Abstract
The objective of this study was to increase understanding about the mechanism by which polyamines (PAs) promote the conversion of embryogenic calli (EC) into somatic embryos in cotton (Gossypium hirsutum L.). We measured the levels of endogenous PAs and H2O2, quantified the expression levels of genes involved in the PAs pathway at various stages of cotton somatic embryogenesis (SE), and investigated the effects of exogenous PAs and H2O2 on differentiation and development of EC. Putrescine (Put), spermidine (Spd), and spermine (Spm) significantly increased from the EC stage to the early phase of embryo differentiation. The levels of Put then decreased until the somatic embryo stage whereas Spd and Spm remained nearly the same. The expression profiles of GhADC genes were consistent with changes in Put during cotton SE. The H2O2 concentrations began to increase significantly at the EC stage, during which time both GhPAO1 and GhPAO4 expressions were highest and PAO activity was significantly increased. Exogenous Put, Spd, Spm, and H2O2 not only enhanced embryogenic callus growth and embryo formation, but also alleviated the effects of D-arginine and 1, 8-diamino-octane, which are inhibitors of PA synthesis and PAO activity. Overall, the results suggest that both PAs and their metabolic product H2O2 are essential for the conversion of EC into somatic embryos in cotton.
Keywords: upland cotton, somatic embryogenesis (SE), polyamines (PAs), hydrogen peroxide (H2O2), polyamine oxidase (PAO), nitric oxide (NO)
Introduction
Plant regeneration through somatic embryogenesis (SE) has greatly aided gene transformation in many plant species, including cotton (G. hirsutum L.). The developmental stages involved in SE are analogous to those involved in zygotic embryogenesis (Silveira et al., 2013); however, somatic embryos have no endosperm and do not undergo some of the early divisions which are characteristic in zygotic embryos (Costa et al., 2014; Soriano et al., 2014). In cotton, plantlets have been regenerated via SE using various combinations of plant growth-regulators, such as 2, 4-dichlorophenoxyacetic acid (2, 4-D), indole-3-butyric acid (IBA), and naphthalene acetic acid (NAA) in combination with kinetin (KT) (Surgun et al., 2014). However, SE in cotton is often hampered by recalcitrance, long culture time, and high frequency of abnormal embryos. Low efficiency of regeneration is another major issue that limits the utilization of Agrobacterium-mediated transformation in cotton. Transcriptomic and proteomic studies have shown that genes and proteins involved in stress response, hormone metabolism, respiration, and photosynthesis play a role in increasing plantlet regeneration via SE in cotton (Ge et al., 2014). In addition, the effects of several genes on SE have been analyzed. For example, auxin-induced expression of WUSCHEL (WUS), a specifier of stem cell fate, was found to be essential for renewal of embryonic stem cells during SE in Arabidopsis (Su et al., 2009). LEC2 plays critical roles during embryo development and is essential for induction of SE in Arabidopsis (Gaj et al., 2005). Understanding the molecular mechanisms of genes involved in SE and their interactions with other factors, such as hormones, can help with the initiation of embryogenic cultures and the enhancement of embryo yield and quality.
Polyamines (PAs), mainly putrescine (Put), spermidine (Spd), spermine (Spm), and the recently discovered thermospermine (T-Spm), are small, aliphatic amines that are widely present in all plant cells (Wojtasik et al., 2015). PAs are involved in many physiological processes, including cell division, rhizogenesis, senescence, floral development, fruit ripening, and the response to biotic and abiotic stress (Alcázar and Tiburcio, 2014). In plants, Put is synthesized by two pathways. One pathway begins with decarboxylation of arginine by arginine decarboxylase (ADC). PA concentrations are enhanced by exogenous arginine (Nieves et al., 2008). Another pathway begins with ornithine, which is converted into Put in a single-step reaction catalyzed by ornithine decarboxylase (ODC). Spermidine is synthesized by spermidine synthase (SPDS) through the addition of an aminopropyl moiety to Put. The aminopropyl moiety is donated by decarboxylated S-adenosylmethionine (dcSAM) which is converted from S-methylmethionine by S-adenosylmethionine decarboxylase (SAMDC). Spermidine then functions as a substrate to synthesize Spm by spermine synthase (SPMS). Thermospermine is an isomer of spermine and assumed to be synthesized by a mechanism analogous to that of Spd biosynthesis (Knott et al., 2007). L-arginine is an important substrate for the biosynthesis of PAs. However, L-arginine can also be used to generate nitric oxide (NO) by nitric oxide synthase (NOS) (Galea et al., 1996). Both H2O2 and NO are important signaling molecules involved in many developmental and physiological processes in plants. The H2O2 is involved in the regulation of root development, seed germination, programmed cell death, and defense responses to pathogen and abiotic stresses (Berna and Bernier, 1999). Studies indicate that H2O2 can be produced either by the NADPH-dependent pathway (Neill et al., 2002a), the antioxidant enzyme system (Alscher et al., 1997), or from PAs (mainly Put, Spd, and Spm) catalyzed by polyamine oxidase (PAO) or diamine oxidase (DAO). NO is a highly diffusible free radical that acts as an intra- and/or inter-cellular messenger to regulate various developmental and biological processes, including root development, seed germination, senescence, respiration, cell death, disease resistance, hormone responses, and abiotic stress responses.
Polyamines have previously been linked to both zygotic embryogenesis and SE. PA concentrations increase during the early stages of SE in conifers but decrease during the late stages (Gemperlová et al., 2009; Paul et al., 2009; Vuosku et al., 2012). Putrescine, spermidine, and spermine have also been shown to significantly improve SE of Theobroma cacao L, Citrus sinensis and Hurst Ecotype (Silva et al., 2009; Wu et al., 2009; Malá, 2012). Elevated levels of Put, Spd, and Spm in embryogenic cells, a result of enhanced expression of enzymes such as SAMDC, ADC and SPDS, suggest that PAs have a role in cellular differentiation during SE (Montague et al., 1978, 1979; Gemperlová et al., 2009; Niemenak et al., 2012). Both ADC mRNA and ADC protein were localized in dividing cells of embryo meristems, suggesting an association between ADC and mitosis (Vuosku et al., 2006). Exogenous Put and Spm enhanced the growth of embryogenic cultures of Araucaria angustifolia and significantly affected endogenous concentrations of PA, IAA and ABA in embryogenic tissues (Steiner et al., 2007). Nevertheless, an inverse correlation was observed between total free PA concentration and embryogenic potential in Pinus nigra Am. Sp. (Noceda et al., 2009). Biosynthesis of PAs is regulated by light in the presence of the plant growth regulators benzylaminopurine and cytokinin during SE in C. canephora (De-la-Peña et al., 2008). The dynamics of protein, sugar, starch, and amino acid were closely related to the accumulation of PAs during SE of A. sellowiana (Cangahuala-Inocente et al., 2014). In addition, genomic DNA of embryogenic tissues of Pinus nigra Am. Sp., i.e., those that are able to produce regenerated plantlets, was found to be lowly methylated (Noceda et al., 2009). These studies have investigated the effects of endogenous or exogenous PAs on SE in different species under different conditions; however, the physiological mechanisms by which PAs promote SE are still largely unclear.
Gossypium hirsutum L. cv. Xinluzao 33 is one of the main cotton cultivars used in Xinjiang Province, China. Regeneration of Xinluzao 33 via SE is problematic because of the lengthy time required for embryogenic callus induction and the low ratio of somatic embryo differentiation. The objectives of this study were (i) to enhance understanding about the relationship among PAs, H2O2, and NO during SE of Xinluzao 33, and (ii) to gain insight into the mechanisms by which PAs promote the conversion of embryogenic calli (EC) into somatic embryos.
Materials and Methods
Tissue Culture and Somatic Embryogenesis in Xinluzao 33
The cotton cultivar used in SE was Xinluzao 33, one of the major commercial cultivars in Xinjiang, China. The method for SE used in this study has been described previously (Sun et al., 2006). Briefly, Xinluzao 33 seeds were decoated, soaked in 0.1% (w/v) Hg2Cl2 for 10 min, and then rinsed three times with sterile, distilled water. The treated seeds were transferred to 100 mL Erlenmeyer flasks containing 25 mL of half-strength MS medium (Murashige and Skoog, 1962) and then incubated in the dark at 28°C for 7 days. Hypocotyls from 7-day-old sterile seedlings were cut into 1 cm segments and then transferred to callus-induction medium for SE. The culture mediums used for SE in this study are described in Supplementary Table S1. All cultures in this study were conducted under 16 h light: 8 h dark at 28°C.
Establishment of Suspension Cultures for Uniform Embryogenic Callus
Embryonic callus (5 g) was collected and inoculated in liquid embryo induction medium, followed by shaking on an orbital shaker at 200 rpm in the dark at 28°C. After 5 days, the cultures were filtered into a flask through a 50-mesh sieve, and the supernatant was removed after 15-min sedimentation. The EC on the bottom of the flask were resuspended in 2 mL of liquid somatic embryo induction medium and used in various experiments.
Exogenous PAs, H2O2, SNP, D-Arg, and 1, 8-DO Treatments
To determine the effects of PAs, H2O2, NO, PA synthesis inhibitor, and PAO inhibitor on the conversion of embryogenic callus into somatic embryos, uniform EC were cultured on somatic embryo induction medium supplemented with 1 mM of putrecine (Put), spermidine (Spd), spermine (Spm), sodium nitroprussiate (SNP; a NO donor) (Paulus et al., 1994), D-arginine (D-Arg, a specific PAs synthesis inhibitor) (Liu et al., 2006), H2O2, or 1,8-diamino-octane (1,8-DO, a specific PAO activity inhibitor) (Rodríguez et al., 2009). All reagents were purchased from Sigma Chemical Co (St. Louis, MO, USA). The 1 mM concentration was chosen based on preliminary gradient experiments (data not shown).
Uniform EC were inoculated at four positions (60 μL/position) in each Petri dish. Each treatment was replicated three times (i.e., three Petri dishes). The cultures were collected after 4 weeks, weighed, suspended in purified water, and then examined with a stereomicroscope to count embryos and cotyledonary embryos (Supplementary Figure S1). Increase in fresh weight (FW) during the culture period was determined by subtracting the initial callus weight from the total FW.
Determination of the Free and Conjugated Polyamine Concentrations
Concentrations of free and conjugated PAs were determined using a modified high performance liquid chromatography (HPLC) method. Tissue samples (1 g) were collected at each stage of SE and then ground in liquid N2. The homogenate was resuspended in 5 mL 10% perchloric acid, incubated on ice for 1.5 h, and then centrifuged at 18514 g for 20 min at 4°C. Seven micro liter benzoyl chloride and 1 mL 2M NaOH were then added to 500 μL of the supernatant. The reactions were allowed to proceed at 37°C for 30 min and then 2 mL ether and 2 mL saturated NaCl were added to the reactions. The reactions were shaken for 5 min and then 1 mL of the ether phase was removed and dried under vacuum. The dried reactions were re-dissolved in 100 μL methanol before HPLC analysis. The HPLC was performed on an Agilent 1200 system (Agilent, USA) with an Agilent XDB-C18 (4.6 mm × 150 mm) column. The HPLC conditions were as follows: liquid phase with a methanol:water ratio of 60:40 (v/v), 1 mL/min of flow rate, 10 μL of sample per injection, detection at 30°C with a wave length of 254 nm, and 30 min of retention time. Peak areas and retention times were measured by comparison with standard Put, Spd, and Spm. The concentrations of PAs (ng of PAs g-1 fresh callus weight) were determined using a standard curve prepared with known amounts of standard Put, Spd, and Spm. The HPLC traces are shown in Supplementary Figure S2. The assays were technically repeated three times.
