Abstract
Key points
Ageing has negative effects on cardiac function.
Endurance training in young subjects is known to improve cardiac function and the ability to deliver blood to exercising tissue.
This study shows that lifelong endurance training maintains cardiac pumping function at a level similar to sedentary young subjects and by the same mechanisms.
Healthy untrained elderly subjects have maintained cardiac pumping function but use compensatory mechanisms similar to what is seen in patients with heart failure.
Healthy ageing should include regular physical activity to maintain cardiac function.
Abstract
Age‐related decline in cardiac function can be prevented or postponed by lifelong endurance training. However, effects of normal ageing as well as of lifelong endurance exercise on longitudinal and radial contribution to stroke volume are unknown. The aim of this study was to determine resting longitudinal and radial pumping in elderly athletes, sedentary elderly and young sedentary subjects. Furthermore, we aimed to investigate determinants of maximal cardiac output in elderly. Eight elderly athletes (63 ± 4 years), seven elderly sedentary (66 ± 4 years) and ten young sedentary subjects (29 ± 4 years) underwent cardiac magnetic resonance imaging. All subjects underwent maximal exercise testing and for elderly subjects maximal cardiac output during cycling was determined using a dye dilution technique. Longitudinal and radial contribution to stroke volume did not differ between groups (longitudinal left ventricle (LV) 52–65%, P = 0.12, right ventricle (RV) 77–87%, P = 0.16, radial 7.9–8.6%, P = 1.0). Left ventricular atrioventricular plane displacement (LVAVPD) was higher in elderly athletes and young sedentary compared with elderly sedentary subjects (14 ± 3, 15 ± 2 and 11 ± 1 mm, respectively, P < 0.05). There was no difference between groups for RVAVPD (P = 0.2). LVAVPD was an independent predictor of maximal cardiac output (R 2 = 0.61, P < 0.01, β = 0.78). Longitudinal and radial contributions to stroke volume did not differ between groups. However, how longitudinal pumping was achieved differed; elderly athletes and young sedentary subjects showed similar AVPD whereas this was significantly lower in elderly sedentary subjects. Elderly sedentary subjects achieved longitudinal pumping through increased short‐axis area of the ventricle. Large AVPD was a determinant of maximal cardiac output and exercise capacity.
Abbreviations
- AVPD
atrioventricular plane displacement
- BSA
body surface area
- CO
cardiac output
- EDV
end‐diastolic volumes
- ESV
end‐systolic volumes
- ICG
indocyanine green
- LA
left atrium
- LV
left ventricle
- LVM
left ventricular mass
- MRI
magnetic resonance imaging
- RA
right atrium
- RV
right ventricle
- SV
stroke volume
- SVlong
longitudinal contribution to stroke volume
- THV
total heart volume
Introduction
Normal healthy ageing decreases ventricular volumes, and systolic and diastolic functions are also decreased (Kitzman et al. 1991; Pearson et al. 1991; Hudsmith et al. 2005; Maceira et al. 2006 a, b). Several studies over the past century have shown that in young and elderly athletes, long‐term endurance training increases cardiac volumes and improves function (Henschen, 1899; Nicolai & Zuntz, 1914; Seals et al. 1994; Bouvier et al. 2001; Scharhag et al. 2002; Arbab‐Zadeh et al. 2004; Steding et al. 2010). However, it is unknown how lifelong endurance training affects cardiac function measured as longitudinal and radial contribution to stroke volume (SV).
Longitudinal pumping has been shown to be of importance for ventricular function, allowing the atria and ventricle to fill reciprocally (Hamilton & Rompf, 1932). With only radial pumping, ejection fraction of the left ventricle would be <30% (Henein & Gibson, 1999). Furthermore, radial pumping is of importance for ventricular filling where the rapid relaxation after radial contraction causes a drop in ventricular pressure and suction of blood into the ventricle from the atrium (Katz, 1930; Brecher, 1956; Yellin et al. 1990). Thus, maintained cardiac pumping mechanics are necessary for optimal cardiac output delivery. Using cardiac magnetic resonance imaging (MRI), these new aspects of the elderly heart can be investigated.
The purpose of this study was therefore to explore the hypothesis that longitudinal and radial contribution to SV is affected by age and that lifelong endurance training can prevent or reduce these effects. We aimed to determine cardiac volumes and longitudinal and radial contributions to SV at rest in three groups: elderly healthy sedentary subjects, elderly athletes with lifelong experience of endurance training and young healthy sedentary subjects. Furthermore, we aimed to investigate the relationship between the functional capacity of the heart to deliver cardiac output during maximal exercise related to cardiac volumes and function.
Methods
The study was approved by the ethics committee of Copenhagen, Denmark (elderly subjects), and the regional ethics committee in Lund, Sweden (young subjects), and was conducted in accordance with the Declaration of Helsinki. All study subjects provided oral and written informed consent. Cardiac MRI examinations of elderly subjects were performed at the Danish Centre for Magnetic Resonance, Hvidovre Hospital, Denmark, and invasive exercise measurements were taken at the Copenhagen Muscle Research Centre, Rigshospitalet, Denmark, on separate days. Cardiac MRI examinations of young subjects were performed at Skåne University Hospital in Lund, Sweden.
