Abstract
Background
B-type natriuretic peptide (BNP) is produced as a biologically inactive prohormone (proBNP1-108), processed, and released as an inactive amino-terminal fragment (NT-proBNP1-76) and a biologically active carboxyl-terminal fragment (proBNP77-108 or BNP32). We hypothesized that simultaneous assessment of proBNP1-108 and active BNP32, as an index of natriuretic peptide processing efficiency, would improve risk stratification in patients with chronic systolic heart failure.
Methods and Results
We quantified plasma proBNP1-108 and BNP32 in 756 subjects in the Penn Heart Failure Study, a prospective cohort of outpatients with predominantly systolic heart failure. Cox models were used to determine the association between biomarker level at time of study entry and incident risk of adverse cardiovascular outcomes. A significant amount of unprocessed proBNP1-108 circulates in patients with systolic heart failure (median 271 pg/ml, interquartile range 65 to 825). Higher levels of proBNP1-108 were associated with an increased risk of all-cause death or cardiac transplantation (adjusted HR 4.9, 95% CI 2.5–9.7, p<0.001 comparing 3rd versus 1st proBNP1-108 tertile). ProBNP1-108 provided additive information to BNP32 risk assessment, particularly in patients with BNP32 less than the median of 125 pg/ml (adjusted HR 1.4, 95% CI 1.2–1.8, p<0.001 per doubling of proBNP1-108).
Conclusions
Circulating proBNP1-108 is independently associated with an increased risk of adverse cardiovascular outcomes in ambulatory patients with chronic systolic heart failure. The combined assessment of BNP32 and proBNP1-108 provides additional information in determining risk of adverse clinical outcomes, particularly in patients with low BNP32 values that might otherwise be reassuring to the clinician.
Keywords: cardiomyopathy, congestive heart failure, natriuretic peptides
Introduction
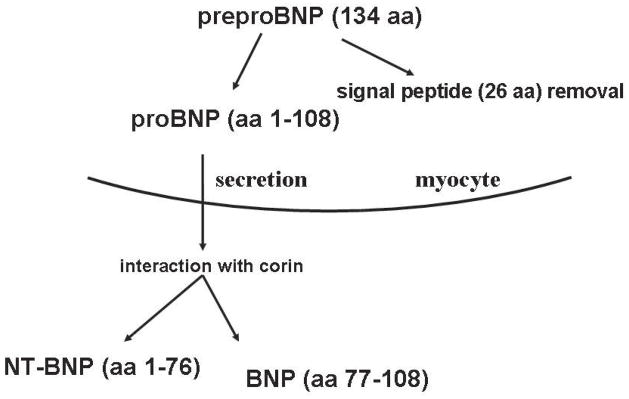
The human gene for brain natriuretic peptide (BNP) encodes a 134–amino acid preproBNP precursor. After removal of a 26-amino acid signal peptide, a 108-amino acid proBNP polypeptide (proBNP1–108) is formed. With further processing of proBNP1-108 by the type 2 transmembrane serine protease corin, two peptides are generated: 1) the physiologically active 32-amino acid carboxyl-terminal BNP molecule (BNP32, also commonly referred to as BNP1-32 or BNP77-108) 1 and 2) an inactive 76–amino acid N-terminal fragment (NT-proBNP1–76) (Figure 1) 2. Although the precise molecular mechanisms of BNP processing remain incompletely understood, the prevailing hypothesis suggests that proBNP undergoes exocytosis from cardiomyocytes, allowing it to interact with the extracellular catalytic domain of corin resulting in BNP32 and NT-proBNP1-76 (Figure 1) 3, 4.
Figure 1.
Schemata of BNP Processing
The natriuretic peptide system exerts compensatory actions in heart failure, including attenuation of adverse neurohormonal activation, balanced venous and arterial vasodilation, reduced endothelin release, enhanced cardiac lusitropy, and intravascular volume regulation by virtue of the ability of BNP to promote natriuresis 5-7. However, it has been recently demonstrated in two independent in vitro systems that BNP must be processed effectively in order to gain biological activity. As compared to processed BNP32, proBNP1-108 has absent 8 or 8-fold lower biological activity 9. Consequently, impaired proBNP processing would be expected to reduce the compensatory actions of the endogenous natriuretic peptide system, and might contribute to heart failure progression and risk for decompensation leading to heart failure hospitalization, death, or cardiac transplantation.
