Abstract
The cardiovascular response to xenobiotic particle exposure has been increasingly studied over the last two decades, producing an extraordinary scope and depth of research findings. With the flourishing of nanotechnology, the term “xenobiotic particles” has expanded to encompass not only air pollution particulate matter (PM) but also anthropogenic particles, such as engineered nanomaterials (ENMs). Historically, the majority of research in these fields has focused on pulmonary exposure and the adverse physiological effects associated with a host inflammatory response or direct particle-tissue interactions. Because these hypotheses can neither account entirely for the deleterious cardiovascular effects of xenobiotic particle exposure nor their time course, the case for substantial neurological involvement is apparent. Indeed, considerable evidence suggests that not only is neural involvement a significant contributor but also a reality that needs to be investigated more thoroughly when assessing xenobiotic particle toxicities. Therefore, the scope of this review is several-fold. First, we provide a brief overview of the major anatomical components of the central and peripheral nervous systems, giving consideration to the potential biologic targets affected by inhaled particles. Second, the autonomic arcs and mechanisms that may be involved are reviewed. Third, the cardiovascular outcomes following neurological responses are discussed. Lastly, unique problems, future risks, and hurdles associated with xenobiotic particle exposure are discussed. A better understanding of these neural issues may facilitate research that in conjunction with existing research, will ultimately prevent the untoward cardiovascular outcomes associated with PM exposures and/or identify safe ENMs for the advancement of human health.
Keywords: particulate matter, engineered nanomaterials, pulmonary system, nervous system, cardiovascular system
specific cardiac and/or vascular outcomes have been associated with pulmonary air pollution particulate matter (PM) exposures for more than two decades (104). Over the years, the definition of PM has evolved to identify specific size fractions: particles with a diameter <10 μm (“coarse” or PM10), particles with a diameter <2.5 μm (“fine” or PM2.5), and particles with a diameter <100 nm (“ultrafine” or PM0.1). This stratification illustrates the conventional wisdom that particle size is a significant determinant of not only respiratory tract deposition but also subsequent toxicity (4, 6, 103). During the later portion of this period, the nanotechnology field exploded and mandated a novel definition: anthropogenic particles with at least one dimension <100 nm (“nanoparticle”). Because PM and nanoparticles share some commonality in size (100 nm) and exposure route (lung), they may be considered “xenobiotic particles,” or particles that are foreign to the body and provoke untoward biologic responses. Whereas mass and anatomical location of pulmonary deposition is important to consider, the higher net surface area per mass concentration found in nanotechnologies or dose metric mass has emphasized the need of nanotoxicology. Xenobiotics have been studied increasingly over these decades to produce an extraordinary scope and depth of research models that incorporate in silico, in vitro, and in vivo approaches with cells, animals, and humans alike.
The scientific method encourages multiple hypotheses to investigate unknown phenomenon. Indeed, this approach has led to the rapid advancement of xenobiotic particle exposure and cardiovascular outcome-based research. It is possible that this rapidity is due to the sustained dominance of only three hypotheses (Fig. 1) in the collective field (17).
Fig. 1.
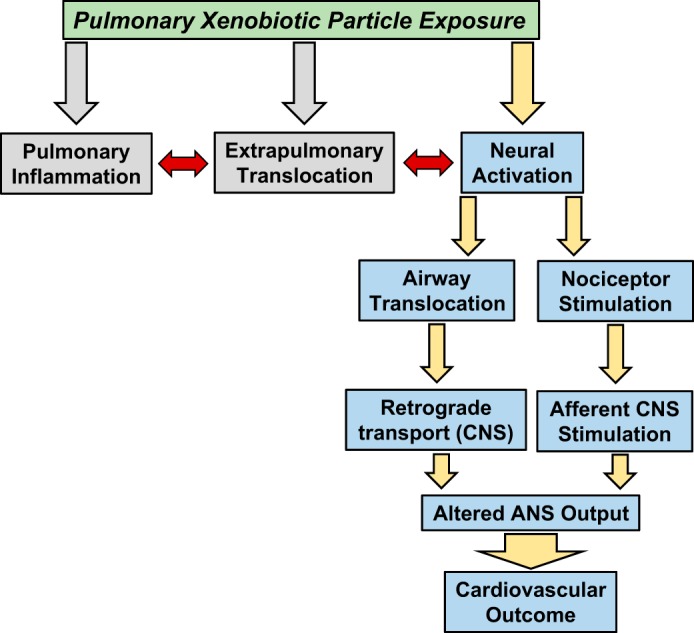
Biological pathways through which inhaled xenobiotic particles are hypothesized to exert extrapulmonary effects. The pathways for pulmonary inflammation and extrapulmonary translocation are not the foci of this review and therefore, are not expanded in the figure. CNS, central nervous system; ANS, autonomic nervous system.
1. Inflammation.
Inhaled xenobiotic particles induce an extrapulmonary response via inflammatory mechanisms that originate in the lung and “spill over” to the systemic circulation. This hypothesis has also been referred to as the “pulmonary shrapnel” hypothesis (Dr. Matthew J. Campen, University of New Mexico, personal communication).
2. Particle-tissue interaction.
Inhaled xenobiotic particles induce an extrapulmonary response by migrating from the lung and interacting directly with a systemic tissue.
3. Neural activation.
Inhaled xenobiotic particles induce an extrapulmonary response by interaction with the central nervous system (CNS) and/or stimulating an autonomic arc between the lung and systemic tissue.
Among these three, hypothesis 1 is, by far, the most widely reported in the literature, followed by hypothesis 2. Hypothesis 3 is the least commonly explored hypothesis in the field and in many research designs, is seldom considered or even mentioned. A recent search of the U.S. National Library of Medicine database (PubMed) provides evidence to support this position. Searching for “particulate matter” or “nanoparticle” combined with “inflammatory” or “neural” produced disparate results. Inflammatory registered 3,117 hits; whereas neural produced only 535. Synonyms, such as “autonomic,” produced similar but lesser results. Teleologically, this disparity implies that inflammation is the most important or required host response after pulmonary xenobiotic particle exposure. However, several lines of evidence refute this notion. First, many particles deposited in the lung are unable to exit the lung for a variety of reasons [e.g., size, solubility, clearance (120)]. Those particles that may exit the lung do not implicitly do so en masse (119). Given the ability for the mammalian physiology to tolerate far worse toxicants at higher concentrations [e.g., cigarette smoke, ozone, polycyclic aromatic hydrocarbons (PAHs), etc.], the likelihood that such a small number of transient particles would be solely responsible for all cardiovascular outcomes is remote. Second, the time course between pulmonary exposure and cardiovascular effect is typically acute (minutes to hours). The development of a robust host response or migration of particles from the lung does not occur within this time frame. Whereas neurological involvement is not only acutely invoked by xenobiotic exposures, it may ultimately expedite afferent signaling (43).
Because inflammation and/or direct particle-tissue interactions cannot entirely account for the cardiovascular responses that follow pulmonary particle exposures, the case for autonomic involvement should be apparent, especially in the early time periods following xenobiotic exposure (74). Indeed, strong evidence exists that elegantly reveals such autonomic responses in experimental animals (25, 52, 125) and humans (16, 85, 123) alike. With the consideration of the existence of such evidence, it is easy to conclude deductively that autonomic involvement may elucidate the unaccounted biological responses reported in controlled air pollution studies (74) (Fig. 2). Seminal research has identified the capacity of inhaled particles to gain access to the olfactory bulb (97, 99). The natural progression of this observation is that inhaled xenobiotics may interact with and/or alter CNS function. Indeed, the CNS, as a target tissue, has been well studied, and federally supported panels/workshops have been organized to understand this effect better (13). As these efforts matured, it became apparent that the cause-effect relationship did not end at the level of the CNS. Unfortunately and despite its importance in regulating and/or mediating most every physiological process, the link between the CNS and cardiovascular dysfunction after xenobiotic inhalation is not as well developed compared with other research branches investigated in this field.
Fig. 2.