Study population
Eight male elderly athletes aged 63 ± 4 years, seven matched sedentary control subjects aged 66 ± 4 years and ten young sedentary subjects aged 29 ± 4 years were included. Elderly athletes were active in road cycling and had a history of more than 5 h of high‐intensity exercise training per week for the last 30 years. Elderly sedentary and young sedentary subjects did not participate in any regular physical training. None of the subjects had been diagnosed with cardiovascular disease, renal dysfunction, insulin resistance, diabetes or hypercholesterolaemia. Resting blood pressure was measured using an automatic cuff (Omron M6 comfort, Kyoto, Japan) or a manual sphygmanometer.
Cardiac MRI
Cardiac MRI was performed in the supine position using a 1.5 T Siemens Avanto scanner (Siemens, Erlangen, Germany) with a body matrix coil or in a 1.5 T Philips Achieva (Philips, Best, The Netherlands) with a 32 channel coil. Images of the heart were acquired using steady‐state free precession sequences with retrospective ECG triggering. After defining the long‐axis orientation of the heart, short‐axis images covering the entire heart from the base of the atria to the apex of the ventricles were obtained. Resting heart rate was obtained from ECG during image acquisition.
Measurements of cardiac volumes
All measurements were performed using the software Segment (Segment 1.9; http://segment.heiberg.se) (Heiberg et al. 2010).
Left and right atrial maximal volumes were defined as the largest volume just before the start of diastolic filling of the ventricles. Volumes were measured in short‐axis images using planimetry and the endocardial borders of the atria were manually defined as previously described (Mosen & Steding‐Ehrenborg, 2014). Left and right ventricular end‐diastolic and end‐systolic volumes (EDV, ESV) and left ventricular mass (LVM) were measured in short‐axis images, manually defining endocardial and epicardial borders of the ventricular myocardium. Papillary muscles were not included in LVM. Total heart volume (THV) was measured in short‐axis images as previously described (Carlsson et al. 2004) and was defined as the volume of all structures within the pericardium, including myocardium, blood pool, atria, pericardial fluid and the proximal parts of the great vessels (Fig. 1).
Figure 1. Example of delineations for total heart volume in short‐axis slices .
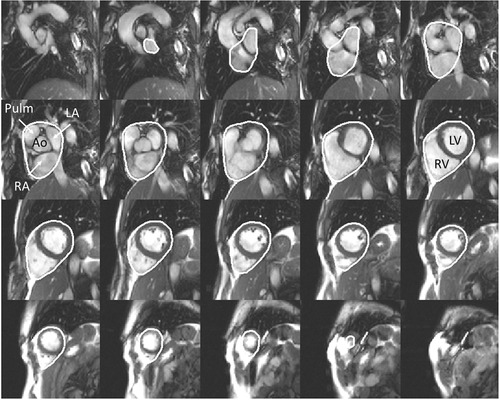
All structures within the pericardial sac were included in the total heart volume: left and right ventricle, left and right atria, aortic and pulmonary trunk. Ao, aorta; Pulm, pulmonary artery; LV, left ventricle; RV, right ventricle; LA, left atria; RA, right atria.
Longitudinal and radial pumping
Atrioventricular plane displacement (AVPD) was determined from long‐axis images (Carlsson et al. 2007 b; Steding‐Ehrenborg et al. 2013). In short, the atrioventricular plane was tracked over the cardiac cycle and AVPD was defined as the distance between the location of the atrioventricular plane in end‐diastole and its location in end‐systole.
SV achieved by longitudinal pumping was calculated by multiplying AVPD with the short‐axis area of the ventricle (SVlong) and longitudinal contribution to SV was determined by dividing SVlong by ventricular SV (LVSVlong(%)) as previously described (Carlsson et al. 2007 b; Steding‐Ehrenborg et al. 2013) Radial pumping was determined from THV variation (Carlsson et al. 2007 a).
Diastolic function measured as peak filling rate and systolic function measured as peak emptying rate of the left ventricle were calculated from the derivative of the time/volume curve (Maceira et al. 2006 a).
Invasive exercise measurements
Prior to the experimental days, elderly subjects completed an incremental (20 W min–1) cycling trial to exhaustion to determine peak workload. Young subjects were retrospectively enrolled and did not participate in invasive measurements.
For determination of indocyanine green (ICG; Pulsion Medical Systems, Feldkirchen, Germany) concentration, a 20 gauge catheter (Arrow, ES‐14150, Reading, PA, USA) was inserted percutaneously using the Seldinger technique into the right femoral artery, 2–5 cm below the inguinal ligament and advanced 8 cm in the proximal direction under local anaesthesia (2% lidocaine). An 18 gauge venous catheter was inserted into an antebrachial vein of the left arm to inject ICG. A three‐lead ECG was applied during catheterization and experimental procedures (Dialogue 2000, Danica, Copenhagen, Denmark), and the ECG and ICG were recorded simultaneously with a PowerLab data acquisition system (ADInstruments, Bella Vista, NSW, Australia).
