Leptin is believed to increase heart rate via adrenergic receptor stimulation. We found evidence that leptin can exert local direct inhibition of heart rate and prolongation of QTc interval via its receptor. The findings offer better understanding of higher incidence of prolonged QT and sudden cardiac death in obesity.
Keywords: leptin, leptin receptor, resting heart rate, QT interval
Abstract
Leptin has been proposed to modulate cardiac electrical properties via β-adrenergic receptor activation. The presence of leptin receptors and adipocytes in myocardium raised a question as to whether leptin can directly modulate cardiac electrical properties such as heart rate and QT interval via its receptor. In this work, the role of local direct actions of leptin on heart rate and ventricular repolarization was investigated. We identified the protein expression of leptin receptors at cell surface of sinus node, atrial, and ventricular myocytes isolated from rat heart. Leptin at low doses (0.1–30 μg/kg) decreased resting heart rate; at high doses (150–300 μg/kg), leptin induced a biphasic effect (decrease and then increase) on heart rate. In the presence of high-dose propranolol (30 mg/kg), high-dose leptin only reduced heart rate and sometimes caused sinus pauses and ventricular tachycardia. The leptin-induced inhibition of resting heart rate was fully reversed by leptin antagonist. Leptin also increased heart rate-corrected QT interval (QTc), and leptin antagonist did not. In isolated ventricular myocytes, leptin (0.03–0.3 μg/ml) reversibly increased the action potential duration. These results supported our hypothesis that in addition to indirect pathway via sympathetic tone, leptin can directly decrease heart rate and increase QT interval via its receptor independent of β-adrenergic receptor stimulation. During inhibition of β-adrenergic receptor activity, high concentration of leptin in myocardium can cause deep bradycardia, prolonged QT interval, and ventricular arrhythmias.
NEW & NOTEWORTHY
Leptin is believed to increase heart rate via adrenergic receptor stimulation. We found evidence that leptin can exert local direct inhibition of heart rate and prolongation of QTc interval via its receptor. The findings offer better understanding of higher incidence of prolonged QT and sudden cardiac death in obesity.
leptin is a 16-kDa adipokine released from adipocytes acting via its receptor. Detection of adipocytes in obese human heart (21, 30) and leptin receptors in human cardiac ventricles (19) has suggested that leptin can have autocrine and paracrine effects on cardiac functions.
Leptin has been demonstrated to play an important role in cardiac contractility (19, 37), development of hypertrophy (12, 19, 37, 39), and apoptosis (20). However, studies of leptin on cardiac electrical properties are rare and limited to indirect effects via adrenergic receptor stimulation. It has been widely accepted that leptin increases the heart rate through increasing sympathetic activity (5, 9, 10). Leptin-mediated increase in sympathetic activity can explain obese subjects with higher heart rate to meet the increased metabolic demands (25), but it cannot explain why many of them exhibit unchanged or decreased resting heart rate (2, 7).
In heart failure patients, fatty infiltration of cardiomyocytes has been demonstrated. The percentage of fat in myocardium is positively correlated with body mass index and an increase in obesity (30). Presence of fat accumulation within myocardium in obese human heart raised the question of whether leptin may have a direct effect on heart rate if leptin receptors are present in the sinus node and on repolarization in the ventricle.
In this work, we provide evidence for the presence of leptin receptors in rat isolated sinus node, atrial, and ventricular myocytes and leptin-induced decrease in heart rate and increase in QT intervals via leptin receptors independent of β-adrenergic receptor stimulation.
MATERIALS AND METHODS
The electrophysiological studies were performed in Sprague-Dawley rats. Obese and lean Zucker rats were used to demonstrate excessive accumulation of adipocytes in the right atrium. The right atria containing the sinus node were from male Sprague-Dawley and male obese and lean Zucker rats. The ventricles were from male Sprague-Dawley rats. The animal protocols in this study were reviewed and approved by the Animal Care and Use Committees of West Virginia University and Chinese Culture University, Taiwan.
Electrocardiograph recording in Sprague-Dawley rats.
Adult (3-mo-old) male Sprague-Dawley rats were anesthetized with inhaled 2% isoflurane mixed with oxygen at a flow rate of 2 l/min. Leptin or isoproterenol was administrated through a catheter (polyurethane, 1 French size) placed in the jugular vein in accordance with Animal Care and Use Committee of Chinese Culture University requirments. The heart rate was recorded by a two-lead vector connected to a computerized, cardiovascular, continuous monitoring system (a PowerLab/4SP analog-to-digital converter; AD Instruments, Colorado Springs, CO) at a 1-KHz sampling rate. The results were collected from five rats.
Isolation of sinus node, atrial, and ventricular myocytes.
The heart was quickly removed from anesthetized rats with pentobarbital sodium (50 mg/kg) and immersed in normal Tyrode solution containing heparin. A blunt end forceps was used to force out the blood. New Tyrode solution was replaced to rinse until clear of blood. Ventricles were removed, and the sinoatrial region was exposed, dissected out, and placed in fresh Tyrode solution gassed with 100% O2 at 37°C.