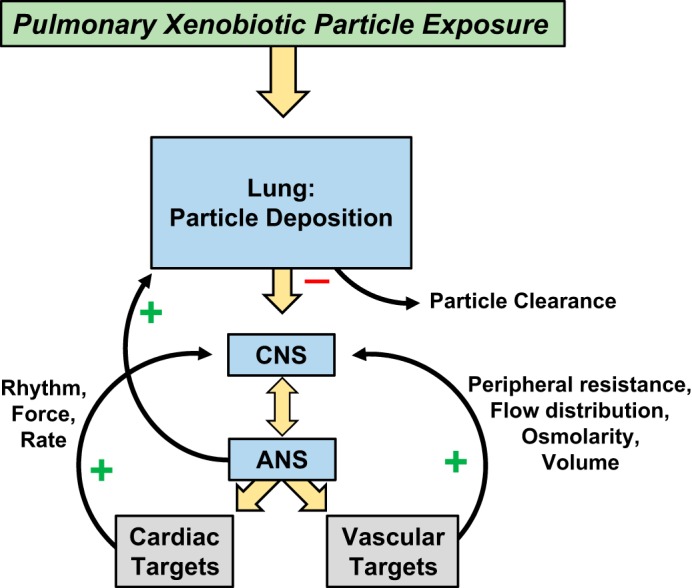
Cardiovascular targets under autonomic stimulatory or inhibitory influence after pulmonary xenobiotic particle exposures. Cardiac targets are under sympathetic and parasympathetic influence. Vascular targets are largely influenced by sympathetic connections.
As with all biological systems, autonomic arcs and influences are far from simple, and they may even interact with other mechanisms (Fig. 1). Additionally, it should be fully appreciated that xenobiotic particle exposures are not limited to the lung. Likewise, extrapulmonary effects are not limited to cardiovascular outcomes, and certainly, mechanistic interactions or redundancies exist. Because of the enormity of potential systemic responses, both independent and dependent, the framework of this review needs to be defined. Therefore, the purpose of this review is to provide a brief history and overview on the autonomic arcs and mechanisms that may be in play after pulmonary xenobiotic particle exposure in cardiovascular tissue. A secondary purpose is to identify how this understudied area can be enhanced to address unique problems, future risks, and hurdles associated with xenobiotic exposures. At this time and in our opinion, the area of greatest need of study/development is how an inhaled particle either stimulates an afferent pathway in the lung and/or alters CNS function characterized by efferent pathways that terminate in cardiovascular tissue.
NEURAL COMPONENTS
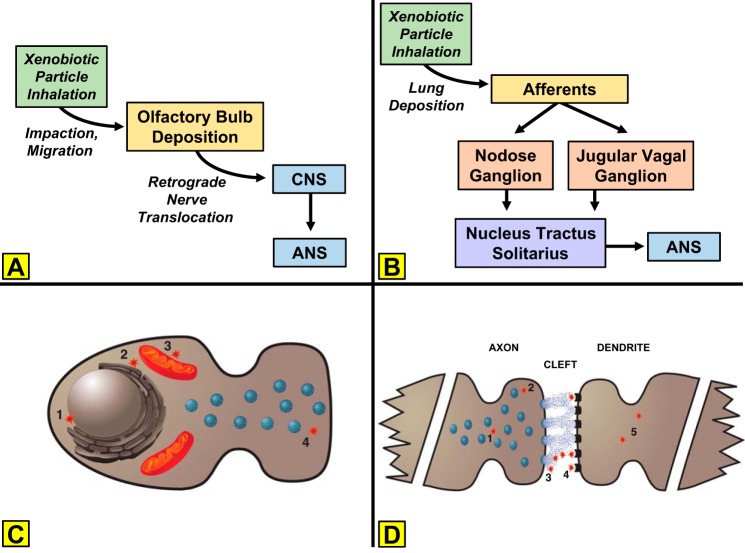
Although the short- and long-term consequences of xenobiotic exposure to the nervous system as a whole are important topics to be vetted, potential CNS and peripheral nervous system components postulated to be involved in the direct uptake of and acute dysfunction caused by inhaled PM, in general, have been the subject of a relatively narrow focus. These include the olfactory and trigeminal neuronal pathways (Fig. 3A) and receptor afferents (such as vagal C-fibers; Fig. 3B), both of which could impact the normal function of the autonomic nervous system (ANS). Credence can also be lent to indirect mechanisms of central dysfunction, which involve translocation of xenobiotics from pulmonary or extrapulmonary tissues to the brain via systemic circulation.
Fig. 3.
Autonomic pathways and cellular mechanisms through which inhaled xenobiotic particles act. A: inhaled particles impact in the upper airway, migrate to the olfactory bulb, and may ultimately translocate to the CNS to alter ANS output. B: alternatively, inhaled particles activate pulmonary afferents that project to ganglia of the nucleus tractus solitarius and alter ANS output. C: within a neuron, xenobiotic particles may alter function by interacting with the nucleus (1), endoplasmic reticulum (2), mitochondria (3), and/or vesicle trafficking/tethering (4). D: within the synapse, transmission may be influenced by interrupting vesicle trafficking (1), vesicle tethering and or neurotransmitter release (2), neurotransmitter binding in the synaptic cleft (3), occupying postsynaptic receptors (4), and/or altering dendrite membrane potentials (5).
Fundamentals of the ANS.
Briefly, the ANS is divided into two divisions: sympathetic and parasympathetic. The structures of both the sympathetic and parasympathetic pathways include preganglionic motor neurons stretching from their CNS origin at the brain stem or spinal cord to a nerve ganglion in the periphery. Here, these neurons form synaptic connections with postganglionic motor neurons (Fig. 3B). These fibers, in turn, innervate target tissues. Anatomically, the two divisions are noted to have separate origins yet reciprocal innervation on many tissues, including those of the cardiovascular system. The effects that are elicited are dependent on two major factors. First, sympathetic postganglionic fibers secrete the catecholamine norepinephrine (NE), whereas parasympathetic fibers secrete ACh (Fig. 3C). Second, NE and ACh rely on various adrenergic and cholinergic receptor populations, respectively, located at the tissues, which dependent on the subtype, can have stimulatory or inhibitory tissue effects (Fig. 3D). The general functions of these divisions are well-established canons in physiology, with the sympathetic division driving “fight-or-flight” responses that are dominant during body stress states and the parasympathetic division associated with restful states. Generally, sympathetic effects are driven by the increased release of NE. In addition, direct sympathetic stimulation of the adrenal gland results in a concomitant release of the analog catecholamine epinephrine. Actions by these catecholamines at the heart result in increased heart rate, conduction velocity, myocyte relaxation rate, and contractility. Cardiac stimulation of adrenergic receptors induces the activation of GTP-binding proteins that stimulate the production of cAMP by adenylyl cyclase, thus resulting in the activation of PKA. This kinase phosphorylates several proteins, including phospholamban, L-type calcium channels, ryanodine receptors, troponin I, and myosin-binding protein C (12). This cascade increases intracellular and sarcoplasmic reticulum levels of Ca2+ and plays an important role in excitation-contraction coupling. Throughout the vasculature, they promote generalized vasoconstriction, resulting in elevated total peripheral resistance (sans specific receptor populations of the skeletal and cardiac muscle tissues where vasodilation is the dominating effect). The overall response leads to an increase in blood pressure. Conversely, at rest, parasympathetic tone outweighs sympathetic. The elevated release of acetylcholine results in reduced heart rate at the heart and vasodilation in the periphery. These effects occur in response to stimulation of muscarinic receptors following release of ACh, which is involved in the regulation of G-protein-coupled, inward-rectifying K+ channels expressed in atrial, sinoatrial, and atrioventricular node cells. ACh also inhibits cAMP-dependent ion channel responses by directly inhibiting adenylyl cyclase or by stimulating phosphodiesterase 2 via production of the vasodilator nitric oxide and cGMP (51). Together with reduced sympathetic tone/NE release, the overall cardiovascular effects include reduced blood pressure and total peripheral resistance.