To determine the effects of Put, Spd, Spm and D-Arg on the PA concentrations, EC were cultured on liquid somatic embryo induction medium supplemented with 1 mM Put, Spd, Spm and D-Arg. The concentration of PAs in the EC was measured 3 days after inoculation. EC without any treatment were used as the control. The samples and treatments were replicated three times and the assays were technically repeated three times.
Determination of H2O2 and NO
Hydrogen peroxide concentrations were determined with an H2O2 determination kit (Jiancheng Biochemistry Company, Nanjing, China) as previously described (Crumbliss et al., 1992). The absorbance of the titanium-peroxide complex was measured at 412 nm. The NO concentrations were determined using an NO determination kit (Jiancheng Biochemistry Company, Nanjing, China) as described by Qian et al. (2006). The absorbance was measured at 550 nm. The concentrations of H2O2 and NO (ng g-1 FW) in the samples were determined using a standard curve prepared with known amounts of H2O2 and NO. The assays were technically repeated three times.
To determine the effects of exogenous H2O2, Put, Spd, Spm, D-Arg and 1, 8-DO on H2O2 concentration, EC were cultured on liquid somatic embryo induction medium supplemented with 1 mM Put, Spd, Spm and D-Arg. The concentration of H2O2 in the EC was measured 3 days after inoculation. EC without any treatment were used as the control. The treatments were replicated three times and the assays were technically repeated three times.
Detection of Hydrogen Peroxide by 3, 3′-Diaminobenzidine (DAB)
In situ detection of H2O2 was performed by DAB staining (Sigma–Aldrich) using a published method (Daudi et al., 2012). The staining reaction was terminated 5 h after DAB infiltration, and then the cultures were fixed in ethanol in a water bath at 95°C for 15 min. The cultures were reimmersed in bleach solution until the chlorophyll was completely bleached. The cultures were then visualized under white light and photographed. A combination of tools from ZEN Imaging Software (ZEISS, Germany) was used to establish the threshold of DAB staining in the cultures and to distinguish the staining from the background. The staining experiments were technically repeated three times.
PAO Enzyme Activity Assay
Polyamine oxidase activity was determined using an ELISA Assay Kit (Jiancheng Biochemistry Company, Nanjing, China) according to the manufacturer’s instructions. The antibodies were produced by the Jiancheng Biochemistry Company (Nanjing, China). The PAs specificity of the PAO enzyme was evaluated by the OD value according to the known concentration of standard PAs. The tissue samples used for PAO enzyme assay were the same as those used for determining PA concentrations. The assays were technically repeated three times.
To determine the effects of Put, D-Arg, and 1,8-DO on PAO activity, EC were cultured on liquid somatic embryo induction medium supplemented with 1 mM Put, D-Arg or 1,8-DO. The PAO activities in the EC were measured 3 days after inoculation. EC without any treatment were used as the control. The treatments were replicated three times and the assays were technically repeated three times.
Gene Identification
To quantify the expression levels of cotton genes encoding ADC, PAO and SAMDC at various stages of cotton SE, protein sequences of the Arabidopsis orthologs [AtADC (NM_127204), AtPAO (NM_121373) and AtSAMDC1 (NC_003074.8)] were used as query to search against the G. raimondii genome (Paterson et al., 2012) using an E-value of 0.99. This analysis identified three ADC-encoding genes, four PAO-encoding genes, and four SAMDC-encoding genes. These genes were used to investigate the expression levels of their orthologs in G. hirsutum. The genes encoding SPDS, SPMS, CAT, NOX, SOD, APX and NOS were determined based on our transcriptome sequencing of upland cotton cv. Xinluzao 33 during SE. The nucleotide sequences of these genes and their gene ID are listed in Supplementary Table S2.
RNA Extraction and Quantitative Real Time PCR
Total RNA was extracted from the samples using a modified CTAB method (Chang et al., 1993). Approximately 2 μg of total RNA was reverse transcribed into cDNA using the Prime Script RT reagent kit (Takara, Japan) with gDNA Eraser (Takara, Japan). The cDNA templates were diluted ten times prior to qPCR. The qRT-PCR experiment was conducted in a Roche LightCycler 480 system (Roche, Switzerland) using the SYBR Premix ExTaqTM kit (Takara, Japan) and the following thermal cycling program: pre-incubation at 95°C for 2 min, followed by 40 cycles of 94°C for 15 s, 56°C for 20 s, and 72°C for 20 s. The relative expression levels were determined using 2-ΔCt with the ubiquitin (GhUBI, XM_012634824) gene as the reference. The primers used in qRT-PCR were designed using Primer Premier 5.0 (Supplementary Table S3). The expression assay of each sample was performed using three biological replicates and each biological replicate was technically repeated three times.
Statistical Analysis
Analysis of variance was performed using SPSS16.0 statistical analysis package. Differences between means were compared by Fisher’s least-significant-difference test at the 5 and 1% probability level.
Results
Polyamines Significantly Increased between the Embryogenic Callus and Early Embryo Differentiation Stages
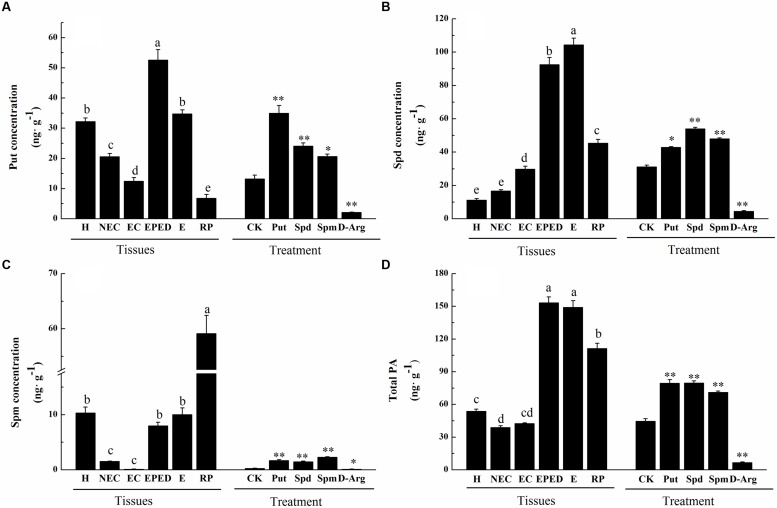
Generally, cotton SE includes four stages, i.e., callus induction, embryogenic callus induction, embryo differentiation, and plant regeneration. To investigate temporal changes of free and conjugated PAs during cotton SE, the concentrations of three common PAs (Put, Spd, and Spm) were analyzed in hypocotyl-derived explants and in samples collected at different stages of cotton SE (Supplementary Figure S3). Compared to non-embryogenic callus, embryogenic callus showed a significant decrease in Put, a significant increase in Spd, and no changed in Spm (Figures 1A–C). Compared to embryogenic callus, a significant increase in all three PAs (Put: 4.2-fold; Spd: 3.1-fold; Spm: 8.1-fold) was observed in the early phase of embryo differentiation (Figures 1A–C). The total concentration of PAs increased 3.6-fold from the embryogenic callus stage to the early phase of embryo differentiation (Figure 1D). The concentrations of these PAs remained high in somatic embryos. The concentrations of Put and Spd declined significantly in the regenerated plantlets whereas the concentration of Spm increased significantly in the regenerated plantlets (Figures 1A–C). Compared to Put and Spd, Spm concentrations remained relatively low throughout most of the SE stages except in the regenerated plantlets. As expected, supplementation of exogenous Put, Spm or Spd significantly increased endogenous Put, Spm and Spd in embryogenic callus. Application of D-Arg, an inhibitor of PA synthesis, significantly decreased endogenous Put, Spm and Spd in embryogenic callus (Figure 1).
FIGURE 1.
Polyamine concentrations at different stages of somatic embryogenesis (SE) in ‘Xinluzao 33’ and the effects of different chemical treatments on polyamine concentrations. (A) Putrescine concentration. (B) Spermidine concentration. (C) Spermine concentration. (D) Total PA. Abbreviations on the x-axis: H, hypocotyl; NEC, non-embryogenic callus; EC, embryogenic callus; EPED, early phase of embryo differentiation; E, embryo; RP, regenerated plantlets. Treatment on the x-axis, CK, control; D-Arg, D-arginine treatment; Put, putrescine treatment; Spd, spermidine treatment; Spm, spermine treatment. Values are the mean + standard error (n = 3). Different lowercase letters above the bars indicate significant differences at P < 0.05 according to LSD multiple range test. ∗ and ∗∗ indicate significant differences compared with the control at P < 0.05 and P < 0.01, respectively, according to LSD multiple range test.
