Abstract
Key points
Previous studies indicate a transient reduction in arterial function following large muscle group exercise, but the mechanisms involved are unknown.
Sympathetic nervous system activation may contribute to such reductions through direct effects in the artery wall, or because of decreases in arterial shear stress.
Administration of prazosin (an α1‐adrenoreceptor blocker) abolished the transient reduction in vascular function observed under placebo conditions following exercise. This effect could not be explained by drug‐induced changes in arterial shear stress.
These results suggest that sympathetic vasoconstriction directly competes with endothelium‐dependent dilator activity to influence post‐exercise vascular function.
These findings have implications for understanding the stimuli responsible for exercise‐induced adaptations in arterial function and health in humans.
Abstract
Transient reduction in vascular function following systemic large muscle group exercise has previously been reported in humans. The mechanisms responsible are currently unknown. We hypothesised that sympathetic nervous system activation, induced by cycle ergometer exercise, would contribute to post‐exercise reductions in flow‐mediated dilatation (FMD). Ten healthy male subjects (28 ± 5 years) undertook two 30 min sessions of cycle exercise at 75% HRmax. Prior to exercise, individuals ingested either a placebo or an α1‐adrenoreceptor blocker (prazosin; 0.05 mg kg−1). Central haemodynamics, brachial artery shear rate (SR) and blood flow profiles were assessed throughout each exercise bout and in response to brachial artery FMD, measured prior to, immediately after and 60 min after exercise. Cycle exercise increased both mean and antegrade SR (P < 0.001) with retrograde SR also elevated under both conditions (P < 0.001). Pre‐exercise FMD was similar on both occasions, and was significantly reduced (27%) immediately following exercise in the placebo condition (t‐test, P = 0.03). In contrast, FMD increased (37%) immediately following exercise in the prazosin condition (t‐test, P = 0.004, interaction effect P = 0.01). Post‐exercise FMD remained different between conditions after correction for baseline diameters preceding cuff deflation and also post‐deflation SR. No differences in FMD or other variables were evident 60 min following recovery. Our results indicate that sympathetic vasoconstriction competes with endothelium‐dependent dilator activity to determine post‐exercise arterial function. These findings have implications for understanding the chronic impacts of interventions, such as exercise training, which affect both sympathetic activity and arterial shear stress.
Abbreviations
- BF
blood flow
- CO
cardiac output
- FMD
flow‐mediated dilatation
- HR
heart rate
- LBNP
lower body negative pressure
- MAP
mean arterial pressure
- MSNA
muscle sympathetic nerve activity
- SNS
sympathetic nervous system
- SR
shear rate
- SRAUC
shear rate area under curve
- SV
stroke volume
- TPRi
total peripheral resistance index
Introduction
Flow‐mediated dilatation (FMD) is a widely used method of assessment of vascular function that provides a surrogate index for arterial health (Takase et al. 1998; Gocke et al. 2003; Thijssen et al. 2011). While the chronic effect of exercise training on FMD has been documented in a wide range of individuals (Maiorana et al. 2001; Green et al. 2004; Watts et al. 2004; Tinken et al. 2008, 2010; Birk et al. 2012), the impact of acute exercise on FMD responses is less clear (Dawson et al. 2013). The acute FMD response to large muscle mass exercise has therefore been a focus for recent studies, with a number of these documenting a transient reduction in FMD (Goel et al. 2007; Dawson et al. 2008; Jones et al. 2010; Johnson et al. 2012 b; Birk et al. 2013), which may be intensity‐dependent (Johnson et al. 2012 b; Birk et al. 2013). In addition, studies focusing on the impact of cycle ergometer exercise have demonstrated reductions in FMD in the upper limb vasculature (Jones et al. 2010; Johnson et al. 2012 b; Birk et al. 2013), which return to baseline levels approximatetly 1 h following the exercise bout, invoking the concept of ‘hormesis’ (Padilla et al. 2011 a). The mechanisms responsible for this apparent reduction in FMD immediately following exercise are currently unknown, but may include oxidative stress, changes in blood pressure, arterial shear rate (SR) (in particular retrograde SR; Dawson et al. 2013), and/or sympathetic nervous system (SNS) activation.
We have previously published studies pertaining to the impact of distinct patterns of blood flow and SR on FMD responses (Thijssen et al. 2009 b, 2014; Tinken et al. 2009; Carter et al. 2013). Retrograde SR has been linked to a pro‐atherogenic phenotype (Chappell et al. 1998; Ziegler et al. 1998; Hastings et al. 2007; Laughlin et al. 2008; Conway et al. 2010) and reductions in arterial diameter (Carter et al. 2013) and FMD (Thijssen et al. 2009 b; Tinken et al. 2009; Schreuder et al. 2014). Exercise that predominently activates the lower limbs, for example cycle ergometry, increases retrograde SR in the brachial artery (Green et al. 2002, 2005; Thijssen et al. 2009 a; Padilla et al. 2011 b), particularly during the initial phase of exercise. It was recently suggested that this may be linked to increased vasoconstrictor outflow (Padilla et al. 2010; Simmons et al. 2011). However, the systemic impact of elevations in retrograde SR mediated by SNS activation during lower limb exercise has not been directly studied.