The divisions of the ANS tend to act synergistically to control cardiovascular mechanisms required to maintain homeostasis. The presence of receptor afferents innervating the aorta and carotid arteries and the respiratory tract provides important input to the cardiopulmonary control centers found in the brain stem. These receptors are sensitive to nociceptive, mechanical, chemical, and biological stimuli and play a crucial role in cardiovascular function via sympathetic- and vagal-feedback mechanisms. The nociceptive-reactive C-fibers induce protective mechanisms, such as bronchial vasodilation, to increase airway blood flow, bradycardia, and reduced contractility (118). Exposure to inhaled PM has been shown to result in dysfunction of these feedback mechanisms. Both direct, inhaled PM exposure and oxidative stress and inflammation (due to PM exposure) in lung tissues cause autonomic dysfunction that is secondary to stimulation of the pulmonary nerve receptors. Compounding this issue are the findings that autonomic dysregulation can itself cause cardiac oxidative stress (31).
Fortunately, systemic control of vital functions does not occur in a vacuum, and compensatory mechanisms ebb and flow over time; however, these safeguards make the identification of physiological perturbations much more difficult to identify. Above, we have described the influence of sympathetic innervation unilaterally from the lung to the cardiovascular system. It is important to keep in mind sympathetic innervation of the lung. Elevated sympathetic activity may lead to bronchiolar dilation and an increased respiratory rate, increasing xenobiotic exposure, thus exacerbating the cycle.
XENOBIOTIC PARTICLES
Exposure models.
Historically, the respiratory tract is deemed to be the major entry site for PM. This has been attributed to environmental airborne pollution exposures and occupational nanoparticulate exposures. These particles encompass a range of sizes as described above, which result in unique depositions throughout the pulmonary system, each with a differing physiological effect (113). Larger course particles have been found to deposit within the nasal and tracheobronchial passages, whereas fine matter deposits throughout the lung. Lastly, ultrafine and nanosized particles deposit preferentially within the alveolar regions. This is of specific relevance to variations in pulmonary receptor populations, neurological input, alveolar gas exchange, and particle translocation. Within the airways, the three major types of receptors are distributed differentially and respond to diverse stimuli: C-nerve fibers, rapidly adapting pulmonary receptors (RARs), and slowly adapting pulmonary receptors (SARs). C-Nerve fibers are present throughout the respiratory tract and initiate chemoreflexes, resulting in cough, bronchoconstriction, and dyspnea in response to chemical irritants (122). Similarly, RARs are located within the larynx, trachea, and bronchi and respond to both mechanical and chemical irritation. Lastly, the lower airways are the only region containing the stretch receptors referred to as SARs, which are characterized by a lower sensitivity to environmental agents than C-fibers and RARs, but nonetheless, they play an important role in controlling respiratory sinus arrhythmia, as well as triggering the cough reflex (64). Given this differential distribution of nerve fibers, as well as particle deposition, it is attractive to speculate that these segmental differences may account for observed differential cardiovascular responses to inhaled xenobiotics.
Whereas there is little doubt that xenoparticles reach all regions of the airway and that exposure results in deleterious effects on the cardiovascular system, there is little data to explain how inhaled particulates influence autonomic nerve activity in this regard. Evidence leads us to conclude that xenoparticles initiate inflammatory responses throughout the respiratory tract, and these processes can affect epithelial and nervous tissues. Indeed, the inflammatory processes occurring in response to ultrafine particles are accompanied by stimulation of mast cells as well as C-fibers (93). Stimulation of these vagal afferents could be linked to autonomic dysfunction. Exposure to concentrated ambient air particulates or single wall carbon nanotubes can induce changes in baroreceptor reflex sensitivity (10) and control (76). Of course, to affect a change requires an anatomical route linking these C-fibers to cardiovascular control mechanisms. A major candidate pathway includes the jugular and nodose ganglia, which receive input from the pulmonary C-fiber populations and project to the cardiovascular control center in the medulla oblongata (48, 55, 112), including the solitary nucleus (Sol) and paratrigeminal nucleus (Pa5) (8, 86, 110, 126) (Fig. 3B). Recently, it was shown that inhalation of ultrafine titanium dioxide and associated elevation in synthesis of the neurotransmitter substance P (SP) in the nodose can alter heart rate and blood pressure responses to adrenergic stimulation (62). However, due to the lack of supporting evidence, the extent of central control over this response requires further analysis. Vagal afferents from the nodose innervate and synapse with SP-immunoreactive structures in the nucleus of the tractus solitarius (66), which is also known to receive input from carotid baro- and chemoreceptors (88) and plays a role in cardiovascular reflexes. Interestingly, a spatial relationship between SP-positive paracellular arborizations and nodose cell soma has also been reported (65, 66). Furthermore, intratracheally instilled microspheres in the 20- to 200-nm range can be taken up and translocated to the nodose and jugular ganglia (60). Potentially, pulmonary C-fibers could form synaptic junctions with baroreceptor traffic and modulate cardiovascular reflexes. Further tract-tracing studies, electron microscopic analyzation, and inhalation experimentation are required to support this, along with differentiating the roles of the jugular and nodose ganglia. Finally, the impact of intratracheal xenobiotic-caused dysfunction on Sol and Pa5 control of cardiovascular homeostasis is of particular importance. In their elegant study using anterograde trans-synaptic viral tracing, McGovern et al. (86) show polysynaptic pathways leading from these medullary areas to various brain stem, thalamic, and hypothalamic areas associated with autonomic (Sol) and somatosensory (Pa5) control (28–30, 34–37, 117, 130, 131). Therefore, dysfunction caused by xenobiotic particle uptake or perturbation of C-fiber signaling of these major regulatory centers could have potentially severe cardiovascular consequences.
In some exposure models (intratracheal), the intranasal cavity is bypassed, eliminating the possibility of olfactory involvement. However, there is clear evidence that a direct route of entry into the CNS can occur from this location (Fig. 3A). Translocation to the CNS via neuronal retrograde transport along the olfactory nerve was described as early as the 1940s for 30 nm poliovirus applied intranasally into primates (58). More recently, ultrafine and nano-sized particles have also been shown to translocate via the olfactory bulb following inhalation (63, 97). Significant concentrations of manganese oxide, titanium dioxide, and iron oxide have been detected in the striatum, frontal cortex, thalamus, hippocampus, cerebellum, and brain stem, thus showing that the trans-synaptic transport via the olfactory neuronal pathway is an efficient route for material translocation of inhaled ultrafine particles into the CNS (42). Separately, uptake and translocation of manganese are also demonstrated by the trigeminal nerve (77), which includes afferents innervating nasal mucosa; however, further analysis of this pathway's importance is required. In addition, it has been estimated that 20% of ultrafine PM deposited in the olfactory mucosa translocates to the olfactory bulb (97), whereas reports of 20–50% of pulmonary deposition remain after clearance (41, 73, 94, 101). Similarly, PM may use a paracellular route via the ethmoid bone and into the cerebrospinal fluid (61). Therefore, xenobiotic particles may travel via retrograde transport to major regulatory sites within the CNS without the need to cross the blood brain barrier (BBB). Autonomic dysregulation of cardiovascular control due to this nose-to-brain route of translocation is a possible outcome, but as mentioned earlier, further studies are necessary to corroborate this hypothesis.
