Abstract
Context:
Polycystic ovary syndrome (PCOS) is a common condition in reproductive-aged women and a major female-specific risk factor of obesity, impaired glucose tolerance, and diabetes.
Objective:
We examined whether the genetic variation predisposing to PCOS affected glycemic changes in women with prior gestational diabetes mellitus (GDM) and whether such an effect was modified by changes in body adiposity, especially during and after pregnancy.
Design, Setting, and Participants:
This is a longitudinal study in Tianjin, China. We genotyped 7 genome-wide association study–identified PCOS single nucleotide polymorphisms and assessed gestational weight gain and changes in glycemic traits and weight at 1 to 5 years postpartum in 1133 women with prior GDM.
Main Outcome Measures:
The main outcome measure was postpartum glycemic changes.
Results:
The PCOS genetic risk score significantly interacted with postpartum weight reduction on changes in fasting glucose and 2-h glucose (P for interaction = .032 and .007; respectively) after multivariable adjustment. In women with postpartum weight reduction of ≥5 kg/y, the genetic risk score was associated with decreased fasting and 2-h glucose, whereas an opposite genetic effect was found in women who lost less weight. The association between postpartum weight reduction and glycemic improvement was more significant among women with a higher genetic risk score.
Conclusions:
In a large cohort of Chinese women with a history of GDM, our data for the first time indicate that the genetic predisposition to PCOS may interact with postpartum weight reduction on long-term glycemic changes, emphasizing the importance of postpartum weight management in prevention of diabetes in this subgroup of women.
Pregnancy is a period when women's weight increases by 20% or more; compelling evidence has suggested that gestational weight gain and postpartum weight reduction can contribute considerably to future glucose regulation (1–3), especially in women with distorted glucose metabolism during pregnancy, such as gestational diabetes mellitus (GDM) (3).
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age (4). Women with PCOS have an increased risk of obesity, GDM, and type 2 diabetes (5–8). Recent genome-wide association studies have identified genetic variants predisposing to PCOS, in concert with environmental risk factors such as obesity (9–12). However, little is known about whether the genetic predisposition to PCOS affects changes in glucose metabolism related to diabetes risk and whether the effect is modified by changes in body adiposity, especially during and after pregnancy.
In thus far one of the largest cohorts of women with a history of GDM, taking advantage of longitudinally collected information on body weight changes during and after pregnancy, we aimed to examine the association of the genetic predisposition to PCOS with postpartum changes in measures of glucose metabolism. We assessed in particular the interactions of gestational weight gain and postpartum weight reduction with genetic predisposition to PCOS on these glycemic changes.
Subjects and Methods
Study population
A retrospective cohort study was performed in 1133 women with prior GDM at 1 to 5 years postpartum using the data for the participants of the Tianjin Gestational Diabetes Mellitus Prevention Program (13–16).
The details of the program have been described previously (13). Since 1999, all pregnant women living in the 6 central urban districts in Tianjin have participated in universal screening for GDM, and the average proportion of screened pregnancies was >91% from 1999 to 2008 (16). All pregnant women at 26 to 30 weeks' gestation participated in a 1-hour 50-g glucose screening test, and those who had a glucose reading of ≥7.8 mmol/L were invited to undergo a 75-g glucose 2-hour oral glucose tolerance test (OGTT) at Tianjin Women's and Children's Health Center (16). GDM was defined using the World Health Organization criteria (17). Women with a 75-g glucose 2-hour OGTT result confirming either diabetes (fasting glucose of ≥7 mmol/L or 2-hour glucose of ≥11.1 mmol/L) or impaired glucose tolerance (2-hour glucose of ≥7.8 and <11.1 mmol/L) were regarded as having GDM. All pregnant women in whom GDM was diagnosed between 2005 and 2009 (N = 4644) were recruited 1 to 5 years after delivery, based on a health care registration system providing health and contact information for mothers with GDM. Participants who met any of the following criteria were excluded: (1) having diagnosed postpartum diabetes; (2) taking medicines known to alter OGTT; (3) having the presence of any chronic diseases that could seriously reduce life expectancy or the ability to participate in the study; and (4) currently being pregnant or planning to become pregnant in the next 2 years. Finally, 1263 women with GDM returned and finished the survey. There were no differences between the women with GDM returning and not returning in the OGTT at 26 to 30 gestational weeks, with regard to age (28.9 vs 28.7 years), fasting glucose (5.34 vs 5.34 mmol/L), 2-hour glucose (9.23 vs 9.16 mmol/L), and the prevalence of impaired glucose tolerance (90.9% vs 91.8%) and diabetes (9.1% vs 8.2%) (14). A total of 1133 women who had genotyping, weight, and glycemic data available were included in the final analysis. The study was approved by the Human Subjects Committee of the Tianjin Women's and Children's Health Center, and informed consent was obtained from each participant.