SNS activation, independent of exercise, has also been linked to a reduction in FMD (Ghiadoni et al. 2000; Hijmering et al. 2002; Lind et al. 2002; Spieker et al. 2002; Dyson et al. 2006). Hijmering et al.(2002) utilised lower body negative pressure (LBNP: −20 mmHg) to increase sympathetic outflow and demonstrated an acute reduction in FMD that was abolished by intra‐arterial infusion of phentolamine. While other studies could not replicate LBNP‐induced reductions in FMD (Dyson et al. 2006), reduced FMD responses have been observed following mental stress (Ghiadoni et al. 2000; Spieker et al. 2002) and cold pressor tests (Lind et al. 2002; Dyson et al. 2006). We recently demonstrated that LBNP of −35 mmHg reduced brachial artery FMD (Thijssen et al. 2014) and that this response was mitigated when the increase in retrograde SR induced by LBNP was abolished by the simultaneous application of a heating stimulus. We concluded that SNS activation induced by LBNP may cause a reduction in FMD which is, at least partly, due to changes in arterial SR patterns. This experiment was performed in resting subjects.
The impact of exercise‐mediated SNS activation on brachial artery FMD remains unclear, as does the extent to which any such impact can be attributed to changes in arterial (especially retrograde) SR. We therefore hypothesised that SNS activation as a result of cycle exercise would contribute to reductions in post‐exercise FMD, an effect mediated by changes in brachial artery SR.
Methods
Ethical approval
All study procedures were approved by the University of British Columbia (Okanagen) institutional ethics committee and adhered to the Declaration of Helsinki. All participants provided written, informed consent before participating in the study.
Participants
Ten healthy, recreationally active male subjects (age: 28 ± 5 years, height: 1.91 ± 0.40 m, weight: 80.1 ± 14.7 kg) volunteered for the study. Subjects were normotensive (systolic blood pressure < 130 mmHg and diastolic blood pressure < 85mmHg), medication free and non‐smokers, with no previous history of cardiovascular disease(s).
Experimental design
Participants were required to report to our laboratory on two occasions. All participants arrived in a fasted state (>6 h) (Thijssen et al. 2011), having avoided strenuous exercise, alcohol and caffeine for a minimum of 8 h prior to testing. Time of day of testing sessions was kept consistent for each participant with a minimum of 48 h and maximum of 7 days between sessions. Ninety minutes before commencing the experimental protocol participants orally ingested a capsule containing either an α1‐adrenoreceptor blocker (prazosin; 0.05 mg kg−1 body mass) or a placebo (empty capsule) and were instructed to lie down. This dosage of prazosin provides ∼80% alpha blockade and has previously been utilised in research studies similar in nature (Jones et al. 2011; Lewis et al. 2013, 2014). Following 90 min of rest, individuals were placed on a semi‐supine bike (Lode Ergometer, Lode, Groningen, Netherlands) and their arm was extended and secured ∼80 deg from the torso. An initial assessment of brachial artery endothelial function, using the flow‐mediated dilatation technique (Thijssen et al. 2011), was then conducted. Each subject then completed 30 min of cycle exercise at 75% of maximum heart rate (mean workloads were 161 ± 16 W in the placebo condition and 140 ± 20 W in the prazosin condition). During every 5 min period of exercise, a 1 min measurement of brachial artery SR and blood flow was recorded, whilst blood pressure parameters were recorded continuously (Finometer Pro, Finapres Medical Systems, Amsterdam, Netherlands). Previous data from our laboratory indicate that the coefficient of variation associated with repeated Finometer measures collected at rest is ∼5% (Lewis et al. 2015) and others have validated model flow approaches during exercise (Sugawara et al. 2003). Post‐exercise FMD was then measured immediately after and 60 min after exercise cessation. The order of the conditions, i.e. prazosin vs. placebo, was randomised.
Experimental measurements
Central haemodynamics
Changes in mean arterial pressure (MAP), heart rate (HR), cardiac output (CO), stroke volume (SV) and total peripheral resistance index (TPRi) were continuously measured using a Finometer PRO (Finapres Medical Systems), with data exported into PowerLab (Labchart 7, AD Instruments, Sydney, Australia).
Assessment of brachial artery endothelial function
After extending the arm to an ∼80 deg angle from the torso, a rapid inflation/deflation pneumatic cuff (D.E. Hokanson, Bellevue, Western Australia) was placed on the forearm, distal to the olecranon process. With the use of a 10–15 MHz multi‐frequency‐linear array probe attached to a high‐resolution ultrasound machine (T3200; Terason, Burlington, MA, USA), a B‐mode image of the brachial artery was obtained. Following optimal acquisition of an image and a 1 min baseline recording of artery diameter and velocity, the forearm cuffs were inflated to 220 mmHg for 5 min. Recordings of diameter and velocity continued until 30 s prior to cuff deflation and continued for 3 min thereafter, in accordance with previous studies (Woodman et al. 2001). This procedure was repeated immediately after and 60 min after cycle ergometer exercise.
