Abstract
Context:
Shift work, which imposes a habitual disruption in the circadian system, has been linked to increased incidence of cardiometabolic diseases, and acute circadian misalignment alters various metabolic processes. However, it remains unclear whether day-to-day circadian dysregulation contributes to these risks beyond poor sleep and other behavioral characteristics.
Objective:
Individuals differ in circadian phase preference, known as chronotype, but may be constrained by modern work obligations to specific sleep schedules. Individuals experience social jetlag (SJL) due to a habitual discrepancy between their endogenous circadian rhythm and actual sleep times imposed by social obligations. Here, we examined whether chronotype and/or SJL associate with components of cardiovascular disease risk beyond the known effects of sleep disturbances, poor health behaviors, and depressive symptomatology.
Design:
Participants were healthy, midlife adults who worked part- or full-time day shifts (n = 447; mean age, 42.7 [range, 30–54] y; 53% female; 83% white). Chronotype was assessed with the Composite Scale of Morningness. SJL was quantified as the difference (in minutes) between the midpoints of actigraphy-derived sleep intervals before work vs non-workdays.
Results:
Multiple regression analyses showed that SJL related to a lower high-density lipoprotein-cholesterol level, higher triglycerides, higher fasting plasma insulin, insulin resistance, and adiposity (P < .05), even after adjustment for subjective sleep quality, actigraphy-derived sleep characteristics, depressive symptomatology, and health behaviors. Evening chronotype associated with lower high-density lipoprotein-cholesterol after adjustment for covariates.
Conclusion:
Our findings suggest that a misalignment of sleep timing is associated with metabolic risk factors that predispose to diabetes and atherosclerotic cardiovascular disease.
Short sleep and poor subjective sleep quality elevate the risk for insulin resistance, the metabolic syndrome, obesity, type 2 diabetes, and incident cardiovascular disease (1–3). Sleep is regulated by both the circadian system and homeostatic drive, which refers to sleep need that accumulates with time spent awake (4). Although sleep disturbances enhance the risk for cardiometabolic diseases, the extent to which circadian rhythm disruptions independently contribute to risk remain unclear.
Physiological processes such as glucose metabolism, core body temperature, and blood pressure (BP) have an intrinsic circadian rhythm that, when disrupted, may contribute to risk for cardiovascular disease. Relative to normal daytime workers, shift workers, who often experience chronic circadian misalignment, are more likely to develop the metabolic syndrome, type 2 diabetes, and coronary heart disease, with relative risk increasing as a function of years spent in shift work (5–7). In an experimental study, healthy adults asked to eat and sleep 12 hours out of phase from their regular schedules exhibited vascular and endocrine abnormalities, such as increased arterial pressure, a reversed daily cortisol rhythm, and postprandial glucose levels in the range of a prediabetic state (8). And in the general population, people are influenced by environmental cues such as work schedules (9) that may enforce less extreme yet habitual misalignment between their intrinsic circadian clock and actual sleep-wake times, again with a potential impact on cardiometabolic risk.
An important individual difference relevant to circadian rhythms is naturally occurring variation in preferred bedtimes and subjective times of peak alertness, also known as an individual's chronotype. Relative to morning types, evening types prefer later bedtimes and later awakening. Evening types are also more likely to be depressed (10), overweight (11), diabetic, and hypertensive (12), in comparison to morning types. However, the extent to which chronotype associates with preclinical endocrine, metabolic, and hemodynamic alterations among otherwise healthy adults is yet to be thoroughly explored.
A second form of circadian disruption, termed “social jetlag” (SJL), describes the chronic jetlag-like phenomenon occasioned by modern work schedules and reflects misalignment between an individual's endogenous circadian clock and actual sleep times (11, 13). More specifically, individuals “travel back and forth” between “time zones” on workdays (socially imposed schedules) and free (ie, non-work) days (14). SJL has been linked to measures of adiposity (11, 15, 16), heart rate (16, 17), and higher cortisol levels in healthy individuals (16) and to higher glycated hemoglobin levels in patients with type 2 diabetes (18). Although these findings link SJL to various metabolic processes, these relationships may stem, in part, from confounding influences of correlated sociodemographic risk factors and poor health behaviors. For instance, an association of SJL with the presence of the metabolic syndrome and with elevated levels of the inflammatory marker C-reactive protein in one recent study eroded to nonsignificance when controlling for either socioeconomic status or cigarette smoking (15). Examining the association between SJL and an array of cardiometabolic parameters while controlling for concomitant risk factors such as socioeconomic status, sleep disturbances, poor health behaviors, and depression (19–21) will help elucidate whether chronotype whether chronotype and/or SJL are independently associated with cardiometabolic risk and, if so, are broadly related or limited to specific metabolic and/or hemodynamic parameters.
