Novel findings include reversal of established hypertrophy, left atrial dilation, and diastolic dysfunction in a model of HCM treated with the antioxidant N-acetylcysteine. This was associated with a reduction in S-glutathionylation of myofilament proteins, which was increased in untreated controls, a decrease in myofilament Ca2+ response, and hastening of cross-bridge kinetics.
Keywords: sarcomeres, cardiac myosin-binding protein C, oxidative stress, diastolic dysfunction, S-glutathionylation
Abstract
S-glutathionylation of cardiac myosin-binding protein C (cMyBP-C) induces Ca2+ sensitization and a slowing of cross-bridge kinetics as a result of increased oxidative signaling. Although there is evidence for a role of oxidative stress in disorders associated with hypertrophic cardiomyopathy (HCM), this mechanism is not well understood. We investigated whether oxidative myofilament modifications may be in part responsible for diastolic dysfunction in HCM. We administered N-acetylcysteine (NAC) for 30 days to 1-mo-old wild-type mice and to transgenic mice expressing a mutant tropomyosin (Tm-E180G) and nontransgenic littermates. Tm-E180G hearts demonstrate a phenotype similar to human HCM. After NAC administration, the morphology and diastolic function of Tm-E180G mice was not significantly different from controls, indicating that NAC had reversed baseline diastolic dysfunction and hypertrophy in our model. NAC administration also increased sarco(endo)plasmic reticulum Ca2+ ATPase protein expression, reduced extracellular signal-related kinase 1/2 phosphorylation, and normalized phosphorylation of phospholamban, as assessed by Western blot. Detergent-extracted fiber bundles from NAC-administered Tm-E180G mice showed nearly nontransgenic (NTG) myofilament Ca2+ sensitivity. Additionally, we found that NAC increased tension cost and rate of cross-bridge reattachment. Tm-E180G myofilaments were found to have a significant increase in S-glutathionylation of cMyBP-C, which was returned to NTG levels upon NAC administration. Taken together, our results indicate that oxidative myofilament modifications are an important mediator in diastolic function, and by relieving this modification we were able to reverse established diastolic dysfunction and hypertrophy in HCM.
NEW & NOTEWORTHY
Novel findings include reversal of established hypertrophy, left atrial dilation, and diastolic dysfunction in a model of HCM treated with the antioxidant N-acetylcysteine. This was associated with a reduction in S-glutathionylation of myofilament proteins, which was increased in untreated controls, a decrease in myofilament Ca2+ response, and hastening of cross-bridge kinetics.
hypertrophic cardiomyopathy (HCM) often progresses to heart failure with preserved ejection fraction (HFpEF), an increasingly common diagnosis characterized by severely impaired diastolic function with little or no effect on systolic cardiac function. The etiology of HFpEF remains unclear, but with many risk factors that range from genetic predisposition, as seen in familial HCM, to common acquired conditions, such as hypertension and hyperlipidemia, HFpEF-related diagnoses are likely to rise in the coming years. However, there are few treatments targeted to relieve the diastolic dysfunction observed in HFpEF (4).
Despite strong implications for a role of redox-related modifications in HCM linked to mutations in sarcomeric proteins, there have been no studies investigating the potential role of reactive oxygen species (ROS)-induced exacerbation of the HCM phenotype at the level of oxidative modification of the myofilament proteins. Our previous studies indicate that ROS-related posttranslational modifications in sarcomeric proteins are likely to be a major mechanism in the diastolic abnormalities associated with familial HCM. These studies reported that ROS, associated with a hypertensive model of HFpEF, induced S-glutathionylation of cardiac myosin-binding protein C (cMyBP-C), which slowed cross-bridge kinetics and increased Ca2+ sensitivity. Ventricular myocytes from the model demonstrated diastolic abnormalities with no change in the Ca2+ transients, suggesting that the diastolic dysfunction was attributed to dysfunction at the level of the sarcomere. Restoring redox balance in this model by relieving NOS uncoupling restored diastolic function to control levels in correlation with a reduction of cMyBP-C S-glutathionylation to basal levels (5, 12).
In experiments reported here, we assessed the redox state and function of a transgenic (Tg) mouse model of familial HCM expressing a mutation in tropomyosin (Tm), where glutamic acid at residue 180 has been exchanged for a glycine (Tm-E180G). Tm-E180G mice develop severe diastolic dysfunction, hypertrophy, and left atrial dilation by 2 wk of age (1). Our previous studies reported that, compared with controls, this model demonstrates a significant increase in myofilament response to Ca2+ and diastolic dysfunction that is likely to trigger maladaptive remodeling (21, 26). Here, our current data show that Tm-E180G hearts display early signs of oxidative stress in the form of increased oxidative modifications of cMyBP-C and activation of the MAPK signaling cascade. Therefore, we hypothesized that treatment with the glutathione precursor N-acetylcysteine (NAC) may reverse the oxidative stress in our model and improve hypertrophy and diastolic dysfunction. NAC administration, beginning at 1 mo of age, reversed established cardiac hypertrophy and diastolic dysfunction in Tm-E180G mice while hastening cross-bridge kinetics and desensitizing the myofilaments to Ca2+ in both NTG and Tm-E180G mice. Our data support the hypothesis that altered redox state is a significant contributor to the familial HCM phenotype and that interventions restoring redox balance may be a viable therapeutic approach.
MATERIALS AND METHODS
All protocols and procedures involving the use of animals were given prior approval by the Institutional Animal Care and Use Committee of the University of Illinois in Chicago (with Association for Assessment and Accreditation of Laboratory Animal Care International accreditation). Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., revised 2011) published by the National Institutes of Health.
Tm-E180G transgenic mice.
We employed a transgenic mouse model in a FVB/N background that displayed a phenotype similar to human HCM, in which 65% of wild-type α-Tm is replaced with α-Tm-E180G (21). Previous studies have reported that from 2 wk of age Tg mice begin to exhibit cardiac hypertrophy, myocyte disarray, interstitial fibrosis, and diastolic dysfunction with preserved systolic function (1). Controls were non-Tg (NTg) FVB/N littermates.
Study design and NAC administration.
One-month-old male and female NTG and Tm-E180G littermates were administered regular drinking water or NAC (Sigma, St. Louis, MO) dissolved in drinking water administered at 250 mg·kg−1·day−1, based on prior data (14), for 4 wk. All mice were subjected to echocardiography before and after the 1-mo treatment. After 1 mo of NAC administration, mice were euthanized using a ketamine-xylazine mix (200 and 100 mg/kg, respectively), and hearts were extracted for functional and biochemical assessment.
