Introduction
Since the discovery that CO2 passes through aquaporin‐1 (AQP1; Nakhoul et al. 1998; Cooper & Boron, 1998), the importance of channel‐ vs. lipid‐mediated gas transport has often been portrayed as an either/or issue. However, depending upon physiological context, the role of channels may be insignificant or dominant.
In a landmark study, Mitchell (1830) examined gas permeation across barriers of natural rubber or animal tissue, rank‐ordered the ‘relative facility of transmission’ of several gases, and recognized that these move independently of one another in a mechanism dependent upon ‘infiltration’ (i.e. solubility) in the organic molecular barrier – the first statement of ‘solubility theory’. Later, Graham showed that permeation across rubber membranes depends on not only solubility but also diffusion through the barrier (Graham, 1866) – the first statement of ‘solubility–diffusion theory’. Meanwhile, Fick proposed his law of mass diffusion, which Wroblewski combined with Henry's law to produce our modern transport equation (Wroblewski, 1879):
Here J is flux, D the diffusion constant in the barrier, s solubility in the barrier, l barrier thickness and Δp trans‐barrier partial pressure difference.
In the late 1890s, Overton used solubility theory to develop his revolutionary hypothesis that the boundary layer of the cytoplasm (‘Grenzschicht des Protoplasten’) – now termed the plasma membrane – is impregnated with lipids. However, modern reference to ‘Overton's rule’
is problematic because (1) the ‘solubility’ rule is really Mitchell's and ignores both (2) D and (3) membrane proteins, which themselves impact J in three ways. First, in the plane of the membrane, proteins impermeable to X displace and organize nearby lipids, reducing P M,X (Wang et al. 2007; Boron, 2010). Second, transporters and channels carry a wide range of lipid‐soluble solutes (Al‐Awqati, 1999). Thus, lipid solubility does not prove permeation via membrane lipid. Third, exomembranous portions of integral membrane proteins can almost completely cover some membranes (Takamori et al. 2006). Boron proposed the ‘access–solubility–diffusion–egress theory’ to account for the resistance of these proteins, and the ordering of water near charged lipid head‐groups, to the entry of a substance into (or exit from) membrane lipid.
The first clear experimental data opposing the solubility–diffusion theory was the demonstration that gastric gland apical membranes have no measurable (Waisbren et al. 1994). In artificial lipid bilayers, a major determinant of may be membrane lipid cholesterol content (C M,chol; Itel et al. 2012; Kai & Kaldenhoff, 2014). In one study, raising C M,chol from 0 to 20% lowered by ≥10‐fold; raising C M,chol from 20 to 70% decreased by an additional 10‐fold (Itel et al. 2012), predominantly due to a decrease in D rather than s. The CO2‐impermeable plant aquaporin NtPIP2;1 reduces of artificial membranes (Kai & Kaldenhoff, 2014) by displacing lipids, further reducing ‘background’ CO2 permeability. True membrane permeability depends on two parallel pathways that sum like parallel electrical conductances: = + . In series with the membrane are unconvected layers (ULs; Fig. 1 A) that reduce the measured apparent membrane permeability ():
Figure 1. Models of CO2 movement across membranes of artificial lipid bilayers (A), as well as plasma membranes with low cholesterol content (B), moderate cholesterol content without (C) and with (D) gas channels, and high cholesterol content without gas channels (E) .