Accordingly, in the present study we investigated whether chronotype and SJL covaried with components of cardiometabolic risk in a nonpatient sample of midlife community volunteers and whether any such associations persisted on adjustment for correlated variation in health practices, including behavioral and subjective measures of other sleep characteristics. In contrast to previous studies, where self-reported sleep schedules were commonly used to assess SJL, here we quantified SJL directly based on instrumental measurements of participants' sleep times.
Subjects and Methods
Subjects
Participants were 490 midlife men and women from the Adult Health and Behavior Project phase 2 (AHAB-II), a study of psychosocial factors, behavioral and biological risk factors, and preclinical vascular disease. AHAB-II was approved by the University of Pittsburgh Institutional Review Board (IRB). AHAB-II participants were recruited between March 2008 and October 2011 through mass mailings of recruitment letters to individuals randomly selected from voter registration and other public domain lists.
To be eligible to participate in AHAB-II, individuals had to be between the ages of 30 and 54 years and working at least 25 hours per week outside the home (this latter restriction was due to a substudy focusing on occupational stress). Individuals were excluded from participation if they: 1) had a history of clinically apparent cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, neurological disorder, lung disease requiring drug treatment, or stage 2 hypertension (systolic/diastolic BP ≥ 160/100 mm Hg); 2) excessively consumed alcohol (≥ five portions, three to four times per week); 3) used fish oil supplements (because of the requirements for another substudy); 4) were prescribed use of insulin, glucocorticoid, antiarrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight loss medications; 5) were pregnant; 6) had less than eighth grade reading skills; or 7) were shift workers. Participants signed an IRB-approved informed consent agreement when enrolled and received compensation up to US $410, depending on the extent of participation in study visits and protocol compliance.
Metabolic and cardiovascular risk components
Hemodynamic and metabolic variables were assessed in the morning after a 12-hour overnight fast. Study staff measured participants' height, weight, pulse rate, and waist circumference; collected a venous blood sample; and recorded medication usage and health status information. After a participant rested for 10 minutes, a registered nurse recorded the average of two seated BP measurements taken from the participant's right arm using a mercury sphygmomanometer (22). Determination of standard serum total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose, and insulin concentrations was performed by the Heinz Nutrition Laboratory, University of Pittsburgh Graduate School of Public Health. Insulin resistance was estimated by the homeostatic model assessment of insulin resistance (HOMA-IR) (23). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation.
Chronotype
The Composite Morningness Scale (CSM) (24) was used to characterize participants' chronotypes. The CSM has both high internal consistency (Cronbach α > 0.80) and test-retest reliability (r > 0.88) (25). Chronotype quantified by the CSM has been validated with respect to variation in the diurnal rhythms of body temperature and subjective alertness (26). Total scores range from 13 (extreme eveningness) to 55 (extreme morningness). Here, chronotype was examined as a continuous score, and the distribution of the CSM total scores was multiplied by −1 so that a high value indicates greater eveningness.
Sleep and SJL
Participants were asked to wear the Actiwatch-16 (Philips Electronics), a wrist accelerometer that samples movement several times per second. Participants wore the Actiwatch 24 hours a day for 7 days and were instructed to keep the watch on even when showering. The monitoring period included at least one night preceding a free (ie, non-work) day to capture differences between sleep intervals preceding workdays and free days. Data were saved in 1-minute epochs and scored with Actiware software (version 5.59) using automated, standard medium thresholds. Sleep onset was defined as a period lasting at least 10 consecutive minutes with < 40 counts of activity (ie, movement) per epoch. Wake onset was defined as 10 consecutive minutes of ≥ 40 activity counts per epoch. Midsleep, defined as the midpoint between sleep onset and wake onset, was calculated separately for each workday and free day and averaged within each day type. SJL was calculated as the absolute difference between the midpoints of sleep on free days vs workdays (11, 13).
Actiwatch data were used to quantify additional sleep characteristics. Sleep duration was defined as the total time between sleep onset and wake onset, averaged across all monitoring nights. Sleep debt was calculated as the difference between average sleep duration on free days and workdays. As an estimate of sleep continuity (and possible sleep disturbances), sleep efficiency was calculated as the percentage of the total rest interval scored as total sleep time minus nonsleep time. Sleep quality was based on the Pittsburgh Sleep Quality Index (PSQI), a self-report questionnaire used to assess sleep quality and disturbance over the past month (27). The PSQI has high internal consistency (Cronbach α = 0.83) and test-retest reliability (r = 0.85) (27). A global PSQI score > 5 indicates poor sleep quality. This threshold has a diagnostic sensitivity of 89.6% and specificity of 86.5%, distinguishing between healthy controls (good sleepers) and patients reporting sleep complaints (poor sleepers) (27).