The overall objective of this study was thus to define the relationship between circulating levels of unprocessed proBNP1-108 and risk of adverse cardiovascular outcomes, including all-cause death, cardiac transplantation, and heart failure hospitalization in a chronic heart failure population. In order to measure proBNP1-108, we utilized a highly specific, novel assay (Bio-Rad Laboratories, Marnes-La-Coquette, France), which demonstrates no cross-reactivity to either BNP32 (aa77-108) or NT-proBNP1-76 (aa1-76) 10. In addition, we also sought to determine the prognostic importance of simultaneous assessment of both unprocessed proBNP1-108 and active, circulating BNP32. We hypothesized that discordance in proBNP1-108 and BNP32 levels represents an abnormality in natriuretic peptide processing and is associated with an increased risk of adverse cardiovascular outcomes.
Methods
Study Population
The Penn Heart Failure Study (PHFS) is an ongoing prospective cohort study of outpatients with chronic heart failure recruited from the Penn Heart Failure and Transplantation program 11, 12. The primary inclusion criterion is a clinical diagnosis of heart failure. Participants are excluded if they have a non-cardiac condition resulting in an expected mortality of less than 6 months, as judged by the treating physician.
At time of study entry, detailed clinical data were obtained using questionnaires administered to the patient and treating physician, with verification via medical records. Variables such as New York Heart Association (NYHA) Class and cardiomyopathy etiology (ischemic versus non-ischemic) were determined by the physician based upon all available clinical data and according to standard heart failure clinical practice guidelines 13. Venous blood samples were obtained at enrollment, processed, and stored at −80°C. Two-dimensional conventional transthoracic echocardiography was performed in all patients at an ICAEL-accredited laboratory typically within 30 days of blood sampling. Ejection fraction (EF) was estimated by a Level III-certified echocardiographer, according to standard clinical protocol.
Follow-up events including all-cause mortality, cardiac transplantation, and hospitalization were prospectively ascertained every 6 months via direct patient contact and verified through death certificates, medical records, and contact with patients’ family members. For hospitalizations, reason for hospitalization was adjudicated by study personnel based upon medical record review and classified as a hospitalization primarily for heart failure, other cardiac conditions, or non-cardiac conditions. The analyses presented in this manuscript were limited to hospitalizations for heart failure only.
All participants provided written, informed consent, and the PHFS protocol was approved by our Institutional Review Board.
ProBNP1-108 and BNP32 Assay
ProBNP1-108 was measured using a highly specific, novel Bio-Rad assay (Marnes-La-Coquette, France) 10. This assay is based on the monoclonal antibody (mAb Hinge76) that recognizes with high affinity the cleavage site of proBNP1–108 (Arg76-Ser77), an epitope present only in the precursor form (Figure 1). By combining the mAb Hinge76 with a polyclonal antibody directed against BNP32, the Bio-Rad intact proBNP1–108 sandwich immunoassay is specific for the presence of proBNP1–108 in plasma samples. There is no significant cross-reactivity with either recombinant NT-proBNP1–76 or synthetic BNP32. The lower limit of detection of proBNP1-108 was 5 pg/ml and upper limit was 12,000 pg/ml. The intra- and inter-assay coefficients of variations (CVs) for the assay were 3.0 to 5.0% and 3.5 to 6.1%, respectively.
