Abstract
Context:
Type 2 diabetes (T2D) is reduced in postmenopausal women randomized to estrogen-based hormone therapy (HT) compared with placebo. Insulin sensitivity is a key determinant of T2D risk and overall cardiometabolic health, and studies indicate that estradiol (E2) directly impacts insulin action.
Objective:
We hypothesized that the timing of E2 administration after menopause is an important determinant of its effect on insulin action.
Design:
We performed a randomized, crossover, placebo-controlled study.
Participants:
Study participants were early postmenopausal (EPM; ≤6 years of final menses; n = 22) and late postmenopausal (LPM; ≥10 years since last menses; n = 24) women naive to HT.
Intervention:
Study interventions included short-term (1 week) transdermal E2 and placebo.
Main Outcomes and Measures:
The study's main outcome was insulin-mediated glucose disposal (glucose disposal rate [GDR]) via hyperinsulinemic-euglycemic clamp.
Results:
Compared to EPM women, LPM women were older (mean ± SD; 63 ± 3 vs 56 ± 4 years, P < .05) and more years past menopause (12 ± 2 vs 3 ± 2 years, P < .05). Body mass index (24 ± 3 vs 25 ± 7 kg/m2) and fat mass (25 ± 7 vs 23 ± 6 kg) did not differ between groups, but fat-free mass (FFM) was lower in LPM women compared to EPM women (40 ± 4 vs 43 ± 5 kg, P < .05). Baseline GDR did not differ between groups (11.7 ± 2.8 vs 11.5 ± 2.9 mg/kg FFM/min). In support of our hypothesis, 1 week of E2 decreased GDR in LPM women compared to an increase in EPM women (+0.44 ± 1.7 vs − 0.76 ± 2.1 mg/kg FFM/min, P < .05).
Conclusions:
There was not an apparent decline in GDR with age or time since menopause per se. However, E2 action on GDR was dependent on time since menopause, such that there was an apparent benefit early (≤6 years) compared to harm later (≥10 years) in menopause. E2-mediated effects on insulin action may be one mechanism by which HT reduces the incidence of T2D in early postmenopausal women.
The question of whether there is an optimal window of opportunity for treating postmenopausal women with exogenous estrogens (to maximize benefit and minimize risk) has received considerable attention in recent years. The so-called “timing hypothesis” originally evolved from animal models of atherosclerotic disease progression (eg, high fat-fed cynomolgus monkeys), in which estrogen treatment was started immediately after ovariectomy or delayed. These studies indicated that estradiol (E2) prevented atherosclerosis only when it was administered early in the disease process (1, 2). Consistent with this, secondary analyses of large randomized clinical trials (RCTs) suggest that initiating estrogen-based hormone therapy (HT) in early postmenopausal (EPM) compared to late postmenopausal (LPM) women impacts the balance of HT benefits and risks (3–6).
The strong preclinical data and secondary observations from clinical trials have led to prospective RCTs designed to evaluate the timing hypothesis. To date, studies have focused on cardiovascular outcomes (7, 8); yet one of the major benefits of initiating HT early in menopause may be type 2 diabetes (T2D) risk reduction (6). Large RCTs have observed a lower incidence of new-onset T2D in women randomized to HT compared with placebo (9–12); meta-analysis of 107 trials estimated the risk reduction to be 30% in nondiabetic postmenopausal women (4). These studies did not directly evaluate whether the effectiveness of HT was influenced by time since menopause per se. However, when T2D incidence was broken down by age in the Women's Health Initiative (WHI) trial, there was a trend for an age-by-treatment interaction, such that the incidence of T2D was lower in younger and higher in older women randomized to HT compared with placebo (11). For EPM women, this translated to a risk reduction in T2D for more than 10 of 1000 women treated with HT for longer than 5 years (6).
It is not known whether the benefit of HT on T2D is through the physiologic action of estrogen on insulin sensitivity. Our previous studies suggested short-term estrogen administration improves insulin action (insulin-stimulated glucose disposal rate; GDR) in EPM women (13), but possibly not in LPM women (14). Thus, this proof-of-concept study was designed to test whether the E2-mediated effects on insulin action depends on the timing of treatment relative to menopause. We hypothesized that E2 would increase GDR in EPM women compared to a decreased GDR in LPM women.