Yet another pathway of CNS infiltration by xenobiotics is that of translocation via the blood circulation. Size is important, as subcutaneous injection of silver nanoparticles, but not microparticles, results in translocation to blood, kidney, liver, spleen, brain, and lung (117). Generally, smaller inhaled nanoparticles were able to translocate and accumulate in secondary target organs, including the brain, with greater efficiency than larger ones (71). As the majority of neurons is located within the brain, a contentious topic is whether the brain is protected behind the BBB from particulate translocation. This barrier protects the CNS and restricts the movement of most substances from the bloodstream into the brain. It is formed by capillary endothelial cells joined together by tight junctions and adjacent astrocytic processes. Passage across the BBB thus requires lipid-mediated simple diffusion, receptor-mediated endocytosis, diffusion through an aqueous channel, or active transport. Since the tight junctions have gaps of only 4–6 nm, paracellular transport is not likely; however, nanoparticles could disrupt tight junctions (32). Rather, transcellular transport is more likely (67). Nevertheless, recent studies have shown that intraperitoneal, intravenous, intracarotid, or intracerebroventricular injections of metal nanoparticles lead to brain edema within 24 h of exposure by disrupting membrane integrity and reducing neurotransmitter levels (106, 108). In a similar study, intraperitoneal administration of metal nanoparticles had minimal effects on BBB function. Li et al. (79) further demonstrate that once/wk for 4 consecutive wk, intratracheally instilled titanium dioxide might pass through the BBB, resulting in oxidative stress brain insult. Nevertheless, it is known that the tight junctions associated with the BBB can be opened only to a limited extent (2); thus only PM with dimensions smaller than ∼20 nm can exploit this pathway to penetrate into the brain (109), and much research has been devoted to engineered nanoparticles with surfactant coatings designed to enhance passage across the BBB for medicinal purposes. Therefore, not only size but also chemical and electrostatic characteristics play a role in BBB infiltration. For example, in vitro, cationized nanoparticles increase endothelial cell permeability and translocate more readily than neutral or anionic nanoparticles (45, 50). In vivo data support this finding, as well as demonstrate that high concentrations of anionic nanoparticles can also translocate across the BBB (69, 81). Additionally, particle translocation to the brain may occur at sites where the BBB is absent, such as the circumventricular organs or in cases where the integrity of the BBB is compromised by pathological events, such as inflammation or neurodegenerative diseases (95). As with other biological membranes, the physicochemical properties of the nanoparticles influence entry and clearance by the vasculature associated with this compartment (70). However, it is also important to note that the systemic toxicological impact of xenobiotic particles is a multidimensional continuum, dependent not only on the physicochemical properties of the toxicant but also on the route and duration of exposure.
The relative toxicity of xenobiotic particles may also be affected by their surface-coating constituents. Specifically, increasing evidence implicates particle-bound endotoxin, a component of the cell wall of Gram-negative bacteria, and trace metals in ambient PM toxicity (83). Leaching out or dissolution of adsorbed trace elements, including semiquinones and PAHs from combustion processes, has been associated with an increasing incidence of respiratory complications (111). The formation of a protein adsorption layer on the surface of nanomaterials introduced in a physiological environment may also influence their bioactivity and toxicity (105).
Particle targets.
With respect to particulate targets of nerve anatomy and physiology, there are numerous sites where particles may influence neuronal activity and have dire consequences on neuronal signaling circuitry, neurotransmitter synthesis, release, and neuronal viability, which may be impacted severely and irreversibly. Within neurons, xenobiotic particles have been shown to interact with cytoskeletal elements and intracellular organelles, ultimately affecting their function, possibly resulting in apoptosis (3, 49, 107, 124). Evidence exists of DNA damage, including single-and double-strand breaks and DNA-protein crosslinks (72); altered overall DNA content (11); as well as mitochondrial DNA methylation (18), following exposure to xenobiotic particles. Furthermore, xenobiotic particles may penetrate to the endoplasmic reticulum and trigger the unfolded protein response, a signaling cascade initiated by the intracellular accumulation of misfolded proteins, as well as oxidative stress (129), a response that may also involve the mitochondria (49, 78, 107, 114) (Fig. 3C).
Within the synapse, xenobiotic particle penetration may influence neuronal transmission by interrupting vesicle trafficking, vesicle tethering, and/or neurotransmitter release (54). At the synaptic cleft, as well as the postsynaptic neuron, neurotransmitter binding to its cognate receptor may be reduced (40), whereas dendrite membrane potentials may also be affected.
Physical exchanges with quanta (the number of neurotransmitter molecules released in a vesicle), postsynaptic receptors, and ionic channels may lead to a decrease in neurotransmitter bioavailability due to particle-quanta binding or physical barrier of the postsynaptic receptor or ionic channels due to particle interactions at the active site (Fig. 3D). Other reasonable outcomes include alterations to neural output, such as increases or decreases in firing rate, neuronal recruitment, and receptor sensitization/excitation.
Neurotransmitter inhibition.
Although not providing direct evidence of xenobiotic translocation, studies of intraperitoneally injected gold nanoparticles demonstrate the ability of nanoparticle exposure to influence enzymatic activity within the brain and reduce serotonin and dopamine levels (108). Furthermore, neuronal remodeling, apoptosis, and autophagy have been described after metal nanoparticle translocation from the periphery across the BBB associated with their high-redox potentials (39). Many in vitro studies have explored selective neural toxicities behind the BBB (14, 82).
Within the CNS, neural communication, memory formation, and learning are mediated primarily by glutamatergic neurons. The central role played by this subset of neurons in excitotoxicity, their prevalence in the CNS, and their vulnerability to oxidative stress make them a likely target for xenobiotic particle toxicity. Glutamate is the main excitatory neurotransmitter within the brain, with almost 85% of the synapses in the cortex glutamatergic (15). Mice exposed to repeated nasal administration of titanium dioxide nanoparticles showed an increase in glutamate synthesis and phosphate-glutaminase activity, as well as a significant reduction in glutamine and glutamine synthetase levels. The associated disturbance of the glutamate-glutamine biosynthetic pathway may cause accumulation of glutamate in the extracellular compartments and lead to excitotoxicity (128). In vitro cultures of hippocampal slices exposed to nanoscale particulate urban air pollutants (<200 nm) showed an increased neurotoxicity by the glutamatergic agonist N-methyl-d-aspartic acid, whereas the glutamate receptor subunit (glutamate receptors of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid subunit 1) was decreased significantly (90). Nasal instillation of titanium dioxide nanoparticles led to a 25–30% loss of neuronal pyramidal cells in the Cornu Ammonis area 1 region (CA1) and in the dentate gyrus associated with morphological and functional changes in the hippocampus (121). Furthermore, a 35% loss of glutamatergic neurons and impaired neurite growth of murine hippocampal slice cultures were seen following exposure to re-aerosolized, ambient nanoparticulate matter (90). Chen and his colleagues (33) reported that multi-wall carbon nanotubes markedly inhibited hippocampal CA1 and CA3 glutamatergic synaptic transmission in vitro, with a decrease in the mean amplitude of excitatory postsynaptic currents within regions of the hippocampus associated with context-specific memory retrieval.
CARDIOVASCULAR OUTCOMES
Heart rate variability (HRV) is a noninvasive technique used to assess autonomic impact on the cardiovascular system. Historically, it has been shown to be a predictor of mortality associated with several conditions, such as myocardial infarction, congestive heart failure, and postcardiac transplants. The theory that exposure to ambient PM may alter the autonomic influence on the heart has led research toward using HRV and ECG morphology analyses as tools to determine the possible adverse consequences of particle exposure (78, 95) (Fig. 2). HRV is defined as the degree of difference in the intervals between successive heartbeats and is an accurate indicator of sympathovagal balance (76). A decrease in HRV is indicative of a decreased vagal tone and a sympathetic dominance. On the other hand, an increase in HRV indicates a dominance of the parasympathetic nervous system on heart rate. Previous studies have linked PM exposure to an increase in nonconducted P-wave arrhythmias (44), QT interval prolongation (80, 127), and pathological changes in T-wave amplitude (53). Particle inhalation may lead to a systemic sympathetic dominance (26) and a decreased parasympathetic cardiac vagal tone, thus decreasing HRV measurements and increasing myocardial vulnerability by triggering episodes of cardiac arrhythmia and ectopic beats, aggravating myocardial ischemia, or altering the dynamics of cardiac repolarization (80). These decreases in HRV may be evident up to 14 h after exposure (27). Therefore, it is conceivable that the skewing of the associated sympathovagal balance is a likely pathophysiological mechanism through which inhaled PM may affect specific homeostatic components and increase cardiovascular morbidity (47). Acute human particulate exposures limited to 2 h, at controlled, lower concentrations, reduced reports of coronary arrhythmias (75).