Brachial artery diameter and blood flow/SR analysis
Brachial artery diameter and velocity were analysed using a custom‐designed edge‐detection and wall‐tracking software, which is largely independent of investigator bias (Woodman et al. 2001), described in detail elsewhere (Black et al. 2008; Thijssen et al. 2011). Diameter measurements collected using this software are more reliable and observer‐independent than manual methods and reduce observer error, while possessing an intra‐observer coefficient of variation of 6.7% (Woodman et al. 2001). Continuous (30 Hz) diameter and velocity data were used to calculate blood flow (BF) (the product of lumen cross‐sectional area and Doppler velocity). SR was calculated as 4 times mean blood velocity divided by vessel diameter. Finally, FMD was calculated in absolute (mm) and relative (%) terms as the increase from resting baseline diameter to peak diameter, as described elsewhere (Black et al. 2008; Thijssen et al. 2011).
Statistics
Statistical analysis was performed using SPSS 22.0 (SPSS, Chicago, IL, USA). All data are reported as mean ± SD unless otherwise stated and statistical significance was assumed at P < 0.05. A two‐way repeated‐measures ANOVA was used to compare mean SR and BF across ‘time’ (time points throughout and following exercise) and between ‘conditions’ (placebo and prazosin). Similar analysis was performed on each of the following variables: antegrade SR and BF, retrograde SR and BF, MAP, HR, SV, CO, TPRi, vascular conductance and brachial artery diameter. Additional two‐way repeated‐measures ANOVAs were completed for the assessment across time (pre, post and post 60) and condition (placebo and prazosin) for baseline brachial artery diameter, change in diameter during the FMD, FMD% and FMD SR area under curve (SRAUC). Post‐hoc analysis was performed using the least significant difference methods. Finally, we analysed the effects of time (pre, post and post60) and condition (placebo and prazosin) for FMD variables, on the change in logarithmically transformed diameter using a linear mixed model using baseline arterial diameter and SRAUC as covariates (Atkinson et al. 2013). This approach accounts for any changes in FMD that may be related to baseline diameter or SR differences between conditions.
Results
There were no differences in pre‐exercise measures of brachial artery diameter, SR, FMD% or SRAUC between the two testing conditions (placebo vs. alpha) (Table 1, all P > 0.05).
Table 1.
Baseline (pre‐exercise) and post‐exercise FMD, shear rate and blood flow, on each day
| Placebo | Prazosin | 2‐way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time × | |||||||||
| Pre | Post | Post 60 | Pre | Post | Post 60 | Condition | Time | Condition | |
| Baseline diameter (mm) | 4.43 ± 0.28 | 4.78 ± 0.66 | 4.41 ± 0.33 | 4.44 ± 0.28 | 4.57 ± 0.34 | 4.50 ± 0.26 | 0.55 | 0.04 | 0.11 |
| Change in diameter during FMD (mm) | 0.22 ± 0.07 | 0.15 ± 0.05 | 0.25 ± 0.07 | 0.21 ± 0.05 | 0.29 ± 0.08 | 0.26 ± 0.13 | 0.07 | 0.16 | 0.01 |
| FMD (%) | 5.09 ± 1.65 | 3.22 ± 1.13 | 5.78 ± 1.69 | 4.70 ± 1.18 | 6.32 ± 1.91 | 5.76 ± 2.82 | 0.06 | 0.11 | 0.01 |
| FMD SRAUC (×103s−1) | 15.87 ± 9.05 | 30.34 ± 9.30 | 17.71 ± 4.36 | 20.15 ± 8.36 | 37.42 ± 11.15 | 26.21 ± 8.12 | 0.01 | <0.001 | 0.41 |
| Corrected FMD (%) | 2.10 ± 0.56 | 1.69 ± 0.62 | 2.47 ± 0.63 | 2.02 ± 0.48 | 2.56 ± 0.86 | 2.43 ± 1.13 | 0.19 | 0.10 | <0.01 |
Exercise haemodynamics
Mean arterial pressure increased in response to exercise in both the placebo and the prazosin conditions (time P < 0.001, Fig. 1). While higher values were evident under the placebo condition during exercise, these differences were statistically insignificant (P = 0.06 for condition; P = 0.12 for interaction effect). HR was significantly elevated throughout exercise, with elevations higher under the prazosin condition (condition effect P = 0.04, Fig. 1). Stroke volume was also elevated throughout exercise, with higher values under the placebo condition (P = 0.03) and no interaction effect (interaction effect P = 0.10). Cardiac output and TPRi did not differ between conditions (Fig. 1).
Figure 1. Mean arterial pressure (MAP), heart rate (HR), stroke volume (SV), cardiac output (CO) and total peripheral resistance index (TPRi) across 30 min of cycle exercise at 75% HRmax .
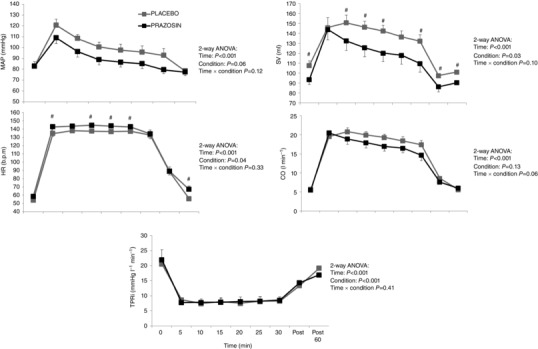
Grey squares denote ‘Placebo’ condition and black squares denote ‘prazosin’ condition. Error bars represent SEM. Statistical significance was assumed at P ≤ 0.05; #statistical significance between conditions. (NB: subjects were exercised at a target HR of 75%HRmax, resulting in mean workloads of 161 ± 16 W in the placebo condition and 140 ± 20 W in the prazosin condition.)