Materials and Methods
Subjects.
We studied 46 postmenopausal women (age, 45–70 years) who were either fewer than 6 years past menopause (EPM; n = 22) or more than 10 years past menopause (LPM; n = 24). Menopause was defined as more than 12 months since final menses or bilateral oophorectomy. All women were naive (never used >6 months) to all formulations of estrogen-based HT. Women were excluded if they underwent menopause (natural or surgical) earlier than age 45 years or had a hysterectomy without bilateral oophorectomy. Women were nonobese (body mass index <30 kg/m2), nonsmokers, sedentary to moderately active, and weight stable (±2 kg over 2 months before enrollment). Women were excluded if they had: diabetes (known diagnosis or fasting glucose ≥126 and/or 2-hour postchallenge glucose ≥200 mg/dL), uncontrolled hypertension (systolic blood pressure >140 and/or diastolic >90 mm Hg), severe hypertriglyceridemia (>400 mg/dL), contraindications to estrogen treatment, or were using any glucose-lowering or insulin-sensitizing medications. Before enrollment, each woman provided informed consent. The protocol was approved by the Colorado Multiple Institutional Review Board.
Body composition assessment.
Total and regional (trunk, leg) fat mass and fat-free mass (FFM) were measured by dual-energy x-ray absorptiometry (Hologic Discovery W, software version 11.2) as previously described (15). CT scans of the abdomen (L2-L3 and L4-L5) and midthigh measured abdominal and femoral subcutaneous fat areas (cm2) as previously described (16). Visceral fat area (cm2) and intermuscular fat area were calculated as the difference between total fat area and subcutaneous fat area.
Weight maintenance/3-day dietary lead-in period.
Before enrollment and throughout testing, all eligible study participants were required to maintain their current body weight ± 2 kg. Three days before each clamp visit, a standardized, eucaloric diet was provided by the University of Colorado Anschutz Medical Campus Clinical Translational Research Center metabolic kitchen to ensure consistent macronutrient intake among the study participants in the days before testing. Composition of the diet was 50% carbohydrate (29% complex/21% simple sugars), 34% fat (10–12%saturated/12–15%monunsaturated/8–10%polyunsaturated), 16% protein, and 100–125 mg cholesterol/1000 kcal. The daily energy requirement for each study participant was determined using the Harris-Benedict equation and an activity factor, as described previously (17). Subjects were not allowed to exercise (other than usual activities of daily living) for the 3 days leading up to the study visit.
Physical activity assessment.
The Yale Physical Activity Survey (18) was administered to assess time spent in a range of activities. Total time (hours/week) for each activity was multiplied by the intensity code (kcal/h) provided by the survey and the sum of all activities (adjusted for season) used to estimate the energy expenditure of daily activities (kcal/day).
Oral glucose tolerance test.
Subjects ate a weight-maintaining diet containing at least 150 g of carbohydrate per day for 3 days before the oral glucose tolerance test. Oral 75 g oral glucose tolerance tests were performed in the morning after an overnight fast (∼12 hours). Venous blood samples were obtained before and at 30, 60, 90, and 120 minutes after glucose load for the determination of plasma glucose and insulin. Volunteers with T2D (American Diabetes Association criteria (19)) were excluded from the study.
Insulin sensitivity assessment.