Echocardiography.
Echocardiographic measurements were performed using a high-resolution transducer (Vevo 770 High Resolution Imaging System with a scan head center frequency of 30 MHz) in isoflurane-anesthetized 2-mo-old mice, as described previously (22). M-mode images of the left ventricle (LV), left ventricle outflow tract (LVOT), and left atrium (LA) were taken from the left parasternal long-axis view. The parasternal short-axis view at the level of the papillary muscles was used to measure the LV internal dimension, and pulse wave Doppler was performed at the apical four-chamber view. The mitral inflow was recorded with the Doppler sample volume at the tip of the mitral valve leaflets. To measure time intervals, the Doppler sample volume was moved toward the LVOT, and both the mitral inflow and LV outflow were obtained in the same recording. Three parameters of the LV diastolic function were evaluated: E/A ratio, E-wave deceleration time, and LV isovolumic relaxation time. Additional information about the diastolic function was obtained with tissue Doppler imaging. Peak myocardial velocities in the early (Em) diastole were obtained with the sample volume at the septal side of the mitral annulus in the four-chamber view. All measurements and calculations were averaged from three consecutive cycles and performed according to the American Society of Echocardiography guidelines (8, 16). Data analysis was performed offline using the Vevo 770 Analytic Software.
Assessment of β-myosin heavy chain expression.
Myofibrils were purified from ∼25.0 mg of liquid nitrogen frozen mouse heart tissue and 1-day-old neonatal mouse heart tissue for the analysis of β-myosin heavy chain expression. The tissue was homogenized twice in standard relaxation buffer (10 mM imidazole, pH 7.2, 75 mM KCl, 2 mM MgCl2, 2 mM EDTA, and 1 mM NaN3) with 1% (vol/vol) Triton X-100. Myofribrils were centrifuged, and the supernatant fraction was removed. The pellets were then washed once in standard relaxation buffer to remove the Triton X-100. The standard relaxation buffers contained both the protease (Sigma, St. Louis, MO) and phosphatase (Calbiochem, Darmsdat, Germany) inhibitors at a 1:100 dilution. The pellet was solubilized in 2× Laemlli sample buffer (Bio-Rad, Hercules, CA), and the protein concentration of the samples was determined with an RCDC assay kit (Bio-Rad). A 6% SDS-PAGE gel was prepared as described previously and run at a constant amperage of 20 mA until the dye front ran off (32). To visualize total protein, Coomassie blue stain was added for 30 min and then destained with 10% methanol and 10% acetic acid and imaged on a ChemiDoc XRS+ (Bio-Rad), using a Coomassie blue filter set. Band densities from gels were determined using ImageLab 3.0 software (Bio-Rad).
Immunoblotting.
Whole heart homogenates and enriched myofibrillar proteins were extracted from ventricles of NTg and Tm-E180G hearts from mice treated both with and without NAC, as described above. Protein expression levels and posttranslational modifications were implicated in oxidative signaling. Phosphorylated and total ERK1/2 (Cell Signaling Technology, Danvers, MA), phosphorylated phospholamban (PLN) Ser16, Thr17, and total PLN (Badrilla, Leeds, UK, and Upstate Biotechnology, Darmsdat, Germany), and sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2; Cell Signaling Technology) were assessed as described previously (24). An enriched fraction of sarcomeric proteins separated by SDS-PAGE and transferred to PVDF was also probed to determine whether there were altered phosphorylation states of cTnI (Ser23 and Ser24; Cell Signaling Technology), p-cMyBP-C Ser282 (Enzo Life Sciences, Farmingdale, NY) and total cMyBP-C (a generous gift from Richard L. Moss), and S-protein glutathione (Virogen, Watertown, MA). Blots were imaged using a Chemidoc XRS+ imager (Bio-Rad, Hercules, CA), and band densities were analyzed using ImageLab software (Bio-Rad).
Analysis of carbonylated proteins.
Carbonylated proteins were detected using the OxyBlot kit (EMD Millepore, Darmsdat, Germany), using enriched myofibrils, according to the manufacturer's instructions. Myofibrils were obtained as described above. The myofibrils were then derivitized using DNPH solution for 15 min at room temperature in the dark, neutralized, and loaded onto SDS-PAGE gels for Western blotting, as described above.
Simultaneous measurement of the force-Ca2+ relationship and cross-bridge kinetics.
Left ventricular papillary muscles were isolated, cut into fiber bundles of ∼200 μm in width and 3–4 mm in length, and detergent extracted (skinned) in a high-relaxing solution (10 mM EGTA, 41.89 mM K-Prop, 100 mM BES, 6.75 mM MgCl2, 6.22 mM Na2ATP, 10 mM Na2CrP, and 5 mM NaN3) with 1% Triton X-100 for 3–4 h at 4°C. Tension and ATPase rate were measured simultaneously using methods and a previously described experimental apparatus (33). The fiber bundles were mounted between a force transducer and displacement motor using aluminum T-clips, and the sarcomere length set to 2.2 μm using He-Ne laser diffraction. The width and diameter were measured at three points along the fiber bundle. Force per cross-sectional area was used to determine tension. The fiber was contracted initially at a saturating Ca2+ concentration (pCa 4.5), and sarcomere length was again adjusted to 2.2 μm. Sarcomere length remained constant throughout the rest of the experiment. ATPase activity was measured at 20°C, as described previously, and calibrated with rapid injections of ADP (0.5 nmol) with a motor-controlled syringe. The fiber was placed in relaxing solution for 2 min and then in the preactivation solution for 2–3 min each time before being placed in the activating solution for 1–2 min (until stabilization of force). The fiber bundle was then quickly returned to the relaxing solution. Various contraction-relaxation cycles were carried out using different ratios of total Ca2+ concentration to total EGTA concentration. The final contraction was again carried out at a saturating Ca2+ concentration. All experiments were carried out at 20°C. The relation between Ca2+ and tension or ATPase activity was fitted using a modified Hill equation (7, 28). We determined pCa values at half-maximum ATPase activity and force generation from mean data normalized to maximum activity. In all experiments, only fiber bundles retaining >80% of their initial maximum tension were included in the analysis.
Statistical analysis.
Statistical analysis was performed using Prism 6.0 software (GraphPad Software, La Jolla, CA), using two-way ANOVA followed by Tukey's post hoc test for multiple comparisons. Factors considered for statistical analysis were genotype and treatment. Data are presented as means ± SE with significance set at P < 0.05.
RESULTS
N-acetylcysteine improves cardiac morphology and diastolic function in hypertrophic cardiomyopathy.