In the periphery, inhaled particles may also interact with specific receptors known as transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 1, present in the respiratory airways and the parenchymal layer of the lungs, causing an increase in parasympathetic tone and HRV (47, 52). Activation of the TRP channels has been implicated further as an initiating trigger between the lung and the nodose ganglion, leading to cardiovascular dysfunction (62). Inhalation of the mucosal irritant acrolein, an unsaturated aldehyde present in cigarette smoke and diesel exhaust, has been correlated with an increase in heart rate, blood pressure, breathing rate, and the HRV parameter low frequency, indicating a sympathetic dominance (100).
Systemic blood pressure is dependent on cardiac output, as well as total peripheral resistance; the influence of xenobiotic particle exposure on the underlying mechanisms of these products is robust. Whereas studies of neurological dysfunction impairing the resistance vasculature remain limited, our work has indicated that inhalation of titanium dioxide nanoparticles leads to a perturbation of physiologic alpha-adrenergic receptor sensitivity, inducing a persistent, sympathetically mediated arteriolar dysfunction (68). Because maintenance of systemic blood pressure is largely influenced by peripheral vascular resistance, the need to evaluate the resistance vasculature directly becomes evident. Assessments, including telemetry-based techniques and methodologies, may elegantly compliment a temporal facet associated with the microvascular alterations described within the literature.
FUTURE DIRECTIONS/STUDIES
The deleterious biological effects of PM exposure on the nervous system require further and more thorough investigation. The paucity of information and contradictory findings associated with the neurotoxic effects of exposure is due, in great part, to the heterogeneous interlaboratory experimental conditions and models used. The lack of a generally acceptable model for PM hazard identification mandates an empirical and methodical approach for the risk assessment (46). Nonetheless, accumulating evidence suggests that unwarranted exposure to PM may negatively impact the CNS and contribute to CNS morbidity (24).
Interactions among mechanisms.
A main focus of this review has been neural interactions and output in response to PM exposure. However, as there is limited direct evidence of particle translocation to the brain or particle deposition within a nerve and given that the temporal association of these interactions has yet to be identified, a combination of hypotheses (inflammation, direct interaction, and neural influence) is most likely. In this case, acute neural inflammation, due to direct particle interactions, may influence sympathetic activity, whereas heightened systemic inflammation (initiated via pulmonary inflammation after exposure) builds, increasing permeability and permitting particle translocation from the pulmonary system. These events would all individually and cumulatively impact the cardiovascular system, with an initial alteration in sympathetic activity, followed by an inflammatory and oxidative storm, culminating in long-term consequences associated with systemic particle deposition.
Furthermore, whereas the theory of neural retrograde transport from the olfactory pathway or pulmonary system is plausible, equally so is the concept of material translocation through the blood or lymph to the brain, leading to neurological outcomes (42).
Neurotoxicity.
The main mechanisms of xenobiotic particle neurotoxicity seem to be associated with oxidative stress and cellular neuroinflammation (15), in addition to cytotoxicity via dysregulation of intracellular ionic milieu balance and subsequent activation of the inflammasome (92). Systemically, in addition to being a byproduct of inflammation, reactive oxygen species (ROS) may be produced from mitochondrial dysfunction of the electron transport chain. This may be related to preferential accumulation of PM within the mitochondria (96) and dysregulation of the respiratory chain complexes I and III in rat brain tissue, leading to an increased generation of ROS (38). These mitochondrial alterations may be long lasting with generational consequences (114).
Within the CNS, damage by means of oxidative stress occurs primarily due to microglial cells (84). Microglia are cells derived from peripheral myeloid cells and make up ∼12% of cells in the brain (14). In response to xenobiotic agents, microglial cells may enter an overactive state and initiate an NADPH oxidase-dependent oxidative burst that leads to the production and accumulation of superoxide anions and ROS. These highly reactive free radicals diffuse from microglial cells and damage the proteins, lipids, and DNA of nearby cells (14). Chronic activation via prolonged exposure to ambient PM has been linked to a neurodegenerative-like phenotype characterized by the accumulation of non-neuritic plaques and formation of neurofibrillary tangles in cortical white matter and subpial regions (19).
Alternate routes.
Whereas the majority of this review focuses on inhalation and pulmonary routes of exposure, given the future product and exposure potential within nanotechnology, we would be remiss not to touch briefly on the alternate routes of nanomaterial exposure.
Further evidence in support of neuronal retrograde transport is that of ocular exposure. The eye is gaining more attention as a possible therapeutic avenue in nanomedicine. The use of nanodrug delivery devices and/or implanting a nanoparticle scaffold are viable treatment options for many ophthalmic conditions (91). Whereas otherwise segregated from the remainder of the body, due to several physiological barriers, there is a singular unifying optic nerve. Similar to the olfactory nerve transmission of information from the nasal passages to the CNS, this nerve is an avenue for further neuronal transport of xenobiotic particles. Nanoparticles in the range of 20–200 nm have been shown to be retained following periocular administration for at least 2 mo, whereas smaller nanoparticles were cleared by the blood and/or lymphatic circulations and localized to organs of the reticuloendothelial system, such as the spleen and the liver (5). This persistence of nanoparticles within specific ocular compartments may allow subsequent and/or chronic translocation into the CNS via retrograde axonal transport within the ocular nerve, potentially leading to long-term neurological dysfunction, even at the low concentrations achieved via this route of exposure.
Percutaneous incorporation of nanomaterials requires a more thorough evaluation, since few and contradictory findings are currently available. Based on the physicochemical properties of the nanomaterials, four pathways of entry across the integumentary system have been identified—intercellular, transcellular, and two transappendageal—via hair follicles and sweat glands (89). Given the physical impedance provided by the epidermal corneal layer, small (<600 Da) molecules may be able to penetrate the skin passively (9). Factors affecting the ability of nanomaterials to penetrate the skin include disruption of the skin barrier, skin pathology, chemical agents, and muscle flexion. Subsequent translocation of dermally administered nanoparticles may affect nerve terminal branches within the epidermal layer. Additionally, given the high population of dermal nociceptors, material may not have to translocate but simply irritate these receptors to initiate local and systemic neurological effects (57). Nonetheless, the dermal concentration of xenobiotic particles necessary to produce such a significant physiological effect is high and may occur only in prolonged exposures.
Neurological disorders.
Chronic or repeated exposures of high-dose xenobiotic mixtures, such as those reported in severely polluted urban areas (Mexico City or Beijing), have led to the deterioration of the BBB activation of the transcription factor NF-κB, involved in the regulation of immune, inflammatory, apoptotic, and mitogenic processes, and neurological degeneration due to repeated inflammation (19, 20). Therefore, an area of research under intense scrutiny involves the association between air pollution and neurodegeneration, as these exposures and neurological outcomes could have symptomatic consequences similar to that of dementia or Alzheimer's disease or a contributing factor to neurodevelopmental disorders, such as autism spectrum disorders. The cardiovascular consequences associated with these neurodegenerative conditions may be caused by a systemic inflammatory response, as well as accumulation of the plasma lipoprotein apolipoprotein E and xenobiotic particles in the cerebral circulation (22).
Fetal/gestational exposures.
Considering the complicated and sensitive nature of pregnancy, exposure during this time should also be considered. Few have demonstrated a link between xenobiotic exposure and untoward maternal and fetal health (7, 23, 115); within these, studies focusing on the vascular and/or neurological effects are severely lacking (56). The neurological outcomes associated with gestational exposures are especially interesting, given that the BBB is being developed during fetal maturation. Therefore, consequences of particle translocation from the maternal to fetal compartment, followed by particle deposition within the brain before BBB completion, are of generational interest. Perinatal exposure has been shown not only to induce deleterious morphological changes within the CNS but also to affect intracellular electrolyte balance and neurotransmitter synthesis/degradation (59). This impingement on vital cellular functions could result in cognitive and behavioral deficits that may manifest themselves in later developmental stages (1). A strong associative link exists between exposure to high concentrations of ambient PM at an early neurodevelopmental age and long-term cognitive deficits in various memory systems (23). Primary sensitization to environmental pollutants may also occur prenatally, with in utero sensitization to environmental antigens resulting in the formation of memory T cells for specific antigens, leading to severe inflammatory responses and morbidity rates on secondary exposure in newborns (87).