Health behaviors
Cigarette smoking, sedentary lifestyle, and poor diet increase the risk for cardiovascular disease, whereas light to moderate alcohol intake and increased physical activity reduce incident cardiovascular disease (28–30). To control for variation in health practices, the following behaviors were included as covariates: smoking status (“never or past smoker” = 0, “current smoker” = 1), alcohol intake (average number of alcoholic beverages that participants reported consuming per week), physical activity (average weekly kilocalories) (31), and food intake (total caloric intake) (32).
The Paffenbarger Physical Activity Questionnaire is a widely used instrument for estimating weekly kilocalories expended (31) from self-reported activities of daily living (eg, stairs climbed, blocks walked) and leisure activities requiring physical exertion (eg, sports, recreational pursuits), indexed to both frequency and duration. As employed in AHAB-II, the Paffenbarger questionnaire was referenced to average weekly levels of physical activity, as experienced over the past year. This instrument has high reliability (33) and convergent validity with several objective measures of physical activity and fitness, including maximal oxygen uptake (34) and body mass index (BMI) (35). The Paffenbarger questionnaire is predictive of health conditions that are related to physical activity, including myocardial infarction (36), and total cholesterol and fasting blood glucose (35). From responses to this questionnaire, an estimate of average weekly energy expenditure, in kilocalories, was calculated (31).
Diet was assessed with the Block Food Frequency Questionnaire (NutritionQuest). This instrument estimates customary intake of diverse nutrients and food groups, with item content developed from the NHANES 1999–2002 dietary recall data (37, 38). Participants completed this online survey with instruction and supervision by research staff trained in the use of Block Dietary Data Systems. The reference period was the previous 4 months.
Depressive symptomatology
Individuals with depression have a greater risk for developing cardiovascular disease relative to nondepressed individuals (39). To adjust for possible confounding effects, depressive symptomatology was measured using the Center for Epidemiological Studies-Depression (CESD) scale (40) and included as a covariate in the current study. This 20-item measure assesses how frequently subjects experienced a range of psychological and physical symptoms of depression during the past week. Responses are on a 4-point scale ranging from 0 (rarely or none of the time [<1 d]) to 3 (most or all of the time [5 to 7 d]). Higher scores indicate more severe depressive symptomatology, with a maximum score of 60. The CESD has high internal consistency (Cronbach α = 0.87) (40). To avoid confounding sleep problems and depressive symptoms, the total score minus the sleep item was used.
Statistical analyses
Data were analyzed with the SPSS software package, version 20 (IBM Corp). Due to a skew in the distribution of SJL scores, these values were normalized before analysis using a square-root transformation. The distributions of triglycerides, fasting insulin, glucose, and HOMA-IR were also skewed and normalized with log10 transformations. In the primary set of analyses, hierarchical linear regressions were conducted to investigate the independent effects of chronotype and SJL on the several metabolic and cardiovascular variables collected here. Participant demographics (age, sex [1 = male, 2 = female], race [1 = white, 2 = nonwhite], educational attainment, and family income), sleep characteristics (sleep duration, sleep debt, sleep efficiency, self-reported sleep quality), and work status (part- vs full-time employment) were included as covariates in each model. Any significant associations were followed up with a second regression model that further controlled for possible confounding effects of health behaviors (physical activity, smoking status, caloric intake, and alcohol consumption) and depressive symptomatology.
Results
Participant characteristics
Of the 490 participants, nine were excluded for use of oral hypoglycemics (n = 2), missing data for chronotype (n = 2), or missing data for one or more cardiometabolic risk factors (n = 5). Of the remaining 481 participants, 18 did not have actigraphy data, and 14 additional participants did not have actigraphy data available for at least one non-workday, which precluded the calculation of sleep debt and SJL, and thus were excluded. Finally, two participants were excluded as statistical outliers for SJL values (>3 SD from the mean of the normalized SJL distribution). The final sample included 447 participants.