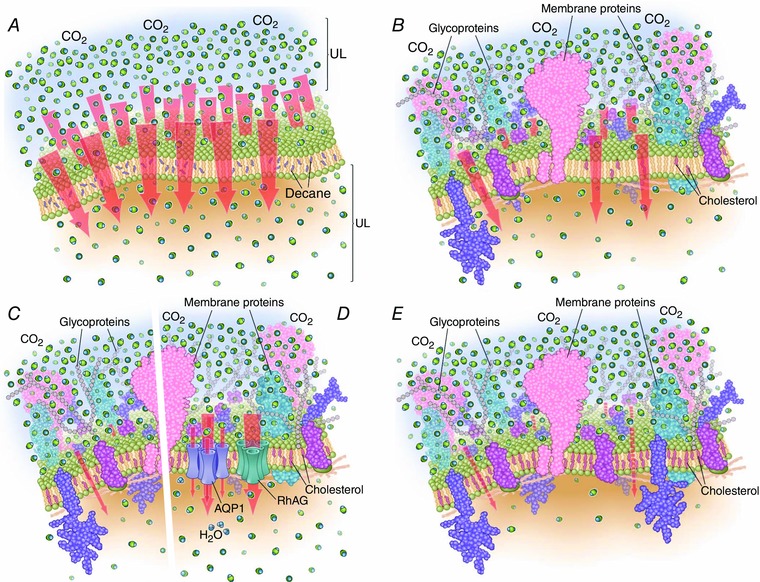
Membrane composition has dramatic effects on CO2 permeability. Red arrows represent CO2 flux. The density of CO2 molecules on upper side of membrane (exaggerated by ∼5‐fold, assuming a membrane thickness of 6 nm and 5% CO2 at room temperature, for illustrative purposes) is the same in all examples; the densities above and below the membranes represent snapshots during the early moments following CO2 addition to extracellular solution. The density of membrane proteins is less than indicated for synaptic vesicles by Takamori et al. (2006). In A, the artificial lipid bilayer contaminated with decane has an extremely high permeability to CO2. The square brackets UL indicate the unconvected layers either side of the membrane. B–E show biological membranes with integral proteins and increasing levels of cholesterol, both of which reduce ‘background’ CO2 permeability (i.e. not due to channels). In B, the presence of gas‐impermeable proteins and low cholesterol concentrations (∼25% of membrane lipid) lowers CO2 permeability and flux. In C, further increasing membrane cholesterol content (∼40%) dramatically decreases CO2 permeability. In D, membranes (e.g. from RBCs) with the same content of integral membrane proteins and cholesterol as in C have a much higher CO2 permeability because specialized channels such as AQP1 and the Rh‐associated glycoprotein (RhAG) augment the passage of CO2. In E, membranes (e.g. apical membranes of proximal colon) with extremely high cholesterol content (∼77%) are expected to have a very low CO2 permeability.
Thus, channels likely contribute more when ULs are thin and is low (as in red blood cells (RBCs)), but less when ULs are thick and/or is high (as in some solid tumour models).
Options in cell membrane design
can span many orders of magnitude. At one end of the spectrum are artificial lipid bilayers, typically loosely packed lipids containing as much as 30% of the solvent n‐decane (Fig. 1 A). Here, = , high enough to make measurement difficult.
Further along the spectrum are plasma membranes from cells with modest C M,chol and abundant integral membrane proteins (Fig. 1 B). Examples include MFC7 breast tumour cells and ascites tumour cells, with C M,chol of ∼25% (Haeffner et al. 1984; Todor et al. 2012). With such a low C M,chol, could be sufficiently high that, even without channels, ascites tumour cells could accommodate their modest of 0.12 ml g−1 min−1 (Warburg, 1956), according to Endeward's analysis (2014).
Even further along the spectrum are Madin‐Darby canine kidney (MDCK) cells, with an intermediate C M,chol (37%), no known gas channels, and a low (Itel et al. 2012) that presumably represents and is sufficient to meet a low metabolic demand (Fig. 1 C). Interestingly, depleting MDCK cells of cholesterol (i.e. raising ) or expressing AQP1 (i.e. raising ) raises .
Falling into the same cholesterol content category (∼40%) as MDCK cell membranes are RBC membranes (Fig. 1 D). Although RBCs have a low , a high gas‐channel content gives them a high (see below).
At the far end of the spectrum are proximal colon apical membranes, with C M,chol of 77%, consistent with the observed low CO2 permeability (Fig. 1 E; Endeward et al. 2014). Apical membranes of gastric glands have no measurable (Waisbren et al. 1994).
Gas channels
In 1998 Boron's laboratory identified the first family of gas channels by showing that CO2 moves through AQP1, heterologously expressed in Xenopus oocytes (Nakhoul et al. 1998; Cooper & Boron, 1998). Both p‐chloromercuriphenylsulfonic acid (pCMBS) (Cooper & Boron, 1998) and DIDS (Endeward et al. 2006) significantly reduce AQP1‐dependent ; because pCMBS but not DIDS reduces water permeability, these agents act via different pathways. AQP1 also conducts NH3 (Nakhoul et al. 2001) and NO (Herrera et al. 2006).