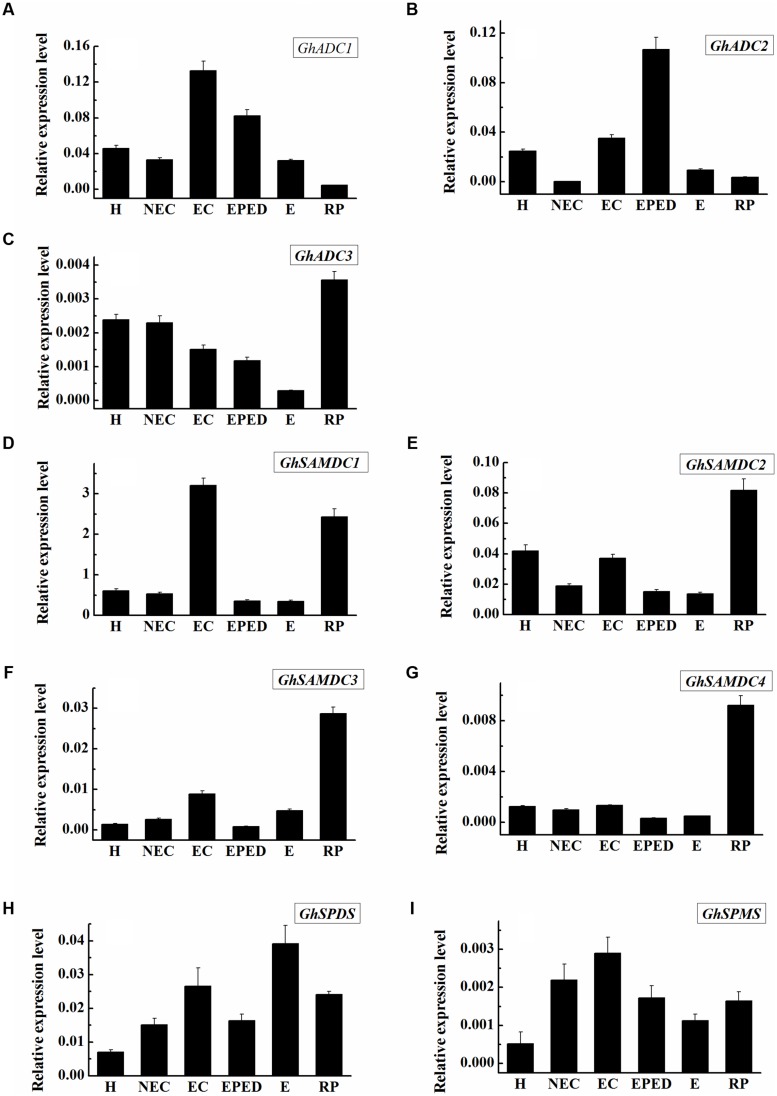
Arginine decarboxylase (ADC) catalyzes the first reaction toward biosynthesis of Put. S-adenosylmethionine decarboxylase (SAMDC) converts dcSAM into Spd by adding an aminopropyl moiety to Put. The main genes for synthesis of Spd and Spm from Put are SPDS and SPMS. We analyzed the expression levels of the genes encoding ADC, SAMDC, SPDS and SPMS at different stages during cotton SE. Of the three ADC-encoding genes, the expression level of GhADC3 was much lower than that of GhADC1 and GhADC2, suggesting that GhADC1 and GhADC2 are the major genes responsible for the biosynthesis of Put in cotton callus tissue. The expression levels of GhADC1 and GhADC2 were highest at the embryogenic callus stage and the early phase of embryo differentiation. There was no clear correlation between the individual expression levels and Put (Figures 2A–C). This suggested that Put concentrations might be determined by the combined action of all GhADC genes. Among the four GhSAMDC genes, the expression of GhSAMDC1 was higher than that of GhSAMDC2, GhSAMDC3 and GhSAMDC4 in all the samples. The highest GhSAMDC1 expression level was in the embryogenic callus, whereas the highest expression of the other three GhSAMDC genes was in regenerated plantlets (Figures 2D–G). The highest SPDS and SPMS expression was in the somatic embryos and embryogenic callus, respectively (Figures 2H,I). Although Spd increased significantly at the early phase of embryo differentiation and the somatic embryo stage, the expression levels of all four GhSAMDC genes were relatively low at these two stages (Figure 1B). These results suggest that Spd concentrations may be determined by Spd synthase (SPDS) rather than by SAMDC.
FIGURE 2.
Expression level of genes encoding SAMDC, ADC, SPDS and SPMS during SE in ‘Xinluzao 33’. The equation 2-ΔCt was applied to calculate the relative expression level using GhUBI as the reference gene. (A–I) Relative expression level of GhSAMDC1/2/3/4, GhADC1/2/3, GhSPDS and GhSPMS. (A) Relative expression level of GhADC1. (B) Relative expression level of GhADC2. (C) Relative expression level of GhADC3. (D) Relative expression level of GhSAMDC1. (E) Relative expression level of GhSAMDC2. (F) Relative expression level of GhSAMDC3. (G) Relative expression level of GhSAMDC4. (H) Relative expression level of GhSPDS. (I) Relative expression level of GhSPMS. Abbreviations on the x-axis: H, Hypocotyl; NEC, Non-embryogenic callus; EC, Embryogenic callus; EPED, Early phase of embryo differentiation; E, Embryo; RP, Regenerated plantlets. Values are the mean + standard error (n = 3).
Exogenous Polyamines Promoted the Conversion of Embryogenic Callus into Somatic Embryos
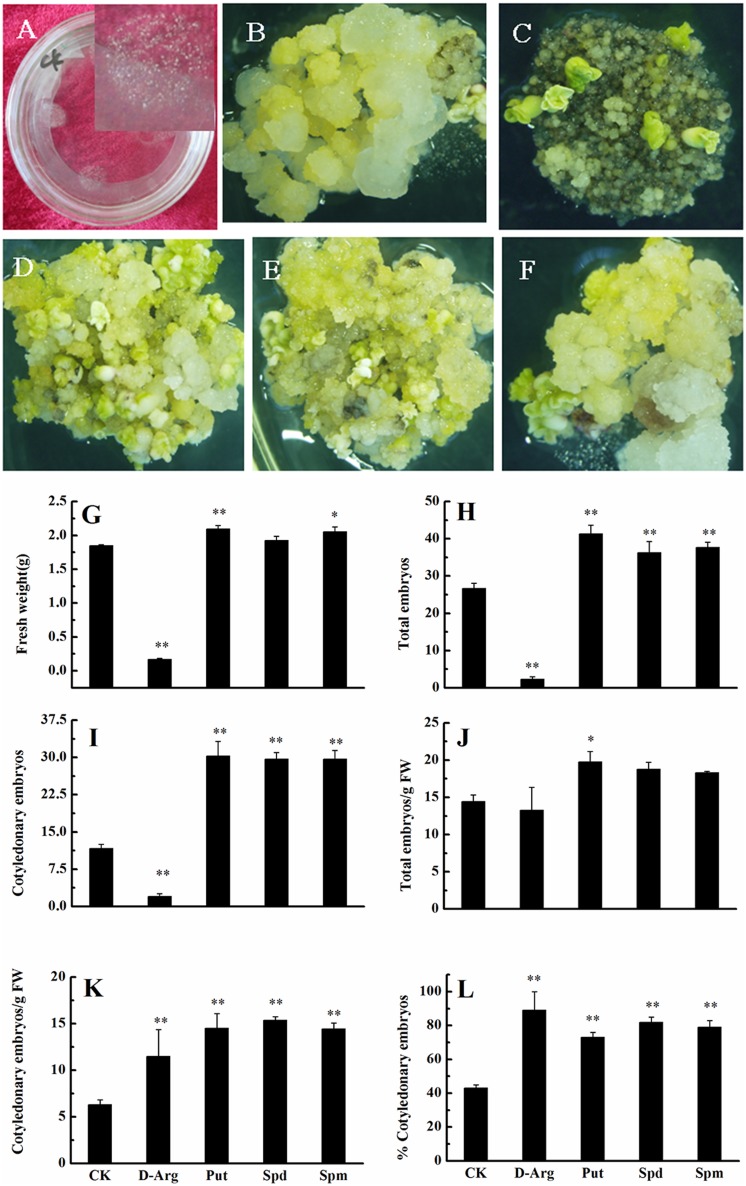
The above results showed that free and conjugated PA concentrations increased significantly during the early phase of embryo differentiation; therefore, our next experiments focused on this stage. The effect of PAs on the conversion of embryogenic callus into somatic embryos was studied by culturing EC on somatic embryo induction medium supplemented with D-Arg (an inhibitor of PAs synthesis), Put, Spd, or Spm. After 4 weeks of cultivation, calli looked moist in the control (i.e., no treatment) (Figures 3A,B). The D-Arg significantly inhibited the embryonic callus growth (Figure 3C), whereas exogenous PAs promoted callus growth (Figures 3D–F). Compared with the control, D-Arg significantly reduced FW, total embryo number, and the number of cotyledonary embryos (Figures 3G–I). In contrast, D-Arg increased the number of cotyledonary embryos/g FW and the percentage of cotyledonary embryos (Figures 3K,L). One explanation is that D-Arg treatment increased the survival rates of embryos. Compared with the control, the three PA treatments significantly increased (∼1.5 fold, P < 0.05) the total embryo number, the number of cotyledonary embryos, the cotylendonary embryo number/g FW, and the percentage of cotyledonary embryos (Figures 3H,I,K,L). The Put treatment also significantly increased the tissue FW and the total embryo number/g FW compared with the control (Figures 3G,J). Overall, these data indicated that application of exogenous PAs promoted the conversion of embryogenic callus into somatic embryos.
FIGURE 3.
Effects of exogenous PA and D-arg on the conversion of embryogenic callus into somatic embryos in ‘Xinluzao 33.’ (A) Initial status of embryogenic callus after passing through a 50 mesh sieve. (B–F) Status of embryogenic callus after 30 days on the somatic embryo induction medium. (B) Untreated medium (control). (C) D-arginine treatment. (D) Put treatment. (E) Spd treatment. (F) Spm treatment. (G–L) Statistic analysis of different treatments. (G) Fresh weight (g). (H) Total embryo number. (I) Cotyledonary embryo number. (J) Total embryo embryo number/g FW. (K) Cotyledonary embryo number/g FW. (L) % Cotyledonary embryos. Abbreviations on the x-axes: CK, control; D-Arg, D-arginine treatment; Put, putrescine treatment; Spd, spermidine treatment; Spm, spermine treatment. Data are mean + standard error (n = 3). ∗ and ∗∗ indicate significant differences compared with the control at P < 0.05 and P < 0.01, respectively, according to LSD multiple range test.
Effects of H2O2 and NO on the Conversion of Embryogenic Callus into Somatic Embryos in Cotton
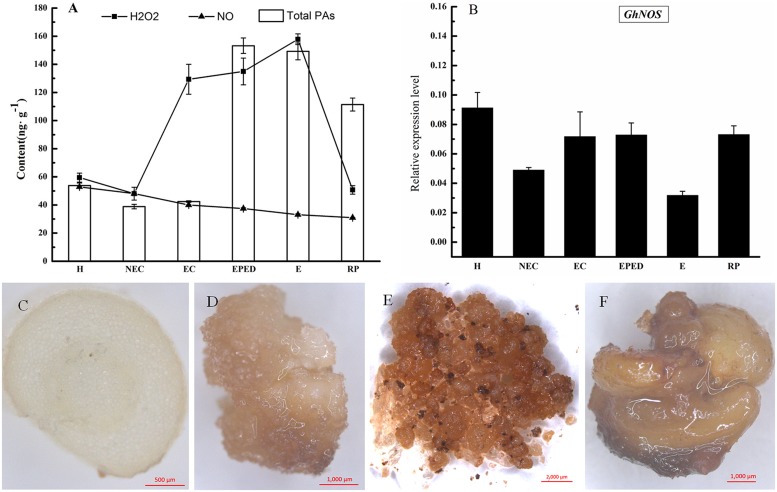
The next step in our study was to determine the role of two signaling molecules in the PAs metabolic pathway (H2O2 and NO) on the conversion of EC into somatic embryos. The concentration of endogenous H2O2 increased 2.7-fold from the non-embryogenic callus stage to the embryogenic callus stage. Endogenous H2O2 concentrations remained high during the early phase of embryo differentiation and the embryo stage and then significantly declined in the regenerated plantlets. Endogenous H2O2 concentrations were greatest at the somatic embryo stage, a critical stage of cotton SE. The temporal changes in H2O2 concentrations were similar to the changes in the concentrations PAs. There was significant positive correlation (r = 0.56, P < 0.05) between the concentrations of PAs and H2O2 (Figure 4A).
FIGURE 4.