Recent epidemiological studies reported decreased placental expression of brain-derived neurotrophic factor and synaptophysin, two important neurodevelopmental genes, with increasing in utero exposure to PM2.5 (102). Prospective birth cohort studies correlated with higher levels of black carbon, an indicator of traffic particles, with decreased cognitive function involving verbal and nonverbal intelligence and memory constructs (116). Similarly, children living in urban Mexico City obtained lower intelligence quotient scores than their control counterparts (21). These findings indicate that the deleterious neurological effects of xenobiotic particle exposure during gestation may lead to visible/quantifiable symptomatology after birth.
In future work, an additional avenue of consideration may be the similarities between transmembrane transporters of compartment membranes, specifically the BBB and the placenta. These barriers both include the P-glycoprotein (P-gp), a transmembrane transporter associated with the binding and transport of pharmaceuticals and other macromolecules but with tissue-specific structure and functionality (132). The transportation of xenobiotic particles via P-gp, specifically across the placenta, is yet to be determined.
CONCLUSIONS
The cardiovascular effects associated with the inflammatory and particle-tissue interaction hypotheses have been studied extensively in the last decade. The momentum acquired by research in this field has paved the way for future studies, especially at the microvascular level, a virtually untrodden path wherein much remains unanswered. Furthermore, notwithstanding the increasing interest in xenobiotic particle toxicology, the neural activation hypothesis lags behind and is seldom studied or even mentioned in scientific literature. This apparent lack of interest is most often a result of the inherent difficulty associated with the evaluation of toxicological effects within the nervous system. Physiological barriers, such as the BBB and the intricate interplay among the various cellular elements within the CNS, impede an empirical assessment of neurological toxicity in vivo. For this reason, the overwhelming majority of current research has adopted extemporaneous in vitro models involving isolated cellular elements or components of the CNS. This reductionist toxicological approach, even though effective at determining a causal association between xenobiotic particle exposure and cytotoxicity, fails to provide a true systems-based mechanism that is applicable to human physiology. The paucity of information and contradictory findings associated with current studies is caused by the heterogeneous experimental conditions and models adopted. For this reason, the development of a systems-based toxicological paradigm will undoubtedly assist this field to overcome its current state of inertia and reach its full potential in the near future.
In conclusion, the extent of the neurological repercussions of pulmonary xenobiotic particle exposures and the associated cardiovascular implications have yet to be defined completely. At a time when the demand for interdisciplinary collaborations is tremendous, future toxicological investigations should also include engineered nanomaterials, and the alternate routes of exposure that diverge from the canonical pulmonary route should also be considered. The use of these materials as a surrogate for environmental PM will enable scientists to address the unique problems, future risks, and hurdles associated with xenobiotic particle exposure. Ultimately, if human endeavors are to be fully protected and enhanced, all mechanistic components must be fully explored. To this end, we encourage fellow investigators to give autonomic influences the full attention necessary to advance human endeavors. It is necessary to bear in mind that the inherent heterogeneity of ambient PM and hence, the complex physiological response associated with its exposure may limit the interpretation and translatability of studies using nanomaterials.
GRANTS
Support for this work was provided by the National Institute of Environmental Health Sciences Grants K99-ES024783 (to P. A. Stapleton) and R01-ES015022 (to T. R. Nurkiewicz) and National Science Foundation Cooperative Agreement (1003907; to T. R. Nurkiewicz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.A.S., A.B.A., S.L.H., and T.R.N. conception and design of research; P.A.S., A.B.A., S.L.H., and T.R.N. prepared figures; P.A.S., A.B.A., S.L.H., and T.R.N. drafted manuscript; P.A.S., A.B.A., S.L.H., and T.R.N. edited and revised manuscript; P.A.S., A.B.A., S.L.H., and T.R.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Mr. Carroll McBride for his expert technical assistance in this study. The authors also thank Ms. Elizabeth Dalton for her graphic arts assistance with Fig. 3.
REFERENCES
- 1.Abu-Taweel GM. Effects of perinatal exposure of lithium on neuro-behaviour of developing mice offspring. Indian J Exp Biol 50: 696–701, 2012. [PubMed] [Google Scholar]
- 2.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 557: 889–907, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afeseh NH, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol 256: 227–240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amatullah H, North ML, Akhtar US, Rastogi N, Urch B, Silverman FS, Chow CW, Evans GJ, Scott JA. Comparative cardiopulmonary effects of size-fractionated airborne particulate matter. Inhal Toxicol 24: 161–171, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis 14: 150–160, 2008. [PMC free article] [PubMed] [Google Scholar]
- 6.Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR, Teeguarden JG. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol 26: 829–842, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backes CH, Nelin T, Gorr MW, Wold LE. Early life exposure to air pollution: how bad is it? Toxicol Lett 216: 47–53, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balan JA, Caous CA, Yu YG, Lindsey CJ. Barosensitive neurons in the rat tractus solitarius and paratrigeminal nucleus: a new model for medullary, cardiovascular reflex regulation. Can J Physiol Pharmacol 82: 474–484, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14: 101–114, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, Lee LM, Okabe K, Verrier RL, Godleski JJ. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect 117: 361–366, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belyanskaya L, Weigel S, Hirsch C, Tobler U, Krug HF, Wick P. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology 30: 702–711, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, az-Sanchez D, Dorman DC, Gold DR, Gray K, Jeng HA, Kaufman JD, Kleinman MT, Kirshner A, Lawler C, Miller DS, Nadadur SS, Ritz B, Semmens EO, Tonelli LH, Veronesi B, Wright RO, Wright RJ. The outdoor air pollution and brain health workshop. Neurotoxicology 33: 972–984, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J 18: 1618–1620, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J Comput Neurosci 10: 71–77, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Brook RD, Bard RL, Morishita M, Dvonch JT, Wang L, Yang HY, Spino C, Mukherjee B, Kaplan MJ, Yalavarthi S, Oral EA, Ajluni N, Sun Q, Brook JR, Harkema J, Rajagopalan S. Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect 122: 624–630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, ez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Byun HM, Panni T, Motta V, Hou L, Nordio F, Apostoli P, Bertazzi PA, Baccarelli AA. Effects of airborne pollutants on mitochondrial DNA methylation. Part Fibre Toxicol 10: 18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderon-Garciduenas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, DEL Tizapantzi MR, Carson JL, Villarreal-Calderon A, Rewcastle B. Air pollution and brain damage. Toxicol Pathol 30: 373–389, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Calderon-Garciduenas L, Kulesza RJ, Doty RL, D'Angiulli A, Torres-Jardon R. Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ Res 137: 157–169, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Calderon-Garciduenas L, Mora-Tiscareno A, Franco-Lira M, Zhu H, Lu Z, Solorio E, Torres-Jardon R, D'Angiulli A. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein epsilon4 children exposed to air pollution. J Alzheimers Dis 45: 757–770, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36: 289–310, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Calderon-Garciduenas L, Torres-Jardon R, Kulesza RJ, Park SB, D'Angiulli A. Air pollution and detrimental effects on children's brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Hum Neurosci 8: 613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26: 133–140, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Costa DL, Farraj AK. Whole and particle-free diesel exhausts differentially affect cardiac electrophysiology, blood pressure, and autonomic balance in heart failure-prone rats. Toxicol Sci 128: 490–499, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavallari JM, Eisen EA, Fang SC, Schwartz J, Hauser R, Herrick RF, Christiani DC. PM2.5 metal exposures and nocturnal heart rate variability: a panel study of boilermaker construction workers. Environ Health 7: 36, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavallari JM, Fang SC, Eisen EA, Schwartz J, Hauser R, Herrick RF, Christiani DC. Time course of heart rate variability decline following particulate matter exposures in an occupational cohort. Inhal Toxicol 20: 415–422, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Cechetto DF, Calaresu FR. Central pathways relaying cardiovascular afferent information to amygdala. Am J Physiol Regul Integr Comp Physiol 248: R38–R45, 1985. [DOI] [PubMed] [Google Scholar]