Two-stage (4 and 40 mU/m2/min), 3.5-hour hyperinsulinemic-euglycemic clamps were performed as previously described (20). Briefly, IV catheters were placed in an antecubital vein (for insulin and glucose infusions) and an arterialized hand vein (for blood sampling). After baseline measurement, a stepped-down priming dose of insulin was infused for 10 minutes and then maintained at a constant rate of 4 mU/m2/min for 80 minutes (stage 1). At 90 minutes, another 10-minute stepped-down priming dose was delivered and then maintained at a constant 40 mU/m2/min for 110 minutes (stage 2). Plasma glucose concentrations were measured bedside every 5 minutes, and the dextrose infusion variably adjusted to maintain euglycemia. Blood samples were collected at baseline (T = −5 minutes) and under steady-state conditions at the end of each insulin stage (T = 60, 75, 90 minutes) and stage (T = 180, 195, and 210 minutes) for later determination of plasma insulin, glucose, glycerol, and free fatty acids (FFA). Whole-body insulin-stimulated GDR (primary outcome) during the final 30 minutes of the higher dose insulin stage was estimated from the steady-state glucose infusion rates, adjusted for fluctuations in plasma glucose concentrations. Whole-body insulin-mediated suppression of lipolysis (secondary outcome) was estimated from the steady-state glycerol concentrations during the low- and high-dose insulin stages. The insulin concentration needed to half-maximally suppress glycerol (EC50) was estimated by curve-fitting (Sigma Plot 12.5) exponential decay curves [y0+(ae-bx), where y0 is max suppression and a and b are fitted parameters] to the basal and two steady-state glycerol and insulin values and EC50 = Ins0+ln2/b, as previously described (21).
E2 treatment.
The clamp visit was repeated on two separate occasions following 1 week of transdermal E2 (0.15 mg; 3 × 0.05 mg patches) and matching transdermal placebo in a randomized, crossover manner. Participants were blinded to treatment. An average of 7 ± 3 weeks separated the two tests.
Blood analyses.
Blood samples were stored at −80 C and analyzed in batch by the Clinical Translational Research Center Core Laboratory. Glucose (Beckman Coulter, Inc.), glycerol (R-Biopharm AG), and FFA (Wako Chemicals) were determined enzymatically; insulin, leptin, and estrone by RIA (EMD Millipore and Diagnostics Systems Lab, respectively); E2 and sex hormone-binding globulin (SHBG) by chemiluminescence (Beckman Coulter, Inc). Sensitivity and precision details can be found at http://www.ucdenver.edu/research/CCTSI/programs-services/ctrc/lab-services.
Statistical analysis.
This study utilized a two-group (LPM vs EPM) repeated measure (E2 vs placebo/control) design. Our a priori-selected primary outcomes were insulin-stimulated glucose disposal (GDR, mg/kg FFM/min) at baseline (placebo/control day) and in response to E2 (ΔGDR, within subject difference between E2 day and control day). Our sample size of 24/group was based on achieving more than 80% power (α = 0.05) to test for group differences (EPM vs LPM) in our primary outcomes. A priori sample size estimates were based on variability previously reported for these outcomes (14, 22). As a secondary insulin resistance outcome, we evaluated insulin suppression of lipolysis (EC50). Baseline group differences (LPM vs EPM) were assessed using t tests. Group differences in treatment-related changes were evaluated using a general linear with repeated measures model. All statistical analyses were done in IBM SPSS Statistics (version 22). Data are presented as mean ± SD unless otherwise specified.
Results
Participation and adherence.
Of 1037 women contacted, 103 consented and were screened for eligibility, 53 were randomized, and 48 (24/group) completed the study. Two women in the EPM group were nonadherent to treatment (verified by self-report and serum sex hormone concentrations) and therefore dropped from analysis.
Baseline subject characteristics.
Compared to EPM women, LPM women were on average 7 years older and 9 years further past menopause (Table 1). All but one woman in each group underwent menopause naturally (no surgery). The EPM and LPM groups included: white, non-Hispanic (n = 19 and 18, respectively); black, non-Hispanic (n = 2 and 1); Hispanic (n = 1 and 2); Asian (n = 0 and 2); and Native American (n = 0 and 1). Body mass index and fat mass did not differ between groups, but FFM was lower in LPM compared to EPM women. There were no differences between groups in abdominal or midthigh fat distribution or in lipid profile. None of the women had impaired fasting glucose or impaired glucose tolerance, but 2-hour glucose and glucose area under the curve values were higher in LPM compared to EPM women; insulin responses to glucose challenge did not differ. Compared to EPM, LPM women tended to have lower serum E2 (P = .06) and higher SHBG (P = .10), but differences did not reach statistical significance (Table 2).
Table 1.