Ripoche et al. (2004) and Khademi et al. (2004) identified another gas‐channel family, the Rhesus (Rh) proteins, by demonstrating permeability to NH3. Work with human RBCs showed that the Rh complex also conducts CO2 (Endeward et al. 2008). Further studies show that each AQP and Rh protein exhibits a characteristic selectivity for CO2 vs. NH3, with some AQPs being impermeable to CO2, NH3, or both (Musa‐Aziz et al. 2009; Geyer et al. 2013 b,c).
Recently, Boron's group identified a third gas‐channel family: the urea transporter UT‐B, which is permeable to NH3 (Geyer et al. 2013 a). The known gas‐channel families consist of physiologically active monomers surrounding a central structure that, for AQPs and Rhs, is a lipophilic pore. We hypothesize that some of these central pores conduct CO2 or other dissolved gases, and that other families of gas channels remain to be identified – known proteins with previously unappreciated gas‐selective pathways. Thus, arguing that particular membranes lack particular channels ignores the possibility of yet‐to‐be‐discovered channels.
Physiological role
The first demonstrated physiological role for channels in gas transport was CO2 uptake (driven by an exceeding small gradient) via NtAQP1 in tobacco plants during photosynthesis (Uehlein et al. 2003). Studies on human RBCs show that DIDS plus the genetic deletion of either AQP1 (Colton‐null) or Rh complex reduces by ≥90% (Endeward et al. 2006, 2008), leaving, at most, 10% for CO2 pathways through lipid. Thus, channels almost certainly make a physiologically important contribution to CO2 exchange in pulmonary and systemic capillaries, particularly during conditions of short transit time, such as exercise (Endeward et al. 2014).
Channels could make significant contributions in other systems with high CO2 fluxes and high AQP levels. Examples include alveolar type I pneumocytes (AQP5; Verkman et al. 2000), astrocytic endfeet/blood–brain barrier (AQP4; Nagelhus et al. 2004) and renal proximal tubules (AQP1; Schnermann et al. 1998).
In conclusion, channels contribute to on a sliding scale that depends on the balance of vs. and ULs. Moving the field forward, and knowing where cells sit on the sliding scale, will require understanding this balance by examining, in simple systems, how physiological ULs and altered membrane composition (i.e. the nature and number of lipids, non‐channel proteins, channels) affect gas fluxes.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment, including a title and a declaration of interest, to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Additional information
Competing interests
None declared.
Funding
This work was supported by grants to WFB from the Office of Naval Research (N00014‐11‐1‐0889, N00014‐14‐1‐0716, N00014‐15‐1‐2060), the NIH (U01‐GM111251), and by the Meyer/Scarpa Chair.
Acknowledgements
We thank Professor Ulrich Hopfer at CWRU for helpful discussions on the Overton papers and Dr. Seong‐Ki Lee for helpfull discussions on the ULs.
Biographies
Gordon Cooper is a Senior Lecturer in Biomedical Science at the University of Sheffield. He has a background in renal and epithelial physiology and his research has focused on the transport of small solutes and gases across biological membranes.

Rossana Occhipinti is Adjunct Instructor in the Department of Physiology and Biophysics at CWRU. Her PhD is in applied mathematics and her current research focuses on mathematical models of CO2 and acid–base transport.
Walter Boron is the Myers/Scarpa Professor and Chair of Physiology and Biophysics at CWRU. He has had a longstanding research interest in transport across cell membranes, especially as it pertains to acids and bases and the regulation of intracellular pH. This work unexpectedly led to the first descriptions of CO2‐impermeable membranes and gas channels.