- 29.Cechetto DF, Calaresu FR. Units in the amygdala responding to activation of carotid baro- and chemoreceptors. Am J Physiol Regul Integr Comp Physiol 246: R832–R836, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Cechetto DF, Ciriello J, Calaresu FR. Afferent connections to cardiovascular sites in the amygdala: a horseradish peroxidase study in the cat. J Auton Nerv Syst 8: 97–110, 1983. [DOI] [PubMed] [Google Scholar]
- 31.Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect 115: 1617–1622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Yokel RA, Hennig B, Toborek M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol 3: 286–295, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Yang JJ, Zhang H, Ren GG, Yang Z, Zhang T. Multi-walled carbon nanotube inhibits CA1 glutamatergic synaptic transmission in rat's hippocampal slices. Toxicol Lett 229: 423–429, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Ciriello J, Calaresu FR. Autoradiographic study of ascending projections from cardiovascular sites in the nucleus tractus solitarii in the cat. Brain Res 186: 448–453, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Ciriello J, Calaresu FR. Distribution of vagal cardioinhibitory neurons in the medulla of the cat. Am J Physiol Regul Integr Comp Physiol 238: R57–R64, 1980. [DOI] [PubMed] [Google Scholar]
- 36.Ciriello J, Calaresu FR. Monosynaptic pathway from cardiovascular neurons in the nucleus tractus solitarii to the paraventricular nucleus in the cat. Brain Res 193: 529–533, 1980. [DOI] [PubMed] [Google Scholar]
- 37.Ciriello J, Calaresu FR. Role of paraventricular and supraoptic nuclei in central cardiovascular regulation in the cat. Am J Physiol Regul Integr Comp Physiol 239: R137–R142, 1980. [DOI] [PubMed] [Google Scholar]
- 38.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int 2014: 736385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cupaioli FA, Zucca FA, Boraschi D, Zecca L. Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol 119–120: 20–38, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Davis DA, Akopian G, Walsh JP, Sioutas C, Morgan TE, Finch CE. Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NO pathway in vitro. J Neurochem 127: 509–519, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng X, Jia G, Wang H, Sun H, Wang X, Yang S, Wang T, Liu Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 45: 1419–1424, 2007. [Google Scholar]
- 42.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdorster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect 114: 1172–1178, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erriquez J, Bolis V, Morel S, Fenoglio I, Fubini B, Quagliotto P, Distasi C. Nanosized TiO2 is internalized by dorsal root ganglion cells and causes damage via apoptosis. Nanomedicine 11: 1309–1319, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Farraj AK, Haykal-Coates N, Winsett DW, Hazari MS, Carll AP, Rowan WH, Ledbetter AD, Cascio WE, Costa DL. Increased non-conducted P-wave arrhythmias after a single oil fly ash inhalation exposure in hypertensive rats. Environ Health Perspect 117: 709–715, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther 291: 1017–1022, 1999. [PubMed] [Google Scholar]
- 46.Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im H, V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 113: 1555–1560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst 5–88, 2000. [PubMed] [Google Scholar]
- 48.Gwyn DG, Wilkinson PH, Leslie RA. The ultrastructural identification of vagal terminals in the solitary nucleus of the cat after anterograde labelling with horseradish peroxidase. Neurosci Lett 28: 139–143, 1982. [DOI] [PubMed] [Google Scholar]
- 49.Hadrup N, Loeschner K, Mortensen A, Sharma AK, Qvortrup K, Larsen EH, Lam HR. The similar neurotoxic effects of nanoparticulate and ionic silver in vivo and in vitro. Neurotoxicology 33: 416–423, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Hardebo JE, Kahrstrom J. Endothelial negative surface charge areas and blood-brain barrier function. Acta Physiol Scand 125: 495–499, 1985. [DOI] [PubMed] [Google Scholar]
- 51.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol 139: 1074–1084, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 119: 951–957, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henneberger A, Zareba W, Ibald-Mulli A, Ruckerl R, Cyrys J, Couderc JP, Mykins B, Woelke G, Wichmann HE, Peters A. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ Health Perspect 113: 440–446, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, Mak JC, Chang RC. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One 7: e36752, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopkins DA, Armour JA. Ganglionic distribution of afferent neurons innervating the canine heart and cardiopulmonary nerves. J Auton Nerv Syst 26: 213–222, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Hougaard KS, Campagnolo L, Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D, Valentino S, Park MV, de Jong WH, Wolterink G, Piersma AH, Ross BL, Hutchison GR, Hansen JS, Vogel U, Jackson P, Slama R, Pietroiusti A, Cassee FR. A perspective on the developmental toxicity of inhaled nanoparticles. Reprod Toxicol 56: 118–140, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Hough LB, Rice FL. H3 receptors and pain modulation: peripheral, spinal, and brain interactions. J Pharmacol Exp Ther 336: 30–37, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howe HA, Bodian D. Second attacks of poliomyelitis: an experimental study. J Exp Med 74: 145–166, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, Zhou M, Liu C, Wang H, Hong F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 31: 8043–8050, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med 159: 1943–1948, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11: 1–18, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Kan H, Wu Z, Lin YC, Chen TH, Cumpston JL, Kashon ML, Leonard S, Munson AE, Castranova V. The role of nodose ganglia in the regulation of cardiovascular function following pulmonary exposure to ultrafine titanium dioxide. Nanotoxicology 8: 447–454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci 48: 464–471, 2012. [DOI] [PubMed] [Google Scholar]
- 64.Kappagoda CT, Ravi K. The rapidly adapting receptors in mammalian airways and their responses to changes in extravascular fluid volume. Exp Physiol 91: 647–654, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Katz DM, Karten HJ. Substance P in the vagal sensory ganglia: localization in cell bodies and pericellular arborizations. J Comp Neurol 193: 549–564, 1980. [DOI] [PubMed] [Google Scholar]
- 66.Kawai Y, Mori S, Takagi H. Vagal afferents interact with substance P-immunoreactive structures in the nucleus of the tractus solitarius: immunoelectron microscopy combined with an anterograde degeneration study. Neurosci Lett 101: 6–10, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol 20: 57–76, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V, Nurkiewicz TR. Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology 6: 724–735, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol 6: 2712–2735, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J Nanosci Nanotechnol 4: 484–488, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol 21 Suppl 1: 55–60, 2009. [DOI] [PubMed] [Google Scholar]
- 72.La Maestra S, Kisby GE, Micale RT, Johnson J, Kow YW, Bao G, Sheppard C, Stanfield S, Tran H, Woltjer RL, D'Agostini F, Steele VE, De Flora S. Cigarette smoke induces DNA damage and alters base-excision repair and tau levels in the brain of neonatal mice. Toxicol Sci 123: 471–479, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 77: 126–134, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Langrish JP, Bosson J, Unosson J, Muala A, Newby DE, Mills NL, Blomberg A, Sandstrom T. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med 272: 224–239, 2012. [DOI] [PubMed] [Google Scholar]
- 75.Langrish JP, Watts SJ, Hunter AJ, Shah AS, Bosson JA, Unosson J, Barath S, Lundback M, Cassee FR, Donaldson K, Sandstrom T, Blomberg A, Newby DE, Mills NL. Controlled exposures to air pollutants and risk of cardiac arrhythmia. Environ Health Perspect 122: 747–753, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Legramante JM, Valentini F, Magrini A, Palleschi G, Sacco S, Iavicoli I, Pallante M, Moscone D, Galante A, Bergamaschi E, Bergamaschi A, Pietroiusti A. Cardiac autonomic regulation after lung exposure to carbon nanotubes. Hum Exp Toxicol 28: 369–375, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Lewis J, Bench G, Myers O, Tinner B, Staines W, Barr E, Divine KK, Barrington W, Karlsson J. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology 26: 113–123, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Li R, Kou X, Geng H, Xie J, Yang Z, Zhang Y, Cai Z, Dong C. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem Res Toxicol 28: 408–418, 2015. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Li J, Yin J, Li W, Kang C, Huang Q, Li Q. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J Nanosci Nanotechnol 10: 8544–8549, 2010. [DOI] [PubMed] [Google Scholar]
