 28.76 ± 11.90 | 38.59 ± 10.18†A | 30.10 ± 10.28†A | ND | 6.62 ± 0.48 | 7.12 ± 0.76 | 7.09 ± 0.84 |
| +l-NAME + Indo | 1.91 ± 5.37* | 31.49 ± 12.56†A | 55.45 ± 6.40†A | 36.23 ± 21.36 | ND | 8.12 ± 0.90* | 8.04 ± 0.56 | 8.35 ± 0.57* |
| +l-NAME + Indo + TEA | 0.00 ± 0.00* | 6.46 ± 5.98 | 33.30 ± 7.68†A,C | 44.73 ± 13.01†A,C | ND | 5.88 ± 0.22 | 7.11 ± 0.64 | 6.71 ± 0.63 |
| +Endothelium removal | 0.37 ± 2.42* | 4.23 ± 2.43 | 46.76 ± 10.49†A,C | 59.25 ± 7.18*†,C | ND | 5.66 ± 0.13 | 7.34 ± 0.52 | 7.70 ± 0.84 |
Data represent means ± SE; n = 7–11 rats/group. ND ND, not determined because of inability to generate sigmoidal curve and calculate the pD2 value due to negligible relaxation.
ER agonist response in Nω-nitro-l-arginine methyl ester (l-NAME), l-NAME + indomethacin (INDO), l-NAME + Indo + tetraethylammonium (TEA)-treated or endothelium-denuded arteries was significantly different (P < 0.05) from the control ER agonist response in intact arteries without blockers;
significantly different (P < 0.05) from corresponding measurements in the aorta and carotid artery.
E2-, PPT-, DPN-, and G1-induced relaxation of KCl contraction.
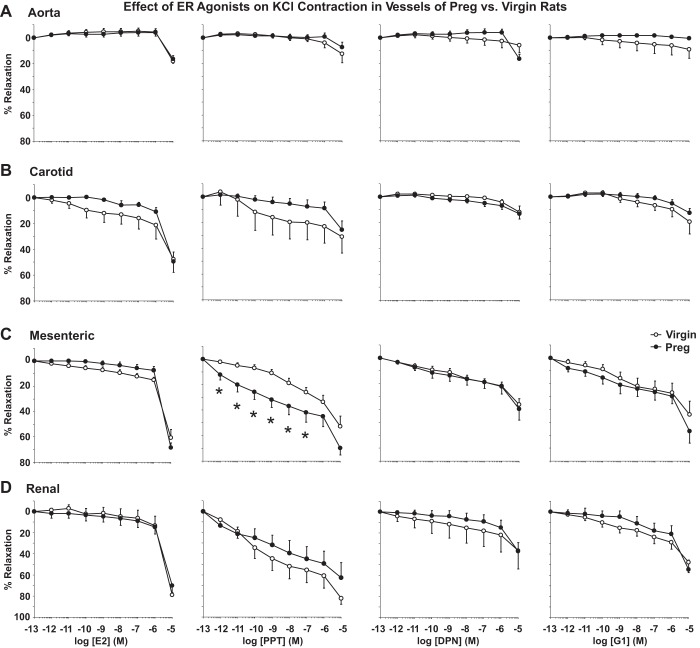
In endothelium-denuded vessels precontracted with high KCl (96 mM) depolarizing solution to induce Ca2+-dependent VSM contraction (61), ER agonists caused slight relaxation in the aorta (Fig. 8A), greater relaxation in the carotid artery (Fig. 8B), and marked relaxation in the mesenteric artery (Fig. 8C) and renal artery (Fig. 8D) of both virgin and pregnant rats (Table 1). ER agonists were more potent in causing relaxation of KCl contraction in the mesenteric and renal artery than in the aorta and carotid artery of virgin rats and, to some extent, pregnant rats (Table 1). PPT-induced relaxation of KCl contraction was greater in the mesenteric artery of pregnant versus virgin rats (Fig. 8C). DPN- and G1-induced inhibition of KCl contraction was not significantly different in the aorta, carotid artery, mesenteric artery, or renal artery of pregnant versus virgin rats (Fig. 8, A–D).
Fig. 8.
Differential effects of E2, PPT, DPN, and G1 on relaxation of the KCl-precontracted aorta (A), carotid artery (B), mesenteric artery (C), and renal artery (D) of virgin versus pregnant rats. Endothelium-denuded segments of the thoracic aorta, carotid artery, mesenteric artery, and renal artery were precontracted with high KCl (96 mM) depolarizing solution to induce a Ca2+-dependent contractile response in vascular smooth muscle. Increasing concentrations (10−12–10−5 M) of E2 (activator of all ERs), PPT (ERα agonist), DPN (ERβ agonist), or G1 (GPER agonist) were added, and the percent relaxation of KCl contraction was measured. Data represent means ± SE; n = 7–10 rats/group. *P < 0.05, pregnant vs. virgin rats.