BNP32 was measured using the Architect™ BNP immunoassay from Abbott Diagnostics (Abbott Park, IL) 11. Of note, current commercial assays for BNP32 demonstrate some cross-reactivity with proBNP1-108. A recent consensus panel estimated 6 to 38% cross-reactivity of the Architect™ BNP32 assay with proBNP1-108 14. Hence, we expect some degree of correlation between these two measurements based on assay characteristics alone. The intra- and inter-assay CVs for the Architect™ BNP32 assay were 0.9 to 5.6% and 1.7 to 6.7%, respectively. The lower limit of detection of BNP32 was 10 pg/ml and upper limit was 4417 pg/ml. Samples below the detectable limit were assigned a value half-way between zero and the lowest detectable limit of each marker 15.
Statistical Analysis
The distribution of both proBNP1-108 and BNP32 were skewed, and the log-transformation of each marker approximated a normal distribution. We examined the data graphically and fit a simple linear regression model in which we were able to quantify the residuals to determine the relationship between the two markers.
Differences in the distribution of clinical variables across tertiles of proBNP1-108 and BNP32 were studied using analysis of variance (ANOVA) for continuous and Pearson Chi-square test for categorical variables. To determine the association between baseline BNP32 or proBNP1-108 level and risk of adverse clinical outcomes, Kaplan-Meier analysis, log-rank tests, and Cox models were used. Two series of univariate and multivariable models were constructed, with proBNP1-108 or BNP32 as the predictor variable and time to the combined endpoint of all-cause death or cardiac transplantation or the composite endpoint of all-cause death, cardiac transplantation, or heart failure hospitalization as the outcome variable. We determined univariate hazard ratios (HR) for each biomarker across categories of biomarker according to tertiles, as well as the continuous form of the variable according to a log base 2 transformation, where the HR represents the effect for a doubling of the original variable. Multiple forms of categorical data including the use of medians, tertiles, quartiles, and quintiles were examined. For multivariable models, confounders were selected using clinical judgment, cross-sectional associations with proBNP1-108, and statistical evidence of potential confounding. Statistical evidence included a univariate association with death/transplant at a p-value<0.20 16.
Given the marked colinearity between proBNP1-108 and BNP32, and consequent limitations in determining the independent effects of either marker by simple confounder adjustment, we used alternative measures to understand the value of proBNP1-108 assessment in the setting of a known BNP32 level. First, in stratified analyses, we determined the association between proBNP1-108 and risk of adverse outcomes according to median BNP32 level. We also formally tested for interaction between the log base 2 transformed proBNP1-108 level and median BNP32 level. Second, we divided patients into subgroups according to their median proBNP1-108 and BNP32 levels to explore the joint effects of proBNP1-108 and BNP32 in determining risk of adverse outcomes. Cox models were used to compare risk, with the reference group being patients with both proBNP1-108 and BNP32 levels less than median. Third, we determined the association between the residuals, generated from the linear regression between proBNP1-108 and BNP32, and risk of adverse outcomes in Cox models.
All analyses were performed using STATA 10.0 (Statacorp, TX). All model assumptions, including proportional hazards and linearity on a log scale were verified. All authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
Study Population
Between December 2003 and October 2007, 756 subjects were enrolled with assessments of both unprocessed proBNP1-108 and processed BNP32. The mean±sd age was 57±14 years, 69% were male, and 82% were Caucasian. The majority of patients (87.6%) suffered from systolic heart failure (87.6%), with a mean EF of 32±16%. The full spectrum of NYHA Class was represented, with the majority (73%) being NYHA Class II or III. One-third of patients had ischemic cardiomyopathy and the remaining two-thirds had a non-ischemic etiology.