Beginning at 1 mo of age, after HCM phenotypic changes were evident, NAC was administered to NTg and Tm-E180G mice (1). We conducted a noninvasive assessment of cardiac morphology and function in Tm-E180G and NTg mice, as summarized in Table 1. M-mode echocardiography demonstrated that Tm-E180G mice have significant left atrial dilation, which was reduced to NTG levels with NAC administration for 1 mo. Hearts of Tm-E180G mice demonstrated an increase in left ventricular mass with no change in internal diameter at diastole, which was characteristic of concentric hypertrophy. Treatment with NAC returned LV mass to NTg levels. Measurements of diastolic function and E/A and E/Em ratios were also decreased to NTg levels. Systolic function was unchanged in Tm-E180G mice.
Table 1.
NAC improves morphology and diastolic function in hypertrophic cardiomyopathy as assessed by echocardiography
| Parameter (Sample Size) | NTg (n = 6) | NTg NAC (n = 9) | Tm-E180G (n = 6) | Tm-E180G NAC (n = 9) |
|---|---|---|---|---|
| Males/females (n) | 3/3 | 3/6 | 3/3 | 5/4 |
| Body weight, g | 23.0 ± 1.73 | 21.7 ± 0.60 | 25.1 ± 1.57 | 21.8 ± 0.95 |
| Heart rate, beats/min | 500 ± 31 | 473 ± 15 | 428 ± 8 | 542 ± 21§ |
| LA size, mm | 1.26 ± 0.06 | 1.36 ± 0.08 | 2.19 ± 0.10**,†† | 1.58 ± 0.06*§ |
| LV mass, mg | 43.90 ± 5.27 | 49.17 ± 5.27 | 65.69 ± 5.21*† | 51.39 ± 3.95 |
| LVID;d, mm | 3.73 ± 0.09 | 3.69 ± 0.11 | 3.75 ± 0.14 | 3.47 ± 0.10 |
| LVID;s, mm | 2.28 ± 0.09 | 2.46 ± 0.10 | 2.26 ± 0.08 | 2.19 ± 0.10 |
| Relative wall thickness, mm | 0.34 ± 0.03 | 0.29 ± 0.03 | 0.37 ± 0.03 | 0.38 ± 0.04 |
| Ejection fraction, % | 69.95 ± 1.44 | 63.77 ± 2.61 | 70.98 ± 0.56 | 71.01 ± 1.47 |
| Fractional shortening, % | 38.89 ± 1.22 | 34.33 ± 1.94 | 39.65 ± 0.45 | 39.72 ± 1.18 |
| E-wave, mm/s | 774 ± 34 | 779 ± 38 | 831 ± 81 | 846 ± 47 |
| A-wave, mm/s | 516 ± 32 | 490 ± 26 | 308 ± 64*† | 540 ± 35§§ |
| Em-wave, mm/s | 26.23 ± 2.77 | 26.74 ± 2.24 | 20.34 ± 1.15*† | 35.85 ± 4.50*†§ |
| E/A ratio | 1.54 ± 0.14 | 1.63 ± 0.09 | 3.03 ± 0.32**,†† | 1.64 ± 0.15§ |
| E/Em ratio | 30.94 ± 3.10 | 30.40 ± 2.20 | 41.44 ± 2.49*† | 28.05 ± 3.30§ |
Values are means ± SE.
NAC, N-acetylcysteine; NTg, nontransgenic; Tm; tropomyosin; LA, left atrium; LV, left ventricular; LVID;d, LV internal dimension at diastole; LVID;s, LV internal dimension at systole.
P < 0.05 and
P < 0.001 vs. NTG;
P < 0.05 and
P < 0.0001 vs. Tm-E180G;
P < 0.01 and
P < 0.001 vs. NTg NAC.
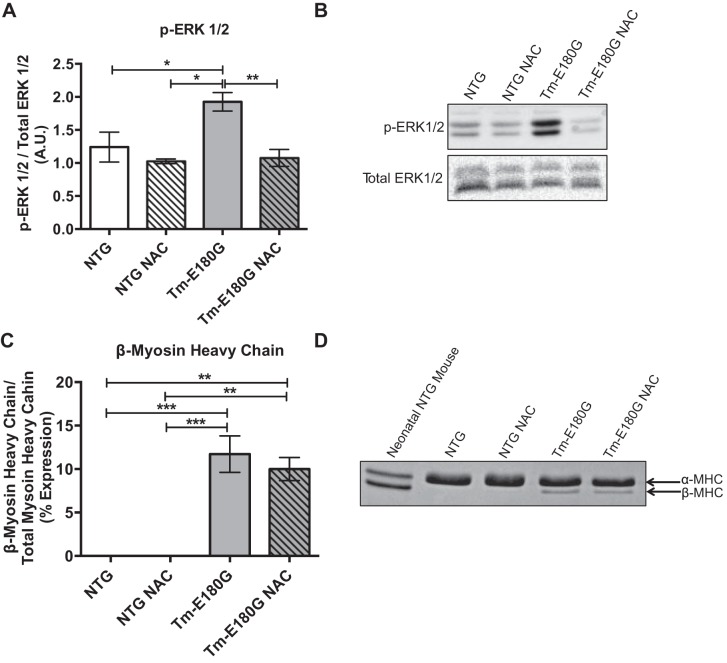
Activation of the MAPK pathway is implicated in the development of both physiological and pathological hypertrophy (6). Additionally, these pathways are activated by elevated ROS (15). Compared with controls, there was an increase in ERK1/2 phosphorylation in hearts from Tm-E180G mice, which returned to NTg levels with NAC administration (Fig. 1, A and B). Interestingly, this reversal of MAPK signaling and hypertrophy did not decrease levels of expression of the neonatal myosin heavy-chain isoform β-myosin heavy chain observed in our model (Fig. 1, C and D).
Fig. 1.