Assessment of weight changes, glycemic traits, and covariates
At the postpartum survey, all participants filled in a questionnaire about sociodemographics, family history of diabetes, history of GDM (values of fasting and 2-hour glucose in the 26–30 gestational weeks' OGTT and the treatment of GDM during the pregnancy), pregnancy outcomes (prepregnancy weight and gestational weight gain), alcohol intake, smoking habits, and physical activity (the frequency and duration of leisure time and sedentary activities). They also completed the 3-day 24-hour food records using the dietary record collection method taught by a dietician. The performance of the questionnaires and the 3-day 24-hour food records has been validated in the China National Nutrition and Health Survey (18, 19).
Body weight and height were measured by trained research doctors using a standardized protocol at the postpartum survey. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. The annual postpartum weight reduction was calculated by dividing the difference between postpartum weight and predelivery weight (prepregnancy weight plus gestational weight gain) by follow-up years. Gestational weight gain was categorized as inadequate, adequate, and excessive according to 2009 Institute of Medicine guideline (20). Adequate gestational weight gain was defined according to prepregnancy BMI as follows: 12.5 to 18 kg (prepregnancy BMI of <18.5 kg/m2), 11.5 to 16 kg (prepregnancy BMI of 18.5–24.9 kg/m2), 7 to 11.5 kg (prepregnancy BMI of 25.0–29.9 kg/m2), and 5 to 9 kg (prepregnancy BMI of ≥30 kg/m2). We also defined adequate gestational weight gain according to the Chinese maternal prepregnancy BMI classification (21) and the 2009 Institute of Medicine guideline as follows: 12.5 to 18 kg (prepregnancy BMI of <18.5 kg/m2), 11.5 to 16 kg (prepregnancy BMI of 18.5–23.9 kg/m2), 7 to 11.5 kg (prepregnancy BMI of 24.0–27.9 kg/m2), and 5–9 kg (prepregnancy BMI of ≥28 kg/m2). Gestational weight gain below or above the recommendation was defined as inadequate or excessive, respectively.
Postpartum blood samples were collected from all participants after an overnight fast of at least 12 hours. Participants were given a 2-hour 75-g OGTT, and fasting glucose and 2-hour glucose were measured on an automatic analyzer (Toshiba TBA-120FR). Hemoglobin A1c (HbA1c) was measured using an automatic glycohemoglobin analyzer (ADAMS A1c HA-8160; Arkray). Changes in glucose and HbA1c were calculated as differences in glucose and HbA1c levels between the postpartum survey and pregnancy (at GDM diagnosis).
Genotyping and computation of genetic risk score
We selected 7 single nucleotide polymorphisms (SNPs) that represent 3 susceptibility loci for PCOS identified by a genome-wide association studies and subsequent replication studies in Han Chinese (Supplemental Table 1) (9). Genomic DNA was extracted from the buffy coat fraction of centrifuged blood using a QIAamp blood maxi kit (QIAGEN). SNPs were genotyped by a quantitative real-time TaqMan PCR (Applied Biosystems). The genotyping success rate was >98%. For quality control, 10% of the samples were genotyped, and the concordance rate was more than 99%. The allele frequencies of all SNPs in total participants were in Hardy-Weinberg equilibrium (all P > .05).
Genetic risk scores were calculated based on the 7 SNPs for PCOS identified in Han Chinese (9), and all SNPs are not in linkage disequilibrium with each other (all pairwise r2 < 0.80). Each SNP was recorded as 0, 1, or 2 according to the PCOS risk allele's number and was weighted according to its relative effect size (β coefficient) (9). The genetic risk score was computed using the equation: genetic risk score = (β1 × SNP1 + β2 × SNP2 + … + βn × SNPn) × (n/sum of the β coefficients), where β is the β coefficient of each SNP for PCOS, n is 7, and sum of the β coefficients is 2.487 in the current analysis.
Statistical analysis
Statistical analyses were performed in SAS 9.1 (SAS Institute). We applied χ2 tests for categorical variables and general linear models for continuous variables to compare proportions and means of characteristics across annual postpartum weight reduction categories (<5, 5–<8, and ≥8 kg/y) and gestational weight gain categories (inadequate, adequate, and excessive). The association of PCOS genetic risk scores with changes in glycemic traits was tested using a general linear regression model. Changes in glycemic traits with each 1 allele increment in the PCOS genetic risk score/each SNP stratified by annual postpartum weight reduction were estimated by using general linear models. An interaction between the genetic risk score/each SNP and annual postpartum weight reduction on change in each glycemic trait was tested by including an interaction term in the models. Covariates considered in the multivariable models included age, follow-up time, prepregnant BMI, dietary fat (% energy), sitting time, and the previous value for the respective glucose trait (variables mentioned above are continuous), and family history of diabetes (yes or no), current smoking (yes or no), current alcohol drinking (yes or no), leisure time physical activity (0, 1–<30, or ≥30 minutes/d), and GDM therapy (yes or no). We also examined the association between annual postpartum weight reduction and changes in glycemic traits according to the tertiles of the genetic risk score/each SNP by using general linear models. Similar analyses were performed to estimate the interactions between the PCOS genetic risk score and gestational weight gain on changes in glycemic traits. We further conducted 2 sensitivity analyses: one was to estimate the interaction between the genetic risk score and gestational weight gain using the Chinese criteria defined gestational weight gain; the other one was to estimate the interactions between the genetic risk score and postpartum weight reduction/gestational weight gain in women within 1.5 years after delivery. P values are two-sided and a P < .05 was considered statistically significant.