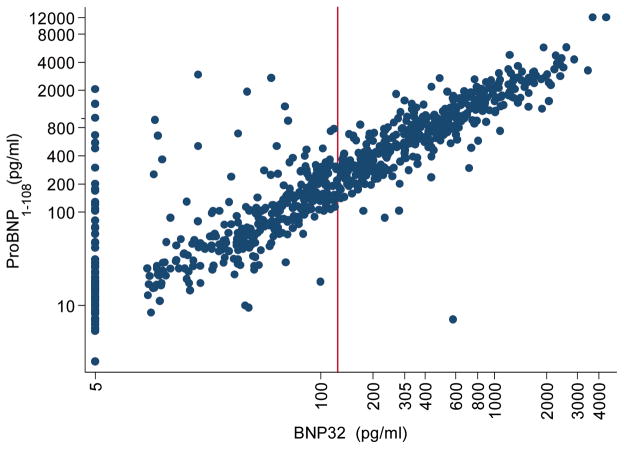
The mean±sd proBNP1-108 was 639±1020 pg/ml and the median interquartile range (IQR) was 271 (65 to 825) pg/ml, with 1% having a proBNP1-108 value less than the detectable limit. The mean±sd BNP32 was 327±509 pg/ml and the median (IQR) was 125 (35 to 395) pg/ml with approximately 12% having a value less than the detectable limit, similar to findings in other ambulatory patients of chronic heart failure 17. As shown in Figure 2 and in our linear regression analyses, BNP32 and proBNP1-108 were strongly correlated (R=0.87, p<0.001). At the same time, there was evidence of discordance between the two markers in a subgroup of patients with BNP32 values less than the median. In particular, at undetectable values of BNP32, there was substantial variability in proBNP1-108. In these patients with a less than detectable BNP32, the mean proBNP1-108 was 124±331 pg/ml.
Figure 2.
Correlation between ProBNP1-108 and BNP32 Spearman ρ=0.89, p<0.001, n=756; Red line denotes median BNP32 125 pg/ml
Patients in the highest ProBNP1-108 tertile were more likely to be older, with a history of hypertension, and worse renal function (Table 1). In addition, these subjects were most likely to have an ischemic cause of their heart failure, worse NYHA class, and lower mean EF.
Table 1.
Clinical Characteristics of 756 Subjects in the Penn Heart Failure Study According to ProBNP1-108 Tertile
| Entire Cohort (n=756) | Tertile 1 (5–107 pg/ml) (n=252) | Tertile 2 (108–572 pg/ml) (n=252) | Tertile 3 (>572 pg/ml) (n=252) | p value* | |
|---|---|---|---|---|---|
| Mean age, yrs (s.d.) | 57 (14) | 51 (13) | 58 (14) | 60 (14) | <0.001 |
| Male, n (%) | 522 (69) | 161 (64) | 176 (70) | 185 (73) | 0.07 |
| Race | |||||
| Caucasian, n (%) | 617 (82) | 206 (82) | 204 (82) | 207 (83) | 0.97 |
| African American | 105 (14) | 35 (14) | 34 (14) | 36 (14) | |
| Other | 28 (4) | 9 (4) | 11 (4) | 8 (3) | |
| Tobacco Use | |||||
| Never, n (%) | 279 (37) | 108 (43) | 75 (30) | 96 (38) | 0.008 |
| Former | 428 (57) | 124 (49) | 158 (63) | 146 (58) | |
| Current | 49 (6) | 20 (8) | 19 (7) | 10 (4) | |
| Hypertension History, n (%) | 360 (48) | 103 (41) | 121 (48) | 136 (54) | 0.01 |
| Diabetes History, n (%) | 210 (28) | 48 (19) | 86 (34) | 76 (30) | <0.001 |