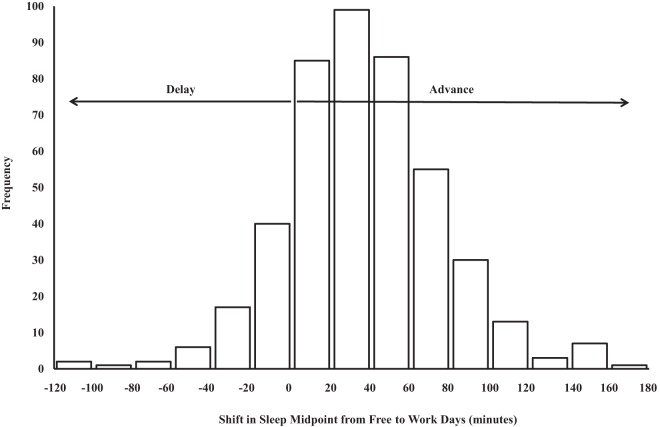
As shown in Figure 1, most participants (84.8%) had a later midsleep on free days compared to workdays, which indicates that these individuals had a circadian phase advance in their sleep timing when transitioning from free days to workdays. In contrast, 68 participants (15.2%) had an earlier midsleep on free days relative to workdays (ie, phase delay when shifting from free days to workdays). Table 1 lists participant characteristics and the bivariate correlation of each characteristic with chronotype and SJL. Greater eveningness correlated moderately with SJL in the current sample (r = 0.13; P = .007). Evening chronotype was associated with younger age, lower family income, later average bed time and later average wake time, greater sleep debt, poorer sleep quality, greater depressive symptomology, and less physical activity (P < .05). SJL was associated with fewer years of schooling, greater sleep debt, poorer sleep quality, and lower physical activity (P < .05). None of the other associations we examined were statistically significant (P > .05).
Figure 1.
Shift in sleep midpoint from free (non-work) days to workdays. Sleep midpoint refers to the midpoint between actigraphy-derived sleep onset and wake onset. Differences shown represent sleep midpoint preceding free days minus sleep midpoint preceding workdays. No participants demonstrated a difference of zero minutes.
Table 1.
Participant Characteristics and their Correlation with Chronotype and Social Jetlag
| Variable | Chronotype r | SJL r | |
|---|---|---|---|
| Demographics | |||
| Age | 42.7 (7.4) | −0.20** | 0.01 |
| Sex | 47.0% male | −0.05a | 0.04a |
| Race | 83.2% white | −0.01a | 0.04a |
| Education, y | 17.0 (2.8) | −0.04 | −0.10* |
| Family income | 17.7% > $110 000 | −0.11* | −0.03 |
| Employment status | 89.7% full-time | 0.03a | −0.08a |
| Marital status | 63.1% married | 0.08a | 0.05a |
| Sleep characteristics | |||
| Chronotype | 39.3 (7.2) | 0.13** | |
| Wake time | 6:31 am (1 h 8 min) | 0.49** | 0.03 |
| Bed time | 11:41 pm (1 h 11 min) | 0.52** | 0.05 |
| Sleep duration | 6.8h (54 min) | −0.06 | −0.03 |
| Sleep debt, h | 1.2 (1.0) | 0.11* | 0.44** |
| Sleep efficiency, % | 83.2 (5.2) | 0.00 | −0.02 |
| PSQI total | 5.0 (2.6) | 0.13** | 0.12** |
| Depression and health behaviors | |||
| CES-D total | 8.5 (7.9) | 0.13** | 0.02 |
| Physical activity, kcal/d | 2810.0 (2132.1) | −0.18** | −0.10* |
| Caloric intake, kcal/d | 1873.6 (829.9) | 0.05 | 0.00 |
| Alcohol intake, drinks/wk | 1.6 (2.5) | 0.07 | 0.03 |
| Smoking status | 11.6% current smoker | 0.05a | 0.08a |
| Health outcomes | |||
| Heart rate, bpm | 33.4 (4.2) | 0.10* | 0.04 |
| Systolic BP, mm Hg | 115.0 (11.1) | −0.03 | −0.06 |
| Diastolic BP, mm Hg | 72.1 (8.2) | −0.01 | 0.02 |
| Total cholesterol, mg/dL | 200.66 (38.7) | 0.00 | 0.09 |
| HDL, mg/dL | 55.8 (15.1) | −0.11* | −0.06 |
| LDL, mg/dL | 122.8 (32.4) | 0.01 | 0.09 |
| Triglycerides, mg/dL | 109 (68.2) | 0.11* | 0.10* |
| Waist circumference, cm | 90.1 (14.2) | 0.05 | 0.12** |
| BMI, kg/m2 | 26.8 (5.2) | 0.04 | 0.15** |
| Fasting glucose, mg/dL | 98.1 (10.2) | 0.06 | 0.09 |
| Fasting insulin, μU/mL | 12.4 (6.2) | 0.06 | 0.09 |
| HOMA-IR | 3.0 (1.7) | 0.07 | 0.10* |
Data are expressed as mean (SD) unless stated otherwise. Correlations with chronotype were conducted with an inversed CSM total score for ease of interpretation (a higher score represents greater eveningness).
Point biserial r conducted with the described category of each variable serving as the comparison group.
P < .05.
P < .01.