Subject Characteristics
| Early Postmenopausal | Late Postmenopausal | |
|---|---|---|
| Age (years) | 56 ± 4 | 63 ± 3a |
| Years past menopause | 3 ± 1 | 12 ± 2a |
| Weight (kg) | 68.9 ± 8.6 | 63.4 ± 7.6a |
| BMI (kg/m2) | 25.7 ± 2.7 | 24.3 ± 3.2 |
| Fat mass (kg) | 25.4 ± 6.6 | 23.0 ± 6.0 |
| Trunk fat mass (kg) | 12.0 ± 3.5 | 11.2 ± 3.7 |
| Leg fat mass (kg) | 9.8 ± 2.9 | 8.4 ± 2.4 |
| Fat-free mass (kg) | 43.5 ± 4.6 | 40.3 ± 3.7a |
| Abdominal SFA (cm2) | 278 ± 94 | 241 ± 78 |
| Abdominal VFA (cm2) | 70 ± 30 | 81 ± 45 |
| Mid-thigh SFA (cm2) | 197 ± 75 | 161 ± 58 |
| Mid-thigh IMFA (cm2) | 17 ± 8 | 14 ± 6 |
| Total cholesterol (mg/dL) | 193 ± 41 | 197 ± 34 |
| HDL cholesterol (mg/dL) | 55 ± 11 | 63 ± 16 |
| LDL cholesterol (mg/dL) | 117 ± 32 | 110 ± 27 |
| Triglycerides (mg/dL) | 104 ± 52 | 113 ± 57 |
| Fasting glucose (mg/dL) | 89 ± 7 | 90 ± 13 |
| 2-hour glucose (mg/dL) | 90 ± 23 | 107 ± 30a |
| Glucose AUC (× 104) | 1.2 ± 0.2 | 1.4 ± 0.4a |
| Fasting insulin (μIU/mL) | 12 ± 4 | 11 ± 4 |
| 2-hour insulin (μIU/mL) | 47 ± 40 | 47 ± 40 |
| Insulin AUC (× 103) | 5.9 ± 3.6 | 6.1 ± 2.1 |
Mean ± sd.
Abbreviations: AUC, area under the curve (2-hour oral glucose tolerance test); BMI, body mass index; HDL, high-density lipoprotein; IMFA, intermuscular fat area; LDL, low-density lipoprotein; SFA, subcutaneous fat area; VFA, visceral fat area.
P < 0.05.
Table 2.
Treatment-Related Changes in Circulating Hormones and Metabolites
| Early Postmenopausal |
Late Postmenopausal |
Treatment |
Treatment × Group |
|||
|---|---|---|---|---|---|---|
| PL | E2 | PL | E2 | P Value | P Value | |
| Baseline fasting | ||||||
| Estradiol (pg/mL) | 19 ± 20 | 175 ± 91 | 10 ± 6 | 144 ± 60 | <.0001 | NS |
| Estrone (pg/mL) | 29 ± 15 | 109 ± 46 | 26 ± 15 | 98 ± 47 | <.0001 | NS |
| SHBG (nm/liter) | 44 ± 19 | 54 ± 17 | 55 ± 24 | 66 ± 29 | <.0001 | NS |
| Leptin (ng/mL) | 17 ± 12 | 20 ± 13 | 15 ± 10 | 18 ± 11 | <.0001 | NS |
| Glucose (mg/dL) | 90 ± 7 | 89 ± 6 | 90 ± 7 | 89 ± 9 | NS | NS |
| Insulin (μIU/mL) | 9 ± 3 | 8 ± 3 | 9 ± 5 | 8 ± 5 | NS | NS |
| Glycerol (mg/dL) | 113 ± 46 | 102 ± 31 | 122 ± 38 | 110 ± 35 | .07 | NS |
| FFA (μmol/L) | 634 ± 159 | 636 ± 140 | 755 ± 216 | 751 ± 186 | NS | NS |
| Stage1 (4 μU/m2/min) | ||||||
| Glucose (mg/dL) | 91 ± 5 | 89 ± 6 | 93 ± 6 | 90 ± 6 | <.05 | NS |
| Insulin (μIU/mL) | 14 ± 7 | 12 ± 3 | 17 ± 16 | 12 ± 3 | .06 | NS |
| Glycerol (mg/dL) | 54 ± 20 | 58 ± 21 | 68 ± 26 | 67 ± 22 | NS | NS |