| Mean creatinine, mg/dL (s.d.) | 1.3 (0.7) | 1.1 (0.3) | 1.2 (0.5) | 1.5 (1.0) | <0.001 |
| Mean body mass Index, kg/m2 (s.d.) | 29 (6) | 30 (7) | 30 (7) | 28 (7) | 0.34 |
| Mean SBP, mmHg (s.d.) | 113 (19) | 116 (16) | 115 (17) | 109 (21) | <0.001 |
| Cardiomyopathy Etiology Ischemic, n (%) | 250 (33) | 31 (12) | 102 (40) | 117 (46) | <0.001 |
| Heart Failure Type Diastolic, n (%) | 94 (12) | 47 (50) | 25 (27) | 22 (23) | 0.01 |
| NYHA | |||||
| I, n (%) | 115 (15) | 77 (31) | 31 (12) | 7 (3) | <0.001 |
| II | 330 (44) | 129 (51) | 115 (46) | 86 (34) | |
| III | 222 (29) | 41 (16) | 82 (33) | 99 (39) | |
| IV | 89 (12) | 5 (2) | 24 (9) | 60 (24) | |
| EF, mean (s.d.) | 32 (16) | 41 (14) | 30 (15) | 24 (16) | <0.001 |
| Cardiac Resynchronization, n (%) | 208 (28) | 38 (15) | 74 (29) | 96 (38) | <0.001 |
| Ace-inhibitor or ARB, n (%) | 648 (86) | 227 (90) | 225 (89) | 196 (78) | 0.001 |
| Beta blocker, n (%) | 626 (83) | 210 (83) | 214 (85) | 202 (80) | 0.35 |
| Mean BNP32, pg/ml (s.d.) | 327 (509) | 33 (47) | 154 (106) | 794 (657) | <0.001 |
ANOVA for continuous variables; Chi-square test for categorical
Association between Circulating ProBNP1-108 and BNP32 Levels and Risk of Adverse Cardiovascular Outcomes
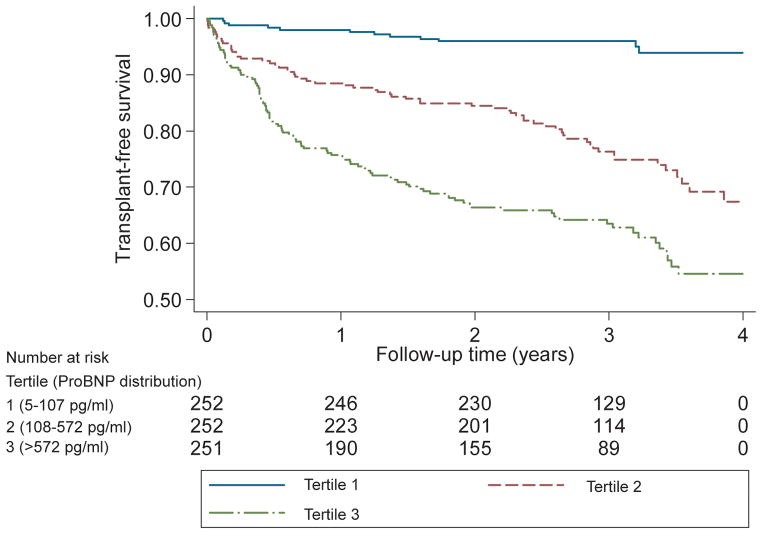
Patients were followed for a median of 2.5 years (IQR 1.1–3.3). During this period, there were 92 deaths, 81 transplants, and 179 first heart failure hospitalizations. For each two-fold increase in proBNP1-108, there was a significantly increased risk of death or cardiac transplantation (unadjusted HR 1.5 (95% CI 1.3–1.6, p<0.001), that remained independent after multivariable adjustment (Table 2). Similarly, compared to patients in the lowest tertile of proBNP1-108 (<108 pg/ml), patients in the highest tertile of proBNP1-108 (>572 pg/ml) had a markedly increased risk of all-cause mortality or cardiac transplantation (Figure 3), with an unadjusted hazard ratio of ratio (HR) of 10.1 (95% CI 5.5–18.4, p<0.001; Table 2). After adjusting for covariates such as demographics, creatinine, ejection fraction, heart failure etiology, and disease severity, the risk of death or transplantation was still significant (HR 4.9, 2.5–9.7, p<0.001; Table 2). Patients with a circulating proBNP1-108 less than 100 pg/ml represented a low risk subset with a small number of adverse events (Table 3).
Table 2.