Metabolic and vascular risk factors
Table 2 reports the effects of chronotype and SJL, as independent predictors, on metabolic and vascular risk parameters. After controlling for participant sleep characteristics and demographic variables (model 1), evening chronotype was related to higher triglycerides and lower HDL cholesterol (P < .05); greater SJL associated positively with triglycerides, fasting insulin, insulin resistance, waist circumference, and BMI, and negatively with HDL-cholesterol (P < .05). Further controlling for health behaviors and depressive symptomatology (model 2) rendered the association of chronotype with triglycerides nonsignificant (P > .05) and with HDL-cholesterol marginally significant (P = .047), whereas all correlates of SJL remained significant (P < .05; Table 3). To illustrate these associations, Table 4 presents mean values of the metabolic variables among individuals experiencing > 60 min of SJL, relative to all other participants. Finally, neither chronotype nor SJL was related significantly to resting heart rate or BP (P > .05).
Table 2.
Relationship of SJL and Chronotype to Components of Metabolic Risk
| Dependent Variable | SJL |
Chronotype |
||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||
| β | P | β | P | β | P | β | P | |
| Heart rate | 0.04 | .397 | 0.02 | .687 | 0.09 | .073 | 0.05 | .300 |
| Systolic BP | −0.05 | .302 | −0.07 | .151 | −0.03 | .602 | −0.05 | .284 |
| Diastolic BP | 0.02 | .672 | 0.01 | .792 | 0.00 | .932 | −0.02 | .672 |
| Total cholesterol | 0.06 | .210 | 0.03 | .535 | 0.04 | .429 | −0.00 | .941 |
| HDL, mg/dL | −0.08 | .069 | −0.09 | .036 | −0.09 | .043 | −0.083 | .047 |
| LDL, mg/dL | 0.06 | .271 | 0.03 | .525 | 0.03 | .534 | 0.00 | .998 |
| Triglycerides, mg/dL | .13 | .009 | .11 | .026 | .12 | .011 | 0.07 | .127 |
| Fasting glucose, mg/dL | 0.07 | .148 | 0.05 | .322 | 0.08 | .099 | 0.04 | .429 |
| Fasting insulin, μU/mL | .12 | .025 | .11 | .033 | 0.04 | .413 | 0.01 | .774 |
| HOMA-IR | .12 | .019 | .11 | .031 | 0.05 | .270 | 0.02 | .676 |
| Waist circumference, cm | .16 | .001 | .15 | .002 | 0.06 | .169 | 0.03 | .502 |
| BMI, kg/m2 | .18 | <.001 | .17 | .001 | 0.05 | .316 | 0.03 | .612 |
Model 1 values represent hierarchical regression analyses controlling for participant demographics and sleep characteristics. Model 2 values represent hierarchical regression analyses controlling for participant demographics, sleep characteristics, health behaviors, and depression. Chronotype was entered as an inversed CSM total score for ease of interpretation (increased score now represents greater eveningness). All outcome variables were entered as continuous variables in the models. Boldface data indicate significant effects (P < .05).
Table 3.
SJL and Metabolic Risk Factors, Adjusted for Demographic and Behavioral Covariates
| Predictor | Dependent Variables β |
|||||
|---|---|---|---|---|---|---|
| Triglycerides | HDL Cholesterol | Fasting Insulin | HOMA-IR | Waist | BMI | |
| Step 1 | ||||||
| Age | 0.16** | 0.03 | −0.04 | 0.01 | 0.17** | 0.11* |
| Sex | −0.26** | 0.51** | −0.09 | −0.12* | −0.40** | −0.12* |
| Race | −0.16** | 0.01 | 0.09 | 0.07 | 0.06 | .11* |
| Education | 0.01 | −0.00 | −0.14** | −0.14** | −0.13** | −0.12* |
| Income | −0.16 | 0.00 | −0.01 | −0.02 | −0.00 | −0.04 |
| Work status | 0.07 | 0.01 | −0.03 | −0.03 | −0.01 | −0.01 |
| Sleep duration | 0.05 | −0.01 | 0.09 | 0.08 | 0.07 | 0.06 |
| Sleep debt | −0.05 | 0.03 | −.13* | −.10* | −.11* | −.12* |
| Sleep efficiency | −0.03 | .10* | −0.07 | −0.09 | −0.08 | −0.09 |
| Sleep quality | 0.03 | 0.06 | .13* | .13* | .10* | 0.09 |
| Step 2 | ||||||
| Depression | 0.01 | −0.06 | 0.01 | 0.01 | −0.03 | −0.02 |
| Alcohol | −0.03 | .24** | −0.03 | −0.01 | −0.02 | −0.02 |
| Physical activity | −.26** | −.13** | −.14** | −.15** | −.18** | −.15** |
| Calories consumed | 0.06 | 0.08 | 0.05 | 0.04 | 0.08 | 0.06 |
| Smoking status | 0.01 | 0.02 | −0.07 | −0.06 | 0.01 | −0.02 |
| Step 3 | ||||||
| SJL | .11* | −0.09* | .11* | .11* | .15** | .17** |
Values represent independent hierarchical linear regression analysis. Sleep duration (hours), sleep debt (minutes), and sleep efficiency (%) are based on sleep actigraphy. Sleep quality is indexed as total PSQI score. Depression denotes total CES-D score minus sleep questions; alcohol, average number of drinks/month; physical activity, total kilocalories/day; calories consumed, total calories/day; and smoking status, current smoker vs ex/never smoker.