Exercise protocol
After 30 min of supine rest, the subjects completed an incremental bicycle ergometer exercise test (Monark 839E or Monark 939 E, Vansbro, Sweden) until exhaustion. For elderly athletes and elderly sedentary subjects, exercise test started at 40–80 W and workload was increased by 40–80 W (i.e. 25, 50, 75 and 100% of the peak workload obtained in the initial cycling trial) every 3 min until exhaustion. For young sedentary subjects, test protocols were based on age, weight and self‐rated fitness level according to clinical practice. Protocols were chosen to yield an exercise duration of ∼8–12 min (Arena et al. 2007). The test continued until exhaustion, i.e. until the subjects stopped due to fatigue or until they could not keep the pedalling rate above 60 r.p.m. despite strong verbal encouragement. In all tests, the respiratory exchange ratio at task failure was >1.15.
Systemic oxygen consumption
Oxygen uptake () was measured continuously during exercise using an automated metabolic cart (Quark CPET system, Cosmed, Rome, Italy; or Oxycon Champion, Jaeger, Hochberg, Germany). The instruments were calibrated with a 3 litre volume syringe and two gases with varying [O2] and [CO2] prior to the experiment. Pulmonary gas measurements were averaged every 15 s (elderly) or 10 s (young).
Cardiac output
A bolus (5–8 mg) of ICG was rapidly infused into the antebrachial vein followed by a 10 ml flush of saline while blood was continuously withdrawn (20 ml min–1) from the femoral artery by an automated pump (Harvard Apparatus, Millis, MA, USA) through a linear photodensitometer (Waters Instruments Inc., Rochester, MN, USA) and the voltage recordings were collected with the data acquisition system. Withdrawn blood was re‐infused into the left antebrachial vein in a closed‐loop system. At the end of each experiment, a three‐point ICG–voltage calibration curve was derived from samples of the participant's blood and known volumes of ICG.
peak/THV ratio
The index peak (ml min−1)/THV (ml) (Engblom et al. 2010) was calculated and used to confirm or refute signs of heart failure in the population.
Statistical analysis
Statistical analysis was done using SPSS 20.0 (IBM, Chicago, IL, USA) and differences between groups were considered statistically significant at P <0.05. The Kruskal–Wallis non‐parametric test was used to identify variables where groups differed and the Mann–Whitney non‐parametric test was used to assess differences between groups. Linear regression analysis was performed to assess correlations between variables. In the elderly population stepwise multiple regression analysis was performed with maximal cardiac output (CO) as the independent variable and LVAVPD, THV, LVM and LVSVlong(%) were tested in the model. Regression analysis was performed separately in both elderly and young subjects with peak as the independent variable with THV, LVM, LVAVPD and LVSVlong(%)tested as dependent variables. Values are presented as mean ± SD unless stated otherwise.
Results
Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| Elderly athletes (n = 8) | Elderly sedentary (n = 7) | Young sedentary (n = 10) | |
|---|---|---|---|
| Age (years) | 64 ± 4 | 66 ± 4 | 29 ± 4 |
| Weight (kg) | 74 ± 8 | 80 ± 7 | 78 ± 8 |
| Height (cm) | 179 ± 7 | 175 ± 10 | 179 ± 3 |
| BSA (m2) | 1.91 ± 0.15 | 1.98 ± 0.11 | 1.95 ± 0.12 |
| SBP (mmHg) | 139 ± 13 | 145 ± 6 | 126 ± 6 |
| DBP (mmHg) | 76 ± 10 | 73 ± 12 | 75 ± 4 |
| peak (ml min−1 kg−1) | 42 ± 9 | 28 ± 7 | 44 ± 6 |
| peak (l min−1) | 3.0 ± 0.7 | 2.3 ± 0.4 | 3.0 ± 0.7 |
BSA, body surface area; DBP, diastolic blood pressure; SBP, systolic blood pressure, peak, peak oxygen uptake.
Cardiac volumes
Ventricular and atrial volumes are presented in Table 2. Resting CO and cardiac index (cardiac index = CO/body surface area (BSA)) measured with cardiac MRI did not differ between groups (CO 6.0 ± 1.3 l min−1 for elderly athletes, 6.3 ± 1.5 l min−1 for elderly sedentary and 6.9 ± 1.1 for young sedentary, P = 0.27; cardiac index 3.1 ± 0.5, 3.2 ± 0.8 and 3.5 ± 0.46 l m−2, P = 0.24 (Fig. 2)). At similar submaximal heart rates (119 ± 6 beats min−1), CO was higher in elderly athletes (18.7 ± 3.3 l min−1) than in sedentary elderly subjects (13.6 ± 1.0 l min−1; P = 0.003). CO during maximal exercise was 23.5 ± 3.3 l min−1 for elderly athletes (n = 8) and 13.6 ± 2.4 l min−1 for elderly sedentary subjects (n = 5; P = 0.003) and consequently also maximal cardiac index differed between groups (Fig. 2). There was no difference in maximal heart rate reached during exercise between the active and sedentary elderly subjects.