Unadjusted and Multivariable Adjusted Risk of All-Cause Death or Cardiac Transplantation According to Tertiles of ProBNP1-108 or BNP32
| ProBNP1-108 | BNP32 | |||||
|---|---|---|---|---|---|---|
| HR, Tertile 2 vs 1 (95% CI)§ | HR, Tertile 3 vs 1 (95% CI)§ | HR per 2-fold increase in proBNP1-108 (95% CI) | HR, Tertile 2 vs 1 (95% CI)§ | HR, Tertile 3 vs 1 (95% CI)§ | HR per 2-fold increase in BNP1-32 (95% CI) | |
| Unadjusted | 5.6 (3.0–10.4) | 10.1 (5.5–18.4) | 1.5 (1.3–1.6) | 3.2 (1.9–5.6) | 7.6 (4.6–12.7) | 1.5 (1.4–1.6) |
| Model 1* | 5.7 (2.9–10.8) | 9.5 (5.0–18.0) | 1.4 (1.3–1.6) | 3.2 (1.8–5.7) | 6.9 (4.0–11.8) | 1.4 (1.3–1.6) |
| Model 2† | 5.0 (2.6–9.7) | 7.8 (4.0–15.4) | 1.4 (1.3–1.5) | 2.9 (1.6–5.2) | 5.5 (3.1–9.8) | 1.4 (1.3–1.5) |
| Model 3‡ | 3.9 (2.0–7.7) | 4.9 (2.5–9.7) | 1.3 (1.2–1.4) | 2.5 (1.4–4.4) | 3.6 (2.0–6.4) | 1.3 (1.2–1.4) |
Model 1 adjusted for age, sex, race, tobacco, creatinine, body mass index
Model 2 adjusted for Model 1 + EF + cardiomyopathy etiology (ischemic versus non-ischemic)
Model 3 adjusted for Model 2 + NYHA Class (I/II versus III/IV)
all p<0.001, including test for trend
Figure 3.
Kaplan-Meier Survival Estimates for All-Cause Death or Cardiac Transplantation According to ProBNP1-108 Tertiles p<0.001 by log rank test
Table 3.
Number of Adverse Cardiovascular Outcomes According to Tertile of ProBNP1-108 or BNP32
| Total* | ProBNP1-108 Tertile | BNP32 Tertile | |||||
|---|---|---|---|---|---|---|---|
| 1 5–107 pg/ml (n=252) | 2 108–572 pg/ml (n=252) | 3 >572 pg/ml (n=251) | 1 4–54 pg/ml (n=252) | 2 55–264 pg/ml (n=252) | 3 >264 pg/ml (n=251) | ||
| All-Cause Death or Cardiac Transplantation | 177 (23.4) | 13 (5.1) | 63 (25.0) | 101 (40.1) | 18 (7.1) | 53 (21.0) | 106 (42.1) |
| All-Cause Death, Cardiac Transplantation, or Heart Failure Hospitalization | 355 (46.9) | 54 (21.4) | 137 (54.4) | 164 (65.1) | 64 (25.4) | 123 (48.9) | 168 (66.7) |
Values expressed as Number (% of tertile)
As expected for highly correlated predictors, there was a similarly increased risk associated with elevated BNP32 levels. For each doubling of BNP32, there was also a 50% increased risk of death or transplant that remained independent after confounder adjustment (Table 2). The risk associated with the highest BNP32 tertile was elevated in both unadjusted (HR 7.6, 4.6–12.7, p<0.001) and adjusted (HR 3.6, 2.0–6.4, p<0.001) models.
We also examined the association between biomarker level and risk of death, transplantation, or heart failure hospitalization. A striking relationship was also observed, although the absolute risk estimates were not as markedly elevated (Table 4). This may be in part secondary to a relatively greater number of events in the lowest tertile groups (Table 3).
Table 4.