| Stage2 (40 μU/m2/min) | ||||||
| Glucose (mg/dL) | 85 ± 6 | 87 ± 5 | 88 ± 6 | 85 ± 6 | NS | NS |
| Insulin (μIU/mL) | 87 ± 20 | 80 ± 18 | 91 ± 24 | 85 ± 25 | <.05 | NS |
| Glycerol (mg/dL) | 31 ± 8 | 34 ± 11 | 38 ± 13 | 33 ± 9 | NS | <.05 |
| GDR (mg/kg/min) | 7.4 ± 2.0 | 7.7 ± 2.1 | 7.3 ± 2.0 | 6.8 ± 1.9 | NS | <.05 |
| GDR (mg/kg FFM/min) | 11.7 ± 2.8 | 12.2 ± 3.1 | 11.5 ± 3.0 | 10.7 ± 2.9 | NS | <.05 |
| Lipolysis EC50 (μIU/mL) | 11.6 ± 3.3 | 10.6 ± 3.5 | 14.1 ± 4.8 | 11.6 ± 4.6 | <.01 | NS |
EC50, insulin concentration needed to half-maximally suppress glycerol (three-stage dose-response); FFA, free fatty acids; FFM, fat-free mass; GDR, glucose disposal rate (stage 2); NS, not significant.
Mean ± sd.
Baseline insulin sensitivity.
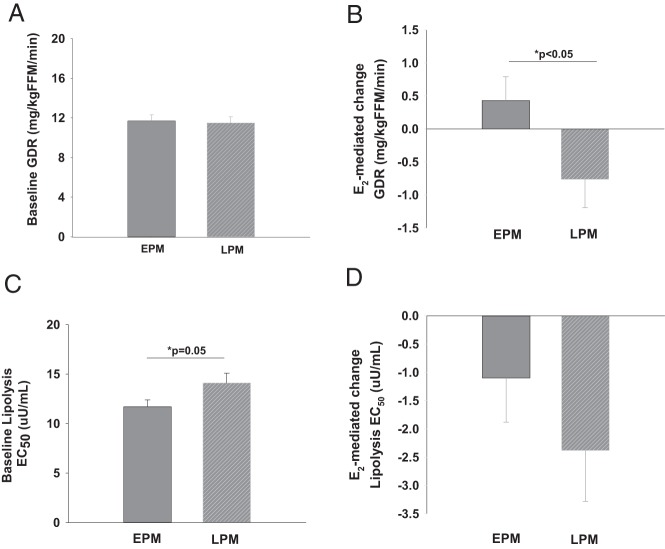
At baseline (control, placebo-treated day) fasting glucose and insulin concentrations were not different between LPM and EPM women (Table 2). Glucoregulatory insulin action (ie, insulin-stimulated GDR) did not differ between groups at baseline, with or without adjustment for group differences in FFM (Table 2, Figure 1A). Fasting glycerol was not different, but fasting FFA was higher (P < .05) in LPM compared with EPM women. Antilipolytic insulin action (ie, lipolysis EC50) was higher (P = .05) in LPM compared to EPM women (Figure 1C).
Figure 1.
Baseline group differences (A, C) and estradiol (E2)-mediated changes (B, D) in insulin-stimulated glucose disposal rate (GDR) and insulin-suppressed glycerol (lipolysis EC50) in early postmenopausal (EPM; ≤6 years past menopause) and late postmenopausal (LPM; ≥10 years past menopause) women (mean ± SE).
E2-mediated changes.
By design, treatment with transdermal E2 for 1 week raised serum E2 and SHBG compared with placebo (Table 2). Treatment with E2 significantly increased serum leptin and tended to decrease basal glycerol, but did not alter fasting glucose or insulin (Table 2). Consistent with our hypothesis, there was a group-by-treatment interaction for glucoregulatory insulin action, such that E2 increased GDR in EPM compared to a decrease in LPM women (Table 2, Figure 1B). The E2-mediated change in GDR was inversely related to time since menopause (r = −0.291, P = .05), but not age (r = −0.141, P = .35). There was a main effect of treatment (but no group-by-treatment interaction) on antilipolytic insulin action, such that lipolysis EC50 was reduced in both groups in response to E2 (Table 2, Figure 1D).