N-acetylcysteine (NAC) reduces ERK1/2 phosphorylation but not β-myosin heavy chain expression. A: tropomyosin (Tm)-E180G mice display increased ERK1/2 phosphorylation, which is reduced to nontransgenic (NTg) levels with NAC administration. B: representative Western blot images of phosphorylated (p)-ERK1/2 and total ERK1/2. C: Tm-E180G mice have increased β-myosin heavy chain expression, which is not reduced with NAC administration. D: representative 6% SDS-PAGE gel image showing β-myosin heavy chain expression. Data were analyzed by 2-way ANOVA, followed by Tukey's post hoc test for multiple comparisons and represented as means ± SE; n = 3–6 hearts/group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
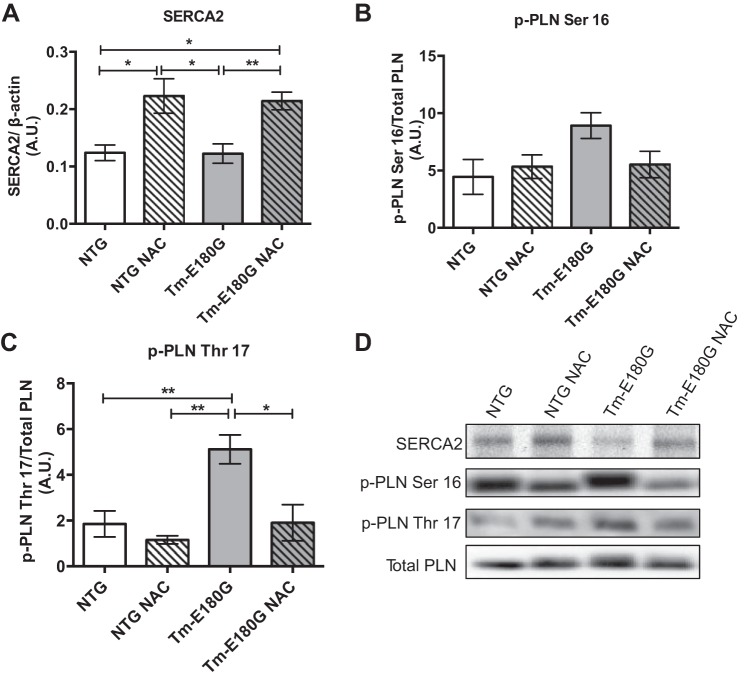
N-acetylcysteine affects SERCA2 expression and phosphorylation of PLN.
We have reported previously that despite changes in phosphorylation of PLN and expression of SERCA2 in ventricular myocytes isolated from Tm-E180G hearts compared with NTg controls, there were no changes in Ca2+ transients (26). However, early adenoviral-mediated overexpression of SERCA2a prevented the development of hypertrophy and fibrosis in Tm-E180G mice (20). Therefore, we tested for NAC-induced alterations in Ca2+ regulatory proteins PLN and SERCA2. NAC treatment increased the expression of total cardiac SERCA2 protein expression in both NTg and Tm-E180G hearts (Fig. 2, A and D). NAC treatment induced phosphorylation of PLN at Ser16 in Tm-E180G hearts to a level close to NTG-levels, but the values failed to reach statistical significance; however, PLN phosphorylation at Thr17 was significantly elevated in the hearts of Tm-E180G mice and returned to control levels following NAC treatment (Fig. 2, B–D).
Fig. 2.
NAC alters expression and posttranslational modification of Ca2+ regulatory proteins. A: NAC-administered mice have significantly increased sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) expression over controls. B: phosphorylation of phospholamban (PLN) at Ser16 is unchanged in all groups. C: p-PLN at Thr17 is significantly increased in Tm-E180G mice and is returned to NTg levels following NAC administration. D: representative Western blot images of SERCA2, p-PLN Ser16, p-PLN Thr17, and total PLN. Data were analyzed by 2-way ANOVA followed by Tukey's post hoc test for multiple comparisons and represented as means ± SE; n = 3–6 hearts/group. *P ≤ 0.05; **P ≤ 0.01.
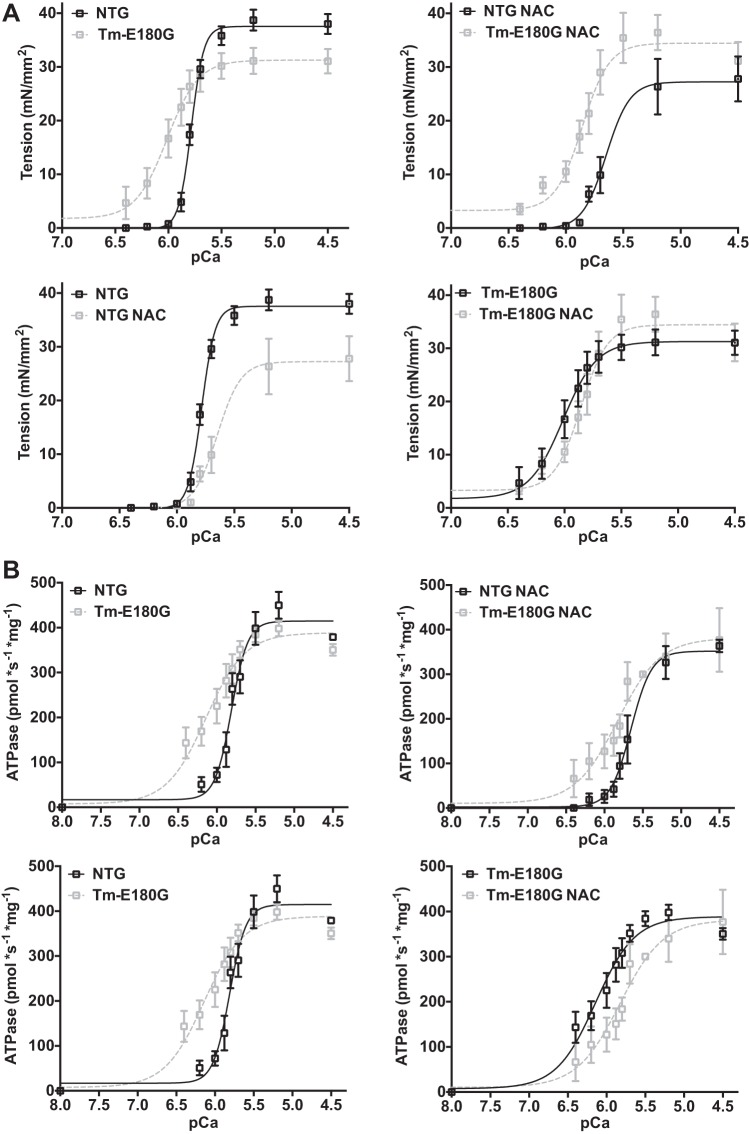
N-acetylcysteine decreases Ca2+ sensitivity and improves cross-bridge kinetics in detergent-extracted fiber bundles.