Determination of H2O2 and NO levels during SE in ‘Xinluzao 33.’ (A) The concentration of H2O2, NO, and total PA at different stages of SE. Abbreviations on the x-axis: H, hypocotyl; NEC, non-embryogenic callus; EC, embryogenic callus; EPED, early phase of embryo differentiation; E, embryo; RP, regenerated plantlets. (B) Expression level of GhNOS during SE. (C–F) Detection of hydrogen peroxide by DAB. (C) Transverse sliced hypocotyl. (D) Non-embryogenic callus; (E) Embryogenic callus; (F) Somatic embryos.
Diaminobenzidine (DAB) can be oxidized by H2O2 to give a dark-brown color. We used DAB staining to visualize the presence and activity of H2O2 in hypocotyl, non-EC, EC and somatic embryos. EC and somatic embryos were stained deep brown (Figures 4E,F), whereas staining in hypocotyls and non-EC were much lighter (Figures 4C,D). These results confirmed a relatively high level of endogenous H2O2 in the EC and somatic embryos. The DAB staining in hypocotyls and non-EC did not strictly match the H2O2 concentrations, probably due to biased sampling of un-uniform non-EC. In contrast, endogenous NO concentrations did not change significantly during cotton SE (Figure 4A). This was consistent with the relatively stable expression level of GhNOS during SE (Figure 4B). There was no significant correlation between the concentrations of PAs and NO.
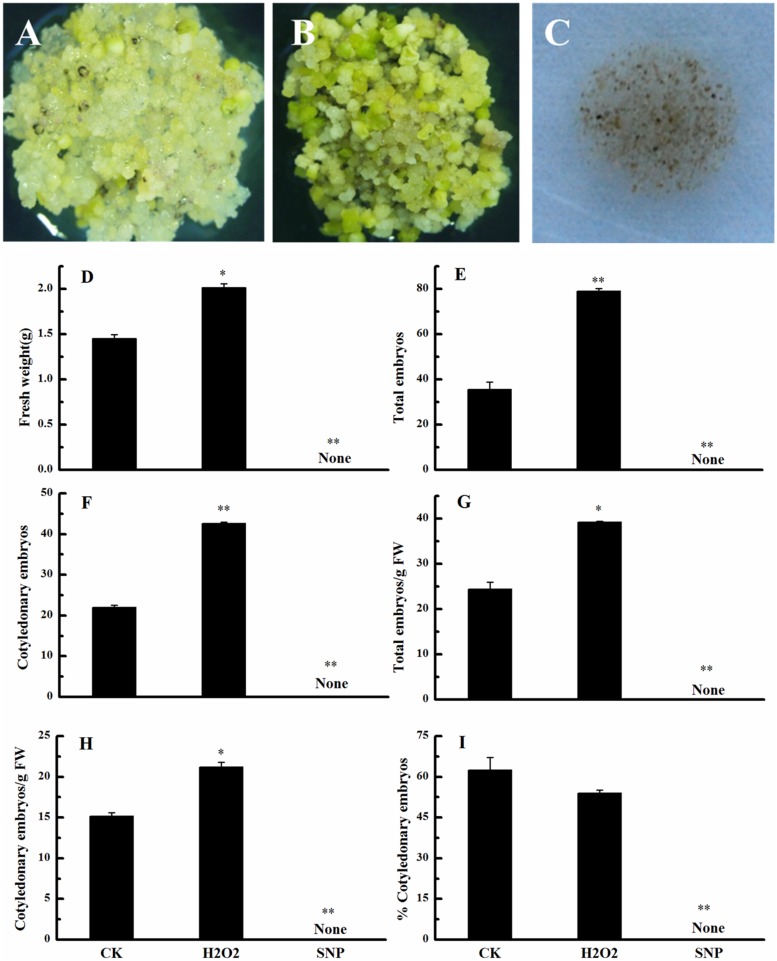
We also investigated the effects of exogenous H2O2 and SNP (an NO donor) on the conversion of embryogenic callus into somatic embryos. Compared with the control, exogenous H2O2 significantly promoted development of the culture (Figures 5A,B), whereas SNP inhibited development (Figure 5C). Exogenous H2O2 significantly increased the FW, the total number of embryos, the number of cotyledonary embryos, total embryo number/g FW, and cotyledonary embryo number/g FW (Figures 5D–H), but had no significant effect on the percentage of cotyledonary embryos (Figure 5I). These results suggested that similar to its metabolic precursor (i.e., PAs), H2O2 promotes the conversion of embryogenic callus into somatic embryos in cotton. Few EC were observed in the SNP treatment. This observation, along with the observation that NO concentrations did not change during cotton SE, suggests that NO has a negative role in cotton SE.
FIGURE 5.
Effects of exogenous H2O2 and NO on the conversion of embryogenic callus into somatic embryos in ‘Xinluzao 33.’ (A–C) Status of embryogenic callus after 30 days on somatic embryo induction medium. (A) Untreated medium (CK). (B) H2O2 treatment. (C) Sodium nitroprussiate (SNP, a NO donor) treatment. (D–I) Statistical analysis of the treatments. (D) Fresh weight (g). (E) Total embryo number. (F) Cotyledonary embryo number. (G) Total embryo number/g FW. (H) Cotyledonary embryo number/g FW. (I) % Cotyledonary embryos. Abbreviations on the x-axes: CK, control; H2O2, H2O2 treatment; SNP, sodium nitroprussiate treatment. Data are mean + standard error (n = 3). ∗ and ∗∗ indicate significant differences at P < 0.05 and P < 0.01, respectively, according to LSD multiple range test.
H2O2 Alleviated the Inhibitory Effect of D-Arg and Promoted the Conversion of Embryogenic Callus into Somatic Embryos
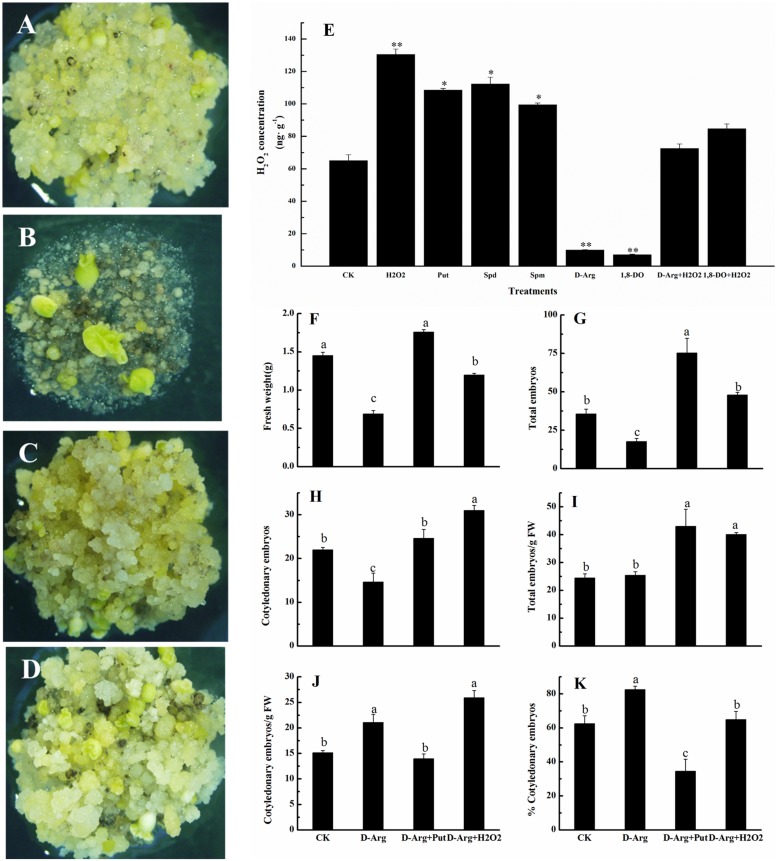
To learn more about the effects of H2O2 and PAs on the conversion of embryogenic callus into somatic embryos, we cultured EC on somatic embryo induction medium containing D-Arg which is an inhibitor of PAs synthesis. Compared with the control (Figure 6A), D-Arg significantly suppressed embryonic callus growth (Figure 6B). The callus FW, the total embryo number, and the number of cotyledonary embryos were significantly less in the D-Arg treatment than in the control (Figures 6F–H). However, the suppressive effects of D-Arg were alleviated when either Put or H2O2 was added to the medium (Figures 6C,D). The callus FW, the total embryo number, the number of cotyledonary embryos, and total embryo number/g FW were significantly higher in the D-Arg + Put and D-Arg + H2O2 treatments than in the D-Arg treatment (Figures 6F–I). Compared with the control, D-Arg increased the number of cotyledonary embryos/g FW and the percentage of cotyledonary embryos. This was similar to the results shown in Figures 3K,L. However, D-Arg + Put and D-Arg + H2O2 had little effect on these variables (Figures 6J,K). It should be noted that the effects of Put and H2O2 on the formation of somatic embryos were not exactly the same. The Put seemed to promote embryos formation whereas H2O2 seemed to promote cotyledonary embryos development (Figures 6G,H). These results further confirmed a role of H2O2 in the conversion of embryogenic callus into somatic embryos.
FIGURE 6.
H2O2 alleviated the inhibitory effect of D-Arg and promoted the conversion of embryogenic callus into somatic embryos in ‘Xinluzao 33.’ (A–D) Status of embryogenic callus after 30 days on the media. (A) Status of embryogenic callus after 30 days on the untreated media as control. (B) D-arginine treatment. (C) D-arginine + Put treatment. (D) D-arginine + H2O2 treatment. (E) H2O2 concentration in the H2O2, Put, Spd, Spm, D-Arg, 1, 8-DO, D-Arg + H2O2 and 1, 8-DO + H2O2 treatments. ∗ and ∗∗ indicate significant differences at P < 0.05 and P < 0.01, respectively, according to LSD multiple range test. (F–K) Statistical analysis of the treatments. (F) Fresh weight (g). (G) Total embryos. (H) Cotyledonary embryos. (I) Total embryos/g FW. (J) Cotyledonary embryos/g FW. (K) % Cotyledonary embryos. The horizontal axis of (F–K) were same, CK: control, D-Arg: D-arginine treatment, D-Arg + Put: media supplement with D-arginine and putrescine, D-Arg + H2O2: media supplemented with D-arginine and H2O2. Values are the mean + standard error (n = 3). Different lowercase letters above the bars indicate significant differences at P < 0.05 according to LSD multiple range tests.
Concentration of H2O2 was also measured in EC growing on medium supplemented with H2O2, Put, Spd, Spm, D-Arg, 1, 8-DO, D-Arg + H2O2 and 1, 8-DO + H2O2. The results showed that H2O2, Put, Spd, or Spm significantly increased H2O2 concentrations, whereas D-Arg and 1, 8-DO significantly reduced H2O2 concentrations. Supplementation of H2O2 in the D-Arg or 1, 8-DO-containing medium alleviated the inhibitory effects of D-Arg and 1, 8-DO on H2O2 production in EC (Figure 6E). These data indicated that accumulation of PAs leads to greater H2O2 concentrations. Furthermore, H2O2 concentrations are reduced by inhibitors of the synthesis or oxidative metabolism. The levels of PA and H2O2 are closely related.