P < .05.
P < .01.
Table 4.
Mean Values (± SD) of Metabolic Parameters as a Function of SJL
| SJL Group |
F(1420) | P Value | η2 | ||
|---|---|---|---|---|---|
| ≤60 min | >60 min | ||||
| n | 326 | 111 | |||
| Triglycerides | |||||
| Log-mean | 1.96 (.22) | 2.03 (.23) | 6.95 | .009 | 0.02 |
| M[g], mg/dL | 91.20 | 107.15 | |||
| HDL cholesterol, mg/dL | 56.59 (15.03) | 54.14 (15.17) | 6.06 | .014 | 0.01 |
| Insulin | |||||
| Log-mean | 1.08 (.16) | 1.13 (.19) | 4.73 | .030 | 0.01 |
| M[g], μU/mL | 12.02 | 13.49 | |||
| HOMA-IR | |||||
| Log-mean | .57 (.14) | .61 (.17) | 4.88 | .028 | 0.01 |
| M[g] | 3.72 | 4.07 | |||
| Waist circumference, cm | 88.86 (14.18) | 94.04 (13.97) | 11.77 | .001 | 0.03 |
| BMI, kg/m2 | 26.33 (4.99) | 28.28 (5.77) | 8.36 | .004 | 0.02 |
Mean values of metabolic risk factors among individuals experiencing SJL >60 vs ≤60 min. F-ratios, P values, and effect sizes are derived from analysis of covariance, controlling for participant demographics, sleep characteristics, health behaviors, and depressive symptomatology. For log-transformed variables (triglycerides, insulin, and HOMA-IR), means are presented in logged values and back-transformed to the raw unit of measurement, expressed as the antilog or geometric mean of the distribution (M[g]). HOMA-IR, insulin resistance.
Discussion
The primary aim of this study was to examine whether chronotype and/or SJL associate with cardiometabolic risk factors. Our results show that a mismatch in sleep timing between workdays and free days linked to greater cardiometabolic risk, specifically with components of glycemic control, serum lipids, and adiposity. In addition, these effects persisted after adjusting for correlated variation in other sleep parameters (ie, duration, sleep efficiency, and sleep quality), and with further adjustment for participant health behaviors (notably, physical activity, caloric intake, and alcohol consumption). To our knowledge, this is the first study to show SJL associated with numerous metabolic risk factors in community-dwelling, midlife adults, independently of poor sleep characteristics and health behaviors.
Consistent with previous reports, chronotype correlated with age in the current sample (r = −0.20) (41). Eveningness tends to peak at approximately 20 years of age, and individuals progressively demonstrate increasing morningness during midlife (41). We found that our sample of middle-aged adults had a small proportion of evening types, such that only 4.9% (n = 22) of the participants met a conventional threshold for evening chronotype (CSM score ≤ 26) (26). It is possible that the low number of evening types in the current sample contributed to the null results. Given that evening chronotypes were more likely to experience SJL compared to morning types (r = 0.13), it is not surprising that the mean SJL was only 44 minutes (SD, 32 min) in the current sample. Previous population studies have reported a wide range of SJL (0 to 4+ h) (11, 13), whereas only 6% (n = 28) of the current sample experienced SJL of 1 hour or more. Two additional participants had SJL > 3.5 hours but were excluded as statistical outliers. It is therefore possible that the associations of SJL with metabolic risk factors seen here may underestimate those in the general population, warranting replication across a wider range of SJL.
We found that SJL was associated with both higher fasting insulin and HOMA-derived insulin resistance. It is important to note that the HOMA-IR index is a steady-state measure of baseline insulin and glucose function, whereas postprandial, dynamic measures such as the oral glucose tolerance test quantify the dynamic interplay of insulin sensitivity and β-cell insulin secretion (42). There is evidence that both fasting and postprandial glucose measures are predictive of type 2 diabetes, and these may represent two distinct risk factors that may contribute to diabetes via different physiological mechanisms (43). Postprandial hyperglycemia predicts increased risk for cardiovascular disease and all-cause mortality, even among individuals with normal fasting glucose and insulin values (44). Because postprandial measures of glucose metabolism are unavailable in this study, it is possible that the effects of chronotype and SJL on glucose metabolism were underestimated. To comprehensively examine whether these forms of circadian disruptions lead to metabolic dysregulation and subsequent cardiovascular risk, future studies should include both fasting and postprandial measures.