DISCUSSION
The main findings of the present study were that 1) expression of ERα and ERβ are greater in the aorta and mesenteric artery and GPER is greater in aorta of pregnant versus virgin rats; 2) pregnancy-associated increases in vascular ERs are mainly in the intima and media, with no change in vessel structure; 3) E2- and PPT-induced relaxation of Phe-induced contraction is greater in the aorta and mesenteric artery, DPN-induced relaxation is not different, and G1-induced relaxation is greater in the aorta of pregnant versus virgin rats; 4) NOS blockade or endothelium removal reduce ER agonist-induced relaxation in the aorta, suggesting an endothelial mechanism, but not in other vessels, suggesting other endothelium-independent mechanisms; and 5) E2, PPT, DPN, and G1 cause relaxation of Ca2+-dependent KCl contraction, with PPT-induced relaxation greater in the mesenteric artery of pregnant versus virgin rats.
Pregnancy- and E2/ER-related adaptations in the uterus, BP, and body weight.
Sex hormones cause periodic changes in the uterine wall during the ovarian cycle and maintain adaptive structural and functional changes in the uterus during pregnancy. The present study supports pregnancy- and E2/ER-related adaptive changes in the uterus because 1) plasma E2 levels were higher in pregnant versus virgin rats; 2) the uterus weight without pups and placentae was approximately ninefold greater in pregnant versus virgin rats; 3) uterine sections were larger and the thickness of the uterine wall, endometrium, myometrium, and perimetrium was greater in pregnant versus virgin rats, supporting uterine wall remodeling; and 4) Western blots and immunohistochemistry revealed greater levels of ERα, ERβ, and GPER in the uterus of pregnant versus virgin rats.
In addition to its effects on the uterus, E2 could cause other physiological changes during pregnancy. Consistent with other reports (70, 84), BP was slightly lower in pregnant versus virgin rats. The body weight even without the uterus was greater in pregnant versus virgin rats, likely due to salt and water retention and increased blood volume and cardiac output. However, the lack of difference in kidney weight in pregnant versus virgin rats suggests that the changes in body weight during pregnancy may not involve renal tissue structural remodeling, an observation that needs to be further examined.
Pregnancy- and E2/ER-related adaptations in systemic vessels.
Previous studies have examined pregnancy-associated changes in the uteroplacental circulation (30, 66, 67) and the effects of E2/ERs on the uterine artery (10, 18, 75). Studies have also shown vasodilator effects of E2 in systemic vessels of nonpregnant animals (15, 24, 31, 53, 76). However, little is known regarding the effects of E2/ERs in the systemic circulation during pregnancy. In addition, while pregnancy-associated changes in the circulation could be partly due to increased E2 levels, the role of vascular ERs is unclear. If vascular ERs play a role in pregnancy-associated changes in the circulation, one would predict changes in the expression and vascular distribution of ERs and, consequently, alterations in vascular structure, vasodilator response to E2, and downstream post-ER signaling mechanisms.
Pregnancy-associated expression of ERα, ERβ, and GPER in specific blood vessels.
With regard to ER expression, previous studies have shown prominent ERα signals in the rat cerebral and coronary artery (16), greater amounts of ERβ than ERα in the baboon carotid artery (1), and GPER immunoreactivity in the carotid artery of Sprague-Dawley rats and the mesenteric artery of female mRen2.Lewis rats (6, 43). Because most of these studies examined one ER subtype in one blood vessel, it has been difficult to relate these findings to each other or to determine the contribution of a specific ER subtype to vasodilation in different vascular beds. Consistent with our recent report (72), we observed differential expression of ERs in the aorta, carotid artery, mesenteric artery, and renal artery of virgin rats, with prominent ERα and ERβ immunoreactivity in the renal artery and increased GPER immunoreactivity in the mesenteric and renal artery of virgin rats. Both Western blots and immunohistochemistry revealed greater amounts of ERα and ERβ in the aorta and mesenteric artery and GPER in the aorta of pregnant versus virgin rats. In addition, among vessels of pregnant rats, ERα and ERβ were greater in the aorta and mesenteric artery and GPER was greater in the aorta than in the carotid artery. These specific increases in vascular ERs may contribute to certain structural or functional adaptations in the vasculature to accommodate the hemodynamic changes during pregnancy, such that the aorta could bear the brunt of the increase in cardiac output, whereas the mesenteric arteries, which harbor a major portion of the systemic circulation, could accommodate the increase in blood volume.