Table 2.
Cardiac volumes and mass determined from cardiac MR
| Elderly athletes (n = 8) | Elderly sedentary (n = 7) | Young sedentary (n = 10) | |
|---|---|---|---|
| THV (ml) | 1040 ± 237 | 806 ± 129 | 783 ± 90 |
| LVEDV (ml) | 212 ± 51 | 160 ± 24 | 183 ± 23 |
| LVSV (ml) | 119 ± 31 | 99 ± 19 | 107 ± 17 |
| LVM (g) | 151 ± 36 | 115 ± 12 | 120 ± 19 |
| RVEDV (ml) | 225 ± 58 | 180 ± 32 | 219 ± 27 |
| RVSV (ml) | 118 ± 30 | 97 ± 15 | 103 ± 15 |
| LAmax (ml) | 123 ± 35 | 90 ± 23 | 77 ± 19 |
| RAmax (ml) | 129 ± 34 | 101 ± 25 | 110 ± 18 |
| LVSVlong (%) | 65 ± 13 | 52 ± 9 | 55 ± 5 |
| RVSVlong (%) | 87 ± 13 | 78 ± 11 | 77 ± 7 |
| LVM/THV (g ml–1) | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.02 |
| LVEDV/THV | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.23 ± 0.01 |
| RVEDV/THV | 0.22 ± 0.02 | 0.22 ± 0.03 | 0.28 ± 0.02 |
| LAmax/THV | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.02 |
| RAmax/THV | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.01 |
| THV/BSA (ml m–2) | 540 ± 98 | 409 ± 72 | 401 ± 35 |
| LVM/BSA (ml m–2) | 78 ± 15 | 59 ± 7 | 61 ± 8 |
| LVEDV/BSA (ml m–2) | 110 ± 21 | 81 ± 14 | 94 ± 10 |
| RVEDV/BSA (ml m–2) | 116 ± 24 | 91 ± 21 | 112 ± 2 |
| LAmax/BSA (ml m–2) | 64 ± 16 | 46 ± 14 | 39 ± 8 |
| RAmax/BSA (ml m–2) | 72 ± 15 | 52 ± 15 | 56 ± 9 |
BSA, body surface area; LA, left atrium; LVEDV, left ventricular end‐diastolic volume; LVM, left ventricular mass; LVSV, left ventricular stroke volume; LVSVlong, longitudinal contribution to left ventricular stroke volume; RA, right atrium; RVEDV, right ventricular end‐diastolic volume; RVSV, right ventricular stroke volume; RVSVlong, longitudinal contribution to right ventricular stroke volume; THV, total heart volume.
Figure 2. Cardiac index at rest and exercise in athletes and sedentary subjects .
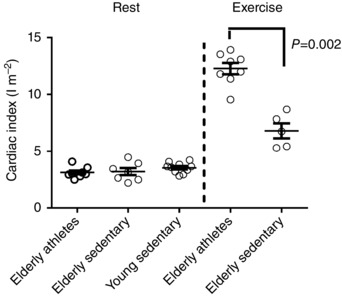
There was no difference between groups at rest. However, during maximal exercise, cardiac index was significantly higher in elderly athletes. Measurements at rest were performed using non‐invasive cardiac MRI, and at maximal exercise using the invasive dye dilution technique.
Longitudinal and radial pumping
LVAVPD was higher in elderly athletes and young sedentary compared to elderly sedentary subjects (14 ± 3, 15 ± 2 and 11 ± 1 mm, respectively, P < 0.05). There was no difference between groups for RVAVPD (elderly athletes 20 ± 4 mm, young sedentary 21 ± 3 mm, elderly sedentary 18 ± 3 mm, P = 0.2) (Fig. 3).
Figure 3. Left and right atrio‐ventricular plane displacement (AVPD) in elderly athletes, elderly sedentary and young sedentary .
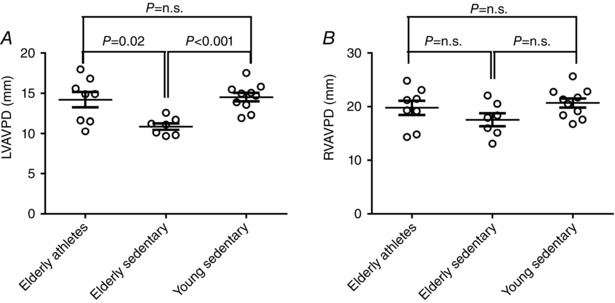
Elderly athletes had a preserved LVAVPD similar to what is seen in young sedentary subjects, whilst elderly sedentary subjects had decreased LVAVPD, A. There was no difference between groups for RVAVPD, B.
LV short‐axis areas used for calculations of longitudinal pumping were smaller in young sedentary compared to both elderly groups (54 ± 12 cm2 in elderly athletes, 46 ± 3 cm2 for elderly sedentary and 40 ± 5 cm2 for young sedentary; P < 0.05) whilst there was no significant difference between elderly groups (P = 0.23). Similarly, RV short‐axis area was smaller in young sedentary compared to elderly (elderly athletes 52 ± 9 cm2, elderly sedentary 46 ± 6 cm2 and young sedentary 38 ± 4 cm2; P < 0.01) whilst there was no difference between elderly groups (P = 0.19).