Polyamine Oxidase (PAO) Plays a Crucial and Positive Role During the Conversion of Embryogenic Callus into Somatic Embryos
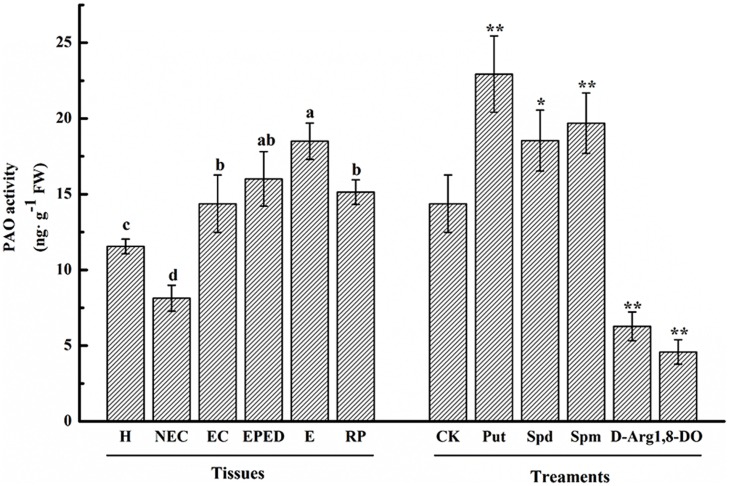
Polyamine oxidase catalyzes the oxidation of PAs to produce H2O2. To investigate the relationship between the activity of PAO and SE, we analyzed the PAO enzyme activities in the various SE stages by ELISA assay. Compared with hypocotyls, PAO activity was significantly reduced in non-embryogenic callus, whereas PAO activity was significantly increased in embryogenic callus, the early phase of differentiated embryos, the somatic embryos and the regenerated plantlets (Figure 7). The highest PAO activity was observed at the somatic embryo stage, which is one of the most important and problematic stages in cotton SE (Figure 7). The PAO activity increased by 1.2, 1.3, and 1.6-fold in Spd-, Spm-, and Put-treated EC, respectively, whereas PAO activity was significantly reduced in the D-Arg- and 1, 8-DO-treated EC (Figure 7).
FIGURE 7.
Polyamine oxidase activity during SE of ‘Xinluzao 33.’ The horizontal axis tissues H, hypocotyl; NEC, non-embryogenic callus; EC, embryogenic callus; EPED, early phase of embryo differentiation; E, embryo; RP, regenerated plantlets. The treatments are liquid embryo induction media supplemented with Put, Spd, Spm, D-arg and 1, 8-DO 3 days after inoculation of embryogenic callus. CK is unamended induction medium. Values are the mean + standard error (n = 3). Different lowercase letters above the bars indicated significant differences in PAO activity among the stages at P < 0.05. ∗ and ∗∗ indicate significant differences at P < 0.05 and P < 0.01, respectively, according to LSD multiple range test.
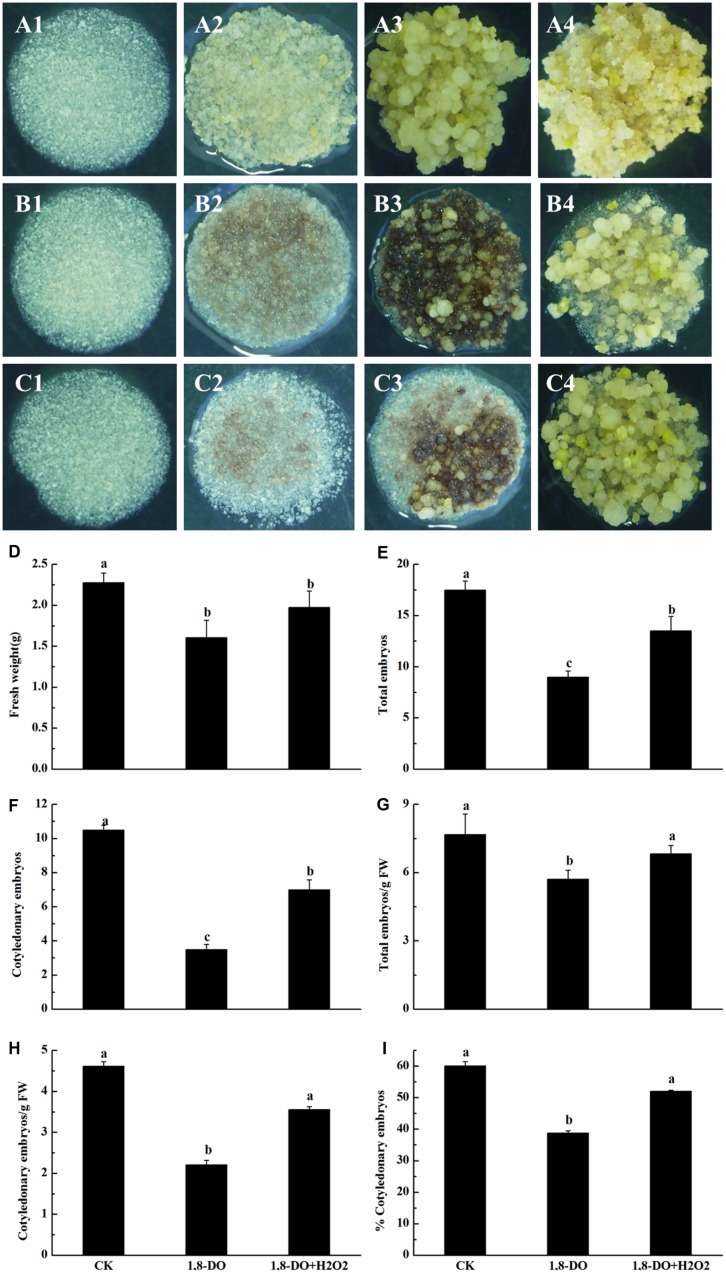
When grown on somatic embryo 0induction medium containing 1, 8-DO, the cultures showed browning and necrosis (Figures 8A,B). There was also a significant decrease in callus FW, total embryo number, cotyledonary embryo number, total embryo number/g FW, cotyledonary embryo number/g FW, and percentage of cotyledonary embryos. These negative effects of 1, 8-DO were lessened when H2O2 was added to the 1, 8-DO-containing medium, with the exception of FW (Figures 8C–I). This was largely due to increased H2O2 concentrations (84.6 g⋅ g-1 FW) in EC grown on 1, 8-DO+H2O2 medium (Figure 6E). These results suggested that the conversion of EC into somatic embryos was significantly reduced when PAO activity was inhibited. The effect was alleviated by application of exogenous H2O2. These results also suggested that PAO-catalyzed production of H2O2 plays a crucial and positive role in the conversion of embryogenic callus into somatic embryos in cotton.
FIGURE 8.
Polyamine oxidase influenced the conversion of embryogenic callus into somatic embryos of ‘Xinluzao 33.’ (A1–4 to C1–4) Represent the status of embryogenic callus on the media at different times during the culture (1: 0 days; 2: 3 days; 3: 10 days; 4: 30 days). (A) Status of embryogenic callus on the untreated media (control). (B) 1, 8-DO treatment. (C) 1, 8-DO + H2O2 treatment. The cultures in 1, 8-DO exhibited browning and underwent necrosis early in the incubation, probably due to loss of efficiency of 1, 8-DO. The cultures recovered after about 10 days and then grew better. (D–I) Statistical analysis of different treatments. (D) Fresh weight (g). (E) Total embryos. (F) Cotyledonary embryos. (G) Total embryos/g FW. (H) Cotyledonary embryos/g FW. (I) % Cotyledonary embryos. The horizontal axis of D-I were same, CK: control, 1, 8-DO: 1, 8-diamino-octane treatment, 1, 8-DO + H2O2: media supplement with 1, 8-diamino-octane and H2O2. Values are the mean + standard error (n = 3). Different lowercase letters above the bars indicate significant differences at P < 0.05 according to LSD multiple range test.
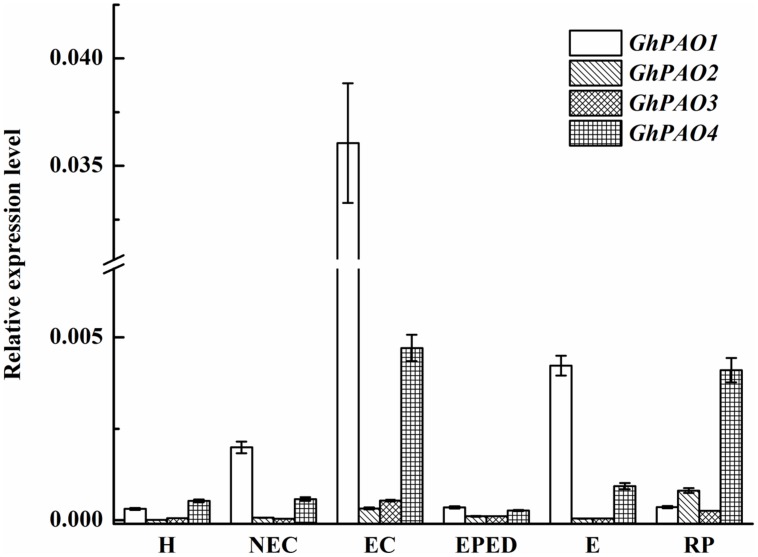
The G. hirsutum genome contains four PAO-encoding genes. GhPAO1 and GhPAO4 were expressed at higher levels than GhPAO2 and GhPAO3 in hypocotyls as well as all callus and embryonic samples (Figure 9). The expression levels of GhPAO1 were consistently higher than that of GhPAO4 in non-embryogenic callus, embryogenic callus, embryos at early phase of differentiation, and somatic embryos, the expression levels of both GhPAO1 and GhPAO4 increased dramatically from the non-embryogenic callus stage to the embryogenic callus stage. GhPAO1 and GhPAO4 expression was highest in embryogenic callus, although a relatively high level of GhPAO1 and GhPAO4 was also observed in somatic embryos and regenerated plantlets (Figure 9). These results suggested that both GhPAO1 and GhPAO4 may play a crucial role in the generation and differentiation of embryogenic callus during cotton SE.
FIGURE 9.
Relative expression of GhPAO1-4 during SE in ‘Xinluzao 33.’ The equation 2-ΔCt was applied to calculate the relative expression level using GhUBI as the reference gene. The horizontal axis: H, hypocotyl; NEC, non-embryogenic callus; EC, embryogenic callus; EPED, early phase of embryo differentiation; E, embryo; RP, regenerated plantlets. Values are the mean + standard error (n = 3).