We considered the possibility that SJL was related to metabolic risk via poor health behaviors. Physical activity was associated with all dependent variables; greater alcohol consumption, with higher glucose and lower HDL-cholesterol; smoking, with higher total cholesterol; and calories consumed, with higher systolic BP (data not shown). Controlling for variability in these health behaviors did not alter observed effects of SJL on the dependent variables, suggesting that these associations are mediated by other mechanisms.
SJL might also contribute to metabolic risk via co-occurring sleep deprivation or poor sleep. In accord with previous reports, we found greater SJL linked to earlier wake times and shorter sleep durations on workdays, as well as later wake times and longer sleep duration on free days (P < .05) (14). The relatively later awakening time on free days may be perpetuating a cycle of circadian misalignment because it delays circadian timing, thus interfering with entrainment to workday schedules (45). Individuals may be sleeping longer on free days to compensate for sleep debt accumulated (4) from the work week. Sleep debt, quantified here as the difference in sleep duration between workdays and free days, was moderately correlated with SJL (r = 0.44). Consistent with previous reports (1, 3), sleep debt in the current sample was associated with various metabolic risk factors, including insulin resistance and measures of adiposity (P < .05). Previous reports have also linked other sleep disorders, such as sleep apnea, with poor health outcomes (46). To examine the effect of sleep disturbances, we quantified sleep efficiency. Sleep efficiency was not correlated with SJL (P > .05), and controlling for this variable did not attenuate any of the observed SJL effects. Given that shift workers were excluded from the study, the presence of shift work sleep disorder was not a confounding variable here. Thus, our findings suggest that SJL heightens metabolic risk through a misalignment in circadian rhythms rather than by sleep disturbances per se.
SJL refers to a mismatch between biological and social timing (14) and may contribute to metabolic risk by desynchronizing the temporal organization of various metabolic processes. At the molecular level, the central clock (in the suprachiasmatic nucleus) and peripheral oscillators throughout the body comprise a self-sustaining network of transcriptional-translational feedback loops that drive 24-hour expression patterns of core clock genes (47). Regions throughout the brain have autonomous circadian oscillators that re-entrain at different rates when they are isolated and then re-exposed to light cues (48). In regard to physiological processes, fat accumulation in adipose tissues, insulin secretion in the pancreas and liver, and food absorption in the intestine all show tissue-specific circadian rhythms (47). Taken together, a disruption in the circadian system triggered by SJL could dysregulate the temporal organization of both central and peripheral rhythms and, in turn, promote metabolic abnormalities.
Our report extends findings from both acute circadian misalignment paradigms and prospective shift-work studies by demonstrating a link between SJL and metabolic abnormalities (8, 11). We found that SJL was specifically related to greater insulin concentrations, insulin resistance, higher levels of triglycerides, and greater waist circumference and BMI. In contrast to previous reports (16, 17), we did not find SJL associated with heart rate. These results suggest that there is a direct effect of habitual misalignments in sleep time on various metabolic parameters and warrant prospective studies to determine the long-term effects of SJL.
The use of actigraphy to quantify SJL is a strength of the current study. Actigraphy provides a behavioral measure of sleep patterns that, unlike self-reported questionnaires, is not influenced by retrospective reporting bias. Nevertheless, some study limitations should be noted. Objective measures of circadian phase were unavailable in this sample, and it is unclear whether the individuals with SJL and/or self-reported evening chronotypes differ in their biological circadian phase. Given that hemodynamic and metabolic factors show circadian rhythms and that individuals vary in the timing of these rhythms, it is possible that our method of collecting samples within the same morning time frame across all participants (ie, irrespective of chronotype) may have led to an underestimation of variability in cardiometabolic risk factors and thus the effects of chronotype. In addition, the current study quantified average caloric intake and energy expenditure but did not examine the timing of these activities. Emerging evidence from both rodent and human studies suggests that food consumption during habitual sleep or rest times (ie, evenings in humans) is associated with greater risk for obesity (49, 50) The timing of meals serves as a behavioral zeitgeber that can entrain peripheral clocks (51). It is thus possible that eating during habitual sleep times may occur as a consequence or perpetuation of SJL and warrants future studies to consider the effects of both disrupted sleep and mealtime rhythms. Lastly, the cross-sectional design of the current study precludes causal inferences and warrants future prospective studies to extend the present findings.