Pregnancy-associated changes in the tissue distribution of vascular ERs.
In addition to changes in ER expression, pregnancy was associated with changes in the vascular tissue distribution of ERs, with increases in ERα and ERβ in the aorta and mesenteric artery being mainly in the intima and media, suggesting effects on the endothelium and VSM. This is consistent with our previous finding of ERα and ERβ in rat aortic VSM cells (47). Other studies have identified GPER in endothelial and VSM cells of the carotid artery of Sprague-Dawley rat and the mesenteric artery of mRen2.Lewis female rats (6, 43). In agreement with these reports, we detected GPER in the intima and media of the aorta, carotid artery, mesenteric artery, and renal artery of virgin rats and increased GPER in the intima and media of the aorta of pregnant rats, suggesting pregnancy-related effects on the endothelium and VSM.
Pregnancy and vascular structure and remodeling.
Studies in the uterine circulation have shown outward, expansive, and hypertrophic remodeling of uterine vessels during pregnancy (65) in part due to E2 effects on the VSM and extracellular matrix (65, 66). The present study in systemic vessels showed increases in ERα and ERβ not only in the intima and media of the aorta and mesenteric artery but also in the adventitia, suggesting potential effects on vessel structure. However, measurement of tissue weight and histological analysis of wall thickness showed no difference in the aorta, mesenteric artery, and renal artery of pregnant versus virgin rats, suggesting little pregnancy-related or ER-mediated structural remodeling in these vessels. In comparison, carotid artery weight and wall thickness were reduced in pregnant versus virgin rats. The carotid artery structural remodeling could be related to a redistribution of blood flow from the cephalic to the uteroplacental circulation to maintain adequate blood supply to the growing fetus. Still, the pregnancy-associated changes in carotid artery structure may not to be related to ERs as no changes in ER amount or distribution were observed in this vessel.
Pregnancy- and E2/ER-related adaptations in vascular function.
To test if the pregnancy-associated changes in ER expression affect ER-mediated vascular activity, we tested the vascular effects of different ER agonists: E2 (all ER subtypes) (53), PPT (ERα) (79, 81), DPN (ERβ) (27), and G1 (GPER) (21, 37). Consistent with our previous report (72), E2- and PPT-induced relaxation was renal artery > mesenteric artery > carotid artery > aorta of virgin rats, supporting a role of ERα in E2-induced relaxation. DPN caused less relaxation than PPT but was still renal artery > mesenteric artery > carotid artery > aorta of virgin rats, suggesting a partial role of ERβ in E2-induced vascular relaxation. These findings agree with reports that ERα and ERβ mediate rapid vasodilator effects of E2 (68, 93), E2 causes relaxation of the rat aorta largely via ERα (5), and PPT causes greater relaxation than DPN in the rat mesenteric artery (57, 72). In contrast, G1 caused small relaxation in all vessels of virgin rats, suggesting a little role for GPER in E2-induced vasodilation. This is consistent with our reports showing that G1 caused little relaxation in the aorta of female Sprague-Dawley rats (47, 72). Other studies have shown G1-induced vasodilation similar to E2 in the carotid and mesenteric artery of Sprague-Dawley rats (6, 25) and in the mesenteric artery of female mRen2.Lewis rats (43). Although we observed greater relaxation to G1 in the mesenteric and renal artery than in the aorta and carotid artery, it was still less than that of E2 or PPT. The differences in the vascular effects of G1/GPER may be related to the rat strain or regional differences in the arterial tree (87).
Importantly, the pregnancy-related changes in ER expression were associated with a distinct relaxation response in different vessels. In line with the increased ERα in the aorta and mesenteric artery, PPT caused greater relaxation in the aorta and mesenteric artery of pregnant versus virgin rats that was similar to that induced by E2, supporting a role for ERα in mediating the vasodilator effects of E2 during pregnancy. In addition, in line with the increased GPER levels in the aorta of pregnant rats, G1 caused greater relaxation in the aorta of pregnant versus virgin rats, highlighting a role of GPER in the adaptive aortic response during pregnancy. The pregnancy-associated changes in GPER-mediated aortic relaxation in the presence of relatively little changes in aortic structure and remodeling provide evidence for important ER-related functional changes rather than simply the mechanical changes that are generally perceived in most conduit arteries due to exposure to the hemodynamic changes during pregnancy.