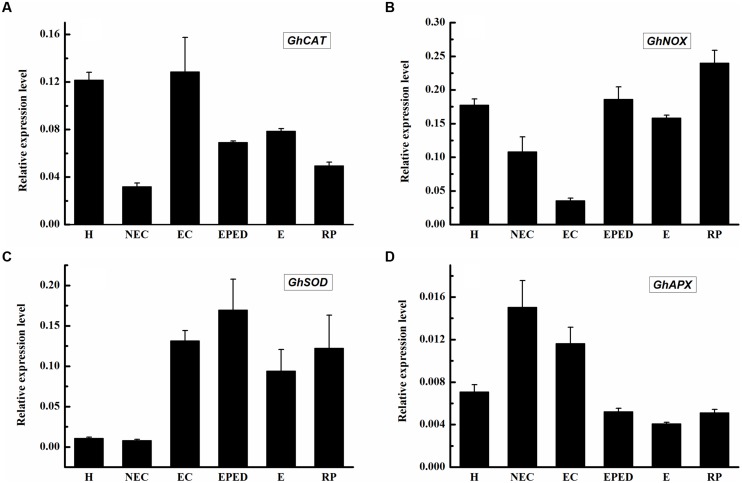
The expression levels of both GhPAO1 and GhPAO4 were quite low in the early phase of embryo differentiation even though H2O2 concentrations were high. This suggested that, in addition to the PAO-catalyzed PA pathway, other H2O2-generating pathways could also contribute to high H2O2 levels observed during cotton SE. These H2O2-generating pathways include the antioxidant enzyme system and the NADPH-dependent pathway. To address this issue, we analyzed the expression levels of genes encoding catalase (CAT), superoxide dismutase (SOD), NADPH oxidase enzyme (NOX), and ascorbate peroxidase (APX). The expression levels of GhCAT, GhSOD and GhNOX were all relatively highly expressed in the early phase of embryos differentiation, the somatic embryos, and the regenerated plantlets (Figures 10A–C). The highest expression level of GhCAT was observed in the embryogenic callus (Figure 10A). This was consistent with high H2O2 concentrations in these samples. The highest GhAPX expression was in the non-embryogenic callus (Figure 10D). Overall, these results support the hypothesis that all three H2O2-generating pathways contribute to high H2O2 concentrations during cotton SE.
FIGURE 10.
Expression level of genes encoding CAT, SOD, APX and NOX during SE in ‘Xinluzao 33’. The equation 2-ΔCt was applied to calculate the relative expression level using GhUBI as the reference gene. (A–D) Relative expression level of GhCAT, GhNOX, GhSOD and GhAPX. (A) Relative expression level of GhCAT. (B) Relative expression level of GhNOX. (C) Relative expression level of GhSOD. (D) Relative expression level of GhAPX. The horizontal axis: H, Hypocotyl; NEC, Non-embryogenic callus; EC, Embryogenic callus; EPED, Early phase of embryo differentiation; E, Embryo; RP, Regenerated plantlets. Values are the mean + standard error (n = 3).
Discussion
Many studies have demonstrate the importance of PAs to SE in several plant species (Feirer et al., 1984; De-la-Peña et al., 2015). A wide range of possible roles of PAs in SE have been suggested. For example, it has been proposed that PAs (i) increase SE by regulating 2, 4-D synthesis and transport (Stuart and Strickland, 1984), (ii) enhance development of the cell cytoskeleton and (iii) generate energy required for cell division and differentiation during SE by interacting with Ca2+ transport (Kauss, 1987; Jansen et al., 1990). PAs have also been considered as hormone-like regulators that influence plant growth and development (Iqbal and Koenig, 1985). Experiments in which either PAs or auxin were exogenously applied demonstrated that PAs may have a synergistic role as auxin (Galston and Sawhney, 1990) to enhance the formation of somatic embryos (Trolinder and Goodin, 1988). It is important to note that PAs can be free, bound to macromolecules (DNA and protein), or attached (i.e., conjugated) to membranes (Kotzabasis et al., 1993). Different forms of PAs may have different functions during SE. Thermospermine is a structural isomer of Spm and it is assumed to be converted from spermidine by ACAULIS5 (ACL5). Genetic and molecular evidence indicates that thermospermine is produced through the action of AtACL5 and required for stem elongation, xylem differentiation and vascular formation in Arabidopsis thaliana (Knott et al., 2007; Tong et al., 2014). In this study we analyzed for thermospermine but did not detect its presence during cotton SE.
Endogenous concentrations of Put, Spd, and Spm increased significantly from the embryogenic callus stage to the early stage of embryo differentiation (Figures 1A–C). Furthermore, H2O2 began increasing before the PAs increased (Figure 4). The increases in Put can be explained by dramatically increased expression levels of GhADC1 and GhADC2, which are responsible for Put synthesis at the embryogenic callus stage and the early embryo differentiation stage (Figure 2). Although ODC seems to be active in cotton (Altman et al., 1982), the GhODC gene has yet to be identified; therefore its expression pattern was not analyzed in this study. Significant increases in PA biosynthesis in maize tumors were correlated with the transcriptional activation of the ZmSAMDC2, ZmSAMDC3 and ADC genes (Rodríguez-Kessler et al., 2008). The changes in PA were related to an increase in the cell division rate. Exogenous application of Put, Spd, Spm, and H2O2 enhanced the growth and the development of somatic embryos, even when PA synthesis was blocked by D-Arg (Figure 6). These results not only confirmed observations reported in previous studies, but also revealed new findings about the roles of Put and H2O2 in the formation of cotton somatic embryos. Specifically, Put seemed to promote embryo formation whereas H2O2 seemed to promote cotyledonary embryos development.
In plants, H2O2 is considered to be one of the most important signaling molecules of abiotic stresses (Neill et al., 2002b). An extracellular oxidative burst is an early plant response to biotic/abiotic stress (Bolwell et al., 2002). Plant regeneration via SE is one of the best examples of plants under serious stress (Jayashree et al., 2003). A role of H2O2 in SE has been demonstrated in Lycium barbaru (Kairong et al., 1999) and M. crystallinum L. (Libik et al., 2005). However, most studies about the role of H2O2 in SE have focused on the antioxidant enzyme system. Relatively little attention has been paid to the role of H2O2 produced by the PA metabolism pathway. Endogenous H2O2 concentrations have been shown to be correlated with the activities of APX and CAT during SE of Astragalus adsurgens Pall (Luo et al., 2001). Similarly, the expression levels of CAT, SOD and APX were observed to be relatively high during SE in L. leptolepis (Zhang et al., 2010). In our study, GhCAT, GhSOD and GhNOX all seemed to have a role in H2O2 production during cotton SE (Figures 10A–C), whereas GhAPX did not (Figure 10D). These results suggested that the antioxidant enzyme system and the NADPH-dependent pathway both contribute to H2O2 synthesis during cotton SE. More importantly, we observed that active PAO was essential for healthy development of somatic embryos (Figures 7 and 8). GhPAO1 and GhPAO4 were highly expressed in the embryogenic callus (Figure 9), suggesting that H2O2 produced by the PAO metabolic pathway could be indispensable for the conversion of non-embryogenic callus to embryogenic callus during cotton SE.
Polyamine metabolism is one of the main sources of reactive oxygen species in plants under stress (Skopelitis et al., 2006). Many reactions in the PA metabolic pathway produce H2O2, especially the pathways metabolized by copper amine oxidase (CuAO) and flavin-containing PAO. Recent studies have cloned the gene(s) encoding PAO, purified the PAO enzyme, and investigated the spectral characteristics, subcellular localization and substrate specificity of PAO (Fincato et al., 2011; Ono et al., 2012; Liu et al., 2014). In plants, PAOs are preferentially associated with the primary and secondary cell walls of tissues undergoing lignification, suberization, and wall stiffening. Biochemical, histochemical, and immunocytochemical studies have confirmed the localization of PAO in primary and secondary cell walls of xylem parenchyma, the endodermis, and the epidermis of maize seedlings (Slocum, 1991). PA Oxidase5 (AtPAO5) regulates Arabidopsis growth and development through thermospermine oxidase activity (Kim et al., 2014). OsPAO7 has been implicated in the biosynthesis of lignin, a major component of secondary wall thickening in anthers (Boerjan et al., 2003). It has been suggested that H2O2 generated by extracellular PAOs could underlie tissue differentiation due to the coordinated processes of cell wall maturation and programmed cell death (Desikan et al., 1998; Del Duca et al., 2014). Studies involving overexpression or down-regulation of apoplastic PAO indicate that H2O2 derived from PA catabolism is important in the induction of either salinity-induced tolerance or programmed cell death in tobacco (Moschou et al., 2008). In addition, a recent study showed that PAO activity could be modulated by interaction between PAs and a regulatory element, such as uORF, located in the 5′-untranslated region of the AtPAO2 gene (Guerrero-González et al., 2014). Hence, it can be stated that a complex interplay of events modulates the levels of both AO and PAs. The rate of their secretion in the cell wall governs the spatio-temporal features of the AO-dependent biosynthesis of extracellular H2O2, which has been shown to play the dual role of (i) triggering peroxidase-mediated wall stiffening events and (ii) signaling the modulation of defense and hypersensitive response (HR)-cell death gene expression (Cona et al., 2006; Kärkönen and Kuchitsu, 2015; Mastracci et al., 2015). In this study, PAO activities significantly increased after the non-embryogenic callus stage (Figure 7). Repressing PAO activity by 1, 8-DO resulted in brown and necrotic cultures (Figure 8B) and a significant decrease in both FW and somatic embryo number (Figures 8D,I). Importantly, the negative effects of 1, 8-DO were reversed by application of exogenous H2O2 (Figure 8C). Consistent with our observation, cell wall-localized Arabidopsis AtAO1 has been reported to be expressed in root cap cells and protoxylem precursors at early stages of vascular tissue differentiation (Sandip et al., 2015). In cell walls, CuAOs and PAOs share overlapping roles as H2O2 sources in developmentally- or light-regulated cell wall maturation events (Cona et al., 2005), as well as in oxidative bursts occurring during defense responses against biotic and abiotic stresses (Cona et al., 2006; Savatin et al., 2014) such as pathogen attack, salt stress and wound healing (Tisi et al., 2008; Mitsuya et al., 2009; Rodríguez et al., 2009; Mastracci et al., 2015). Our results indicate that active PAO and H2O2 are both essential in cotton SE, which is a severe biotic stress. To our knowledge this is the first report about the role of PAO in the conversion of EC into somatic embryos.
Nitric oxide is another signal molecule involved in the PA metabolic pathway; however, it is not known if NO has a role in SE. One previous study indicated that endogenous NO emission decreased significantly during the early stages of SE of Araucaria angustifolia treated with glutathione (Vieira et al., 2012). In contrast, Put increased NO emission in embryogenic suspension cultures of A. angustifolia (Silveira et al., 2006). The addition of SNP, a donor of NO, increased cell elongation and the expression of the receptor kinase (SERK) protein during SE of Medicago sativa L (Ötvös et al., 2005). In our study, NO concentrations tended to decrease during cotton SE; however, the declines were not very large (Figure 4A). Furthermore, cotton SE was completely inhibited when 1 mM SNP was added to the somatic embryo induction medium (Figure 4C). The function of NO-related chemicals and genes involved in NO-PA metabolism should be studied in the future.