Brachial artery SR and blood flow
Mean SR was elevated significantly in both conditions during exercise (P < 0.001) with a significant condition × time interaction (P = 0.01). Post hoc tests revealed significantly higher mean SR at the 5 min exercise time point under the prazosin condition (P = 0.02, Fig. 2), with no significant differences at any other exercise time point.
Figure 2. Mean, antegrade and retrograde shear rate (SR) comparisons across 30 min of cycle exercise at 75% HRmax under ‘Placebo’ (grey squares) and ‘prazosin’ (black squares) .
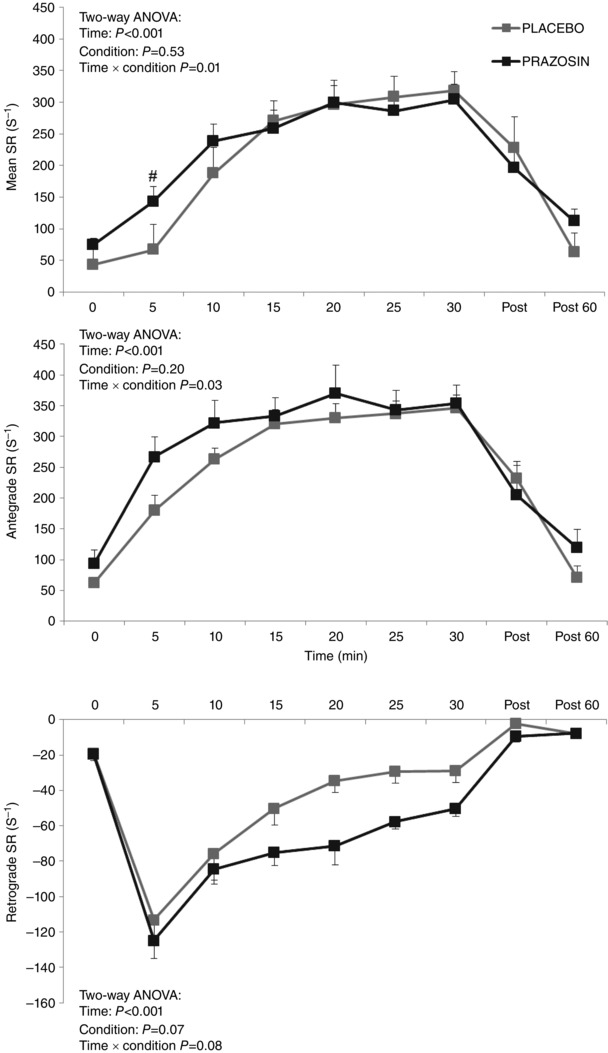
Error bars represent SEM. Statistical significance was assumed at P ≤ 0.05; #statistical significance between conditions. (NB: subjects were exercised at a target HR of 75%HRmax, resulting in mean workloads of 161 ± 16 W in the placebo condition and 140 ± 20 W in the prazosin condition.)
Antegrade SR increased in both conditions during exercise (time P < 0.001), with a significant condition × time interaction (P = 0.03). Similar to mean SR, post hoc tests demonstrated a significantly higher antegrade SR at the 5 min exercise time point in the prazosin condition. Retrograde SR also increased significantly in both conditions (time both P < 0.001), although no difference was evident in this increase between the conditions (interaction effect P = 0.08, Fig. 2).
Both mean and antegrade BF increased with exercise in both conditions (both P < 0.001) with both demonstrating a significant condition × time interaction (P = 0.02 and P = 0.01, respectively). Post hoc tests revealed that at the 5 and 10 min exercise time points, both mean and antegrade flows were higher in the prazosin condition. Finally, retrograde BF increased under both conditions (P < 0.001), with no condition or interaction effects apparent (P = 0.22 and P = 0.14, respectively).
Vascular conductance increased in both conditions (P < 0.001) but this increase was not significant between the two conditions (condition effect P = 0.24, Fig. 3). Finally, brachial artery diameter increased during the exercise session in both conditions (P < 0.001) but this effect was not different between the two conditions (condition effect P = 0.80, Fig. 3).
Figure 3. Blood flow, conductance, brachial artery velocity and arterial diameter across 30 min of cycle exercise at 75% HRmax under ‘Placebo’ (grey squares) and ‘prazosin’ (black squares) .