Results
Table 1 presents the characteristics of women with prior GDM according to annual postpartum weight reduction and gestational weight gain. Compared with women with annual postpartum weight reduction of <5 kg/y, women with annual postpartum weight reduction of ≥8 kg/y were younger, had a shorter follow-up time, and had higher predelivery weight but lower postpartum weight (all P ≤ .001). Greater postpartum weight reduction was associated with lower levels of postpartum fasting glucose, 2-hour glucose, and HbA1c (all P < .001). Women with excessive gestational weight gain compared with those with inadequate gestational weight gain appeared to be younger, have a longer follow-up time, have greater weight at prepregnancy, predelivery, and postpartum and lower levels of 2-hour glucose both at GDM diagnosis and postpartum, and have a lower proportion of education attainment of ≥16 years and a longer sitting time (all P < .05). The PCOS genetic risk score was not significantly different among the 3 categories of annual postpartum weight reduction or gestational weight gain. No other differences in characteristics across the categories of weight reduction categories or gestational weight gain were observed.
Table 1.
Characteristics of Women With Prior GDM
| Annual Postpartum Weight Reduction |
P | Gestational Weight Gain |
P | |||||
|---|---|---|---|---|---|---|---|---|
| <5 kg/Year | 5–<8 kg/Year | ≥8 kg/Year | Inadequate | Adequate | Excessive | |||
| n | 403 | 363 | 367 | 157 | 384 | 592 | ||
| Age, y | 32.7 ± 3.5 | 32.6 ± 3.6 | 31.9 ± 3.3 | .001 | 33.0 ± 3.5 | 32.6 ± 3.5 | 32.1 ± 3.5 | <.001 |
| Follow-up time, y | 2.8 ± 1.0 | 2.3 ± 0.7 | 1.7 ± 0.4 | <.001 | 2.2 ± 0.9 | 2.2 ± 0.8 | 2.4 ± 0.9 | .013 |
| BMI, kg/m2 | ||||||||
| Prepregnancy | 23.2 ± 3.5 | 23.0 ± 3.6 | 23.0 ± 3.2 | .618 | 22.1 ± 2.9 | 22.3 ± 2.9 | 23.8 ± 3.4 | <.001 |
| Predelivery | 28.8 ± 4.1 | 29.4 ± 3.6 | 30.7 ± 3.9 | <.001 | 25.6 ± 2.7 | 27.6 ± 2.4 | 31.9 ± 3.4 | <.001 |
| Postpartum | 25.5 ± 4.4 | 23.8 ± 3.4 | 23.0 ± 3.4 | <.001 | 22.7 ± 3.3 | 22.9 ± 3.1 | 25.4 ± 4.1 | <.001 |
| Weight, kg | ||||||||
| Prepregnancy | 59.4 ± 9.3 | 59.0 ± 8.8 | 59.4 ± 8.9 | .957 | 56.4 ± 8.1 | 56.8 ± 8.1 | 61.7 ± 9.2 | <.001 |
| Predelivery | 73.8 ± 11.2 | 75.6 ± 10.4 | 79.0 ± 10.5 | <.001 | 65.2 ± 7.3 | 70.4 ± 7.1 | 82.6 ± 9.3 | <.001 |
| Postpartum | 65.4 ± 11.7 | 61.2 ± 9.8 | 59.4 ± 9.4 | <.001 | 58.0 ± 9.0 | 58.4 ± 8.5 | 65.6 ± 11.2 | <.001 |
| Fasting glucose, mmol/L | ||||||||
| At GDM diagnosis | 5.3 ± 0.8 | 5.3 ± 0.8 | 5.3 ± 0.8 | .692 | 5.2 ± 0.8 | 5.3 ± 0.8 | 5.4 ± 0.8 | .037 |
| Postpartum | 5.6 ± 1.2 | 5.3 ± 0.8 | 5.2 ± 0.6 | <.001 | 5.6 ± 1.4 | 5.3 ± 0.8 | 5.4 ± 0.9 | .109 |
| 2-h glucose, mmol/L | ||||||||
| At GDM diagnosis | 9.2 ± 1.3 | 9.2 ± 1.3 | 9.2 ± 1.3 | .391 | 9.3 ± 1.4 | 9.2 ± 1.3 | 9.1 ± 1.2 | .014 |
| Postpartum | 7.8 ± 3.0 | 6.9 ± 2.4 | 6.4 ± 1.7 | <.001 | 7.6 ± 3.3 | 7.0 ± 2.3 | 7.0 ± 2.4 | .048 |
| HbA1c, % | ||||||||
| At GDM diagnosis | 5.8 ± 0.6 | 5.8 ± 0.6 | 5.8 ± 0.6 | .520 | 5.8 ± 0.6 | 5.8 ± 0.6 | 5.8 ± 0.6 | .359 |
| Postpartum | 5.8 ± 0.9 | 5.7 ± 0.8 | 5.5 ± 0.6 | <.001 | 5.7 ± 0.9 | 5.6 ± 0.6 | 5.7 ± 0.8 | .580 |