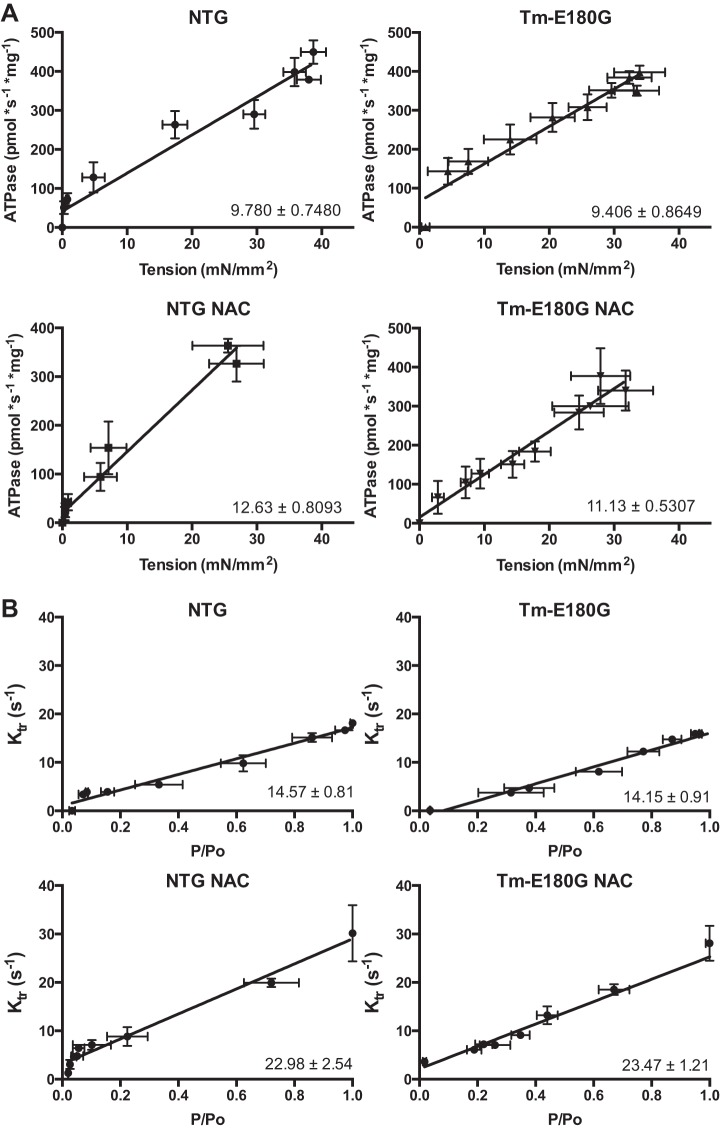
We employed detergent-extracted (skinned) fiber bundles from NTg and Tm-E180G hearts to determine the effect of antioxidant treatment on Ca2+ dependence of isometric tension, actomyosin ATPase rate, and the kinetics of tension redevelopment (ktr), a measurement of the rate of entry of cross-bridges into the force-generating state. Results are summarized in Table 2. Consistent with previous reports (21), there was a significant increase in Ca2+ sensitivity of tension, as determined from the pCa at half-maximal tension, in Tm-E180G skinned fiber bundles compared with NTg controls. After 1 mo of NAC administration, fiber bundles from both NTG and Tm-E180G hearts demonstrated a decrease in Ca2+ sensitivity of tension and ATPase. Maximum tension and ATPase rate were unchanged in fibers from both NTg and Tg hearts treated with NAC (Fig. 3A). Compared with NTg controls, the Ca2+ sensitivity of ATPase rate was increased significantly in Tm-E180G fibers, and NAC administration significantly reduced the Ca2+ sensitivity of the rate of ATP hydrolysis in both groups (Fig. 3B). NAC administration also resulted in a significant increase in ktr, the rate of cross-bridge reattachment, measured as a slope of a linear regression of ktr values plotted against relative tension (P/Po). Measurements of ktr at minimum P/Po revealed no changes between the groups. However, ktr at maximum P/Po revealed significant changes in NAC-administered NTg and Tm-E180G fibers, suggesting that as tension generation increases, NAC increases the rate of cross-bridge reattachment. The slope of the relation between tension and ATPase rate is a measurement of tension cost, is related to the rate of cross-bridge detachment, and followed the same trend as ktr values, where tension cost was increased in fibers excised from NAC-administered mice. (Fig. 4, A and B). Thus, NAC administration results in desensitization of the myofilaments to Ca2+ and increase in cross-bridge kinetics.
Table 2.
NAC decreases Ca2+ sensitivity while hastening cross-bridge kinetics
| Parameter (Sample Size, Fibers) | NTg (n = 7) | NTg NAC (n = 6) | Tm-E180G (n = 7) | Tm-E180G NAC (n = 6) |
|---|---|---|---|---|
| Maximum tension, mN/mm2 | 36.07 ± 1.84 | 27.78 ± 4.18 | 33.06 ± 2.27 | 31.03 ± 3.54 |
| pCa50 of tension | 5.78 ± 0.01 | 5.55 ± 0.06* | 6.01 ± 0.05**,†† | 5.84 ± 0.05†,§ |
| Max ATPase, pmol·s−1·mg−1 | 379 ± 0.07 | 364 ± 14.02 | 351 ± 12.91 | 377 ± 0.71 |
| pCa50 of ATPase | 5.73 ± 0.05 | 5.68 ± 0.03 | 6.17 ± 0.07**,†† | 5.86 ± 0.09§ |
| Tension cost | 9.78 ± 0.74 | 12.63 ± 0.80** | 9.41 ± 0.74†† | 11.13 ± 0.53**,§§ |
| ktr Slope, s−1/(P/Po) | 14.57 ± 0.81 | 22.98 ± 2.54** | 14.15 ± 0.91†† | 23.47 ± 1.21**,§§ |
| ktr (s−1) at minimum P/Po | 3.76 ± 0.30 | 3.62 ± 0.88 | 3.17 ± 0.50 | 4.90 ± 1.02 |
| ktr (s−1) at maximum P/Po | 17.63 ± 0.74 | 28.20 ± 4.55* | 13.77 ± 0.92†† | 27.93 ± 1.77*,§§ |
Values are means ± SE.
pCa50, pCa at half-maximal tension; ktr, kinetics of tension redevelopment.
P < 0.05 and
P < 0.001 vs. NTg;
P < 0.05 and
P < 0.001 vs. Tm-E180G; †P < 0.05 and
P < 0.001 vs. NTg NAC.
Fig. 3.
NAC desensitizes the myofilament to Ca2+ in NTg and Tm-E180G myofilaments. A: comparison of pCa-tension relationships of NTg, NTg NAC, Tm-E180G, and Tm-E180G NAC mice. B: comparison of Ca2+ sensitivity of ATPase rates among NTg, NTg NAC, Tm-E180G, and Tm-E180G NAC mice. Data are represented as means ± SE; n = 6–7 fibers, 3–5 hearts, per group.
Fig. 4.