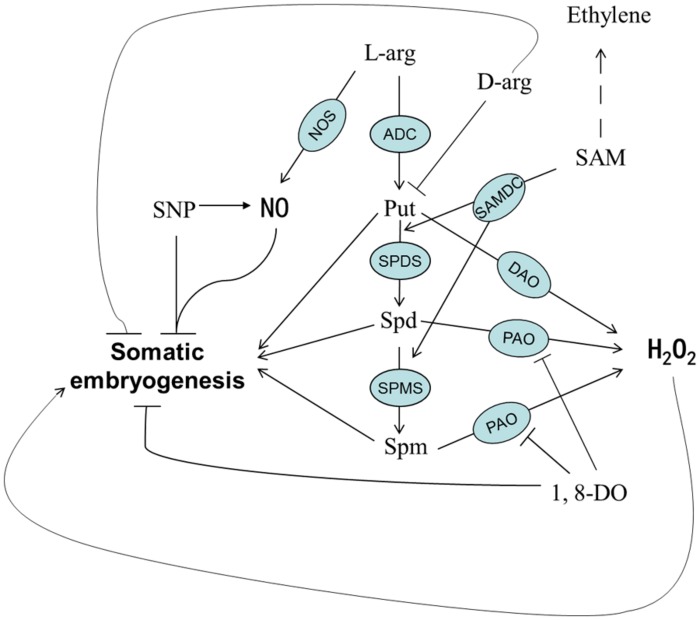
Based on previous knowledge and the results of this study, we developed a working model showing the relationship between SE and chemicals involved in PA biosynthesis (Figure 11). PAs and H2O2 are the key components of this model and are essential for normal SE. The differentiation and the development of embryonic callus are both inhibited when the synthesis of PAs is suppressed or PAO activity is blocked. The H2O2 detected during SE could have been generated from multiple pathways; however, the PA metabolic pathway and PAO seem to be critical factors affecting cotton SE.
FIGURE 11.
A proposed model of PA metabolism and possible involvement of PA-related chemicals during SE in cotton.
Author Contributions
Conceived and designed the experiments: W-HC, H-GZ, and JS Performed the experiments: W-HC, F-LW, and X-QC. Analyzed the data: W-HC. Contributed reagents/materials/analysis tools: JS, Y-QS. Wrote the paper: W-HC, Q-HZ.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Bo Liu (Section of Plant Biology, University of California, USA) for his kind assistance in experiments and critical reading of the manuscript. We also thank Dr. William J. Gale (College of Agriculture, Shihezi University, China) for critical reading and professional editing of the manuscript. This work was supported by the National Natural Science Foundation of China (Grant No. 31301363), National Natural Science Foundation of China Key Program (Grant No. U1303281) and Special Program of Xinjiang Production and Construction Corps: Germplasm and Functional Gene Discovery (Grant No. 2012BB049).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.01063
References
- Alcázar R., Tiburcio A. F. (2014). Plant polyamines in stress and development: an emerging area of research in plant sciences. Front. Plant Sci. 5:319 10.3389/fpls.2014.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher R. G., Donahue J. L., Cramer C. L. (1997). Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 100 224–233. 10.1111/j.1399-3054.1997.tb04778.x [DOI] [Google Scholar]
- Altman A., Friedman R. A., Levin N. (1982). Arginine and ornithine decarboxylases, the polyamine biosynthetic enzymes of mung bean seedlings. Plant physiol. 69 876–879. 10.1104/pp.69.4.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A., Bernier F. (1999). Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol. Biol. 39 539–549. 10.1023/A:1006123432157 [DOI] [PubMed] [Google Scholar]
- Boerjan W., Ralph J., Baucher M. (2003). Lignin biosynthesis. Annu. Rev. Plant Biol. 54 519–546. 10.1146/annurev.arplant.54.031902.134938 [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Bindschedler L. V., Blee K. A., Butt V. S., Davies D. R., Gardner S. L., et al. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53 1367–1376. 10.1093/jexbot/53.372.1367 [DOI] [PubMed] [Google Scholar]
- Cangahuala-Inocente G. C., Silveira V., Caprestano C. A., Floh E. I., Guerra M. P. (2014). Dynamics of physiological and biochemical changes during somatic embryogenesis of Acca sellowiana. In Vitro Cell. Dev. Biol. Plant 50 166–175. 10.1007/s11627-013-9563-3 [DOI] [Google Scholar]
- Chang S., Puryear J., Cairney J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11 113–116. 10.1007/BF02670468 [DOI] [Google Scholar]
- Cona A., Moreno S., Cenci F., Federico R., Angelini R. (2005). Cellular re-distribution of flavin-containing polyamine oxidase in differentiating root and mesocotyl of Zea mays L. seedlings. Planta 221 265–276. 10.1007/s00425-004-1435-y [DOI] [PubMed] [Google Scholar]
- Cona A., Rea G., Angelini R., Federico R., Tavladoraki P. (2006). Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11 80–88. 10.1016/j.tplants.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Costa L. M., Marshall E., Tesfaye M., Silverstein K. A., Mori M., Umetsu Y., et al. (2014). Central cell–derived peptides regulate early embryo patterning in flowering plants. Science 344 168–172. 10.1126/science.1243005 [DOI] [PubMed] [Google Scholar]
- Crumbliss A., Perine S., Stonehuerner J., Tubergen K., Zhao J., Henkens R., et al. (1992). Colloidal gold as a biocompatible immobilization matrix suitable for the fabrication of enzyme electrodes by electrodeposition. Biotechnol. Bioeng. 40 483–490. 10.1002/bit.260400406 [DOI] [PubMed] [Google Scholar]
- Daudi A., Cheng Z., O’brien J. A., Mammarella N., Khan S., Ausubel F. M., et al. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24 275–287. 10.1105/tpc.111.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Duca S., Serafini-Fracassini D., Cai G. (2014). Senescence and programmed cell death in plants: polyamine action mediated by transglutaminase. Front. Plant Sci. 5:120 10.3389/fpls.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-la-Peña C., Galaz-Avalos R. M., Loyola-Vargas V. M. (2008). Possible role of light and polyamines in the onset of somatic embryogenesis of Coffea canephora. Mol. Biotechnol. 39 215–224. 10.1007/s12033-008-9037-8 [DOI] [PubMed] [Google Scholar]
- De-la-Peña C., Nic-Can G. I., Galaz- valos R. M., Avilez-Montalvo R., Loyola-Vargas V. M. (2015). The role of chromatin modifications in somatic embryogenesis in plants. Front. Plant Sci. 6:635 10.3389/fpls.2015.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R., Reynolds A., Hancock J., Neill S. (1998). Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 330 115–120. 10.1042/bj3300115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feirer R. P., Mignon G., Litvay J. D. (1984). Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223 1433–1435. 10.1126/science.223.4643.1433 [DOI] [PubMed] [Google Scholar]
- Fincato P., Moschou P. N., Spedaletti V., Tavazza R., Angelini R., Federico R., et al. (2011). Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 62 1155–1168. 10.1093/jxb/erq341 [DOI] [PubMed] [Google Scholar]
- Gaj M. D., Zhang S., Harada J. J., Lemaux P. G. (2005). Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222 977–988. 10.1007/s00425-005-0041-y [DOI] [PubMed] [Google Scholar]
- Galea E., Regunathan S., Eliopoulos V., Feinstein D., Reis D. (1996). Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem. J. 316 247–249. 10.1042/bj3160247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston A. W., Sawhney R. K. (1990). Polyamines in plant physiology. Plant Physiol. 94 406–410. 10.1104/pp.94.2.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Zhang C., Wang Q., Yang Z., Wang Y., Zhang X., et al. (2014). iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J. Proteome Res. 14 268–278. 10.1021/pr500688g [DOI] [PubMed] [Google Scholar]
- Gemperlová L., Fischerová L., Cvikrová M., Malá J., Vondráková Z., Martincová O., et al. (2009). Polyamine profiles and biosynthesis in somatic embryo development and comparison of germinating somatic and zygotic embryos of Norway spruce. Tree Physiol. 29 1287–1298. 10.1093/treephys/tpp063 [DOI] [PubMed] [Google Scholar]
- Guerrero-González M. L., Rodríguez-Kessler M., Jiménez-Bremont J. F. (2014). uORF, a regulatory mechanism of the Arabidopsis polyamine oxidase 2. Mol. Biol. Rep. 41 2427–2443. 10.1007/s11033-014-3098-5 [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Koenig H. (1985). Polyamines appear to be second messengers in mediating Ca2+ fluxes and neurotransmitter release in potassium-depolarized synaptosomes. Biochem. Biophys. Res. Commun. 133 563–573. 10.1016/0006-291X(85)90943-X [DOI] [PubMed] [Google Scholar]
- Jansen M. A., Booij H., Schel J. H., De Vries S. C. (1990). Calcium increases the yield of somatic embryos in carrot embryogenic suspension cultures. Plant Cell Rep. 9 221–223. 10.1007/BF00232184 [DOI] [PubMed] [Google Scholar]
- Jayashree R., Rekha K., Venkatachalam P., Uratsu S., Dandekar A., Jayasree P. K., et al. (2003). Genetic transformation and regeneration of rubber tree (Hevea brasiliensis Muell. Arg) transgenic plants with a constitutive version of an anti-oxidative stress superoxide dismutase gene. Plant Cell Rep. 22 201–209. 10.1007/s00299-003-0666-x [DOI] [PubMed] [Google Scholar]
- Kairong C., Gengsheng X., Xinmin L., Gengmei X., Yafu W. (1999). Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci. 146 9–16. 10.1016/S0168-9452(99)00087-4 [DOI] [Google Scholar]
- Kärkönen A., Kuchitsu K. (2015). Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112 22–32. 10.1016/j.phytochem.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Kauss H. (1987). Some aspects of calcium-dependent regulation in plant metabolism. Annu. Rev. Plant Physiol. 38 47–71. 10.1146/annurev.pp.38.060187.000403 [DOI] [Google Scholar]
- Kim D. W., Watanabe K., Murayama C., Izawa S., Niitsu M., Michael A. J., et al. (2014). Polyamine oxidase5 regulates Arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 165 1575–1590. 10.1104/pp.114.242610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott J. M., Römer P., Sumper M. (2007). Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett. 581 3081–3086. 10.1016/j.febslet.2007.05.074 [DOI] [PubMed] [Google Scholar]
- Kotzabasis K., Christakishampsas M. D., Roubelakisangelakis K. A. (1993). A narrow-bore HPLC method for the identification and quantitation of free, conjugated, and bound polyamines. Anal. Biochem. 214 484–489. 10.1006/abio.1993.1526 [DOI] [PubMed] [Google Scholar]
- Libik M., Konieczny R., Pater B., Ślesak I., Miszalski Z. (2005). Differences in the activities of some antioxidant enzymes and in H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep. 23 834–841. 10.1007/s00299-004-0886-8 [DOI] [PubMed] [Google Scholar]
- Liu J.-H., Nada K., Honda C., Kitashiba H., Wen X.-P., Pang X.-M., et al. (2006). Polyamine biosynthesis of apple callus under salt stress: importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 57 2589–2599. 10.1093/jxb/erl018 [DOI] [PubMed] [Google Scholar]
- Liu T., Kim D. W., Niitsu M., Maeda S., Watanabe M., Kamio Y., et al. (2014). Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 55 1110–1122. 10.1093/pcp/pcu047 [DOI] [PubMed] [Google Scholar]
- Luo J.-P., Jiang S.-T., Pan L.-J. (2001). Enhanced somatic embryogenesis by salicylic acid of Astragalus adsurgens pall: relationship with H2O2 production and H2O2-metabolizing enzyme activities. Plant Sci. 161 125–132. 10.1016/S0168-9452(01)00401-0 [DOI] [Google Scholar]