| HbA1c, mmol/mol | ||||||||
| At GDM diagnosis | 41 ± 4 | 41 ± 4 | 41 ± 4 | .520 | 41 ± 4 | 41 ± 4 | 41 ± 4 | .359 |
| Postpartum | 41 ± 5 | 39 ± 5 | 37 ± 4 | <.001 | 39 ± 5 | 38 ± 4 | 39 ± 5 | .580 |
| Education | ||||||||
| <13 years | 100 (24.8) | 73 (20.2) | 77 (21.0) | .235 | 34 (21.7) | 63 (16.5) | 153 (25.8) | <.001 |
| 13–<16 years | 277 (68.7) | 263 (72.7) | 254 (69.2) | 108 (68.6) | 277 (72.3) | 409 (69.1) | ||
| ≥16 years | 26 (6.5) | 26 (7.2) | 36 (9.8) | 15 (9.6) | 43 (11.2) | 30 (5.1) | ||
| Family history of diabetes | 117 (29.0) | 101 (27.8) | 128 (34.9) | .086 | 50 (31.9) | 120 (31.3) | 176 (29.7) | .818 |
| Current smoking | 9 (2.2) | 2 (0.6) | 9 (2.5) | .061 | 5 (3.2) | 4 (1.0) | 11 (1.9) | .222 |
| Current alcohol drinkers | 97 (24.1) | 77 (21.2) | 72 (19.6) | .315 | 31 (19.8) | 80 (20.8) | 135 (22.8) | .623 |
| Leisure time physical activity | ||||||||
| 0 min/d | 312 (77.4) | 288 (79.3) | 291 (79.3) | .445 | 120 (76.4) | 305 (79.4) | 466 (78.7) | .714 |
| 1–<30 min/d | 77 (19.1) | 69 (19.0) | 70 (19.1) | 35 (22.3) | 69 (18.0) | 112 (18.9) | ||
| ≥30 min/d | 14 (3.5) | 6 (1.7) | 6 (1.6) | 2 (1.3) | 10 (2.6) | 14 (2.4) | ||
| Sitting time, h/d | 3.2 ± 2.2 | 3.2 ± 2.1 | 3.2 ± 2.1 | .910 | 2.8 ± 1.9 | 3.1 ± 1.9 | 3.4 ± 2.3 | <.001 |
| Dietary intakes | ||||||||
| Energy consumption, kcal/d | 1651 ± 426 | 1671 ± 422 | 1674 ± 453 | .454 | 1684 ± 425 | 1658 ± 415 | 1664 ± 447 | .742 |
| Fiber, g/d | 12.2 ± 4.7 | 12.3 ± 4.8 | 12.1 ± 4.7 | .873 | 12.5 ± 4.7 | 12.3 ± 4.7 | 12.0 ± 4.7 | .162 |
| Fat, % energy | 33.5 ± 6.1 | 33.4 ± 6.4 | 33.4 ± 6.6 | .872 | 32.9 ± 6.1 | 33.4 ± 6.4 | 33.6 ± 6.4 | .197 |
| PCOS genetic risk score | 6.74 ± 2.15 | 6.76 ± 2.13 | 6.78 ± 2.19 | .841 | 7.0 ± 2.2 | 6.7 ± 2.0 | 6.7 ± 2.2 | .418 |
Data are means ± SD for continuous variables or % (n) for categorical variables. P values were calculated by χ2 tests for categorical variables and general linear models for continuous variables.
The distribution of the genetic risk score and its association with changes in glycemic traits are shown in Supplemental Figure 1. The genetic risk score showed no significant, positive association with changes in fasting glucose (multivariable adjusted β = 0.01 mmol/L), 2-hour glucose (multivariable adjusted β = 0.04 mmol/L), and HbA1c (multivariable adjusted β = 0.02%). We next examined the association of the genetic predisposition to PCOS with changes in glycemic traits according to annual postpartum weight reduction (Table 2). We found that postpartum weight reduction significantly interacted with the genetic risk score on changes in fasting glucose and 2-hour glucose (multivariable adjusted P for interaction = .032 and .007, respectively). With increments of the genetic risk score, fasting glucose and 2-hour glucose decreased in women with postpartum weight reduction of 5 to <8 kg/y and ≥8 kg/y, but increased in women with postpartum weight reduction of <5 kg/y. No significant genetic effect on change in HbA1c was observed in any of the 3 categories of postpartum weight reduction (all P > .05).