For isolation of sinus node and atrial myocytes, the sinoatrial region was digested in a Ca2+-free Tyrode solution containing 0.4 mg/ml Librase Blendyme 4 (Roche Applied Sciences) for ∼20 min at 37°C. After digestion, the tissue was trimmed into strips of ∼1 mm in width and 3–4 mm in length in Ca2+-free Tyrode solution. The digested tissue was then placed in Krafte-brühe (KB) solution. The sinus node myocytes were dissociated by gently puffing KB solution onto the tissue. Normal Tyrode solution contained (in mM) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5.5 d-glucose, and 5 HEPES-NaOH, pH 7.4. KB solution contained (in mM) 50 l-glutamic acid, 80 KOH, 40 KCl, 3 MgSO4, 25 KH2PO4, 10 HEPES, EGTA 1, 20 taurine, and 10 glucose, pH 7.2.
The ventricular myocytes were prepared using a combination of collagenase perfusion and mechanical shaking, as described previously (18). Briefly, the aorta was cannulated, and the heart was perfused with oxygenated Ca2+-free Tyrode solution at 35–37°C for 5–10 min, followed by oxygenated Ca2+-free Tyrode solution containing 0.1 mg/ml Liberase Blendzyme 4 (Roche). The heart was then left in oxygenated Ca2+-free Tyrode solution for 15 min. The ventricles were minced into small pieces in KB solution and dispersed through gentle shaking. The isolated myocytes were stored in KB solution at room temperature for 1 h before experiments.
Immunofluorescence microscopy of cardiac myocytes.
Isolated sinus node myocytes were placed on glass slides and left to settle for ≥1 h before fixation in 4% paraformaldehyde for 20 min at room temperature. Paraformaldehyde was removed, and myocytes were washed for three times with PBS. The cells were then permeablized with 0.5% Triton-X 100 in PBS for 2 min and rinsed with PBS for three times. Then the cells were blocked for 30 min at room temperature using PBS containing 2% BSA. Primary antibodies against HCN4 and ObRb were prepared by 1:100 dilution in the blocking solution, in which the cells were incubated over two nights at 4°C. After three times of washes with PBS, the secondary antibodies (1:1,000, conjugated with fluorescent probe and Alexa Fluor 488 and 555; Invitrogen) were added and incubated for 1 h in the dark at room temperature. Following the final rinses with PBS and subsequently distilled water, the glass slides were cover-slipped, using 10 μl of Prolong Gold with DAPI (Invitrogen). This mounting medium required curing overnight in the dark at room temperature. The slides were then ready for examination using confocal laser scanning LSM510 microscopy (Carl Zeiss). All imaging experiments were performed at room temperature.
Patch clamp recording of action potential in Sprague-Dawley rat ventricular myocytes.
Whole cell patch clamp studies were carried out for action potential recordings in ventricular myocytes isolated from Sprague-Dawley rats. Action potential was recorded at current clamp mode with normal Tyrode and pipette solutions. The Tyrode solution contained (in mM) 143 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.25 NaH2PO4, and 5 HEPES; pH was adjusted to 7.4 by NaOH. The pipette solution contained (in mM) 120 KCl, 1 CaCl2, 5 MgCl2, 5 Na2ATP, 11 EGTA, 10 HEPES, and 11 glucose; pH was adjusted to 7.3 by KOH. The current stimulus was 0.5 to 1 nA for 2–5 ms. Action potentials were recorded at 35 ± 1°C and maintained by a temperature controller (Cell MicroControls).
Statistical analysis.
Data are shown as means ± SE. Student's t-test and two-way ANOVA (for more than 2 groups) were used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Presence of adipocytes and leptin receptor in myocardium.
Figure 1 shows the presence of adipocytes near the sinoatrial node (SAN) in the right atrium of lean Zucker (Fig. 1A, white arrow) and Sprague-Dawley rat hearts (Fig. 1B, white arrow). For demonstration of excessive accumulation of adipocytes in the right atrium caused by obesity, we also examined adipocytes in the right atrium of obese Zucker rats, as shown in Fig. 1C. The adipocytes were confirmed by Oil Red staining (Fig. 1D). The amount of adipocytes within the right atrium near SAN area is nearly 20-fold higher in obese Zucker rat than in lean Zucker and Sprague-Dawley rats (obese Zucker rat 1,906 ± 144, lean Zucker rat 49 ± 17, Sprague-Dawley rat 17 ± 3; n = 5).
Fig. 1.
A–C: presence of adipocytes in the right atrium near the sinoatrial node (SAN; bright area) of lean Zucker (LZR; A), Sprague-Dawley (SD; B), and obese Zucker rats (OZR; C). D: adipocytes were stained with Oil Red. E: statistics of adipocytes in OZR (gray bar), LZR (open bar), and SD rats (black bar). *Statistically significant difference compared with LZR or SD rat (n = 5).