Discussion
This study is the first to demonstrate that E2 increases insulin-stimulated glucose disposal when administered to early postmenopausal women (ie, within 6 years of menopause) compared to a decrease when administered to late postmenopausal women (ie, more than 10 years past menopause). These data suggest that the physiologic effect of E2 on glucoregulatory insulin action (glucose disposal) depends on the timing of treatment relative to menopause. In contrast, the effect of E2 on antilipolytic insulin action (insulin suppression of lipolysis) did not differ by time since menopause.
The timing hypothesis.
Multiple lines of evidence (preclinical, observational, and RCTs) support a divergent effect of HT on various health outcomes (eg, heart disease, stroke) when treatment is started early vs late in menopause (23). Importantly, meta-analysis of studies that have evaluated mortality suggest there is a consistent reduction in overall mortality (approximately 25%) when HT is initiated early in menopause (24). To date, preclinical studies (1, 2) and RCTs (7, 8) have focused on atherosclerotic disease progression as the primary pathophysiology behind the differential effect of HT on health outcomes in early vs late menopause. This is not surprising given the documented vascular effect of estrogens (25). However, estrogens also play a prominent role in insulin action and glucose homeostasis (26). To our knowledge, no studies have evaluated the impact of HT timing on insulin resistance (whole body or tissue-specific) despite the relative importance of insulin action on multiple risk factors underlying cardiometabolic health, including vascular function (27, 28).
Estrogens and diabetes risk reduction.
Secondary analysis of large RCTs suggests new incidence of T2D is reduced in women randomized to HT compared with placebo (9–11), though evidence has been inconsistent (29, 30). Nevertheless, meta-analysis of 107 studies (49,973 patient years) followed for 1.5 years on average showed HT reduced abdominal obesity (6.8%), insulin resistance (12.9%; homeostasis model assessment of insulin resistance), and new incidence of T2D (30%) (4). The impact of time since menopause was not evaluated. However, when the WHI trial evaluated T2D incidence broken down by age, a crude index of time since menopause in women who go through menopause naturally (a majority in WHI), there was a weak (P = .11) age-by-treatment interaction, such that the incidence of T2D was lower in younger (age, 50–69 years) and higher in older (age, 70–79 years) women randomized to HT compared with placebo (11). Furthermore, a population-based prospective cohort study of early postmenopausal women (n = 8483) observed a 69% and 47% lower conversion to T2D in postmenopausal women who used HT continuously (throughout the 5-year follow-up) or part-time (<2.5 during follow-up) compared to never users, respectively (31). Taken together, these studies suggest estrogen-based HT reduces T2D, particularly in early postmenopausal women who have not been estrogen deficient for any length of time.
Physiologic role of E2 on glucoregulatory insulin action.
It is unlikely that estrogen-based HT would ever be clinically indicated for primary prevention of T2D, but understanding the pathophysiology behind the impact of estrogens on glucoregulatory insulin action remains highly clinically important. Our data suggest one of the benefits of postmenopausal HT on T2D may be through the physiologic action of estrogens on insulin-stimulated glucose uptake. Animal studies suggest there are multiple mechanisms by which E2 may improve insulin-mediated glucose uptake (26). E2 appears to have an effect on insulin signaling and glucose transport in adipose tissue and muscle (32, 33). E2 has also been shown to directly increase insulin receptor number and specific insulin binding in a tissue-specific and dose-dependent manner (34, 35). Studies of human tissue ex vivo suggest E2 acts on IRS-1, Akt, and insulin-stimulated tyrosine phosphorylation (36). Evidence also suggests E2 may impact whole-body glucose disposal indirectly through multiple mechanisms (eg, hepatic glucose production, lipid homeostasis, inflammatory cytokines) (26). Taken together, the preclinical evidence supports an effect of E2 on glucoregulatory insulin action. Consistent with this, our early clinical studies suggested a beneficial effect of short-term E2 on insulin-stimulated GDR in early postmenopausal women (13), but further preliminary data suggested a potentially harmful effect in late postmenopausal women (14). Based on these observations, our current study was designed specifically to test whether E2 action on GDR was dependent on time since menopause; results suggested benefit early (6 or fewer years) in menopause, but harm later (10 or more years) in menopause.