In summary, the current study presents the first evidence that behaviorally quantified SJL is related to a cluster of metabolic risk factors in midlife adults. Our results, along with other recent reports, suggest that a misalignment of the biological and socially influenced sleep timing is an additional factor contributing to risk for developing obesity, type 2 diabetes, and atherosclerotic cardiovascular disease and highlight the potential for sleep and circadian-focused interventions in preventative health care.
Acknowledgments
We thank Barbara Anderson, PhD, and Melissa Delaney, BA, of the Department of Psychology, University of Pittsburgh, for coordination of data collection and actigraphy data management. We also thank Annette Wood, MS, of the Department of Psychiatry, University of Pittsburgh Medical Center, for her guidance in processing and scoring the actigraphy data.
This work was supported by National Institutes of Health Grant PO1 HL040962 (to S.B.M.).
Author Contributions: P.M.W. researched the data and wrote the manuscript. B.P.H. contributed to the analytical plan and discussion and reviewed/edited the manuscript. T.W.K. and M.F.M. reviewed and edited the manuscript. S.B.M. contributed to the analytical plan and discussion, and edited/reviewed the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostatic model assessment of insulin resistance
- LDL
- low-density lipoprotein
- SJL
- social jetlag.
References
- 1. Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–223. [DOI] [PubMed] [Google Scholar]
- 3. Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. [DOI] [PubMed] [Google Scholar]
- 4. Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 5. De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854. [DOI] [PubMed] [Google Scholar]
- 6. Puttonen S, Kivimäki M, Elovainio M, et al. Shift work in young adults and carotid artery intima-media thickness: The Cardiovascular Risk in Young Finns study. Atherosclerosis. 2009;205:608–613. [DOI] [PubMed] [Google Scholar]
- 7. Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45:948–952. [DOI] [PubMed] [Google Scholar]
- 10. Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28:771–778. [DOI] [PubMed] [Google Scholar]
- 11. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. [DOI] [PubMed] [Google Scholar]
- 12. Merikanto I, Lahti T, Puolijoki H, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30:470–477. [DOI] [PubMed] [Google Scholar]
- 13. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. [DOI] [PubMed] [Google Scholar]
- 14. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. [DOI] [PubMed] [Google Scholar]
- 15. Parsons MJ, Moffitt TE, Gregory A, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond). 2015;39:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutters F, Lemmens SG, Adam TC, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29:377–383. [DOI] [PubMed] [Google Scholar]
- 17. Kantermann T, Duboutay F, Haubruge D, Kerkhofs M, Schmidt-Trucksäss A, Skene DJ. Atherosclerotic risk and social jetlag in rotating shift-workers: First evidence from a pilot study. Work. 2013;46:273–282. [DOI] [PubMed] [Google Scholar]
- 18. Reutrakul S, Hood MM, Crowley SJ, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36:2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1998;280:356–362. [DOI] [PubMed] [Google Scholar]
- 20. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 21. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 24. Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–738. [DOI] [PubMed] [Google Scholar]
- 25. Di Milia L, Adan A, Natale V, Randler C. Reviewing the psychometric properties of contemporary circadian typology measures. Chronobiol Int. 2013;30:1261–1271. [DOI] [PubMed] [Google Scholar]
- 26. Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Pers Individ Dif. 2001;30:293–301. [Google Scholar]
- 27. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 28. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. [DOI] [PubMed] [Google Scholar]
- 29. Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 30. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. [DOI] [PubMed] [Google Scholar]
- 31. Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. [DOI] [PubMed] [Google Scholar]
- 32. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 33. Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46:1403–1411. [DOI] [PubMed] [Google Scholar]
- 34. Nowak Z, Plewa M, Skowron M, Markiewicz A, Kucio C, Osiadło G. Paffenbarger Physical Activity Questionnaire as an additional tool in clinical assessment of patients with coronary artery disease treated with angioplasty. Kardiol Pol. 2010;68:32–39. [PubMed] [Google Scholar]
- 35. Choo J, Elci OU, Yang K, et al. Longitudinal relationship between physical activity and cardiometabolic factors in overweight and obese adults. Eur J Appl Physiol. 2010;108:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chomistek AK, Chiuve SE, Jensen MK, Cook NR, Rimm EB. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc. 2011;43:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. [DOI] [PubMed] [Google Scholar]
- 38. Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 39. De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24:412–424. [DOI] [PubMed] [Google Scholar]
- 40. Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 41. Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 42. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 43. Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–1914. [DOI] [PubMed] [Google Scholar]
- 44. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 45. Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep Biol Rhythms. 2008;6:172–179. [Google Scholar]
- 46. Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abe M, Herzog ED, Yamazaki S, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–1381. [DOI] [PubMed] [Google Scholar]
- 51. Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. In: Kramer A, Merrow M, eds. Circadian Clocks. Berlin Heidelberg, Germany: Springer-Verlag; 2013:3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]