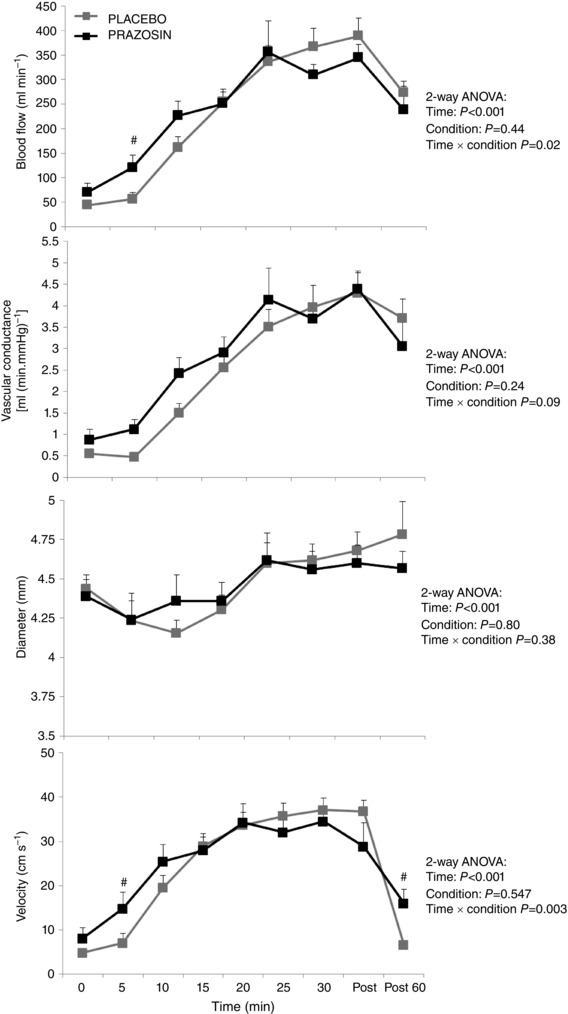
Error bars represent SEM. Statistical significance was assumed at P ≤ 0.05; #statistical significance between conditions. (NB: subjects were exercised at a target HR of 75%HRmax, resulting in mean workloads of 161 ± 16 W in the placebo condition and 140 ± 20 W in the prazosin condition.)
FMD parameters
Two‐way ANOVA performed on baseline brachial artery diameter revealed a significant time effect (P = 0.04), but no condition or interaction effects (Table 1). A significant time × condition interaction was evident for FMD (%) (P = 0.01, Fig. 4). Posthoc tests indicated a significant FMD reduction immediately following the 30 min exercise intervention (P = 0.03) under the placebo condition, whereas an increase in FMD was observed with prazosin (P = 0.004, Fig. 4). In terms of the post‐cuff deflation SR stimulus to FMD [FMD SRAUC (103 s−1)], prazosin was associated with elevated values both before and after exercise (condition effect P< 0.01). However, when corrected for changes in both diameter and SRAUC [Corrected FMD (%), Table 1], the interaction effect for FMD remained (P < 0.01).
Figure 4. Brachial artery flow‐mediated dilatation (FMD) prior to, immediately post and 60 min following 30 min cycle exercise at 75%HRmax .

A, placebo condition (grey bars); B, prazosin condition (black bars). Error bars represent SEM. Statistical significance was assumed at P ≤ 0.05; *statistical significance from ‘Pre’ value. (NB: subjects were exercised at a target HR of 75%HRmax, resulting in mean workloads of 161 ± 16 W in the placebo condition and 140 ± 20 W in the prazosin condition.)
Discussion
In keeping with several previous studies examining large muscle group exercise (Goel et al. 2007; Dawson et al. 2008; Jones et al. 2010; Johnson et al. 2012 b; Birk et al. 2013), in the present study we observed a transient post‐exercise reduction in brachial artery FMD following 30 min of cycle ergometer exercise. This effect was abolished by the ingestion of prazosin, a specific α1‐adrenoceptor blocker, suggesting that exercise‐induced increases in SNS activation contribute to the acute effects of exercise on arterial function in vivo.
While an acute impact of exercise on arterial function has been previously reported (Goel et al. 2007; Dawson et al. 2008; Jones et al. 2010; Johnson et al. 2012 b; Birk et al. 2013), these studies did not fully address the mechanisms responsible. The impact of SNS activation on FMD may be due to direct interactions between neurotransmitters and endothelium‐dependent vasodilators (Kolo et al. 2004) or secondary impacts of sympathetic vasoconstriction on arterial SR during exercise, or both. In this context, we recently demonstrated that FMD reduction during LBNP was ameliorated by applying a forearm heating stimulus that normalised the brachial artery retrograde
SR (Thijssen et al. 2014). These findings suggested that changes in FMD may, at least partly, result from LBNP effects on retrograde shear rather than direct impacts on endothelium‐mediated dilatation per se. In the present study, the impact of prazosin on FMD cannot be ascribed to impacts of this drug on SR responses during exercise, as it was associated with small but significant increases in retrograde shear across the exercise period. These findings therefore infer that the α1‐blockade may have direct impacts on the nitric oxide–dilator pathway and FMD response during large muscle group lower limb exercise. Indeed, several studies in animals have indicated that nitric oxide interacts with noradrenaline, thereby directly modifying arterial vasoactivity (Vo et al. 1991; Chowdhary & Townend, 1999; Zanzinger, 1999; Kolo et al. 2004). Our data suggest that a balance exists between SNS‐mediated vasoconstriction and endothelium‐dependent dilatation, with large muscle group lower limb exercise associated with a transient increase in systemic SNS activation, and consequent reduction in FMD. Prazosin‐induced blockade of α1‐adrenoceptors altered this balance in favour of nitric oxide‐mediated vasodilatation and consequently increased FMD, as observed immediately following exercise in the present experiment.