Physiologic role of E2 on antilipolytic insulin action.
In the current study, 1 week of transdermal E2 reduced the insulin concentration needed to half-maximally suppress lipolysis (ie, increased antilipolytic insulin sensitivity), irrespective of time since menopause. The current results are in contrast to our previous study in which we did not observe an acute effect of estrogen on insulin suppression of lipolysis (20). However, in that study, we used conjugated estrogens delivered acutely (IV bolus of Premarin) and studied only early postmenopausal women. The physiologic effect on insulin suppression of lipolysis may be specific to E2 because earlier studies demonstrated an effect of transdermal E2, but not oral Premarin, to improve insulin suppression of lipolysis (37). The impact of time since menopause on E2-mediated insulin suppression of lipolysis remains unclear. There was a trend for a greater improvement in late, compared to early, postmenopausal women, but this was likely due to their greater antilipolytic insulin resistance at baseline.
Potential mechanisms.
The reason for the switch from a benefit of E2 on glucoregulatory insulin action early, compared to harm later, in menopause is not known, but may be through changes in estrogen receptor (ER) expression with increasing duration of estrogen deficiency. Two isoforms of ER (ERα and ERβ) have been identified, and E2 binds to each with equal affinity (38), but the relative expression of each isoform varies among tissues and likely changes with prolonged estrogen deficiency (39). Moreover, E2 binding to ERα generally appears beneficial to glucose homeostasis, whereas binding to ERβ appears harmful (33). Thus, the prevailing balance of ERα and ERβ in glucoregulatory tissues may dictate the net action of E2 on whole-body glucose disposal (26). Future studies will be needed to determine whether ER expression differs between early and late postmenopausal women and whether this impacts E2-mediated changes in insulin action.
Study limitations and future directions.
We cannot rule out the possibility that group differences are a function of age rather than duration of estrogen deficiency. A strength of the study is that all women were naive to any use of HT throughout menopause, so their time since menopause was equivalent to duration of estrogen deficiency. However, the late postmenopausal women were, on average, 7 years older than the early postmenopausal group, and age is highly correlated with time since menopause. That said, the E2-mediated change in insulin-stimulated glucose disposal was significantly related to time since menopause but not to age, so the impact of age per se may be minimal. Moreover, groups were similar in total and regional adiposity as well as baseline insulin sensitivity, so the E2-mediated changes were not explained by these outcomes. Nevertheless, future studies of age-matched postmenopausal women with variable history of early HT use (eg, HT naive vs HT past users) are needed to clarify whether duration of estrogen deficiency determines E2 action, independent of age. Such studies will also help to clarify whether use of HT early in menopause can mitigate the adverse effects of E2 on insulin action later in menopause.
Conclusion
This proof-of-concept study demonstrated that the physiologic action of E2 on insulin-stimulated glucose disposal was dependent on time since menopause, such that there was a benefit early (≤6 years) compared to harm later (≥10 years) in menopause. An E2-mediated improvement in insulin action may be one mechanism by which HT reduces the incidence of T2D in early postmenopausal women; this effect of E2 may be reversed in late postmenopausal women. The physiologic data from this small, well-controlled clinical study should not be interpreted as evidence to support the use of E2 early in menopause to prevent T2D. Instead, the data further our understanding of how timing of E2 administration relative to menopause impacts E2 action.
Acknowledgments
The authors thank the staff members of the University of Colorado Anschutz Medical Campus Clinical Translational Research Center, Department of Radiology, and Energy Balance Core of the Nutrition and Obesity Research Unit for their assistance in conducting this study. The authors also thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts.
The following awards from the National Institutes of Health supported this research: R01 DK088105, P50 HD073063, T32 DK007446, P30 DK048520, UL1 TR000154.