It could be argued that the observed greater ER agonist-induced relaxation in smaller vessels (mesenteric and renal artery) compared with larger vessels (aorta and carotid artery) is related to more feasible diffusion of the compounds through the fewer cell layers in the small vessels. However, the enhanced PPT-, DPN-, and G1-induced relaxation in small vessels such as the renal artery of virgin rats was associated with specific increases in the protein amount and immunoreactivity of ERα, ERβ, and GPER, respectively. Furthermore, the vasorelaxation effects of ER agonists were enhanced in specific vessels of pregnant compared with virgin rats, and the enhanced relaxation coincided with parallel increases in the expression of specific ER subtypes, making it more likely that the observed differences in the responsiveness to ER agonists among different vessels and in vessels of pregnant versus virgin rats are due to differential expression/activity of vascular ER subtypes.
ER-mediated endothelium-dependent and -independent vasodilation during pregnancy.
ER-mediated vasodilation depends not only on ER amount but also on the post-ER signaling mechanisms in the endothelium and VSM. To test the role of the endothelium in ER-mediated relaxation, we first confirmed a functional endothelium and showed that ACh-induced relaxation was not different in vessels of pregnant versus virgin rats. In many vessels, ACh-induced relaxation involves the NO-cGMP pathway. In addition, our previous studies in the aorta, carotid artery, and renal artery of virgin rats confirmed that ACh-induced relaxation was associated with increased NO production and was inhibited by the NOS inhibitor l-NAME (72). In contrast, in the mesenteric artery, a large portion of ACh-induced relaxation remained in the presence of l-NAME and the COX inhibitor indomethacin and was abolished by the K+ channel blocker TEA, suggesting a role of a hyperpolarization pathway (49, 51).
E2 induces vasodilation through genomic and nongenomic endothelium-dependent and endothelium-independent pathways (53, 64). E2 activates endothelium-dependent NO-cGMP, PGI2-cAMP, and EDHF relaxation pathways in a blood vessel- and ER-specific manner (35, 78). E2 via ERα increases endothelial NOS expression and NO production in bovine pulmonary artery endothelial cells (38, 48, 80). In addition, G1-induced relaxation in the rat carotid artery is abolished by endothelium removal or l-NAME, suggesting GPER-mediated endothelium- and NO-dependent relaxation (6). E2 also increases COX-1 expression and PGI2 synthesis in pulmonary artery endothelial cells (32). The present experiments on the aorta of pregnant rats suggest that ER agonist-induced relaxation may involve endothelium-dependent NO because 1) E2-, PPT-, DPN-, and G1-induced relaxation was inhibited by l-NAME; 2) blockade of COX and PGI2 production using indomethacin and the hyperpolarization pathway using TEA did not cause further inhibition of ER agonist-induced aortic relaxation; and 3) endothelium removal abolished E2-, PPT-, DPN-, and G1-induced aortic relaxation. The endothelium-dependent relaxation effects of PPT and G1 are in line with the immunohistochemical detection of ERα and GPER in the intima of the aorta of pregnant rats. In comparison, ER-mediated relaxation in the carotid, mesenteric, and renal artery appears to mainly involve an endothelium-independent mechanism in VSM because 1) E2-, PPT-, DPN-, and G1-induced relaxation of the carotid, mesenteric, and renal artery was not blocked by l-NAME, indomethacin, TEA, or endothelium removal; 2) ER agonist-induced relaxation was still greater in the mesenteric and renal artery and in the carotid artery than in the aorta even after treatment with blockers of endothelium-derived vasodilators or endothelium removal; and 3) immunohistochemistry showed ERα, ERβ, and GPER in the tunica media and VSM of the aorta, carotid artery, mesenteric artery, and renal artery of pregnant rats. These data are consistent with reports that E2 causes relaxation of the endothelium-denuded aorta and coronary artery (13, 14, 31), and inhibits Phe- and PGF2α-induced contraction in VSM cells from the rat aorta and porcine coronary artery (60, 61).
ER-mediated inhibition of Ca2+-dependent contraction during pregnancy.