By virtue of its α‐blocking action, prazosin is a vasodilator and it is possible that the stimulus to brachial arterial dilatation following cuff deflation during the FMD tests was affected by this drug. Indeed, directly measured SRAUC following cuff deflation was higher in the prazosin condition, although no interaction effect existed for SRAUC between the conditions and the magnitude of change in SR after exercise was similar between the conditions (from 15.87 to 30.34 × 103 s−1: 90% increase; 20.15 to 37.42 × 103 s−1: a 86% increase). Furthermore, the significant impact of prazosin on FMD% persisted after we corrected for differences in the SR stimulus before and after exercise. It seems unlikely that the effect of prazosin on FMD that we observed can be fully or even largely explained by effects on the SR stimulus per se.
Vascular function and FMD can be modulated by inflammation and oxidative stress and several studies have reported improvements in FMD following treatments that ameliorate oxidation and inflammation (Ellis et al. 2000; Silvestro et al. 2002; Donato et al. 2010; Johnson et al. 2012 a,2013; Wray et al. 2012). Improvements in FMD following the ingestion of anti‐oxidants have been observed in the elderly (Donato et al. 2010; Johnson et al. 2012 a), patients with hypertension (Plantinga et al. 2007), peripheral artery disease (Silvestro et al. 2002) and heart failure (Ellis et al. 2000). Conversely, studies in healthy individuals have typically not demonstrated increases in FMD with administration of oral antioxidants (Richardson et al. 2007; Donato et al. 2010), although Johnson and colleages suggested that vitamin C supplementation in the presence of increased retrograde SR induced by either cuff placement (Johnson et al. 2013) or cycle exercise (Johnson et al. 2012 a) can enhance FMD responses. We cannot exclude the possibility that the exercise performed in our study had impacts on inflammatory or oxidative factors. However, the acute impact of prazosin is specific to α1‐adrenoceptor blockade and we are unaware of any direct effects of this agent on inflammation or oxidative stress. The complete reversal in FMD we observed with prazosin strongly implies that interaction between SNS activation and nitric oxide plays a role in the acute impact of exercise on arterial function in humans.
An important limitation of our study was that no direct assessment of SNS activation was undertaken. It is not technically possible to perform muscle sympathetic nerve activity (MSNA) assessments from the common peroneal nerve during cycle exercise, and upper limb MSNA assessments are also challenging to maintain at higher cycle exercise intensities. While it is possible that any impact of whole body exercise would be greater during the immediate post‐exercise period than at later time points (e.g. 1 h), and some human evidence exists to support this contention (Cleroux et al. 1992), there is also evidence that MSNA is lower following large muscle mass exercise (Halliwill et al. 1996; Kulics et al. 1999) and this question has not been resolved in the literature. Another limitation is that prazosin provides specific α1‐receptor blockade and therefore we cannot exclude the possibility that differential β‐adrenoceptor‐mediated dilatation or α2‐mediated vasoconstriction occurred in the downstream microvasculature in our study. Some studies suggest that functional sympatholysis is mainly due to blunting of α2‐adrenoceptor‐mediated vasoconstriction (Wray et al. 2004) although both α1‐ and α2‐adrenergic receptor‐mediated vasoconstriction are blunted in contracting human skeletal muscle (Rosenmeier et al. 2003). Our findings indicate that, at the level of the conduit arteries, α1‐blockade impacts on FMD and this effect is not due to changes in arterial shear stress (as a result of downstream vascular effects), given that the impact of prazosin on flows and shear were directionally opposite to those that might enhance FMD. Nonetheless, there remains a possibility that increases in retrograde shear with prazosin may be due to greater α2‐mediated constriction when α1‐receptors are blocked, and multiple blockade with non‐specific α‐ and β‐blockade could be addressed in future studies.
A final limitation of our study relates to differences between the conditions in exercise intensity. We exercised subjects to a target HR (75% HRmax), due mainly to our previous study (Birk et al. 2013) which indicated that exercise at ∼70%HRmax resulted in a significant decrease in FMD following cycling. We did not anticipate that prazosin would induce differences in workload of ∼20 W between the conditions. This could theoretically contribute to the differences we observed in post‐exercise FMD. However, in the study by Birk et al.(2013), cycling performed at lower workloads resulted in a decrease in FMD, whereas in our study post‐exercise FMD increased when prazosin was administered. Furthermore, a correlation performed on differences between conditions in workload and exercise‐impact on FMD revealed no relationship (r = 0.18, P = 0.62). We suggest that, while distinct workloads may have contributed to some extent to the FMD differences between conditions, it seems that a primary impact of prazosin on nitric oxide‐mediated dilator function predominated.
Conclusions
Our findings suggest that changes in vascular function following cycle exercise reflect a balance between sympathetically driven vasoconstriction and nitric oxide‐mediated vasodilatation. The acute impairment in FMD observed following exercise in the current study suggests that cycle exercise, at the intensity used in this study, transiently alters this balance in favour of SNS‐mediated vasoconstriction. When taken in context with previous studies utilising LBNP, the findings of the present study suggest that a direct interaction between noradrenaline and endothelium‐dependent dilators, and/or SNS‐mediated changes in SR patterns, can both influence artery function in vivo. The contribution of these direct and indirect pathways may differ according to which stimulus of SNS activation is adopted: previous studies suggest that LBNP contributes to FMD changes via shear stress‐related mechanisms, whereas our current results cannot be explained via this secondary impact.