This study can be found under clinicaltrials.gov number NCT01605071.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- E2
- estradiol
- ER
- estrogen receptor
- EPM
- early postmenopausal
- FFA
- free fatty acid
- FFM
- fat-free mass
- GDR
- glucose disposal rate
- HT
- hormone therapy
- LPM
- late postmenopausal
- RCTs
- randomized controlled trials
- SHBG
- sex hormone-binding globulin
- T2D
- type 2 diabetes
- WHI
- Women's Health Initiative.
References
- 1. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–384. [DOI] [PubMed] [Google Scholar]
- 2. Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20:342–353. [DOI] [PubMed] [Google Scholar]
- 3. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35–44. [DOI] [PubMed] [Google Scholar]
- 4. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. [DOI] [PubMed] [Google Scholar]
- 5. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 6. Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260. [DOI] [PubMed] [Google Scholar]
- 8. Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonds DE, Lasser N, Qi L, Brzyski R, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia. 2006;49:459–468. [DOI] [PubMed] [Google Scholar]
- 10. Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. [DOI] [PubMed] [Google Scholar]
- 12. Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–558. [DOI] [PubMed] [Google Scholar]
- 13. Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285:E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Pelt RE, Schwartz RS, Kohrt WM. Insulin secretion and clearance after subacute estradiol administration in postmenopausal women. J Clin Endocrinol Metab. 2008;93:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrin Metab. 2002;282:E1023–E1028. [DOI] [PubMed] [Google Scholar]
- 16. Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kien CL, Ugrasbul F. Prediction of daily energy expenditure during a feeding trial using measurements of resting energy expenditure, fat-free mass, or Harris-Benedict equations. Am J Clin Nutr. 2004;80:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–642. [PubMed] [Google Scholar]
- 19. American Diabetes Association. Screening for diabetes. Diabetes Care. 2002;25:S21–S24. [DOI] [PubMed] [Google Scholar]
- 20. Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring). 2006;14:2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stumvoll M, Jacob S, Wahl HG, et al. Suppression of systemic, intramuscular, and subcutaneous adipose tissue lipolysis by insulin in humans. J Clin Endocrinol Metab. 2000;85:3740–3745. [DOI] [PubMed] [Google Scholar]
- 22. Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- 23. Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol. 2014;142:68–75. [DOI] [PubMed] [Google Scholar]
- 24. Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122:1016–1022.e1. [DOI] [PubMed] [Google Scholar]
- 25. Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Natali A, Toschi E, Baldeweg S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. [DOI] [PubMed] [Google Scholar]
- 29. Manson JE, Rimm EB, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2:665–673. [DOI] [PubMed] [Google Scholar]
- 30. Gabal LL, Goodman-Gruen D, Barrett-Connor E. The effect of postmenopausal estrogen therapy on the risk of non-insulin-dependent diabetes mellitus. Am J Public Health. 1997;87:443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pentti K, Tuppurainen M, Honkanen R, Sandini L, Kroger H, Alhava E, Saarikoski S. Hormone therapy protects from diabetes: the Kuopio Osteoporosis Risk Factor and Prevention Study. Eur J Endocrinol. 2009;160:979–983. [DOI] [PubMed] [Google Scholar]
- 32. Muraki K, Okuya S, Tanizawa Y. Estrogen receptor alpha regulates insulin sensitivity through IRS-1 tyrosine phosphorylation in mature 3T3–L1 adipocytes. Endocr J. 2006; [DOI] [PubMed] [Google Scholar]
- 33. Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297:E124–E133. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen SB, Borglum JD, Moller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol. 1992;85:13–19. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez C, Alonso A, Grueso NA, Esteban MM, Fernandez S, Patterson AM. Effect of treatment with different doses of 17-beta-estradiol on the insulin receptor. Life Sci. 2002;70:1621–1630. [DOI] [PubMed] [Google Scholar]
- 36. Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Sullivan AJ, Ho KKY. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1783–1788. [DOI] [PubMed] [Google Scholar]
- 38. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. [DOI] [PubMed] [Google Scholar]
- 39. Wend K, Wend P, Krum SA. Tissue-specific effects of loss of estrogen during menopause and aging. Front Endocrinol (Lausanne). 2012;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]