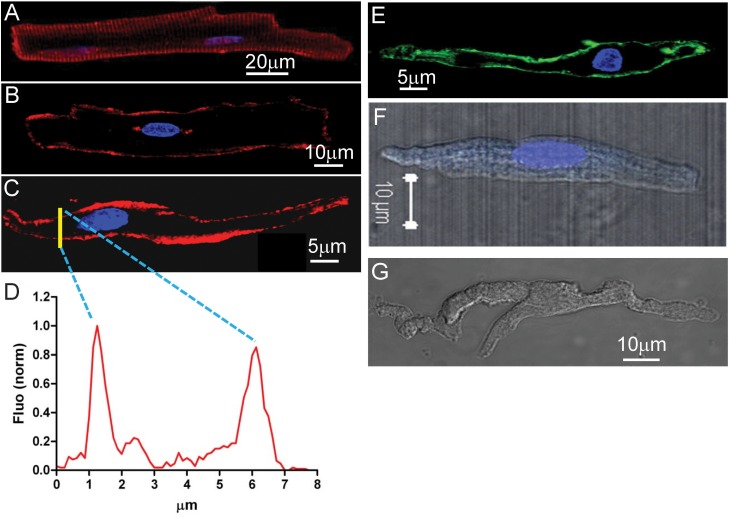
Confocal immunofluorescence microscopy imaging revealed the protein expression of leptin receptor at the cell surface of a ventricular myocyte (Fig. 2A, red), an atrial myocyte (Fig. 2B, red), and a sinus node myocyte (Fig. 2C, red) isolated from Sprague-Dawley rat heart. The distribution of fluorescence in a section (Fig. 2C, yellow line) was plotted in Fig. 2D. Two characteristic peaks indicate the surface expression of leptin receptors. The positive control using HCN4 as a sinus node marker at the cell surface (4) is shown in Fig. 2E, and negative controls are shown in Fig. 2, F (no HCN4 antibody) and G (no leptin receptor antibody). Similar results were reproduced for 10 myocytes in sinus node, atria, and ventricles. Increased accumulation of adipocytes releases more leptin, which should be able to bind its receptor for direct local actions.
Fig. 2.
Leptin receptor expression at surface of cardiac myocytes isolated from in SD rat. A: a ventricular myocyte (transverse scanning image focused on cell surface). B: an atrial myocyte (transverse scanning image focused on the nucleus). C: a sinus node myocyte (transverse scanning image focused on the nucleus). Leptin receptor is shown in red, and the nucleus is shown in blue (DAPI). D: yellow line in C marks a section of transverse scanning image to reveal the distribution of fluorescence shown in the graph. Two peaks indicate the plasma membrane expression of leptin receptors. E: HCN4 expression at cell surface of a sinus node myocyte. F: lack of HCN4 expression without use of HCN4 antibody in an atrial myocyte as a negative control. G: lack of leptin receptor expression without use of leptin receptor antibody in sinus node myocytes as a negative control.
Leptin decreases the resting heart rate.
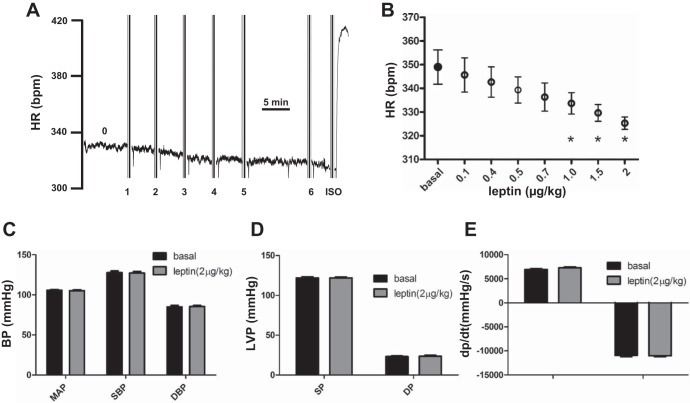
Figure 3A shows a representative ECG heart rate. Compared with the basal heart rate (labeled 0), leptin at doses ranging from 100 to 500 ng/kg (doses 1 and 2, 100 ng/kg; dose 3, 200 ng/kg; dose 4, 300 ng/kg; doses 5 and 6, 500 ng/kg) gradually decreased heart rate. Isoproterenol (ISO; 400 ng/kg) was used as a positive control to demonstrate the normal responsiveness of ISO-mediated acute increase in heart rate. Averaging from five rats, leptin at low doses from 0.1 to 2 μg/kg induced a progressive decrease in heart rate (Fig. 3B). However, statistically significant inhibition of heart rate by leptin occurred only at doses of 1 μg/kg and above (P < 0.05) (basal: 349 ± 7.2 beats/min; 0.1 μg/kg: 345.7 ± 7.2 beats/min; 0.4 μg/kg: 342.7 ± 6.4 beats/min; 0.5 μg/kg: 339.3 ± 5.5 beats/min; 0.7 μg/kg: 336.3 ± 5.9 beats/min; 1.0 μg/kg: 333.7 ± 4.5 beats/min; 1.5 μg/kg: 329.7 ± 3.5 beats/min; 2.0 μg/kg: 325.3 ± 2.6 beats/min). Leptin at low doses (2.0 μg/kg) did not affect blood pressure (P > 0.05) [basal: mean arterial pressure (MAP) = 105.7 ± 1.0 mmHg, systolic blood pressure (SBP) = 127.7 ± 2.1 mmHg, and diastolic blood pressure (DBP) = 85.0 ± 1.7 mmHg; leptin: MAP = 105.3 ± 1.0 mmHg, SBP = 127.3 ± 1.8 mmHg, and DBP = 85.5 ± 1.2 mmHg; Fig. 3C], left ventricular pressure [basal: systolic pressure (SP) = 121.9 ± 1.6 mmHg and diastolic pressure (DP) = 23.2 ± 0.8 mmHg; leptin: SP = 121.9 ± 1.1 mmHg and DP = 23.6 ± 1.2 mmHg; Fig. 3D], or ventricular contractility (basal: +dp/dt = 6,928 ± 180 mmHg/s and −dp/dt = −10,921 ± 289 mmHg/s; leptin: +dp/dt = 7,288 ± 187 mmHg/s and −dp/dt = −11,008 ± 206 mmHg/s; Fig. 3E).