Table 2.
Changes in Glycemic Traits With Genetic Risk to PCOS by Annual Postpartum Weight Reduction
| Changes in Glycemic Traits | <5 kg/Y |
5–<8 kg/Y |
≥8 kg/Y |
P for Interaction | |||
|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | ||
| Increment of each genetic risk allele | |||||||
| Fasting glucose, mmol/L | |||||||
| Unadjusted | 0.050 (0.029) | .083 | −0.054 (0.023) | .019 | −0.041 (0.021) | .051 | .008 |
| Multivariable-adjusteda | 0.045 (0.027) | .094 | −0.038 (0.019) | .047 | −0.011 (0.014) | .426 | .032 |
| 2-h glucose, mmol/L | |||||||
| Unadjusted | 0.117 (0.063) | .063 | −0.040 (0.056) | .480 | −0.097 (0.046) | .036 | .006 |
| Multivariable-adjusteda | 0.130 (0.060) | .032 | −0.023 (0.054) | .675 | −0.077 (0.039) | .049 | .007 |
| HbA1c, % | |||||||
| Unadjusted | 0.006 (0.027) | .813 | −0.030 (0.024) | .215 | −0.003 (0.019) | .875 | .837 |
| Multivariable-adjusteda | 0.005 (0.025) | .853 | −0.010 (0.021) | .634 | 0.011 (0.014) | .424 | .816 |
| Each C allele of the DENND1A gene variant (rs10986105) | |||||||
| Fasting glucose, mmol/L | |||||||
| Unadjusted | 0.226 (0.163) | .166 | 0.001 (0.127) | .997 | −0.202 (0.112) | .072 | .026 |
| Multivariable-adjusteda | 0.281 (0.152) | .064 | 0.109 (0.105) | .300 | −0.085 (0.076) | .265 | .025 |
| 2-h glucose, mmol/L | |||||||
| Unadjusted | 0.576 (0.358) | .109 | 0.180 (0.311) | .564 | −0.721 (0.247) | .004 | .003 |
| Multivariable-adjusteda | 0.738 (0.341) | .031 | 0.349 (0.298) | .243 | −0.555 (0.209) | .008 | .003 |
| HbA1c, % | |||||||
| Unadjusted | 0.140 (0.160) | .383 | −0.053 (0.133) | .692 | 0.145 (0.102) | .155 | .863 |
| Multivariable-adjusteda | 0.182 (0.143) | .204 | 0.060 (0.114) | .596 | 0.112 (0.072) | .124 | .656 |
β represents the change in each glycemic trait per increment of 1 genetic risk allele or each C allele (risk allele) of the rs10986105 genotype.
Adjustment for age, follow-up time, prepregnant BMI, dietary fat (% energy), sitting time, and the previous value for the respective glucose trait (continuous variables) and family history of diabetes, current smoking, current alcohol drinking, leisure time physical activity, and GDM therapy (categorical variables).
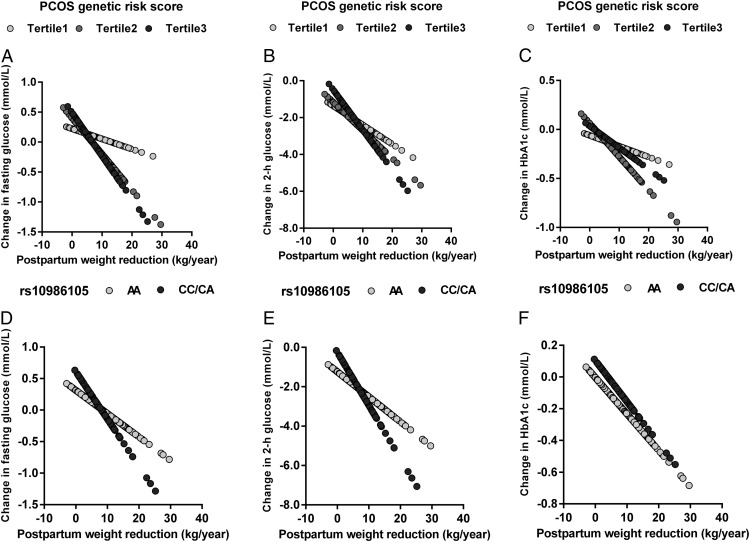
Each 1-kg/y postpartum weight reduction was associated with a decrease of −0.04 mmol/L in fasting glucose, −0.15 mmol/L in 2-hour glucose, and −0.02% in HbA1c (multivariable adjusted P < .001, < .001, and = .013, respectively). We further examined the linear relationship between postpartum weight reduction and predicated changes in fasting glucose and 2-hour glucose according to tertiles of the PCOS genetic risk score. In tertile 1 (the lowest), tertile 2, and tertile 3 (the highest) of the genetic risk score, each per 1-kg/y postpartum weight reduction was associated with −0.02 (P = .144), −0.06 (P < .001), and −0.07 (P < .001) mmol/L reductions in fasting glucose, respectively, and with −0.10 (P < .001), −0.15 (P < .001), and −0.22 (P < .001) mmol/L reductions in 2-hour glucose, respectively (Figure 1).