Left and right longitudinal contribution to SV did not differ between groups (LV, P = 0.12; RV, P = 0.16). For the LV, longitudinal contribution ranged between 52 and 65% in the whole population, and RV longitudinal was 77–87% (Table 2). THV variation (%) and thus radial contribution to SV was similar in all groups (elderly athletes 8.1 ± 1.8%, elderly sedentary 8.6 ± 1.4% and young sedentary 7.9 ± 2.8%, P = 1.0).
Furthermore, peak filling rate and peak emptying rate did not differ between groups (peak filling rate of elderly athletes was 516 ± 138 ml s−1, elderly sedentary 550 ± 95 ml s−1 and young sedentary 645 ± 127 ml s−1, P = 0.26; and peak emptying rate for elderly athletes was 615 ± 143 ml s−1, elderly sedentary 670 ± 104 ml s−1 and young sedentary 715 ± 148 ml s−1, P = 0.27).
Determinants of CO and oxygen uptake
In elderly subjects, LVAVPD was an independent predictor of maximal CO (R 2 = 0.61, P < 0.01, β = 0.78). THV, LVM and LVSVlong(%) did not add further value to the model. THV was an independent predictor of peak (R 2 = 0.53, P < 0.01, β = 0.73) and LVM, LVAVPD or LVSVlong(%) did not add to the model.
THV normalized for body surface area (THV/BSA) correlated with peak (ml min−1 kg−1) (R 2 = 0.58, P = 0.001) and with maximal CO (R 2 = 0.59, P < 0.01) (Fig. 4).
Figure 4. Total heart volumes versus peak oxygen uptake and maximal cardiac output .
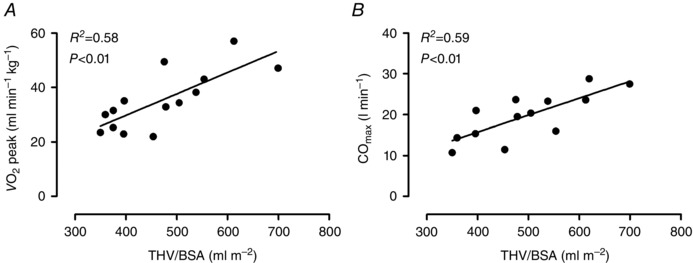
A, the relationship between total heart volumes normalized for body surface area (THV/BSA) and peak oxygen uptake ( peak). B, the relationship between THV/BSA and maximal cardiac output (COmax) determined by the dye dilution technique. Only elderly subjects underwent invasive examinations and are included in the figures.
peak/THV ratio
The peak/THV ratio as a measurement of normal cardiac function ranged between 2.5 and 3.6 in elderly athletes, 2.4 and 3.3 in elderly sedentary, and 4.0 and 4.7 in young sedentary subjects.
Discussion
The presented measurements of longitudinal and radial contributions to SV in this study provide novel insights into changes in heart function in elderly normal subjects and athletes. Against our hypothesis, there was no difference in longitudinal and radial contribution to SV between elderly athletes and elderly sedentary or between elderly and young sedentary subjects. However, how the groups achieved their longitudinal pumping differed. The lower LVAVPD seen in elderly sedentary subjects was compensated for by a larger short‐axis area of the ventricle. As longitudinal pumping is calculated by multiplying AVPD by short‐axis area, differences between groups were masked when comparing only the longitudinal contribution to SV. This compensatory mechanism was previously shown in patients with heart failure (Carlsson et al. 2007 b). The finding of the same compensatory mechanism in normal subjects raises important questions regarding the nature of healthy ageing. In the absence of regular physical activity, the sedentary group has developed a cardiac pumping mechanic that is considered pathological when found in combination with other symptoms of heart failure. Although beyond the scope of this study, this suggests that healthy ageing needs to include physical exercise to be considered truly healthy. However, note that there are no concerns that the untrained elderly population in the present study suffers from early stage heart failure as their exercise capacity was within expected limits, and the index of peak/THV that has been shown to discriminate between normal hearts and failing hearts was within normal range (Engblom et al. 2010). Furthermore, results show that resting LVAVPD is an important predictor of a high CO. Thus, lifelong endurance exercise preserves the function of the atrioventricular plane, enabling the heart to deliver large volumes of blood and preserve exercise capacity at levels similar to sedentary young subjects.
Cardiac pumping
The importance of maintained atrioventricular plane function can be understood through the mechanism of the heart's reciprocal filling and emptying. The movement of the atrioventricular plane is important not only for ejection of blood but also for filling of the atria (Hamilton & Rompf, 1932; Steding‐Ehrenborg et al. 2013). During ventricular systole the movement of the plane towards the apex of the heart causes pressure to drop in the atria and blood is aspirated into the atria from the caval and pulmonary veins (Steding‐Ehrenborg et al. 2013). Thus, when the ventricle is empty, the atria fill (Hamilton & Rompf, 1932). With reciprocal filling the heart maintains a constant volume (Bowman & Kovacs, 2003) which, according to Lundbäck (1986), may save energy for the heart as no energy is lost to pulling on surrounding tissue.