References
- Al‐Awqati Q (1999). One hundred years of membrane permeability: does Overton still rule? Nat Cell Biol 1, E201–E202. [DOI] [PubMed] [Google Scholar]
- Boron WF (2010). Sharpey–Schafer Lecture: Gas channels. Exp Physiol 95, 1107–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GJ & Boron WF (1998). Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol 275, C1481–C1486. [DOI] [PubMed] [Google Scholar]
- Endeward V, Al‐Samir S, Itel F & Gros G (2014). How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Front Physiol 4, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Cartron J‐P, Ripoche P & Gros G (2008). RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22, 64–73. [DOI] [PubMed] [Google Scholar]
- Endeward V, Musa‐Aziz R, Cooper GJ, Chen L‐M, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF & Gros G (2006). Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20, 1974–1981. [DOI] [PubMed] [Google Scholar]
- Geyer RR, Musa‐Aziz R, Enkavi G, Mahinthichaichan P, Tajkhorshid E & Boron WF (2013. a). Movement of NH₃ through the human urea transporter B: a new gas channel. Am J Physiol Renal Physiol 304, F1447–F1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer RR, Musa‐Aziz R, Qin X & Boron WF (2013. b). Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am J Physiol Cell Physiol 304, C985–C994. [DOI] [PubMed] [Google Scholar]
- Geyer RR, Parker MD, Toye AM, Boron WF & Musa‐Aziz R (2013. c). Relative CO2/NH3 permeabilities of human RhAG, RhBG and RhCG. J Membr Biol 246, 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T (1866). On the absorption and dialytic separation of gases by colloid septa. Philos Trans R Soc Lond 156, 399–439. [Google Scholar]
- Haeffner EW, Hoffmann CJ, Stoehr M & Scherf H (1984). Cholesterol‐induced growth stimulation, cell aggregation, and membrane properties of ascites tumor cells in culture. Cancer Res 44, 2668–2676. [PubMed] [Google Scholar]
- Herrera M, Hong NJ & Garvin JL (2006). Aquaporin‐1 transports NO across cell membranes. Hypertension 48, 157–164. [DOI] [PubMed] [Google Scholar]
- Itel F, Al‐Samir S, Öberg F, Chami M, Kumar M, Supuran CT, Deen PMT, Meier W, Hedfalk K, Gros G & Endeward V (2012). CO2 permeability of cell membranes is regulated by membrane cholesterol and protein gas channels. FASEB J 26, 5182–5191. [DOI] [PubMed] [Google Scholar]
- Kai L & Kaldenhoff R (2014). A refined model of water and CO₂ membrane diffusion: effects and contribution of sterols and proteins. Sci Rep 4, 6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi S, O'Connell J 3rd, Remis J, Robles‐Colmenares Y, Miercke LJW & Stroud RM (2004). Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 305, 1587–1594. [DOI] [PubMed] [Google Scholar]
- Mitchell JK (1830). On the penetrativeness of fluids. Am J Med Sci 13, 36–67. [Google Scholar]
- Musa‐Aziz R, Chen L‐M, Pelletier MF & Boron WF (2009). Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106, 5406–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM & Ottersen OP (2004). Aquaporin‐4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 129, 905–913. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF & Boron WF (1998). Effect of expressing the water channel aquaporin‐1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274, C543–C548. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Hering‐Smith KS, Abdulnour‐Nakhoul SM & Hamm LL (2001). Transport of NH3/NH4 + in oocytes expressing aquaporin‐1. Am J Physiol Renal Physiol 281, F255–F263. [DOI] [PubMed] [Google Scholar]
- Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y & Cartron J‐P (2004). Human Rhesus‐associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci USA 101, 17222–17227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA & Verkman AS (1998). Defective proximal tubular fluid reabsorption in transgenic aquaporin‐1 null mice. Proc Natl Acad Sci USA 95, 9660–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F & Jahn R (2006). Molecular anatomy of a trafficking organelle. Cell 127, 831–846. [DOI] [PubMed] [Google Scholar]
- Todor IN, Lukyanova NY & Chekhun VF (2012). The lipid content of cisplatin‐ and doxorubicin‐resistant MCF‐7 human breast cancer cells. Exp Oncol 34, 97–100. [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F & Kaldenhoff R (2003). The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425, 734–737. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Matthay MA & Song Y (2000). Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol 278, L867–L879. [DOI] [PubMed] [Google Scholar]
- Waisbren SJ, Geibel JP, Modlin IM & Boron WF (1994). Unusual permeability properties of gastric gland cells. Nature 368, 332–335. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cohen J, Boron WF, Schulten K & Tajkhorshid E (2007). Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J Struct Biol 157, 534–544. [DOI] [PubMed] [Google Scholar]
- Warburg O (1956). On the origin of cancer cells. Science 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Wroblewski S (1879). On the nature of the absorption of gases. Nature 21, 190–192. [Google Scholar]