Figure 1.
Predicted changes in fasting glucose, 2-hour glucose, and HbA1c according to annual postpartum weight reduction by PCOS genetic risk score and DENND1A gene variant (rs10986105) genotype. The slope represents the β coefficient. A, Change in fasting glucose according to annual postpartum weight reduction by PCOS genetic risk score. B, Change in 2-hour glucose according to annual postpartum weight reduction by PCOS genetic risk score. C, Change in HbA1c according to annual postpartum weight reduction by PCOS genetic risk score. D, Change in fasting glucose according to annual postpartum weight reduction by rs10986105 genotype. E, Change in 2-hour glucose according to annual postpartum weight reduction by rs10986105 genotype. F, Change in HbA1c according to annual postpartum weight reduction by rs10986105 genotype.
Each 1-kg gestational weight gain was associated with a decrease of −0.02 mmol/L in fasting glucose, −0.05 mmol/L in 2-hour glucose, and −0.01% in HbA1c (multivariable adjusted P < .001, < .001 and = .009, respectively). The genetic risk score was associated with increased fasting glucose in women with inadequate gestational weight gain but with decreased fasting glucose in women with excessive gestational weight gain after multivariable adjustment (P for interaction = .035). There was no significant interaction between gestational weight gain and the genetic risk score in relation to changes in 2-hour glucose and HbA1c (Table 3). Sensitivity analyses applying the Chinese maternal prepregnancy BMI classification defined gestational weight gain showed similar results (Supplemental Table 2).
Table 3.
Changes in Glycemic Traits With Genetic Risk to PCOS by Gestational Weight Gain
| Changes in Glycemic Traits | Inadequate |
Adequate |
Excessive |
P for Interaction | |||
|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | ||
| Fasting glucose, mmol/L | |||||||
| Unadjusted | 0.048 (0.046) | .303 | −0.015 (0.022) | .510 | −0.033 (0.020) | .092 | .057 |
| Multivariable-adjusteda | 0.056 (0.047) | .232 | 0.005 (0.017) | .788 | −0.022 (0.016) | .180 | .035 |
| 2-h glucose, mmol/L | |||||||
| Unadjusted | 0.083 (0.106) | .435 | 0.061 (0.061) | .318 | −0.072 (0.042) | .090 | .040 |
| Multivariable-adjusteda | 0.051 (0.106) | .630 | 0.056 (0.052) | .283 | −0.050 (0.039) | .202 | .114 |
| HbA1c, % | |||||||
| Unadjusted | −0.006 (0.037) | .877 | 0.013 (0.022) | .582 | −0.026 (0.019) | .178 | .371 |
| Multivariable-adjusteda | 0.001 (0.035) | .968 | 0.028 (0.017) | .093 | −0.011 (0.016) | .491 | .468 |
β represents the change in each glycemic trait per increment of 1 genetic risk allele of the genetic risk score.
Adjustment for age, follow-up time, prepregnant BMI, fat (% energy), sitting time, and the previous value for the respective glucose trait (continuous variables for above variables) and family history of diabetes, current smoking, current alcohol drinking, leisure time physical activity, and GDM therapy (categorical variables for above variables).
We then analyzed the 7 SNPs individually. SNP rs10986105 within the DENND1A gene showed significant interactions with annual postpartum weight reduction on changes in fasting glucose and 2-hour glucose (multivariable adjusted P for interaction = .025 and .003, respectively) (Table 2). With an increment of each C allele (risk allele), fasting glucose and 2-hour glucose decreased in women with postpartum weight reduction of ≥8 kg/y but increased in women with postpartum weight reduction of <5 kg/y. No significant genetic effect on change in HbA1c was observed in any of the 3 categories of postpartum weight reduction (all P > .05). In addition, the associations of predicted decreases in fasting glucose and 2-hour glucose with annual weight reduction (per 1 kg/y) were stronger in rs10986105 genotype CC/CA (β = −0.08 and −0.27 mmol/L for fasting glucose and 2-hour glucose, respectively) than that in AA (β = −0.04 and −0.13 mmol/L for fasting glucose and 2-hour glucose, respectively) (Figure 1). Other SNPs were not shown to have significant interactions with postpartum weight reduction on the glycemic traits.