The myofibres causing longitudinal pumping are complemented by oblique fibres that cause a circumferential rotational contraction of the left ventricle during systole (Streeter et al. 1969). This rotational contraction known as the left ventricular twist is, together with radial pumping, highly important for maintaining diastolic filling through the mechanism of diastolic suction (Rademakers et al. 1992). The myofibres contain titin, a protein that acts like a spring that recoils back and untwists when the myocardium relaxes (Granzier & Labeit, 2004). This causes a rapid drop in ventricular pressure and consequently inflow of blood from the atrium (Katz, 1930; Brecher, 1958; Courtois et al. 1988). Nakai et al. (2006) showed a close relationship between radial deformation and twisting, and they also investigated the effects of age in a cross‐sectional study design of untrained young, middle aged and elderly subjects using two‐dimensional speckle tracking echocardiographic imaging. Left ventricular diastolic untwisting was shown to decrease with age, which may be explained by the age‐related changes in heart rate and contractility (Dong et al. 1999). With an increase in age, the ventricle becomes stiffer (Fujimoto et al. 2012), which decreases contractility as well as relaxation and consequently diastolic suction, making the ventricle more dependent on atrial contraction for optimal filling.
Effects of long‐term exercise on cardiac pumping
To our knowledge, no previous study has investigated the effects of long‐term exercise on radial pumping. In the present study, there was no difference between groups for radial pumping at rest. However, Weiner et al. (2010) performed a 90 day interventional exercise study on young male, relatively inexperienced athletes and showed improved twisting mechanics during systole as well as improved untwisting during diastole. Furthermore, ventricular twisting has been linked to preload and afterload, where increased preload increases twisting and increased afterload conversely causes a decrease in twisting (Dong et al. 1999). As long‐term endurance training is known to increase blood volume and thus preload, as well as to decrease blood pressure, and thus decrease afterload, an increased ventricular twist is also probable in elderly athletes.
Cardiac pumping is also affected by ventricular compliance as adequate compliance is necessary to ensure appropriate filling of the ventricle at low filling pressures as well as to allow for increased ejection via the Frank–Starling mechanism (Arbab‐Zadeh et al. 2004). Arbab‐Zadeh et al. (2004) compared the effects of healthy sedentary ageing with life‐long endurance training and showed that a sedentary lifestyle is associated with decreased compliance and diminished diastolic performance, whereas endurance training preserves compliance. The study by Arbab‐Zadeh was performed on Master athletes who had been active for more than 20 years and who were now running on average 32 miles a week. Although it may not be necessary to train at the level of Master athletes, Bhella et al. (2014) showed that 30 min of exercise, 4–5 days per week over a lifetime is needed to sufficiently prevent most of the decreases in left ventricular compliance with ageing.
The population of the present study did not show signs of stiff ventricles as there were no differences in radial contribution to SV (reflecting twisting mechanics as suggested by Nakai et al. 2006) or peak filling rate. However, these measurements were performed with cardiac MRI at rest and to fully establish differences in ventricular compliance cardiac catheterization is needed. During exercise, elderly athletes displayed a higher exercise capacity, which was attributed to a preserved AVPD but may also be affected by a larger twisting reserve as well as better ventricular compliance.
The effects of exercise training on AVPD have previously been studied both at rest and with exercise in young populations. Wisloff et al. (2001) assessed AVPD during exercise in young female cross‐country skiers and found that AVPD fell significantly during exercise and concluded that AVPD is not an important mechanism for enhanced cardiac pumping during exercise. However, Slordahl et al. (2004) performed a short training study on sedentary young women lasting 8 weeks and found no change in AVPD at rest, although a significant increase in AVPD was seen during exercise at 85–90% of maximal heart rate. Similar results were shown by Sundstedt et al. (2008) in a study of young male endurance athletes in whom mitral AVPD increased from rest to peak exercise by 68 and 49% in the septal and lateral borders, respectively. This is in line with the results of the present study of elderly subjects showing the importance of atrioventricular plane function during maximal exercise.
Chronic effects of training on AVPD have been shown by Carlhall et al. (2001) in which endurance trained young men had significantly higher mitral AVPD compared to untrained controls and strength trained athletes, and these results were supported by Steding‐Ehrenborg et al. (2013) who showed larger LVAVPD in male athletes compared to controls. In the present study, elderly athletes did not differ from young sedentary subjects, but compared to age‐matched controls LVAVPD was higher whereas there was no difference for RVAVPD. To the best of our knowledge, this is the first study to investigate AVPD in elderly athletes and further studies with a longitudinal design and including men and women of higher ages as well as testing both at rest and during exercise are needed to fully establish the effects of exercise on AVPD and its importance for performance.