- Malá J., Gemperlová L., Cvikrová M., Máchová P. (2012). Role of Polyamines in Efficiency of Norway Spruce (Hurst Ecotype) Somatic Embryogenesis. Rijeka: INTECH Open Access Publisher. [Google Scholar]
- Mastracci T. L., Robertson M. A., Mirmira R. G., Anderson R. M. (2015). Polyamine biosynthesis is critical for growth and differentiation of the pancreas. Sci. Rep. 5:13269 10.1038/srep13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya Y., Takahashi Y., Berberich T., Miyazaki A., Matsumura H., Takahashi H., et al. (2009). Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J. Plant Physiol. 166 626–643. 10.1016/j.jplph.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Montague M. J., Armstrong T. A., Jaworski E. G. (1979). Polyamine metabolism in embryogenic cells of Daucus carota II. Changes in arginine decarboxylase activity. Plant Physiol. 63 341–345. 10.1104/pp.63.2.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. (1978). Polyamine metabolism in embryogenic cells of Daucus carota I. Changes in intracellular content and rates of synthesis. Plant Physiol. 62 430–433. 10.1104/pp.62.3.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou P. N., Paschalidis K. A., Delis I. D., Andriopoulou A. H., Lagiotis G. D., Yakoumakis D. I., et al. (2008). Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20 1708–1724. 10.1105/tpc.108.059733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Neill S., Desikan R., Hancock J. (2002a). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5 388–395. 10.1016/S1369-5266(02)00282-0 [DOI] [PubMed] [Google Scholar]
- Neill S. J., Desikan R., Clarke A., Hurst R. D., Hancock J. T. (2002b). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53 1237–1247. 10.1093/jexbot/53.372.1237 [DOI] [PubMed] [Google Scholar]
- Niemenak N., Awah T. M., Lieberei R. (2012). Establishment of suspension culture in Theobroma cacao and polyamines associated with cacao embryogenesis. Plant Growth Regul. 67 1–8. 10.1007/s10725-011-9653-3 [DOI] [Google Scholar]
- Nieves N., Sagarra F., González R., Lezcano Y., Cid M., Blanco M., et al. (2008). Effect of exogenous arginine on sugarcane (Saccharum sp.) somatic embryogenesis, free polyamines and the contents of the soluble proteins and proline. Plant Cell Tissue Organ Cult. 95 313–320. 10.1007/s11240-008-9445-2 [DOI] [Google Scholar]
- Noceda C., Salaj T., Pérez M., Viejo M., Cañal M. J., Salaj J., et al. (2009). DNA demethylation and decrease on free polyamines is associated with the embryogenic capacity of Pinus nigra Arn. cell culture. Trees 23 1285–1293. 10.1007/s00468-009-0370-8 [DOI] [Google Scholar]
- Ono Y., Kim D. W., Watanabe K., Sasaki A., Niitsu M., Berberich T., et al. (2012). Constitutively and highly expressed Oryza sativa polyamine oxidases localize in peroxisomes and catalyze polyamine back conversion. Amino Acids 42 867–876. 10.1007/s00726-011-1002-3 [DOI] [PubMed] [Google Scholar]
- Ötvös K., Pasternak T. P., Miskolczi P., Domoki M., Dorjgotov D., Bottka S., et al. (2005). Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J. 43 849–860. 10.1111/j.1365-313X.2005.02494.x [DOI] [PubMed] [Google Scholar]
- Paterson A. H., Wendel J. F., Gundlach H., Guo H., Jenkins J., Jin D., et al. (2012). Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 492 423–427. 10.1038/nature11798 [DOI] [PubMed] [Google Scholar]
- Paul A., Mitter K., Raychaudhuri S. S. (2009). Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia L. Plant Cell Tissue Organ Cult. 97 303–311. 10.1007/s11240-009-9529-7 [DOI] [Google Scholar]
- Paulus W. J., Vantrimpont P. J., Shah A. M. (1994). Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation 89 2070–2078. 10.1161/01.CIR.89.5.2070 [DOI] [PubMed] [Google Scholar]
- Qian L.-B., Wang H.-P., Qiu W.-L., Huang H., Bruce I. C., Xia Q. (2006). Interleukin-2 protects against endothelial dysfunction induced by high glucose levels in rats. Vascul. Pharmacol. 45 374–382. 10.1016/j.vph.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Rodríguez A. A., Maiale S. J., Menéndez A. B., Ruiz O. A. (2009). Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J. Exp. Bot. 60 4249–4262. 10.1093/jxb/erp256 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Kessler M., Ruiz O. A., Maiale S., Ruiz-Herrera J., Jiménez-Bremont J. F. (2008). Polyamine metabolism in maize tumors induced by Ustilago maydis. Plant Physiol. Biochem. 46 805–814. 10.1016/j.plaphy.2008.05.012 [DOI] [PubMed] [Google Scholar]
- Sandip A. G., Carucci A., Renato A., Tisi A., Franchi S., Tavladoraki P., et al. (2015). The apoplastic copper amine oxidase AtAO1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Plant Physiol. 168 690–707. 10.1104/pp.15.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatin D. V., Gramegna G., Modesti V., Cervone F. (2014). Wounding in the plant tissue: the defense of a dangerous passage. Front. Plant Sci. 5:470 10.3389/fpls.2014.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva T. E., Cidade L. C., Alvim F. C., Cascardo J. C., Costa M. G. (2009). Studies on genetic transformation of Theobroma cacao L: evaluation of different polyamines and antibiotics on somatic embryogenesis and the efficiency of uidA gene transfer by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. 99 287–298. 10.1007/s11240-009-9603-1 [DOI] [Google Scholar]
- Silveira V., De Vita A. M., Macedo A. F., Dias M. F. R., Floh E. I. S., Santa-Catarina C. (2013). Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tissue Organ Cult. 114 351–364. 10.1007/s11240-013-0330-2 [DOI] [Google Scholar]
- Silveira V., Santa-Catarina C., Tun N. N., Scherer G. F., Handro W., Guerra M. P., et al. (2006). Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci. 171 91–98. 10.1016/j.plantsci.2006.02.015 [DOI] [Google Scholar]
- Skopelitis D. S., Paranychianakis N. V., Paschalidis K. A., Pliakonis E. D., Delis I. D., Yakoumakis D. I., et al. (2006). Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18 2767–2781. 10.1105/tpc.105.038323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D. (1991). “Tissue and subcellular localization of polyamines and enzymes of polyamine metabolism,” in Biochemistry and Physiology of Polyamines in Plants, eds Slocum R. D., Flores H. E. (Boca Raton, FL: CRC Press; ), 93–103. [Google Scholar]
- Soriano M., Li H., Jacquard C., Angenent G. C., Krochko J., Offringa R., et al. (2014). Plasticity in cell division patterns and auxin transport dependency during in vitro embryogenesis in Brassica napus. Plant Cell 26 2568–2581. 10.1105/tpc.114.126300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner N., Santa-Catarina C., Silveira V., Floh E. I., Guerra M. P. (2007). Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryogenic cultures. Plant Cell Tissue Organ Cult. 89 55–62. 10.1007/s11240-007-9216-5 [DOI] [Google Scholar]
- Stuart D. A., Strickland S. G. (1984). Somatic embryogenesis from cell cultures of medicago sativa LI the role of amino acid additions to the regeneration medium. Plant Sci. Lett. 34 165–174. 10.1016/0304-4211(84)90140-8 [DOI] [Google Scholar]
- Su Y. H., Zhao X. Y., Liu Y. B., Zhang C. L., O’neill S. D., Zhang X. S. (2009). Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 59 448–460. 10.1111/j.1365-313X.2009.03880.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zhang X., Huang C., Guo X., Nie Y. (2006). Somatic embryogenesis and plant regeneration from different wild diploid cotton (Gossypium) species. Plant Cell Rep. 25 289–296. 10.1007/s00299-005-0085-2 [DOI] [PubMed] [Google Scholar]
- Surgun Y., Yilmaz E., Çöl B., Bürün B. (2014). Callus induction, in vitro shoot development and somaclonal variations in cotton (Gossypium hirsutum L.). J. Appl. Biol. Sci. 8 62–68. [Google Scholar]
- Tisi A., Angelini R., Cona A. (2008). Wound healing in plants: cooperation of copper amine oxidase and flavin-containing polyamine oxidase. Plant Signal. Behav. 3 204–206. 10.4161/psb.3.3.5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W., Yoshimoto K., Kakehi J.-I., Motose H., Niitsu M., Takahashi T. (2014). Thermospermine modulates expression of auxin-related genes in Arabidopsis. Front. Plant Sci. 5:94 10.3389/fpls.2014.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trolinder N. L., Goodin J. (1988). Somatic embryogenesis in cotton (Gossypium) I. Effects of source of explant and hormone regime. Plant Cell Tissue Organ Cult. 12 31–42. 10.1007/BF00043105 [DOI] [Google Scholar]
- Vieira L. D. N., Santa-Catarina C., De Freitas Fraga H. P., Dos Santos A. L. W., Steinmacher D. A., Schlogl P. S., et al. (2012). Glutathione improves early somatic embryogenesis in Araucaria angustifolia (Bert) O. Kuntze by alteration in nitric oxide emission. Plant Sci. 195 80–87. 10.1016/j.plantsci.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Vuosku J., Jokela A., Läärä E., Sääskilahti M., Muilu R., Sutela S., et al. (2006). Consistency of polyamine profiles and expression of arginine decarboxylase in mitosis during zygotic embryogenesis of Scots pine. Plant Physiol. 142 1027–1038. 10.1104/pp.106.083030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuosku J., Suorsa M., Ruottinen M., Sutela S., Muilu-Mäkelä R., Julkunen-Tiitto R., et al. (2012). Polyamine metabolism during exponential growth transition in Scots pine embryogenic cell culture. Tree Physiol. 32 1274–1287. 10.1093/treephys/tps088 [DOI] [PubMed] [Google Scholar]
- Wojtasik W., Kulma A., Namysl K., Preisner M., Szopa J. (2015). Polyamine metabolism in flax in response to treatment with pathogenic and non-pathogenic fusarium strains. Front. Plant Sci. 6:291 10.3389/fpls.2015.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.-B., Wang J., Liu J.-H., Deng X.-X. (2009). Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J. Plant Physiol. 166 52–62. 10.1016/j.jplph.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Zhang S.-G., Han S.-Y., Yang W.-H., Wei H.-L., Zhang M., Qi L.-W. (2010). Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tissue Organ Cult. 100 21–29. 10.1007/s11240-009-9612-0 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.