Sensitivity analyses in women within the first 1.5 years after delivery (n = 193) showed that the associations were in the same direction but nonsignificant because of the small sample size compared with those in overall participants. Each 1 kg/y postpartum weight reduction was associated with a decrease of −0.03 mmol/L in fasting glucose, −0.10 mmol/L in 2-hour glucose, and −0.01% in HbA1c (multivariable adjusted P = .013, < .001, and = .31, respectively). Each 1-kg gestational weight gain was associated with a decrease of −0.01 mmol/L in fasting glucose, −0.05 mmol/L in 2-hour glucose, and −0.01% in HbA1c (multivariable adjusted P = .15, .009, and .21, respectively). The interactions between the genetic risk score and postpartum weight reduction/gestational weight gain were not significant in this small sample size (Supplemental Tables 3 and 4).
Discussion
In this study of a cohort of Chinese women with prior GDM, we examined the associations of the genetic predisposition to PCOS, postpartum weight reduction, and gestational weight gain with postpartum changes in glycemic traits. We found significant bidirectional interactions between postpartum weight reduction and the genetic risk score on postpartum changes in fasting glucose and 2-hour glucose.
In the present study, we found that the PCOS genetic effect on the glycemic measures significantly varied among women with different degrees of postpartum weight reduction. The PCOS genetic risk score was related to increased fasting and 2-hour glucose levels among women with less postpartum weight reduction but was associated with decreased levels of these markers among women with greater postpartum weight reduction. Intriguingly, we found that the gene-postpartum weight reduction interactions were bidirectional: the relation between postpartum weight reduction and glycemic traits also significantly differed according to the levels of the genetic risk score. Women with a higher genetic risk score showed a more pronounced relationship between postpartum weight reduction and glycemic improvement than women with a lower genetic risk score. As a common reproductive condition, PCOS is characterized by heterogeneous symptoms of chronic oligoovulation or anovulation, androgen excess, ovulatory dysfunction, and polycystic ovaries (22). Patients with PCOS have profound insulin resistance, defects in insulin secretion, and elevated risk of glucose intolerance, GDM, and diabetes (23–25). Previous studies have shown that postpartum weight reduction was associated with improvements in glycemic traits, especially fasting and 2-hour glucose (3, 26). Our data indicate that women with a higher genetic predisposition to PCOS might be more susceptible to such beneficial effects of postpartum weight reduction.
Our data suggest that the genetic interactions with postpartum weight reduction on the glycemic measures might be mainly driven by the variant in the DENND1A gene. Notably, the DENND1A variant (rs10986105) has been identified as the strongest association in women of European ancestry (27) and the second strongest risk variant associated with PCOS in Han Chinese women (9). The DENND1A gene encodes DENN/MADD domain-containing 1A (DENND1A), which is highly expressed in ovarian theca cells and the adrenal zona reticularis, both androgen-producing tissues (28). Interestingly, quantitative trait analyses have linked the DENND1A locus with glucose and insulin levels, as well as body weight in humans. Such observations provide a potential biologic basis for the interactions observed in the present study (29). However, considering the multiple comparison issue, we acknowledged that the significant interaction of the DENND1A gene was probably observed by chance, and the results should be interpreted with caution.
Compared with postpartum weight reduction, gestational weight gain showed a less significant interaction with the genetic predisposition to PCOS on postpartum changes in glycemic traits. Gestational weight gain had a marginally significant interaction with the genetic risk score on change in postpartum fasting glucose after multivariable adjustment but had no significant interaction with the genetic risk score on postpartum changes in 2-hour glucose and HbA1c. The less significant interaction was probably due to an indirect relationship between gestational weight gain and postpartum glucose regulation. Gestational weight gain is suggested to be a risk factor for postpartum glucose intolerance or type 2 diabetes (30, 31); however, the adverse effects of gestational weight gain are probably mediated through the pathway of weight before pregnancy, postpartum weight retention, and later weight gain after delivery (31).
To the best of our knowledge, this is the first study to examine the interaction between genetic predisposition to PCOS and postpartum weight reduction on postpartum changes in glycemic traits in women with prior GDM. Our data provide a novel perspective to understand the association between postpartum body weight management and glycemic improvement on a genetic background. A major strength of this study is that the findings are based on longitudinal measures of weight and glycemic traits from thus far one of the largest cohorts of women with a history of GDM. However, several limitations need to be acknowledged. First, we did not collect sufficient reproductive data such as postpartum depression, which may be associated with postpartum weight reduction and glucose metabolism. And although urban residents were subject to a strict one-child restriction according to the family planning policy, we cannot exclude the possibility that very rare women might have subsequent pregnancies, because of a lack of information on subsequent pregnancies after the index GDM pregnancy. Second, our study participants were restricted to Chinese women, and further studies in prospective cohorts are warranted to verify our findings in other demographic or ethnic populations. And third, because of the limited power to detect the relatively modest effects conferred by each SNP, most of the individual SNPs showed consistent but nonsignificant interactions with postpartum weight reduction in relation to changes in glycemic traits. Thus, a genetic risk score that aggregates the effects of multiple genetic SNPs has been demonstrated as a useful method for assessing gene-environment interactions (32).