Fig. 3.
Low-dose leptin reduced heart rate. A: reduction in heart rate. Basal heart rate (HR) is labeled 0. Leptin doses were labeled as follows: doses 1 and 2, 100 ng/kg; dose 3, 200 ng/kg; dose 4, 300 ng/kg; doses 5 and 6, 500 ng/kg. B: statistical data on reduction of heart rate by leptin at various low doses. Leptin (2 μg/kg) on blood pressure (BP; C), left ventricular pressure (LVP; D), and ventricular contractility (E). *Statistically significant change compared with basal condition (n = 4). ISO, isoproterenolol; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SP, systolic pressure; DP, diastolic pressure.
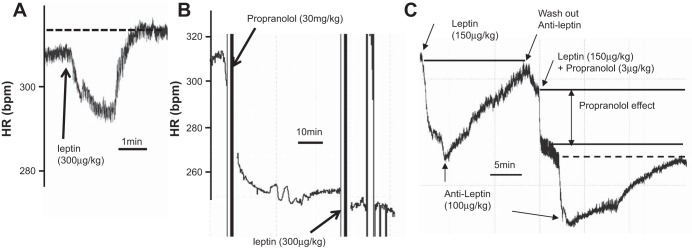
Increasing the leptin dose to 300 μg/kg induced a biphasic effect, an initial decrease followed by an increase in heart rate (Fig. 4A). We noticed that a new steady-state resting heart rate was higher than that before leptin administration (Fig. 4A, dashed line). Wondering whether the increase phase might be mediated by adrenergic receptor stimulation, we applied a nonselective β-adrenergic receptor blocker, propranolol, to block the adrenergic stimulation (16). In the presence of high-dose (30 mg/kg) propranolol, leptin at 300 μg/kg only decreased the heart rate (Fig. 4B).
Fig. 4.
Effects of high-dose leptin on HR. A: 300 μg/kg leptin induced a prominent biphasic effect on HR. Arrow indicates the time point when leptin was administrated. Dashed line indicates the HR at steady state after leptin-induced increase on heart rate. B: in the presence of propranolol, 300 μg/kg leptin induced a reduction of HR. Arrows indicate the time points when propranolol (top arrow) and leptin (bottom arrow) were administrated. C: leptin antagonist (100 μg/kg) reversed leptin-induced reduction of HR in the absence and presence of propranolol. Prior to leptin (A and C) or propranolol administration (B) is the basal HR.
To seek additional supporting evidence that leptin-induced inhibition of heart rate is via its own receptor, we used a leptin receptor antagonist, leptin triple mutant L39A/D40A/F41A, that abolishes leptin interaction with the immunoglobulin-like domain, thus preventing receptor activation (8). Figure 4C shows that the decreased heart rate induced by leptin can be reversed by the anti-leptin antagonist. After administrating a low dose (3 μg/kg) of propranolol with leptin, two phases of inhibition in heart rate were observed. The first decrease was induced by propranolol. The second-phase decrease of heart rate was induced by leptin since this decrease can be reversed by anti-leptin to the level of propranolol effect (Fig. 4C, dashed line). We have similar observations in a total of five rats.
We also studied the effect of leptin on isolated right atrium that contained sinus node. Leptin at 20 nM reversibly inhibited the spontaneous beating rate by 28% (control 134.5 ± 5.0 beats/min, leptin 96.5 ± 5.2 beats/min, washout 113.3 ± 5.7 beats/min; n = 8, P < 0.05). At increased concentration (50 nM), leptin induced a sinus arrest. Leptin at 50 nM also blunted isoproterenol stimulation of the sinus node beating rate. Similar results with 50 nM leptin were observed in four additional preparations.
Leptin increases QT interval and ventricular action potential duration.
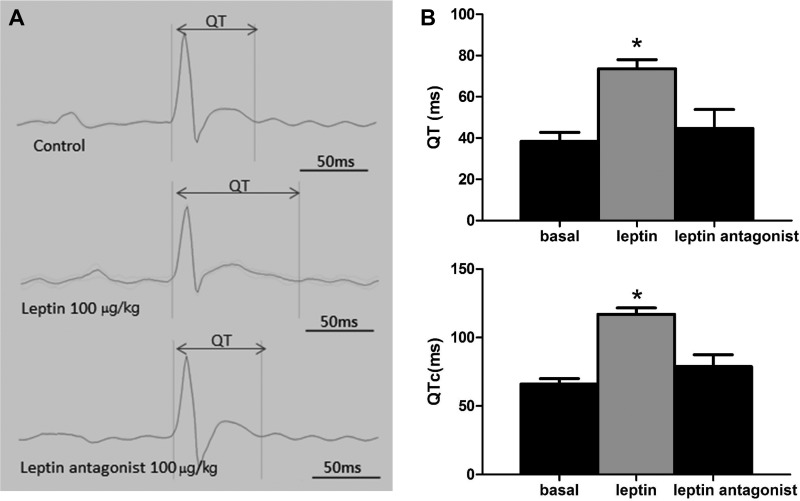
Since the slow heart rate is frequently associated with prolonged QT interval, we next examined the effect of leptin on QT interval. Figure 5 shows that leptin at 100 μg/kg increased the QT interval (Fig. 5A, middle) compared with the control (Fig. 5A, top). Leptin antagonist did not increase QT intervals (Fig. 5A, bottom). On average, QT was increased by 192% (basal 38.35 ± 4.33 ms, leptin 73.65 ± 4.38 ms; n = 5, P < 0.05) (Fig. 5B, top) and unaltered by leptin antagonist (basal 38.35 ± 4.33 ms, leptin antagonist 43.19 ± 3.21 ms; n = 5, P > 0.05); the heart rate-corrected QT interval (QTc) was increased by 177% (basal 66.09 ± 4.01 ms, leptin 117.05 ± 4.68 ms; n = 5, P < 0.05) (Fig. 5B, bottom) and unaltered by leptin antagonist (basal 66.09 ± 4.01 ms, leptin antagonist 71.31 ± 3.70 ms; n = 5, P > 0.05). The resting heart rate was reduced by 9% (basal 340.8 ± 4.6 beats/min, leptin 310.0 ± 2.9 beats/min; n = 5, P < 0.05).