Additional information
Competing interests
None of the authors has any competing interests to disclose.
Author contributions
C.L.A., P.N.A., D.H.J.T. & D.J.G. conceived and designed the experiments. C.L.A. contributed to the collection and analysis of data along with writing of the manuscript, with the help of D.J.G., N.C.L., H.H.C., D.H.J.T. and P.N.A. All authors helped draft and/or revise the manuscript critically and have approved the final submitted version.
Funding
D.J.G. is funded by the Australian Research Council (DP 130103793).
References
- Atkinson G, Batterham AM, Thijssen DH & Green DJ (2013). A new approach to improve the specificity of flow‐mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 31, 287–291. [DOI] [PubMed] [Google Scholar]
- Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH & Green DJ (2012). Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 112, 1653–1658. [DOI] [PubMed] [Google Scholar]
- Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DH & Green DJ (2013). Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med 34, 409–414. [DOI] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DHJ & Green DJ (2008). Importance of measuring the time‐course of flow‐mediated dilation (FMD) in humans. Hypertension, 51, 203–210. [DOI] [PubMed] [Google Scholar]
- Carter HH, Dawson EA, Birk GK, Spence AL, Naylor LH, Cable NT, Thijssen DH & Green DJ (2013). Effect of SR manipulation on conduit artery dilation in humans. Hypertension 61, 143–150. [DOI] [PubMed] [Google Scholar]
- Chappell DC, Varner SE, Nerem RM, Medford RM & Alexander RW (1998). Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82, 532–539. [DOI] [PubMed] [Google Scholar]
- Chowdhary S & Townend JN (1999). Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin Sci (Lond) 97, 5–17. [PubMed] [Google Scholar]
- Cleroux J, Kouame N, Nadeau A, Coulombe D & Lacourciere Y (1992). Aftereffects of exercise on regional and systemic hemodynamics in hypertension. Hypertension 19, 183–191. [DOI] [PubMed] [Google Scholar]
- Conway DE, Williams MR, Eskin SG & McIntire LV (2010). Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time‐average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol 298, H367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson EA, Green DJ, Cable NT & Thijssen DH (2013). Effects of acute exercise on flow mediated dilatation (FMD) in healthy humans. J Appl Physiol 115, 1589–1598. [DOI] [PubMed] [Google Scholar]
- Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, Gaze D, Shave RE, Wilson M, George KP & Green DJ (2008). Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 105, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Bailey DM, Wray DW & Richardson RS (2010). Exercise‐induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298, H671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson KS, Shoemaker JK & Hughson RL (2006). Effect of acute sympathetic nervous system activation on flow‐mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290, H1446–1453. [DOI] [PubMed] [Google Scholar]
- Ellis GR, Anderson RA, Lang D, Blackman DJ, Morris RH, Morris‐Thurgood J, McDowell IF, Jackson SK, Lewis MJ & Frenneaux MP (2000). Neutrophil superoxide anion‐generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short‐ and long‐term vitamin C therapy. J Am Coll Cardiol 36, 1474–1482. [DOI] [PubMed] [Google Scholar]
- Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A & Deanfield JE (2000). Mental stress induces transient endothelial dysfunction in humans. Circulation 102, 2473–2478. [DOI] [PubMed] [Google Scholar]
- Gocke N, Keaney JF, Hunter LM, Watkins MT, Nedelijkovic ZS, Menzoian JO & Vita JA (2003). Predictive value of non‐invasively determined endothelial dysfunction for long‐term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41, 1769–1775. [DOI] [PubMed] [Google Scholar]
- Goel R, Majeed F, Vogel R, Corretti MC, Weir M, Mangano C, White C, Plotnick GD & Miller M (2007). Exercise‐induced hypertension, endothelial dysfunction, and coronary artery disease in a marathon runner. Am J Cardiol 99, 743–744. [DOI] [PubMed] [Google Scholar]
- Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G & Walsh JH (2005). Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Cheetham C, Reed C, Dembo L & O'Driscoll G (2002). Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during lower limb exercise. J Appl Physiol 93, 361–368. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana AJ, O'Driscoll G & Taylor R (2004). Topical review: effect of exercise training on endothelium‐derived nitric oxide function in humans. J Physiol 561, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA & Eckberg DL (1996). Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NE, Simmers MB, McDonald OG, Wamhoff BR & Blackman BR (2007). Atherosclerosis‐prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro‐inflammatory priming. Am J Physiol Cell Physiol 293, C1824–C1833. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ & Rebelink TJ (2002). Sympathetic activation markedly reduces endothelium‐dependent, flow‐mediated vasodilation. J Am Coll Cardiol 39, 683–688. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD & Wallace JP (2012. a). Brachial artery flow‐mediated dilation following exercise with augmented oscillatory and retrograde shear rate. Cardiovasc Ultrasound 10, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD & Wallace JP (2013). Vitamin C prevents the acute decline of flow‐mediated dilation after altered shear rate patterns. Appl Physiol NutrMetab 38, 268–274. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Padilla J & Wallace JP (2012. b). The exercise dose affects oxidative stress and brachial artery flow‐mediated dilation in trained men. Eur J Appl Physiol 112, 33–42. [DOI] [PubMed] [Google Scholar]
- Jones H, Green DJ, George K & Atkinson G (2010). Intermittent exercise abolishes the diurnal variation in endothelial‐dependent flow‐mediated dilation in humans. Am J Physiol RegulIntegr Comp Physiol 298, R427–432. [DOI] [PubMed] [Google Scholar]
- Jones H, Lewis NC, Green DJ, Ainslie PN, Lucas SJ, Tzeng YC, Grant EJ & Atkinson G (2011). α1‐Adrenoreceptor activity does not explain lower morning endothelial‐dependent, flow‐mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 300, R1437–1442. [DOI] [PubMed] [Google Scholar]