NAC increases cross-bridge kinetics in NTg and Tm-E180G myofilaments. A: in untreated NTg and Tm-E180G mice, there is no difference in tension cost. Upon NAC administration, there was a significant increase in tension cost of both NTg and Tm-E180G mice. B: NAC increases the rate of tension redevelopment (ktr) in both NTg and Tm-E180G mice. Data are represented as means ± SE; n = 6–7 fibers, 3–5 hearts, per group.
N-acetylcysteine alters phosphorylation and oxidative modifications of myofilament proteins.
Regulatory redox mechanisms that involve posttranslational modifications may account for the alterations in the cross-bridge kinetics found in Tm-E180G hearts. Therefore, we assessed the phosphorylation and S-glutathionylation levels of myofilament proteins (5, 12). Previously, we identified three S-glutathionylation sites on cMyBP-C (19).
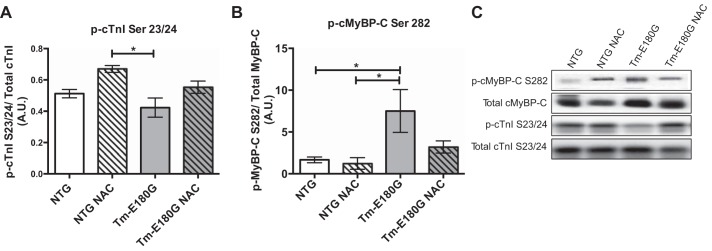
At 2 mo of age, phosphorylation of cMyBP-C Ser282 was increased in Tm-E180G mice, which was reduced upon NAC administration (Fig. 5, B and C). Phosphorylation of cTnI at Ser23/24 was decreased in Tm-E180G mice compared with NTG NAC-administered mice. NAC was associated with increased cTnI phosphorylation in Tm-E180G mice administered NAC; however, this value was not significant from controls (Fig. 5, A and C). No other differences in myofilament protein phosphorylation were found using ProQ diamond phosphoprotein stain (data not shown).
Fig. 5.
NAC alters posttranslational modification of myofilament proteins. A: phosphorylation of cTnI in Tm-E180G mice was decreased compared with NTg NAC mice. B: phosphorylation of cMyBP-C was increased in Tm-E180G mice. C: representative Western blot images of p-cMyBP-C Ser282, total cMyBP-C, p-cTnI Ser23/24, and total cTnI. Data were analyzed by 2-way ANOVA followed by Tukey's post hoc test for multiple comparisons and represented as means ± SE; n = 4–6 hearts/group. *P ≤ 0.05.
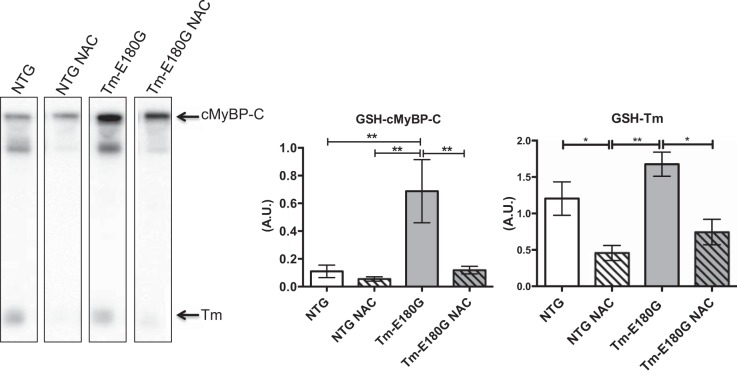
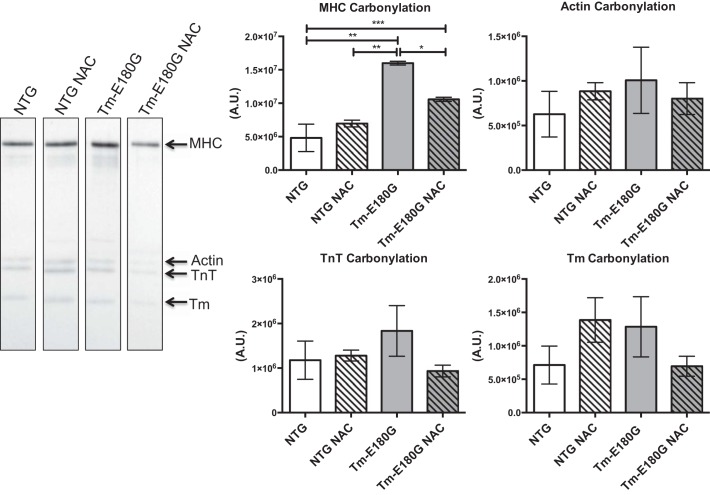
Functionally, S-glutathionylation results in increased Ca2+ sensitivity with depressed cross-bridge kinetics. Therefore, we investigated whether S-glutathionylation of cMyBP-C contributes to diastolic dysfunction in HCM (5, 12). We found that Tm-E180G mice exhibit a significant increase in S-glutathionylation of cMyBP-C. NAC reduced the levels of S-glutathionylation of cMyBP-C to NTg control levels (Fig. 6). Further analysis revelaed that S-glutathionylation of Tm was significantly reduced in NAC-administered NTG and Tm-E180G mice. Additionally, we performed dinitorphenol (DNP) derivitization to determine the extent of carbonylation of myofilament proteins. Western blot analysis revealed that there was a significant increase in carbonylation of myosin heavy chain (MHC) in Tm-E180G mice compared with NTG and NTG NAC mice, which was reversed to NTG levels upon NAC administration (Fig. 7). No other changes in myofilament protein carbonylation were observed.
Fig. 6.
NAC decreased S-glutathionylation of cMyBP-C and tropomyosin. cMyBP-C glutathionylation was significantly increased in Tm-E180G mice, which was reduced to NTg control and NTg NAC-levels after NAC administration. Tropomyosin S-glutathionylation was significantly reduced in NTg NAC- and Tm-E180G NAC-administered mice compared with NTg and Tm-E180G controls. Data were analyzed by 2-way ANOVA followed by Tukey's post hoc test for multiple comparisons and represented as means ± SE; n = 4 hearts/group. *P ≤ 0.05; **P ≤ 0.01.
Fig. 7.