Fig. 5.
Leptin-induced increase in QT interval. A: basal QT interval (top), increased QT interval induced by 100 μg/kg leptin (middle), and unaltered QT interval by leptin antagonist (100 μg/kg; bottom). B: average QT (top) and HR-corrected QT (QTc; bottom) intervals by leptin and its antagonist with statistical significance. *Comparison with the control group.
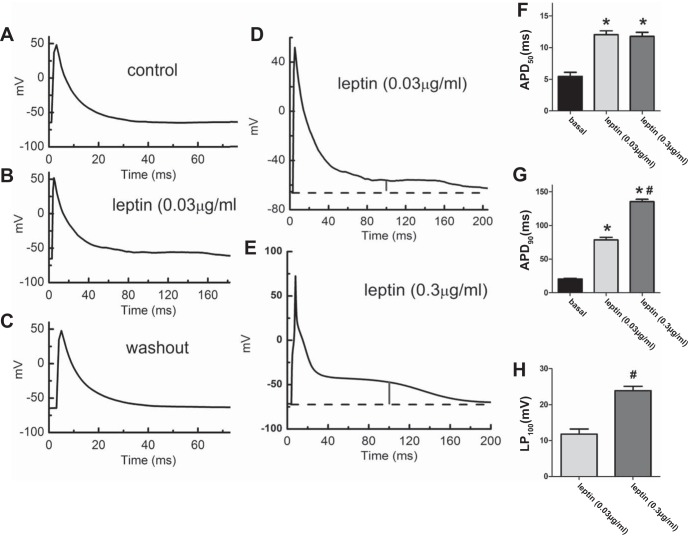
To explore the direct evidence that leptin-mediated prolongation QT interval is via its receptor in cardiac myocytes, we investigated the effects of leptin on isolated ventricular myocytes. Figure 6 shows that in isolated ventricular myocytes, leptin at a concentration of 0.03 μg/ml reversibly increased the duration of action potential by inducing a late-plateau (LP) phase during repolarization (Fig. 6B) compared with control (Fig. 6A) and washout (Fig. 6C). This LP phase (marked by a vertical line in Fig. 6D) can be enhanced further by increasing leptin concentration to 0.3 μg/ml (Fig. 6E). On average, leptin at 0.03 μg/ml increased APD50 (50% of repolarization) by 118% (basal 5.5 ± 0.6 ms, leptin 12.0 ± 0.6 ms; n = 6, P < 0.05; Fig. 6F) and APD90 (90% of repolarization) by 282% (basal 20.6 ± 1.2 ms, leptin 78.7 ± 3.8 ms; n = 6, P < 0.05; Fig. 6G). Leptin at 0.3 μg/ml did not further increase APD50 (0.03 μg/ml: 12.0 ± 0.6 ms; 0.3 μg/ml: 11.8 ± 0.7 ms; n = 6, P > 0.05; Fig. 6F) but did further increase APD90 (0.03 μg/ml: 78.7 ± 3.8 ms; 0.3 μg/ml: 135.3 ± 3.4 ms; n = 6, P < 0.05; Fig. 6G). This may be caused by the leptin-induced late-plateau phase (Fig. 6H).
Fig. 6.
Leptin reversibly increased action potential duration in rat ventricular myocytes. Representative action potentials were elicited by current clamp in a ventricular myocyte before (A), during (B), and after washout (C) of leptin (0.03 μg/ml). Leptin induced a late-plateau (LP) phase in another ventricular myocyte at the concentrations of 0.03 μg/ml (D) and 0.3 μg/ml (E), respectively. The vertical bar marks the height of the LP at t = 100ms. Dashed lines indicate resting membrane potentials. Leptin increased action potential duration (APD) at 50% repolarization (APD50; F), APD90 (G), and LP100 (H). *Statistically significant compared with basal (F and G); #statistically significant compared with leptin at 0.03 μg/kg (G and H).
Leptin induces premature ventricular beats in the absence of propranolol, sinus pauses, and ventricular tachycardia in the presence of propranolol.