- 80.Liao D, Shaffer ML, Rodriguez-Colon S, He F, Li X, Wolbrette DL, Yanosky J, Cascio WE. Acute adverse effects of fine particulate air pollution on ventricular repolarization. Environ Health Perspect 118: 1010–1015, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target 12: 635–641, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Loeffler DA, Demaggio AJ, Juneau PL, Havaich MK, Lewitt PA. Effects of enhanced striatal dopamine turnover in vivo on glutathione oxidation. Clin Neuropharmacol 17: 370–379, 1994. [DOI] [PubMed] [Google Scholar]
- 83.Long CM, Suh HH, Kobzik L, Catalano PJ, Ning YY, Koutrakis P. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: in vitro effects of endotoxin and other particulate properties. Environ Health Perspect 109: 1019–1026, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect 115: 1631–1637, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, Stone PH, Gold DR. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med 67: 625–630, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, Mazzone SB. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci 35: 7041–7055, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, Tsai WY, Whyatt RM, Perera FP, Ford JG. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med 164: 995–1001, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. J Physiol 223: 525–548, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monteiro-Riviere NA, Inman AO. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon 44: 1070–1078, 2006. [Google Scholar]
- 90.Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, Kam W, Hsu YT, Winkler JW, Chen JC, Petasis NA, Baudry M, Sioutas C, Finch CE. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect 119: 1003–1009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morrison PW, Khutoryanskiy VV. Advances in ophthalmic drug delivery. Ther Deliv 5: 1297–1315, 2014. [DOI] [PubMed] [Google Scholar]
- 92.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nemmar A, Delaunois A, Nemery B, Dessy-Doize C, Beckers JF, Sulon J, Gustin P. Inflammatory effect of intratracheal instillation of ultrafine particles in the rabbit: role of C-fiber and mast cells. Toxicol Appl Pharmacol 160: 250–261, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol 5: 1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2: 8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823–839, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16: 437–445, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65: 1531–1543, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Perez CM, Ledbetter AD, Hazari MS, Haykal-Coates N, Carll AP, Winsett DW, Costa DL, Farraj AK. Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Toxicol Sci 132: 467–477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 7: 1179–1194, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saenen ND, Plusquin M, Bijnens E, Janssen BG, Gyselaers W, Cox B, Fierens F, Molenberghs G, Penders J, Vrijens K, De Boever P, Nawrot TS. Fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRAGE Birth Cohort Study. Environ Health Perspect 123: 834–840, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samet JM, Graff D, Berntsen J, Ghio AJ, Huang YC, Devlin RB. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal Toxicol 19 Suppl 1: 29–32, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol 142: 23–35, 1995. [DOI] [PubMed] [Google Scholar]
- 105.Shannahan JH, Podila R, Aldossari AA, Emerson H, Powell BA, Ke PC, Rao AM, Brown JM. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci 143: 136–146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharma HS, Ali SF, Tian ZR, Hussain SM, Schlager JJ, Sjoquist PO, Sharma A, Muresanu DF. Chronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51. J Nanosci Nanotechnol 9: 5073–5090, 2009. [DOI] [PubMed] [Google Scholar]
- 107.Sheng L, Ze Y, Wang L, Yu X, Hong J, Zhao X, Ze X, Liu D, Xu B, Zhu Y, Long Y, Lin A, Zhang C, Zhao Y, Hong F. Mechanisms of TiO2 nanoparticle-induced neuronal apoptosis in rat primary cultured hippocampal neurons. J Biomed Mater Res A 103: 1141–1149, 2015. [DOI] [PubMed] [Google Scholar]
- 108.Siddiqi NJ, Abdelhalim MA, El-Ansary AK, Alhomida AS, Ong WY. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflammation 9: 123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simko M, Mattsson MO. Risks from accidental exposures to engineered nanoparticles and neurological health effects: a critical review. Part Fibre Toxicol 7: 42, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sousa LO, Lindsey CJ. Cardiovascular and baroreceptor functions of the paratrigeminal nucleus for pressor effects in non-anaesthetized rats. Auton Neurosci 147: 27–32, 2009. [DOI] [PubMed] [Google Scholar]
- 111.Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic Biol Med 31: 1132–1138, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Stansfeld CE, Wallis DI. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol 54: 245–260, 1985. [DOI] [PubMed] [Google Scholar]
- 113.Stapleton PA, Minarchick VC, McCawley M, Knuckles TL, Nurkiewicz TR. Xenobiotic particle exposure and microvascular endpoints: a call to arms. Microcirculation 19: 126–142, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stapleton PA, Nichols CE, Yi J, McBride CR, Minarchick VC, Shepherd DL, Hollander JM, Nurkiewicz TR. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology 9: 941–951, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stapleton PA, Nurkiewicz TR. Maternal nanomaterial exposure: a double threat to maternal uterine health and fetal development? Nanomedicine (Lond) 9: 929–931, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suglia SF, Wright RO, Schwartz J, Wright RJ. Association between lung function and cognition among children in a prospective birth cohort study. Psychosom Med 70: 356–362, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, Yuan F, Xi T. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol 9: 4924–4932, 2009. [DOI] [PubMed] [Google Scholar]
- 118.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178: 406–413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallenborn JG, Kovalcik KD, McGee JK, Landis MS, Kodavanti UP. Systemic translocation of (70)zinc: kinetics following intratracheal instillation in rats. Toxicol Appl Pharmacol 234: 25–32, 2009. [DOI] [PubMed] [Google Scholar]
- 120.Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol Sci 98: 231–239, 2007. [DOI] [PubMed] [Google Scholar]
- 121.Wang JX, Liu Y, Jiao F, Lao F, Li W, Gu YQ, Li YF, Ge CC, Zhou GQ, Li B, Zhao YL, Chai ZF, Chen CY. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 254: 82–90, 2008. [DOI] [PubMed] [Google Scholar]
- 122.Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect 109 Suppl 4: 579–584, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. The relationship between traffic-related air pollutants and cardiac autonomic function in a panel of healthy adults: a further analysis with existing data. Inhal Toxicol 23: 289–303, 2011. [DOI] [PubMed] [Google Scholar]
- 124.Xu F, Piett C, Farkas S, Qazzaz M, Syed NI. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol Brain 6: 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, Spino C, Brook RD, Harkema JR, Rajagopalan S. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 122: 79–86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu YG, Lindsey CJ. Baroreceptor-sensitive neurons in the rat paratrigeminal nucleus. Auton Neurosci 105: 25–34, 2003. [DOI] [PubMed] [Google Scholar]
- 127.Yue W, Schneider A, Stolzel M, Ruckerl R, Cyrys J, Pan X, Zareba W, Koenig W, Wichmann HE, Peters A. Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res 621: 50–60, 2007. [DOI] [PubMed] [Google Scholar]
- 128.Ze Y, Hu R, Wang X, Sang X, Ze X, Li B, Su J, Wang Y, Guan N, Zhao X, Gui S, Zhu L, Cheng Z, Cheng J, Sheng L, Sun Q, Wang L, Hong F. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res A 102: 470–478, 2014. [DOI] [PubMed] [Google Scholar]
- 129.Zhang R, Piao MJ, Kim KC, Kim AD, Choi JY, Choi J, Hyun JW. Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int J Biochem Cell Biol 44: 224–232, 2012. [DOI] [PubMed] [Google Scholar]
- 130.Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrical stimulation of thalamic nucleus submedius area on the rat tail flick reflex. Brain Res 696: 205–212, 1995. [DOI] [PubMed] [Google Scholar]
- 131.Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain 72: 127–135, 1997. [DOI] [PubMed] [Google Scholar]
- 132.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 38: 802–832, 2008. [DOI] [PubMed] [Google Scholar]