NAC decreases myosin heavy chain carbonylation. Myosin heavy chain carbonylation was increased significantly in Tm-E180G mice, which was reduced to NTg control and NTg NAC levels after NAC administration. Actin, troponin T, and Tm carbonylation were unchanged. Data were analyzed by 2-way ANOVA followed by Tukey's post hoc test for multiple comparisons and represented as means ± SE; n = 3 hearts/group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
DISCUSSION
Our data are the first to report that NAC administration is able to reverse an increase in myofilament Ca2+ sensitivity and diastolic dysfunction associated with S-gluathionylation of cMyBP-C in HCM. Moreover, we report novel evidence that administration of NAC alters expression of SERCA2 as well as phosphorylation of PLN. Previous studies reported that exogenous NAC treatment of models with HCM linked to a cTnT mutation is able to decrease fibrosis and hypertrophy (5, 12, 14). Additional studies were completed in rabbits containing an HCM-linked mutation on cardiac myosin heavy chain, where NAC administration was shown to have similar beneficial effects as in the cTnT murine model (10). Our studies significantly extend these findings in that in addition to reversing the hypertrophy and increase in left atrial size, we report evidence for a mechanistic basis for the reversal of diastolic abnormalities in HCM at the level of the myofilaments. The improvement of the diastolic abnormality was associated with the effects of NAC treatment on reducing the elevated Ca2+ sensitivity of Tm-E180G skinned fiber bundles and the hastening of cross-bridge kinetics.
A novel finding related to the improvement of diastolic function is a reversal of S-glutathionylation of cMyBP-C. Our previous studies reported a strong correlation of increased cMyBP-C S-glutathionylation with slowing of cross-bridge kinetics and an increase in myofilament response to Ca2+ in a model of diastolic heart failure (5, 12). In the present study, we did not find a significant decrease in ktr in skinned fiber bundles from Tm-E180G mice compared with NTg controls. We did find that NAC administration hastened ktr and increased tension cost in both NTg and Tm-E180G skinned fiber bundles, suggesting that the improvement in diastolic function is related to a reduction in oxidative stress and an associated increase in cross-bridge kinetics.
The functional significance of cMyBP-C has generally been considered as a “brake” on the reaction of cross-bridges with the thin filament and as a desensitizer of the myofilaments to Ca2+ (23). In Tm-E180G mice, we report differential phosphorylation of cMyBP-C, where phosphorylation of Ser282 is increased. Although we observed a trend toward normalization in phosphorylation of Ser282 in Tm-E180G mice, it was not significant. Additionally, this Ca2+-desensitizing phosphorylation of cMyBP-C is present despite the constitutive increase in myofilament Ca2+ responsiveness we observed, suggesting that S-glutathionylation of cMyBP-C is dominant in its Ca2+-sensitizing effect. Previously, we have identified that S-glutathionylation of cMyBP-C occurs at cysteine residues (C479, C627, C655) in the C3, C4, and C5 domains, whereas phosphorylation is known to occur at the M domain of the COOH terminus (19). Similar to phosphorylation, S-glutathionylation imparts a negative charge (27). Phosphorylation is thought to induce a Ca2+-desensitizing effect by inducing steric hindrance of the myosin SI catalytic unit (18). S-glutathionylation may exert its dominant effect by performing the opposite, modifying the radial disposition of cMyBP-C toward the thick filament, where the C5 domain has been shown to interact away from myosin (19). A direct effect may be exerted by the increase in MHC carbonylation in Tg mice that was reversed with NAC administration. Reports have suggested MHC carbonylation is involved in the desensitization of the myofilament to Ca2+ and depression in cardiac function (25). However, there is no consensus as to the functional effects of myofilament protein carbonylation, and we note that many studies are not extended to other oxidative modifications of myofilament proteins. Combinational control of cross-bridge kinetics and Ca2+ sensitivity by various posttranslational modifications is likely to play an effect, as it has been demonstrated in other thin filament proteins under scenarios of phosphorylation (17). Such an investigation in oxidative modifications is likely to be difficult, as approaches involving mutagenesis to mimic these posttranslational modifications are not available. Ultimately, future studies will shed light on the structural/functional changes due to cMyBP-C and myosin posttranslational modification.
Less clear is the effect of S-glutathionylation on tropomyosin. Previously, we have shown that Tm is able to be S-glutathionylated after in vitro incubation with oxidized glutathione (19). To our knowledge, this work is the first to suggest S-glutathionylation of Tm in vivo. The Tm-E180G mutation results in decreased thermal stability and increased flexibility, and it has been suggested that this could contribute to the increase in Ca2+ sensitivity by allowing the interaction of strongly bound cross-bridges with actin in the absence of Ca2+ (9, 11, 13). Tm contains one cysteine at residue 190, which is important for the maintenance of the coiled-coil structure by stabilizing ionic interactions throughout the molecule. Experiments performed in vitro where 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was used to disrupt the disulfide bond created at C190, which is important in maintaining the coiled-coil structure of Tm, result in a decreased affinity of Tm for actin. The structural consequences of this “cross-linking”, or disruption of the disulfide bond, induce partial unfolding of the molecule at physiological temperatures and increased regional flexibility, most likely through a destabilization of the structure, similar to and in the same region as the Tm-E180G mutation (31). S-glutathionylation at C190 may also disrupt the disulfide bond, resulting in the same effect as DTNB, causing a local destabilization of the molecule and promoting Ca2+ sensitivity. This hypothesis could explain the decrease in Ca2+ sensitivity observed in NTg NAC-administered mice, as we report a significant decrease in S-glutathionylation of NTg NAC mice compared with NTg controls, although unlike the transgenic groups, no change was seen in S-glutathionylation of cMyBP-C. The same effect on S-glutathionylation of Tm was seen in Tm-E180G NAC mice.
Peña et. al. (20) showed that early adenoviral overexpression of SERCA2a is able to delay the development of fibrosis and hypertrophy in Tm-E180G mice. However, SERCA2a overexpression was unable to reverse myofilament modifications and baseline diastolic dysfunction in this model. Additionally, mRNA levels of heart failure markers ANP, BNP, and β-myosin heavy chain were unchanged as the SERCA2a-overexpressing Tm-E180G mice aged. Although we also found that β-myosin heavy chain expression was unchanged with NAC, in our present work we found both a functional and morphological improvement with NAC administration. Unexpectedly, we found a significant increase in SERCA2 protein expression in both groups treated with NAC along with reversal of hypertrophy in Tm-E180G mice. The mechanism by which NAC increases SERCA2 expression is unclear. Supporting our findings, in a model of inflammatory signaling-mediated diastolic dysfunction, SERCA2a expression was reduced. The authors who made this observation attributed this reduction in SERCA2a to activation of NF-κB. Treating cells and rats with NF-κB blockers, as well as statins, improved SERCA2a expression in these models (30). Additionally, NAC administration prevented the activation of NF-κB in the hearts of brain-dead Ba-Ma miniature pigs (34).