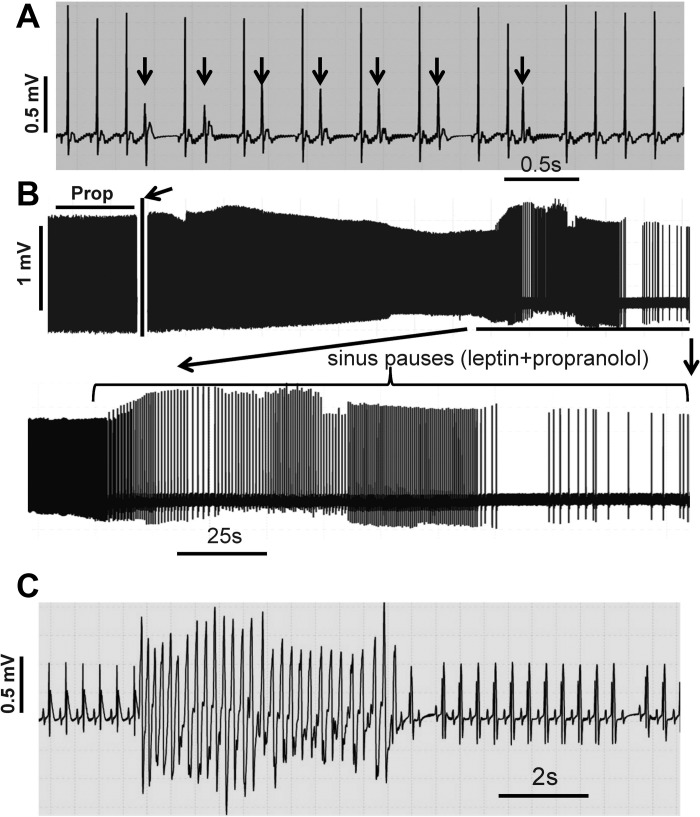
Changes in heart rate cannot only affect QT interval but also trigger premature ventricular contractions or premature ventricular beats (24, 35). Figure 7 shows that leptin at 50 μg/kg induced premature ventricular beats (Fig. 7A, arrows). No premature ventricular beats were observed during leptin administration at a low dose (e.g., 1 μg/kg). In the presence of propranolol (30 mg/kg), a high dose (150 μg/kg) of leptin triggered sinus pauses (Fig. 7B). Further increases in leptin dose (300 μg/kg) induced ventricular tachycardia within 5 min in the presence of propranolol (30 mg/kg) (Fig. 7C). High-dose leptin-induced ventricular arrhythmias were observed in four of 16 rats.
Fig. 7.
High-dose leptin-induced cardiac arrhythmias. A: leptin at 50 μg/kg induced premature ventricular beats (arrows). B: in the presence of propranolol (30 mg/kg), leptin at 150 μg/kg triggered sinus pauses (bottom). Arrows indicate the time point when leptin was administrated. C: in the presence of propranolol (30 mg/kg), leptin at 300 μg/kg induced ventricular tachycardia. All experiments were repeated in 3–4 rats. Prop, propranolol.
DISCUSSION
To the best of our knowledge, this is the first study to demonstrate a local direct action of leptin on cardiac electrical properties. Plasma leptin levels are at least fourfold higher in obese subjects over lean controls (6). However, local leptin concentration in the myocardium is unknown, although we expected a much higher leptin level in obese than in lean subjects. Long QT and associated cardiac events are common in obese patients. Thus, our findings may have clinical significance.
Previously, it was demonstrated that chronic infusion of leptin at 0.1 μg/kg into carotid artery or femoral vein for 7 days had no effect on heart rate (28). Infusion of leptin at 1 μg/kg for 7 days began to induce an increase in heart rate (28). This leptin-mediated stimulation of heart rate was blunted by adrenergic receptor blockade (5). It was thus concluded that leptin increases heart rate via sympathetic receptor activation (5), which is in agreement with the established observation that leptin increases sympathetic activity (10). The literature also reported that the threshold dose of leptin to trigger norepinephrine release was about 1 μg/kg when leptin was introduced via intracerebroventriuclar route (27). We found that at low doses, leptin decreases heart rate within minutes. When administered intravenously, leptin was reported to activate sympathetic activity at a threshold dose of 100 μg/kg (10).
So far, six isoforms of leptin receptor have been identified (23, 33). The transcripts of three isoforms (ObRa, ObRb, and ObRe) of leptin receptor have been identified in the rat heart (26). ObRa is the short form of leptin receptor that lacks the intracellular signaling domain; its function is unclear (23). ObRb is the long form containing the intracellular signaling domain, or the functional form of leptin receptor (23). ObRe is the soluble form of leptin receptor that leptin can bind to but lacks the transmembrane and the intracellular signaling domains; it can be found in the cytoplasm bound with leptin (23).
In this work, we detected the leptin receptor protein at the plasma membrane of sinus node, atrial, and ventricular myocytes from the Sprague-Dawley rat. Cellular distribution of fluorescence is an effective way to study cell surface expression of membrane proteins such as ion channels and receptors (13, 34). The presence of adipocytes and leptin receptors in the heart strongly suggests a possibility that leptin can exert a local, direct effect on cardiac functions.
At low doses (100 ng/kg to 2 μg/kg), leptin decreased the heart rate. At higher doses (>100 μg/kg), leptin induced an initial decrease and then increase biphasic effect. Our interpretation is that leptin exerted two opposing effects via separate mechanisms, direct leptin receptor-mediated inhibition and indirect adrenergic receptor-mediated stimulation of heart rate. Because leptin was administered to the jugular vein, its direct inhibitory effect occurred prior to its indirect stimulatory effect on heart rate. When high-dose propranolol was applied before leptin, no leptin-mediated increase in heart rate occurred. These data strongly indicated that in the absence of β-adrenergic receptor activation, leptin can locally inhibit the resting heart rate. The direct evidence for this local inhibition of heart rate by leptin via its receptor was the reversal of leptin-mediated inhibition by leptin antagonist (Fig. 4) and inhibition of spontaneous beating of the isolated sinus node.