VSM contraction in response to α-adrenergic agonists such as Phe is triggered by increases in intracellular free Ca2+ concentration ([Ca2+]i) due to initial Ca2+ release from the intracellular stores and maintained Ca2+ influx from the extracellular space (4, 36). The observation that ER agonists decreased Phe-induced maintained contraction in endothelium-denuded vessels suggests inhibition of Ca2+ entry into VSM. In addition, high KCl causes vascular contraction mainly by inducing membrane depolarization and Ca2+ entry through voltage-gated channels (61). In the present study, ER agonists inhibited KCl-induced contraction in the endothelium-denuded carotid, mesenteric, and renal artery of virgin and pregnant rats, supporting inhibition of Ca2+ entry into VSM. This is consistent with reports that E2 decreased KCl-induced Ca2+ influx in the endothelium-denuded rat aorta, coronary artery, and rat aortic VSM cells (13, 22, 61) and inhibited Phe-induced capacitative Ca2+ influx in the rat aorta (8) and that E2, PPT, and DPN caused relaxation of KCl-induced contraction in endothelium-denuded cephalic, thoracic, and abdominal vessels (72) and vasodilation and decreased [Ca2+]i in mesenteric vessels of female rats (51). Importantly, PPT-induced relaxation of KCl-induced contraction was greater in the mesenteric artery of pregnant versus virgin rats, suggesting specific enhancement of ERα-mediated inhibition of Ca2+ entry mechanisms of vasoconstriction in the mesenteric circulation during pregnancy.
Mechanism of ER-mediated decrease in Ca2+ entry in VSM.
An important question is how ER agonists decrease Ca2+ entry into VSM. Some studies have suggested indirect mechanisms whereby ER agonists activate VSM K+ channels and cause VSM hyperpolarization, which, in turn, inhibits voltage-gated Ca2+ channels (2, 46, 85, 88, 91). This is unlikely because ER agonists inhibited KCl contraction, and the high KCl gradient should prevent outward movement of K+ through K+ channels. ER-mediated decrease in VSM Ca2+ entry could also involve adenylate cyclase/cAMP/PKA or guanylate cyclase/cGMP/PKG pathways (2, 17, 34, 45, 83, 88, 90). However, our recent study showed that the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) did not reverse ER agonist-induced relaxation or decreased [Ca2+]i in mesenteric vessels, suggesting little role for guanylate cyclase/cGMP/PKG (51). Alternatively, the ER-mediated decrease in Ca2+ entry may involve direct effects on VSM Ca2+ channels. This is consistent with reports that ER agonists decreased [Ca2+]i in mesenteric vessels of female rats preconstricted with high KCl and the L-type Ca2+ channel agonist Bay K 8644 (51) and that E2 caused relaxation of endothelium-denuded aortic rings precontracted with Bay K 8644 or high KCl that was not modified by K+ channel blockers, inhibited basal and Bay K 8644-stimulated l-type Ca2+ current with no effect on basal K+ current (7), decreased [Ca2+]i in porcine coronary artery and rat aortic VSM cells (60, 61), and inhibited voltage-dependent L-type Ca2+ current in cultured A7r5 rat aortic VSM cells (62, 92). It appears that ER agonist-induced inhibition of Ca2+ entry in blood vessels of pregnant rats involves both indirect and direct action on Ca2+ channels, and the mechanisms of ER-mediated decrease in VSM [Ca2+]i may vary depending on the blood vessel, the vascular region, and the animal species studied.
Limitations.
Other considerations include, first, although the ERβ amount and immunoreactivity in the endothelium and VSM of the aorta and mesenteric artery were greater in pregnant rats than in virgin rats, no differences in the vasodilator effects of the ERβ agonist DPN were observed. ERβ mediates both genomic and nongenomic vascular effects (68, 93), and while the lack of difference in the rapid vasodilator effects of DPN suggests no difference in ERβ-mediated nongenomic pathways, it does not rule out possible enhancement of ERβ-mediated genomic effects during pregnancy. Second, although no pregnancy-associated changes in ER amount/distribution were observed in the carotid artery, the E2- and PPT-induced maximum relaxation of Phe-induced contraction was enhanced in the carotid artery versus the aorta of pregnant rats treated with blockers of endothelium-derived vasodilators or endothelium denuded, and maximum relaxation of KCl contraction by all ER agonists was greater in the endothelium-denuded carotid artery versus the aorta of pregnant rats. Whether these findings are related to the observed changes in carotid artery histology and its potential remodeling remain to be examined. Third, maximal effects of E2 were observed at micromolar concentrations, which are higher than physiological nanomolar plasma E2 levels, and the observed small levels of plasma E2 even in pregnant rats. Because E2 is lipophilic, its plasma levels may not reflect its vascular tissue level, and prolonged exposure to small E2 