Left and right ventricular volumes and mass in the controls of the present study are comparable with previous studies of normal values for this age group (Hudsmith et al. 2005; Maceira et al. 2006 a). Furthermore, THV, and ventricular and atrial volumes in athletes were comparable to what has been shown in cardiac MRI studies of young athletes (Steding et al. 2010; Mosen & Steding‐Ehrenborg, 2014). When volumes where normalized to THV there were no differences between groups, which confirms that after lifelong endurance training, athletes’ heart are physiologically enlarged with all four chambers having adapted in a balanced way.
Limitations
Two different MR scanners were used in this study. However, as both had field strength of 1.5 T and comparable sequences were used for image acquisition this is unlikely to have affected the results. Furthermore, the study population of elderly subjects was small and a larger sample size would have strengthened the results for invasive measurements. However, for MR parameters normalized for BSA, the study population was large enough to achieve a calculated power of 82%. Young subjects were retrospectively enrolled and did not undergo invasive measurements of CO and it is therefore not possible to conclude what variable is the main determinant of maximal CO in this group.
Conclusion
This study compares novel quantitative measurements of longitudinal and radial function between elderly with lifelong experience of endurance training, healthy sedentary elderly and young sedentary subjects. Longitudinal and radial contribution to SV did not differ between groups. However, how longitudinal pumping was achieved differed between elderly athletes and young subjects compared to elderly sedentary subjects. Elderly athletes and young sedentary subjects showed a similar AVPD whilst this was significantly lower in elderly sedentary subjects. Instead, elderly sedentary subjects compensated for decreased AVPD with increased short‐axis area of the ventricle. Furthermore, resting LVAVPD was shown to be an independent predictor of maximal CO.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
K.S.E. and S.M. conceptualized the study. K.S.E. and P.A. collected MR data and K.S.E. analysed the images. S.M., J.A.C. and R.B. collected and analysed data from exercise tests and CO measurements. K.S.E., S.M. and R.B. drafted the manuscript. All authors have read and critically revised the final version of the manuscript.
Funding
This study was supported by grants provided to Professor Bengt Saltin by the Novo Nordisk Foundation. K.S.E. was funded by a post‐doc scholarship from the Swedish Heart Association. The research on young healthy subjects was part of a larger study funded by the Swedish Research Council, the Swedish National Centre for Research in Sports, the Swedish Heart and Lung Foundation, the Medical Faculty at Lund University, Sweden, and the Region of Scania, Sweden.
Acknowledgements
We would like to acknowledge Professor Bengt Saltin who conceptualized the MR study, provided financial support, assisted in data collection and analysis and revised early drafts of the manuscript. He sadly died before the manuscript reached its final stages. We also thank Lars G. Hanson at Hvidovre Hospital, Denmark, Jaya Rosenmeier at Copenhagen University Hospital, Denmark, and Ann‐Helen Arvidsson and Christel Carlander, Skåne University Hopsital Lund, Sweden, for assistance with data collection and administrative tasks.
References
- Arbab‐Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D & Levine BD (2004). Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, Collins E & Fletcher G (2007). Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 116, 329–343. [DOI] [PubMed] [Google Scholar]
- Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick‐Ranson G, Palmer MD, Boyd KN, Adams‐Huet B & Levine BD (2014). Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 64, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier F, Saltin B, Nejat M & Jensen‐Urstad M (2001). Left ventricular function and perfusion in elderly endurance athletes. Med Sci Sports Exerc 33, 735–740. [DOI] [PubMed] [Google Scholar]
- Bowman AW & Kovacs SJ (2003). Assessment and consequences of the constant‐volume attribute of the four‐chambered heart. Am J Physiol Heart Circ Physiol 285, H2027–2033. [DOI] [PubMed] [Google Scholar]
- Brecher GA (1956). Experimental evidence of ventricular diastolic suction. Circ Res 4, 513–518. [DOI] [PubMed] [Google Scholar]
- Brecher GA (1958). Critical review of recent work on ventricular diastolic suction. Circ Res 6, 554–566. [DOI] [PubMed] [Google Scholar]
- Carlhall CJ, Lindstrom L, Wranne B & Nylander E (2001). Atrioventricular plane displacement correlates closely to circulatory dimensions but not to ejection fraction in normal young subjects. Clin Physiol 21, 621–628. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Cain P, Holmqvist C, Stahlberg F, Lundback S & Arheden H (2004). Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol 287, H243–250. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Ugander M, Heiberg E & Arheden H (2007. a). The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am J Physiol Heart Circ Physiol 293, H636–644. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Ugander M, Mosen H, Buhre T & Arheden H (2007. b). Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 292, H1452–1459. [DOI] [PubMed] [Google Scholar]