Leptin-mediated increase in action potential duration of isolated ventricular myocytes provides the direct evidence that supports the underlying mechanism of leptin-induced prolongation of QT interval being a local direct action of leptin via its receptor. It is important to note that leptin induced a concentration-dependent late-plateau phase in the ventricular myocyte action potential (Fig. 6). Although the mechanism is unknown at this point, there must be an increased net inward current induced by leptin during repolarization. Previously, we have reported a disrupted L-type calcium channel inactivation as one ionic mechanism that underlies the prolonged action potential duration in obese Zucker rats (18). Whether leptin can significantly alter the gating properties of L-type calcium channels and/or other ion channels (such as IKr and IKs) and exchangers (such as the Na+/Ca2+ exchanger) important to repolarization remains to be investigated.
Perhaps the most intriguing findings are that high-dose leptin can sometimes induce arrhythmias, including premature ventricular beats (Fig. 7A), sinus pauses (Fig. 7B), and ventricular tachycardia (Fig. 7C), especially under β-adrenergic receptor inhibition. This observation may have clinical implications since many overweight/obese patients are under β-blocker treatment of hypertension (36), which simultaneously suppresses heart rate.
Why can deep bradycardia become a risk factor in ventricular arrhythmias? Slower resting heart rate leads to longer QT interval. In normal heart, sympathetic stimulation shortens, whereas parasympathetic stimulation prolongs ventricular action potential duration and the QT interval (40). It has been recognized that bradycardia can cause tachycardia, a phenomenon called bradycardia-tachycardia syndrome (1, 38). In patients with deep bradycardia caused by dysfunctional HCN4 pacemaker channel, prolonged QT interval and polymorphic ventricular tachycardia have also been observed (31, 32).
Higher incidence of prolonged QT, ventricular arrhythmias, and sudden cardiac death has been well documented in obese patients in the absence of structural cardiac defects and malfunction (15, 22). Premature ventricular contraction occurs 10 times more frequently in obese subjects than in lean controls (22). Prolonged QT interval is a cardiac electrical disorder that predicts late development of ventricular arrhythmias and sudden cardiac death (11). Long QT has been positively correlated with the body mass index (BMI) and occurs in early development of type 2 diabetes that occurs mostly in obese populations (14, 17, 25, 29). The most recent report from the Multi-Ethnic Study of Atherosclerosis has provided strong evidence that, in addition to a positive correlation between QT interval and BMI, even a small increase in QT interval (10 ms) can impose a significant effect on future increased incidents of cardiovascular events, although the mechanism is presently unknown (3). Although obese subjects are generally thought to have higher heart rates to meet the increased metabolic demands (25), many of them exhibit unchanged or decreased resting heart rate (2, 7), which cannot be explained by leptin-mediated sympathetic stimulation. Our data indicated the existence of a local direct leptin-signaling pathway via its receptor in addition to the known indirect leptin signaling via sympathetic tone. A combination of direct (via leptin receptor) and indirect (via β-adrenergic receptor) effects of leptin not only provides an explanation for slow heart rate and long QT in obese the Zucker rat (18) but can also explain all three alterations of heart rate in obese patients. High concentration of leptin can increase sympathetic activity, leading to increased heart rate. When direct and indirect actions of leptin are cancelled with each other, little change in heart rate will be observed. If sympathetic signaling is impaired, whether due to a genetic or drug effect, leptin can decrease heart rate. Our data have also suggested leptin as a possible molecular link between BMI and QT interval, which may help understanding of the high incidence of long QT in obese patients (25).
CONCLUSION
We present evidence to support our hypothesis that leptin can exert a local direct inhibition of resting heart rate and prolongation of QT interval via its receptor in cardiac myocytes. Our findings support the notion that leptin can increase heart rate via sympathetic activity but only at high concentrations mediated by adrenergic receptor activation. During inhibition of sympathetic tone, high-concentration leptin can directly increase QT intervals and trigger arrhythmias such as premature ventricular beats, sinus pauses, and ventricular tachycardia. At this point, we do not know the underlying ionic mechanisms that mediate leptin's local direct actions on cardiac electrical properties. Investigating leptin signaling in sinus node and ventricular myocytes and underlying ionic mechanisms will represent future research direction.
GRANTS
This work has been supported by National Institutes of Health (NIH) Division of Research Resources Grant 5P20-RR-016477 to the West Virginia IDeA Network for Biomedical Research Excellence (WV-INBRE), National Institute of General Medical Sciences Grant U54-GM-104942, an American Heart Association (AHA) Grant-in-Aid (13GRNT16420018), an AHA Predotoral Fellowship (10PRE3530011; to J. Huang), and the Office of Research and Graduate Programs/Health Sciences Center at West Virginia University. Microscope experiments and image analysis were performed in the West Virginia University Microscope Imaging Facility, supported in part by the Mary Babb Randolph Cancer Center and NIH Division of Research Resources Grant P20-RR-016440. The research performed by Y. -C. Lin was supported by the National Science Council (NSC) of the Executive Yuan, Taiwan (NSC 101-2320-B-034-001).