In conclusion, we found that the genetic predisposition to PCOS might affect a postpartum change in glycemia, and a postpartum weight reduction significantly interacted with the PCOS genetic factors on postpartum changes in glycemic traits in Chinese women with prior GDM. A higher genetic predisposition to PCOS was associated with increased glycemic traits in women who lost less weight but associated with decreased glycemic traits in women who lost greater weight. On the other hand, women with a higher genetic predisposition showed a more pronounced association between postpartum weight reduction and glycemic improvement than women with a lower genetic predisposition. Our data highlight the importance of postpartum weight management in postpartum glycemic improvement in women genetically predisposed to PCOS.
Acknowledgments
We are grateful to all participants in the study for their dedication and contribution to the research.
The study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Program for Collaborative Research between China and Europe, the National Natural Science Foundation of China (Grant 81502821), and the Tianjin Public Health Bureau. L.Q. is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, and DK078616), the Boston Obesity Nutrition Research Center (DK46200), and the United States–Israel Binational Science Foundation (Grant 2011036). G.H. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases under Award R01DK100790.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- GDM
- gestational diabetes mellitus
- HbA1c
- hemoglobin A1c
- OGTT
- oral glucose tolerance test
- PCOS
- polycystic ovary syndrome
- SNP
- single nucleotide polymorphism.
References
- 1. Ferrara A, Hedderson MM, Albright CL, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care. 2011;34:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kew S, Ye C, Hanley AJ, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37:1998–2006. [DOI] [PubMed] [Google Scholar]
- 3. Liu H, Zhang C, Zhang S, et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obesity (Silver Spring). 2014;22:1560–1567. [DOI] [PubMed] [Google Scholar]
- 4. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. [DOI] [PubMed] [Google Scholar]
- 5. Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod. 2003;18:1438–1441. [DOI] [PubMed] [Google Scholar]
- 6. Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep. 2006;6:77–83. [DOI] [PubMed] [Google Scholar]
- 7. Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16:1995–1998. [DOI] [PubMed] [Google Scholar]
- 8. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–363. [DOI] [PubMed] [Google Scholar]
- 9. Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. [DOI] [PubMed] [Google Scholar]
- 10. Shi Y, Zhao H, Shi Y, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020–1025. [DOI] [PubMed] [Google Scholar]
- 11. Hwang JY, Lee EJ, Jin Go M, et al. Genome-wide association study identifies GYS2 as a novel genetic factor for polycystic ovary syndrome through obesity-related condition. J Hum Genet. 2012;57:660–664. [DOI] [PubMed] [Google Scholar]
- 12. Lee H, Oh JY, Sung YA, et al. Genome-wide association study identified new susceptibility loci for polycystic ovary syndrome. Hum Reprod. 2015;30:723–731. [DOI] [PubMed] [Google Scholar]
- 13. Hu G, Tian H, Zhang F, et al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. 2012;98:508–517. [DOI] [PubMed] [Google Scholar]
- 14. Li W, Zhang S, Liu H, et al. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care. 2014;37:2533–2539. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Liu H, Zhang S, et al. Obesity index and the risk of diabetes among Chinese women with prior gestational diabetes. Diabet Med. 2014;31:1368–1377. [DOI] [PubMed] [Google Scholar]
- 16. Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. 2011;28:652–657. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 18. Li YP, He YN, Zhai FY, et al. Comparison of assessment of food intakes by using 3 dietary survey methods [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:273–280. [PubMed] [Google Scholar]
- 19. Ma G, Luan D, Li Y, et al. Physical activity level and its association with metabolic syndrome among an employed population in China. Obes Rev. 2008;9:113–118. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 21. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 22. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 23. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. [DOI] [PubMed] [Google Scholar]
- 24. Lo JC, Feigenbaum SL, Escobar GJ, Yang J, Crites YM, Ferrara A. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care. 2006;29:1915–1917. [DOI] [PubMed] [Google Scholar]
- 25. Reyes-Muñoz E, Castellanos-Barroso G, Ramírez-Eugenio BY, et al. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil Steril. 2012;97:1467–1471. [DOI] [PubMed] [Google Scholar]
- 26. Ehrlich SF, Hedderson MM, Quesenberry CP, Jr, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med. 2014;31:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welt CK, Styrkarsdottir U, Ehrmann DA, et al. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab. 2012;97:E1342–E1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAllister JM, Legro RS, Modi BP, Strauss JF., 3rd Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medical College of Wisconsin, Bioinformatics Program, HMGC. QTL report providing DENND1A phenotype and disease descriptions, mapping, as well as links to markers and candidate genes. 2014. Available from http://rgd.mcw.edu/rgdweb/search/qtls.html?term=%20DENND1A%20[gene]&speciesType=1.
- 30. Al Mamun A, Mannan M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PLoS One. 2013;8:e75679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mannan M, Doi SA, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71:343–352. [DOI] [PubMed] [Google Scholar]
- 32. Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]