- Kolo LL, Westfall TC & Macarthur H (2004). Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 286, H296–303. [DOI] [PubMed] [Google Scholar]
- Kulics JM, Collins HL & DiCarlo SE (1999). Postexercise hypotension is mediated by reductions in sympathetic nerve activity. Am J Physiol 276, 27–32. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC & Bender SB (2008). Importance of hemodynamic forces as signals for exercise‐induced changes in endothelial cell phenotype. J Appl Physiol 104, 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N, Messinger L & Ainslie P (2014). Effect of acute hypoxia on the time course change in flow‐mediated dilation: effect of sympathetic nerve activity. FASEB J 28, http://www.fasebj.org/content/28/1_Supplement/708.4.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NC, Ainslie PN, Atkinson G, Jones H, Grant EJ & Lucas SJ (2013). Initial orthostatic hypotension and cerebral blood flow regulation: effect of α1‐adrenoreceptor activity. Am J Physiol Heart Circ Physiol 304, H147–154. [DOI] [PubMed] [Google Scholar]
- Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T & Ainslie PN (2015). Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129, 169–178. [DOI] [PubMed] [Google Scholar]
- Lind L, Johansson K & Hall J (2002). The effects of mental stress and the cold pressure test on flow‐mediated vasodilation. Blood Press 11, 22–27. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor RR & Green DJ (2001). The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38, 860–866. [DOI] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce‐Esquivel AA, Whyte JJ & Laughlin MH (2011. a). Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 26, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH & Fadel PJ (2011. b). Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH & Fadel PJ (2010). Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298, H1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S & Salvetti A (2007). Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens 20, 392–397. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S & Bailey DM (2007). Exercise‐induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292, H1516–1522. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ & Joyner MJ (2003). α1‐ and α2‐adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder TH, Green DJ, Hopman MT & Thijssen DH (2014). Acute impact of retrograde shear rate on brachial and superficial femoral artery flow‐mediated dilation in humans. Physiol Rep 2, e00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L & Brevetti G (2002). Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 165, 277–283. [DOI] [PubMed] [Google Scholar]
- Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH & Fadel PJ (2011). Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF & Noll G (2002). Mental stress induces prolonged endothelial dysfunction via endothelin‐A receptors. Circulation 105, 2817–2820. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R & Matsuda M (2003). Non‐invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179, 361–366. [DOI] [PubMed] [Google Scholar]
- Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F & Kurita A (1998). Endothelium‐dependent flow‐mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82, 1535–1539. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Atkinson CL, Ono K, Sprung VS, Spence AL, Pugh CJA & Green DJ (2014). Sympathetic nervous system activation, arterial shear rate, and flow‐mediated dilation. J Appl Physiol 116, 1300–1307. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Black MA, Pyke K, Padilla J, Atkinson GA, Harris RA, Parker B, Widlansky ME, Tschakovsky ME & Green DJ (2011). Assessment of flow mediated dilation (FMD) in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300, H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DHJ, Dawson EA, Black MA, Hopman MTE, Cable NT & Green DJ (2009. a). Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc 41, 1072–1079. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Dawson EA, Tinken TM, Cable NT & Green DJ (2009. b). Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53, 986–992. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DH, Black MA, Cable NT & Green DJ (2008). Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586, 5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins ND, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT & Green DJ (2009). Impact of shear rate modulation on vascular function in humans. Hypertension 54, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins ND, Dawson EA, Cable NT & Green DJ (2010). Shear stress mediates vascular adaptations to exercise training in humans. Hypertension 55, 312–318. [DOI] [PubMed] [Google Scholar]
- Vo PA, Reid JJ & Rand MJ (1991). Endothelial nitric oxide attenuates vasoconstrictor responses to nerve stimulation and noradrenaline in the rat tail artery. Eur J Pharmacol 199, 123–125. [DOI] [PubMed] [Google Scholar]
- Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O'Driscoll G & Green DJ (2004). Exercise training normalises vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 43, 1823–1827. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA & Green D (2001). Improved analysis of brachial artery ultrasound using a novel edge‐detection software system. J Appl Physiol 91, 929–937. [DOI] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven P & Sander M (2004). Inhibition of α‐adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555, 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MAH, Ives SJ, Barrett‐O'Keefe Z & Richardson RS (2012). Acute reversal of endothelial dysfunction in the elderly following antioxidant consumption. Hypertension 59, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanzinger J ( 1999). Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc Res 43, 639–649. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Bouzourene K, Harrison VJ, Brunner HR & Hayoz D (1998). Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18, 686–692. [DOI] [PubMed] [Google Scholar]