DISCLOSURES
The authors declare that they have no conflicts of interest, financial or otherwise, to declare.
AUTHOR CONTRIBUTIONS
Y.-C.L., J.H., and H.-G.Y. performed experiments; Y.-C.L., J.H., and H.-G.Y. analyzed data; Y.-C.L., J.H., S.H., K.H.M., R.H., M.E.D., and H.-G.Y. interpreted results of experiments; Y.-C.L., J.H., and H.-G.Y. prepared figures; Y.-C.L., J.H., S.H., K.H.M., R.H., M.E.D., and H.-G.Y. edited and revised manuscript; Y.-C.L., J.H., S.H., K.H.M., R.H., M.E.D., and H.-G.Y. approved final version of manuscript; H.-G.Y. conception and design of research; H.-G.Y. drafted manuscript.
ACKNOWLEDGMENTS
We thank Yu-Xiao Pei and Po-Ming Chang for their assistance in ECG data analysis.
Current address of J. Huang: Department of Neurology and Center for Neuroscience and Regeneration Research, Yale University School of Medicine, New Haven, CT 06510; and Rehabilitation Research Center, Veterans Affairs Connecticut Healthcare System, West Haven, CT 06516.
REFERENCES
- 1.Adan V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 67: 1725–1732, 2003. [PubMed] [Google Scholar]
- 2.Andalib A, Brugada R, Nattel S. Atrial fibrillation: evidence for genetically determined disease. Curr Opin Cardiol 23: 176–183, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Beinart R, Zhang Y, Lima JA, Bluemke DA, Soliman EZ, Heckbert SR, Post WS, Guallar E, Nazarian S. The QT interval is associated with incident cardiovascular events: the MESA study. J Am Coll Cardiol 64: 2111–2119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brioschi C, Micheloni S, Tellez JO, Pisoni G, Longhi R, Moroni P, Billeter R, Barbuti A, Dobrzynski H, Boyett MR, DiFrancesco D, Baruscotti M. Distribution of the pacemaker HCN4 channel mRNA and protein in the rabbit sinoatrial node. J Mol Cell Cardiol 47: 221–227, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obes Rev 6: 275–281, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Gertler A, Solomon G. Leptin-activity blockers: development and potential use in experimental biology and medicine. Can J Physiol Pharmacol 91: 873–882, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep 2: 311–318, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension 30: 619–623, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hedley PL, Jorgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, Kanters JK, Corfield VA, Christiansen M. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat 30: 1486–1511, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Hou N, Luo MS, Liu SM, Zhang HN, Xiao Q, Sun P, Zhang GS, Luo JD, Chen MS. Leptin induces hypertrophy through activating the peroxisome proliferator-activated receptor alpha pathway in cultured neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiol 37: 1087–1095, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Lin YC, Hileman S, Martin KH, Hull R, Yu HG. PP2 Prevents beta-Adrenergic Stimulation of Cardiac Pacemaker Activity. J Cardiovasc Pharmacol 63: 533–543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jermendy G, Koltai MZ, Pogatsa G. QT interval prolongation in type 2 (non-insulin-dependent) diabetic patients with cardiac autonomic neuropathy. Acta Diabetol Lat 27: 295–301, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J 115: 869–875, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol 91: 137–142, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Ren H, Xu ZR, Liu YJ, Yang XP, Liu JQ. Prevalence and risk factors of prolonged QTc interval among Chinese patients with type 2 diabetes. Exp Diabetes Res 2012: 234084, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YC, Huang J, Kan H, Castranova V, Frisbee JC, Yu HG. Defective calcium inactivation causes long QT in obese insulin-resistant rat. Am J Physiol Heart Circ Physiol 302: H1013–H1022, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circ Heart Fail 2: 676–683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGaffin KR, Zou B, McTiernan CF, O'Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res 83: 313–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med 144: 517–524, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Messerli FH, Nunez BD, Ventura HO, Snyder DW. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med 147: 1725–1728, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Forleo C, Luzzi G, Totaro P, De Nicolo M, Rizzon P. Dependency of premature ventricular contractions on heart rate. Am Heart J 133: 153–161, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113: 898–918, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Purdham DM, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol 287: H2877–H2884, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48: 1787–1793, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409–414, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Stern S, Sclarowsky S. The ECG in diabetes mellitus. Circulation 120: 1633–1636, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res 101: 759–767, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Hirano Y, Higashiuesato Y, Aizawa Y, Hayashi T, Inagaki N, Tana T, Ohya Y, Takishita S, Muratani H, Hiraoka M, Kimura A. Role of HCN4 channel in preventing ventricular arrhythmia. J Hum Genet 54: 115–121, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y, Yasunami M, Takishita S, Yamashina A, Ohe T, Sunamori M, Hiraoka M, Kimura A. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem 279: 27194–27198, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Villanueva EC, Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 32, Suppl 7: S8–S12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HG, George MS, Kim J, Wang C, Pitt GS. Ca2+/calmodulin regulates trafficking of Ca(V)1.2 Ca2+ channels in cultured hippocampal neurons. J Neurosci 27: 9086–9093, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkle RA. The relationship between ventricular ectopic beat frequency and heart rate. Circulation 66: 439–446, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev 11: CD002003, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res 101: 545–559, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P, Wang Z, Kuo CT, Nattel S. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation 119: 1576–1585, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res 77: 64–72, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 3: 108–113, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]