concentrations in vivo could lead to gradual tissue accumulation that eventually reaches levels similar to those used in the acute studies. In this respect, ex vivo studies may require higher E2 concentrations to bind to plasma membrane lipids and both physiologically active and other ER variants. Fourth, splice variants or isoforms of ER have been described. Most ERα variants differ in their 5′-untranslated region, not in the coding sequence. The biological functions of ERα isoforms are unclear, but they may heterodimerize with full-length ERα and repress activation function (AF)-1-mediated activity. Additionally, surface membrane binding sites for E2 have been identified in cells transfected not only with full-length ER-α66 but also with truncated isoforms ER-α46 and ER-α36 (41). The localization of ERα isoforms in the plasma membrane may help to further elucidate the ERα nongenomic signaling mechanisms and the role of MAPK/ERK and phosphatidylinositol 3-kinase/Akt pathways (42). Multiple ERβ isoforms also exist as a result of either alternative splicing of the last coding exon 8, deletion of one or more coding exons, or alternative usage of untranslated exons in the 5′-region. Among ERβ isoforms, five full-length transcripts (ERβ1-ERβ5) have been reported in humans (28). Future studies should further examine the expression and distribution of different ER isoforms in different blood vessels and the potential changes in the expression of these isoforms during pregnancy. Finally, pregnancy is associated with increases not only in E2 but also progesterone. Progesterone receptors have been identified in the endothelium and VSM (53, 54), and progesterone induces genomic and nongenomic vascular effects (53, 64) and decreases [Ca2+]i (60), PKC (56), and Rho kinase in VSM (63). The pregnancy-associated changes in vascular progesterone receptors should be examined.
Conclusions and perspectives.
During pregnancy, vascular ERs exhibit regional changes in their expression, vasodilator activity, and signaling mechanisms with specific increases in ERα and ERβ in the endothelium and VSM of the aorta and mesenteric artery and GPER in the aorta. The pregnancy-associated increases in aortic and mesenteric artery ERα expression coupled with ERα-mediated endothelium-dependent relaxation in the aorta and endothelium-independent inhibition of Ca2+ entry in the mesenteric artery support a role of aortic and mesenteric arterial ERα in pregnancy-associated systemic vasodilation. GPER may contribute to aortic vasodilation via an endothelium-dependent mechanism, while the enhanced ERβ expression could mediate other genomic vascular effects during pregnancy. The observed changes in the expression/activity of ER subtypes in systemic arteries appear to be different from those reported in the local uterine circulation. A review of earlier studies showed that ERα ligands are more potent than ERβ ligands in increasing uterine blood flow in nonpregnant sheep, supporting the premise that E2-mediated uterine vasodilatation may be mediated more by ERα than ERβ (69). Similar to the present observations in the aorta and mesenteric artery, ER expression/activity also appears to be enhanced in the uterine artery during gestation, although the dominant ER subtype may be different. While some studies (11, 40) have shown intensive immunoreactive signals for both ERα and ERβ in uterine artery tissue sections from late-pregnant sheep, another study (69) has suggested that ERβ may be the main receptor subtype modulated in the uterine artery during gestation. In addition, a study (12) of the uterine circulation in pregnant women has shown that E2, PPT, and DPN caused significant relaxation of isolated myometrial arteries with relaxation of E2 > DPN > PPT, whereas G1 did not cause significant relaxation. These observations highlight the importance of further examining ER distribution, activity, and downstream signaling mechanisms in the systemic versus uterine circulation. Nevertheless, the data support the contention that pregnancy-associated changes in vascular ER distribution/activity in the systemic versus the uterine circulation could play a role in the regulation of regional blood flow and distribution of blood in the maternal circulation by promoting vasodilation, opposing vasoconstriction, and maintaining normotension during normal pregnancy. Future studies should investigate possible changes in vascular ERs in hypertensive pregnancy and the potential usefulness of ER agonists in promoting vasodilation and improving maternal hemodynamics and BP in preeclamptic pregnancies. Due to their vasodilator effects, specific ER agonists could reduce BP while avoiding the adverse effects of E2 on uterine and breast cancer, inflammation, and plasma lipids. In addition, specific ER agonists could modulate vascular ER activity in specific blood vessels without affecting other vessels in the systemic circulation.