- Courtois M, Kovacs SJ, Jr & Ludbrook PA (1988). Transmitral pressure–flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation 78, 661–671. [DOI] [PubMed] [Google Scholar]
- Dong SJ, Hees PS, Huang WM, Buffer SA, Jr , Weiss JL & Shapiro EP (1999). Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol 277, H1053–1060. [DOI] [PubMed] [Google Scholar]
- Engblom H, Steding K, Carlsson M, Mosen H, Heden B, Buhre T, Ekmehag B & Arheden H (2010). Peak oxygen uptake in relation to total heart volume discriminates heart failure patients from healthy volunteers and athletes. J Cardiovasc Magn Reson 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick‐Ranson G, Palmer D & Levine BD (2012). Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 590, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier HL & Labeit S (2004). The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res 94, 284–295. [DOI] [PubMed] [Google Scholar]
- Hamilton WF & Rompf JH (1932). Movement of the base of the ventricle and the relative constancy of the cardiac volume. Am J Physiol 102, 559–565. [Google Scholar]
- Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H & Arheden H (2010). Design and validation of Segment ‐ freely available software for cardiovascular image analysis. BMC Med Imaging 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henein MY & Gibson DG (1999). Normal long axis function. Heart 81, 111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen ES (1899). Skiddlauf und skidwettlauf. Eine medizinische sportstudie. Jena Fischer Verlag, Mitt. Med. Klin, Uppsala. [Google Scholar]
- Hudsmith LE, Petersen SE, Francis JM, Robson MD & Neubauer S (2005). Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 7, 775–782. [DOI] [PubMed] [Google Scholar]
- Katz L (1930). The role played by the ventricular relaxation process in filling the ventricle. Am J Physiol 95, 542–553. [Google Scholar]
- Kitzman DW, Sheikh KH, Beere PA, Philips JL & Higginbotham MB (1991). Age‐related alterations of Doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol 18, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Lundbäck S (1986). Cardiac pumping and function of the ventricular septum. Acta Physiol Scand 127, 8–101. [PubMed] [Google Scholar]
- Maceira AM, Prasad SK, Khan M & Pennell DJ (2006. a). Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8, 417–426. [DOI] [PubMed] [Google Scholar]
- Maceira AM, Prasad SK, Khan M & Pennell DJ (2006. b). Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady‐state free precession cardiovascular magnetic resonance. Eur Heart J 27, 2879–2888. [DOI] [PubMed] [Google Scholar]
- Mosen H & Steding‐Ehrenborg K (2014). Atrial remodelling is less pronounced in female endurance‐trained athletes compared with that in male athletes. Scand Cardiovasc J 48, 20–26. [DOI] [PubMed] [Google Scholar]
- Nakai H, Takeuchi M, Nishikage T, Kokumai M, Otani S & Lang RM (2006). Effect of aging on twist‐displacement loop by 2‐dimensional speckle tracking imaging. J Am Soc Echocardiogr 19, 880–885. [DOI] [PubMed] [Google Scholar]
- Nicolai GF & Zuntz N (1914). Füllung und Enteerung des Herzens bei Ruhe and Arbeit. Berl Klein Wschr 128, 821–824. [Google Scholar]
- Pearson AC, Gudipati CV & Labovitz AJ (1991). Effects of aging on left ventricular structure and function. Am Heart J 121, 871–875. [DOI] [PubMed] [Google Scholar]
- Rademakers FE, Buchalter MB, Rogers WJ, Zerhouni EA, Weisfeldt ML, Weiss JL & Shapiro EP (1992). Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 85, 1572–1581. [DOI] [PubMed] [Google Scholar]
- Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B & Kinermann W (2002). Athletes heart. Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 40, 1856–1863. [DOI] [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB & Ehsani AA (1994). Enhanced left ventricular performance in endurance trained older men. Circulation 89, 198–205. [DOI] [PubMed] [Google Scholar]
- Slordahl SA, Madslien VO, Stoylen A, Kjos A, Helgerud J & Wisloff U (2004). Atrioventricular plane displacement in untrained and trained females. Med Sci Sports Exerc 36, 1871–1875. [DOI] [PubMed] [Google Scholar]
- Steding‐Ehrenborg K, Carlsson M, Stephensen SS & Arheden H (2013). Atrial aspiration from pulmonary and caval veins is caused by ventricular contraction and secures 70% of the total stroke volume independent of resting heart rate and heart size. Clin Physiol Funct Imaging 33, 233–240. [DOI] [PubMed] [Google Scholar]
- Steding K, Engblom H, Buhre T, Carlsson M, Mosen H, Wohlfart B & Arheden H (2010). Relation between cardiac dimensions and peak oxygen uptake. J Cardiovasc Magn Reson 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter DD, Jr , Spotnitz HM, Patel DP, Ross J, Jr & Sonnenblick EH (1969). Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24, 339–347. [DOI] [PubMed] [Google Scholar]
- Sundstedt M, Hedberg P & Henriksen E (2008). Mitral annular excursion during exercise in endurance athletes. Clin Physiol Funct Imaging 28, 27–31. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Hutter AM, Jr , Wang F, Kim J, Weyman AE, Wood MJ, Picard MH & Baggish AL (2010). The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging 3, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Helgerud J, Stoylen A & Ellingsen O (2001). Atrioventricular plane displacement in female endurance athletes. Med Sci Sports Exerc 33, 1503–1510. [DOI] [PubMed] [Google Scholar]
- Yellin E, Nikolic S & Frater R (1990). Left ventricular filling dynamics and diastolic function. Prog Cardiovasc Dis 32, 247–271. [DOI] [PubMed] [Google Scholar]