In summary, we have demonstrated that antioxidant administration improves diastolic dysfunction, hypertrophy, Ca2+ regulatory protein expression, cross-bridge cycling kinetics, and posttranslational modification of myofilament proteins to relieve maladaptation in HCM. Although much work has been done toward generating drugs acting as Ca2+ sensitizers or sarcomere activators, no Ca2+-desensitizing therapies exist (3, 29). Our work provides novel evidence for a Ca2+-desensitizing therapy of an approved drug. We hypothesize that desensitization of the myofilaments to Ca2+ and improvement of diastolic dysfunction can be attributed to the reduction in S-glutathionylation of myofilament proteins. Therefore, our work suggests that reducing the oxidative load on the cell may be an effective treatment for HCM. We look forward to the results of a clinical trial that is underway investigating the effect of NAC on human populations with HCM induced by sarcomeric mutations (clinical trial identifier NCT01537926).
GRANTS
Our work was supported by National Institute of Health Grants P01-HL-62426 (Project 1; to R. J. Solaro and B. M. Wolska) and T32-HL-007692, an American Physiological Society Porter Fellowship (to T. Wilder), and American Heart Association Grant 15PRE22180010 (to D. M. Ryba).
DISCLOSURES
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
T.W., D.M.R., D.F.W., B.M.W., and R.J.S. conception and design of research; T.W. and D.M.R. performed experiments; T.W., D.M.R., and R.J.S. analyzed data; T.W., D.M.R., D.F.W., B.M.W., and R.J.S. interpreted results of experiments; T.W. and D.M.R. prepared figures; T.W., D.M.R., and R.J.S. drafted manuscript; T.W., D.M.R., and R.J.S. edited and revised manuscript; T.W., D.M.R., D.F.W., B.M.W., and R.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Richard L. Moss for the generous gift of total cMyBP-C antibody. We are grateful to Paul T. Mungai for helpful comments and conversation.
REFERENCES
- 1.Alves ML, Dias FA, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RT 3rd, Sadayappan S, Robbins J, Wieczorek DF, Solaro RJ, Wolska BM. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet 7: 132–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves ML, Gaffin RD, Wolska BM. Rescue of familial cardiomyopathies by modifications at the level of sarcomere and Ca2+ fluxes. J Mol Cell Cardiol 48: 834–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 32: 670–679, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC Jr, Solaro RJ. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol 56: 44–54, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122: 2727–2735, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem 281: 13471–13477, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Lehrer SS, Geeves MA. The myosin-activated thin filament regulatory state, M(−)-open: a link to hypertrophic cardiomyopathy (HCM). J Muscle Res Cell Motil 35: 153–160, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, Willerson JT, Betocchi S, Wickline SA, Efimov IR, Marian AJ. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation 119: 1398–1407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loong CK, Zhou HX, Chase PB. Familial hypertrophic cardiomyopathy related E180G mutation increases flexibility of human cardiac alpha-tropomyosin. FEBS Lett 586: 3503–3507, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity/novelty and significance. Circ Res 110: 841–850, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly S, Lehrer SS. Long-range effects of familial hypertrophic cardiomyopathy mutations E180G and D175N on the properties of tropomyosin. Biochemistry 51: 6413–6420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol 47: 827–834, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matos TJ, Duarte CB, Goncalo M, Lopes MC. Role of oxidative stress in ERK and p38 MAPK activation induced by the chemical sensitizer DNFB in a fetal skin dendritic cell line. Immunol Cell Biol 83: 607–614, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10: 165–193, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol 72: 177–185, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer BM, Sadayappan S, Wang Y, Weith AE, Previs MJ, Bekyarova T, Irving TC, Robbins J, Maughan DW. Roles for cardiac MyBP-C in maintaining myofilament lattice rigidity and prolonging myosin cross-bridge lifetime. Biophys J 101: 1661–1669, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel BG, Wilder T, Solaro RJ. Novel control of cardiac myofilament response to calcium by S-glutahionylation at specific sites of myosin binding protein C. Front Physiol 4: 336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peña JR, Szkudlarek AC, Warren CM, Heinrich LS, Gaffin RD, Jagatheesan G, del Monte F, Hajjar RJ, Goldspink PH, Solaro RJ, Wieczorek DF, Wolska BM. Neonatal gene transfer of Serca2a delays onset of hypertrophic remodeling and improves function in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 49: 993–1002, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro JR, Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol 33: 1815–1828, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation 121: 410–418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadayappan S, de Tombe PP. Cardiac myosin binding protein-C as a central target of cardiac sarcomere signaling: a special mini review series. Pflugers Arch 466: 195–200, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF. Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem 288: 28925–28935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao CH, Rozanski GJ, Nagai R, Stockdale FE, Patel KP, Wang M, Singh J, Mayhan WG, Bidasee KR. Carbonylation of myosin heavy chains in rat heart during diabetes. Biochem Pharmacol 80: 205–217, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan KA, Arteaga GM, Hinken AC, Dias FA, Ribeiro C, Wieczorek DF, Solaro RJ, Wolska BM. Functional effects of a tropomyosin mutation linked to FHC contribute to maladaptation during acidosis. J Mol Cell Cardiol 50: 442–450, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelton MD, Chock BP, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Takeda S, Kobayashi T, Taniguchi H, Hayashi H, Maeda Y. Structural and functional domains of the troponin complex revealed by limited digestion. Eur J Biochem 246: 611–617, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Tardiff JC, Carrier L, Bers DM, Poggesi C, Ferrantini C, Coppini R, Maier LS, Ashrafian H, Huke S, van der Velden J. Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc Res 105: 457–470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai CT, Wu CK, Lee JK, Chang SN, Kuo YM, Wang YC, Lai LP, Chiang FT, Hwang JJ, Lin JL. TNF-α down-regulates sarcoplasmic reticulum Ca2+ ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-κB to promoter response element. Cardiovasc Res 105: 318–329, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Walsh T, Wegner A. Effect of the state of oxidation of cysteine 190 of tropomyosin on the assembly of the actin-tropomyosin complex. Biochim Biophys Acta 626: 79–87, 1980. [DOI] [PubMed] [Google Scholar]
- 32.Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149–151, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, de Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express beta-tropomyosin. Circ Res 84: 745–751, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Zhai W, Feng R, Huo L, Li J, Zhang S. Mechanism of the protective effects of N-acetylcysteine on the heart of brain-dead Ba-Ma miniature pigs. J Heart Lung Transplant 28: 944–949, 2009. [DOI] [PubMed] [Google Scholar]