GRANTS
This work was supported by National Institutes of Health Grants HL-65998, HL-98724, HL-111775, and HD-60702. K. Mata was a visiting scholar from the Department of Pathology, Faculty of Medicine of Ribeirão Preto, University of São Paulo (São Paulo, Brazil), and a recipient of a fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo Research Foundation (São Paulo, Brazil). W. Li was a visiting scholar from Tongji Hospital, Huazhong University of Science & Technology (Wuhan, Hubei Province, China), and a recipient of a scholarship from the China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.M., O.M.R., and R.A.K. conception and design of research; K.M.M., W.L., and O.M.R. performed experiments; K.M.M., W.L., O.M.R., W.T.S., L.A.O., and R.A.K. analyzed data; K.M.M., W.L., O.M.R., and R.A.K. interpreted results of experiments; K.M.M., W.L., O.M.R., and R.A.K. prepared figures; K.M.M. and R.A.K. drafted manuscript; K.M.M. and R.A.K. edited and revised manuscript; R.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. M. Q. Mazzuca for assistance in measurements of blood pressure and data analysis.
REFERENCES
- 1.Aavik E, du Toit D, Myburgh E, Frosen J, Hayry P. Estrogen receptor beta dominates in baboon carotid after endothelial denudation injury. Mol Cell Endocrinol 182: 91–98, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Alda JO, Valero MS, Pereboom D, Gros P, Garay RP. Endothelium-independent vasorelaxation by the selective alpha estrogen receptor agonist propyl pyrazole triol in rat aortic smooth muscle. J Pharm Pharmacol 61: 641–646, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 38: 730–735, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-α agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther 313: 1203–1208, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 298: H1055–H1061, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Cairrao E, Alvarez E, Carvas JM, Santos-Silva AJ, Verde I. Non-genomic vasorelaxant effects of 17β-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacol Sin 33: 615–624, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo C, Ceballos G, Rodriguez D, Villanueva C, Medina R, Lopez J, Mendez E, Castillo EF. Effects of estradiol on phenylephrine contractility associated with intracellular calcium release in rat aorta. Am J Physiol Cell Physiol 291: C1388–C1394, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res 87: E44–52, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension 56: 750–757, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology 145: 113–125, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran JJ, Nicholson C, Sweeney M, Charnock JC, Robson SC, Westwood M, Taggart MJ. Human uterine and placental arteries exhibit tissue-specific acute responses to 17β-estradiol and estrogen-receptor-specific agonists. Mol Hum Reprod 20: 433–441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews JK, Khalil RA. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol 19: 1034–1040, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol 26: 707–715, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca2+ entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 34: 931–936, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Dan P, Cheung JC, Scriven DR, Moore ED. Epitope-dependent localization of estrogen receptor-α, but not -β, in en face arterial endothelium. Am J Physiol Heart Circ Physiol 284: H1295–H1306, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol Heart Circ Physiol 272: H2765–H2773, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta C, Chen M, Zhang H, Yang S, Zhang L. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-α gene in ovine uterine arteries via heightened promoter methylation. Hypertension 60: 697–704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension 44: 789–795, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148: 3236–3245, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Freay AD, Curtis SW, Korach KS, Rubanyi GM. Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta. Role of nuclear estrogen receptor and Ca2+ uptake. Circ Res 81: 242–248, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Gaspar R, Foldesi I, Havass J, Marki A, Falkay G. Characterization of late-pregnant rat uterine contraction via the contractility ratio in vitro significance of α1-adrenoceptors. Life Sci 68: 1119–1129, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Gisclard V, Miller VM, Vanhoutte PM. Effect of 17β-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther 244: 19–22, 1988. [PubMed] [Google Scholar]
- 25.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17β-estradiol on function and expression of estrogen receptor α, estrogen receptor β, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49: 1358–1363, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206: 13–22, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Herrington DM, Braden GA, Williams JK, Morgan TM. Endothelial-dependent coronary vasomotor responsiveness in postmenopausal women with and without estrogen replacement therapy. Am J Cardiol 73: 951–952, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension 58: 1132–1139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17β-oestradiol in vitro. Br J Pharmacol 104: 1033–1037, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun SS, Chen Z, Pace MC, Shaul PW. Estrogen upregulates cyclooxygenase-1 gene expression in ovine fetal pulmonary artery endothelium. J Clin Invest 102: 176–183, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol 280: C34–C45, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Keung W, Chan ML, Ho EY, Vanhoutte PM, Man RY. Non-genomic activation of adenylyl cyclase and protein kinase G by 17β-estradiol in vascular smooth muscle of the rat superior mesenteric artery. Pharmacol Res 64: 509–516, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol 86: 1627–1642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalil RA, van Breemen C. Intracellular free calcium concentration/force relationship in rabbit inferior vena cava activated by norepinephrine and high K+. Pflugers Arch 416: 727–734, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Kleuser B, Malek D, Gust R, Pertz HH, Potteck H. 17β-Estradiol inhibits transforming growth factor-β signaling and function in breast cancer cells via activation of extracellular signal-regulated kinase through the G protein-coupled receptor 30. Mol Pharmacol 74: 1533–1543, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol Lung Cell Mol Physiol 273: L119–L126, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and -9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol 89: 370–385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-α and -β in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod 72: 530–537, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Lin AH, Li RW, Ho EY, Leung GP, Leung SW, Vanhoutte PM, Man RY. Differential ligand binding affinities of human estrogen receptor-α isoforms. PLoS One 8: e63199, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J, Wang ZY, Sun QY. ER-α36, a variant of ER-α, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One 5: e9013, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2 Lewis female rat. J Cardiovasc Pharmacol 57: 598–603, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am J Physiol Endocrinol Metab 305: E113–E118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81: 99–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor β contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol 577: 945–955, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem 26: 457–470, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 81: 355–362, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca2+]i in mesenteric microvessels of pregnant rats. Br J Pharmacol 169: 1335–1351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension 64: 632–643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzuca MQ, Mata KM, Li W, Rangan SS, Khalil RA. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]i in mesenteric microvessels of female rat. J Pharmacol Exp Ther 352: 291–304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol 588: 1997–2010, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90: 3F–6F, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86: 58–64, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minshall RD, Miyagawa K, Chadwick CC, Novy MJ, Hermsmeyer K. In vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J 12: 1419–1429, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Montgomery S, Shaw L, Pantelides N, Taggart M, Austin C. Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility. Br J Pharmacol 139: 1249–1253, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murata T, Dietrich HH, Xiang C, Dacey RG Jr. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke 44: 779–785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol 280: R87–R99, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Murphy JG, Khalil RA. Decreased [Ca2+]i during inhibition of coronary smooth muscle contraction by 17β-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther 291: 44–52, 1999. [PubMed] [Google Scholar]
- 61.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834–C844, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 17β-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol 294: 625–635, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290: C661–C668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation 21: 38–47, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992. [PubMed] [Google Scholar]
- 68.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90: 1087–1092, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Pastore MB, Jobe SO, Ramadoss J, Magness RR. Estrogen receptor-α and estrogen receptor-β in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med 30: 46–61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol Ther 65: 215–239, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol 109: 350–353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J Cardiovasc Pharmacol 62: 26–40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand 88: 639–646, 2009. [DOI] [PubMed] [Google Scholar]
- 75.Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vasc Pharmacol 38: 115–125, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99: 2429–2437, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serock MR, Wells AK, Khalil RA. Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov 3: 165–186, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Smiley DA, Khalil RA. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr Med Chem 16: 1863–1887, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 43: 4934–4947, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Sumi D, Ignarro LJ. Estrogen-related receptor α1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci USA 100: 14451–14456, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology 140: 800–804, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Tanbe AF, Khalil RA. Circulating and vascular bioactive factors during hypertension in pregnancy. Curr Bioact Comp 6: 60–75, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teoh H, Man RY. Enhanced relaxation of porcine coronary arteries after acute exposure to a physiological level of 17β-estradiol involves non-genomic mechanisms and the cyclic AMP cascade. Br J Pharmacol 129: 1739–1747, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol 24: 11–14, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors α and β mediating acute vasodilation of epicardial coronary arteries. Hypertension 49: 1364–1370, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 7: 79, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Drongelen J, Hooijmans CR, Lotgering FK, Smits P, Spaanderman ME. Adaptive changes of mesenteric arteries in pregnancy: a meta-analysis. Am J Physiol Heart Circ Physiol 303: H639–H657, 2012. [DOI] [PubMed] [Google Scholar]
- 88.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res 77: 936–942, 1995. [DOI] [PubMed] [Google Scholar]
- 89.Yener T, Turkkani Tunc A, Aslan H, Aytan H, Cantug Caliskan A. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat Histol Embryol 36: 75–77, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Yu X, Li F, Klussmann E, Stallone JN, Han G. G protein-coupled estrogen receptor 1 mediates relaxation of coronary arteries via cAMP/PKA-dependent activation of MLCP. Am J Physiol Endocrinol Metab 307: E398–E407, 2014. [DOI] [PubMed] [Google Scholar]
- 91.Yu X, Ma H, Barman SA, Liu AT, Sellers M, Stallone JN, Prossnitz ER, White RE, Han G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am J Physiol Endocrinol Metab 301: E882–E888, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang F, Ram JL, Standley PR, Sowers JR. 17β-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol Cell Physiol 266: C975–C980, 1994. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. [DOI] [PubMed] [Google Scholar]