Unadjusted and Multivariable Adjusted Risk of All-Cause Death, Cardiac Transplantation, or First Heart Failure Hospitalization According Tertiles of ProBNP1-108 or BNP32
| ProBNP1-108 | BNP32 | |||||
|---|---|---|---|---|---|---|
| HR, Tertile 2 versus 1 (95% CI)§ | HR, Tertile 3 versus 1 (95% CI)§ | HR per 2-fold increase in proBNP1-108 (95% CI) | HR, Tertile 2 versus 1 (95% CI)§ | HR, Tertile 3 versus 195% CI§ | HR per 2-fold increase in BNP1-32 (95% CI) | |
| Unadjusted | 3.1 (2.3–4.3) | 4.4 (2.3–4.3) | 1.3 (1.2–1.3) | 2.2 (1.6–3.0) | 3.8 (2.9–5.1) | 1.3 (1.2–1.3) |
| Model 1* | 3.1 (2.2–4.2) | 4.3 (3.1–6.0) | 1.3 (1.2–1.3) | 2.2 (1.6–3.0) | 3.7 (2.7–5.0) | 1.2 (1.2–1.3) |
| Model 2† | 2.7 (1.9–3.8) | 3.4 (2.3–4.8) | 1.2 (1.2–1.3) | 1.9 (1.4–2.7) | 2.8 (2.0–3.9) | 1.2 (1.1–1.3) |
| Model 3‡ | 2.4 (1.7–3.3) | 2.5 (1.7–3.6) | 1.2 (1.1–1.2) | 1.8 (1.3–2.5) | 2.1 (1.5–3.0) | 1.1 (1.1–1.2) |
Model 1 adjusted for age, sex, race, tobacco, creatinine, body mass index
Model 2 adjusted for Model 1 + EF + cardiomyopathy etiology (ischemic versus non-ischemic)
Model 3 adjusted for Model 2 + NYHA Class (I/II versus III/IV)
all p<0.001, including test for trend
Association between Circulating ProBNP1-108 and Risk of Adverse Cardiovascular Outcomes According to BNP32 Level
As noted above, approximately 12% of the cohort, or 88 patients, had a BNP32 level that was below detection. In this subgroup, there were 3 adverse events (death/transplantation), and among these three patients, proBNP1-108 values were substantially elevated at 548, 1440, and 2043 pg/ml and in the upper quartile of proBNP1-108. Furthermore, we determined that the relationship between proBNP1-108 and risk of death or transplantation differed significantly according to BNP32 level (interaction p=0.003). In patients with a BNP32 less than the median of 125 pg/ml, a 2-fold increase in proBNP1-108 was associated with an elevated risk of death or transplantation in both unadjusted and adjusted models (adjusted HR 1.5, 1.3–1.8, p=0.001) (Table 5). However, in patients with BNP32 levels greater than the median, this association was less pronounced and significant in unadjusted, but not after multivariable adjustment (adjusted HR 1.1, 0.9–1.3 p=0.35) (Table 5). We also examined the interaction between proBNP1-108 and BNP32 on a continuous scale, and found a highly significant interaction in unadjusted and adjusted models (p=0.001 for both). This model was also consistent in demonstrating a more pronounced effect of proBNP1-108 when BNP32 was low.
Table 5.
Association between ProBNP1-108 and Risk of All-Cause Death or Cardiac Transplantation According to Median BNP32 Level
| BNP32 Level | N | Unadjusted HR per 2-fold increase in proBNP1-108 (95% CI) | Interaction p-value | Adjusted HR per 2-fold increase in proBNP1-108 (95% CI)* | Interaction p-value |
|---|---|---|---|---|---|
| BNP32 < 125pg/ml | 378 | 1.7 (1.4–2.0) | 0.004 | 1.5 (1.3–1.8) | 0.003 |
| BNP32 > 125pg/ml | 377 | 1.2 (1.1–1.4) | 1.1 (0.9–1.2) |
Adjusted for all covariates in Table 2, Model 3
In examining the association between biomarker level and risk of death, transplantation, or heart failure hospitalization, we observed a similar relationship (Table 6). The risk of an event associated with proBNP1-108 was greater in those subjects with a BNP32 level less than the median (adjusted HR 1.3, 1.2–1.4, p<0.001), compared to those subjects with BNP32 levels greater than the median (adjusted HR 1.0, 0.9–1.1, p=0.52). Again, our interaction was highly significant using BNP32 on a categorical and continuous scale (adjusted p<0.001 for both).
Table 6.
Association between ProBNP1-108 and Risk of All-Cause Death, Cardiac Transplantation, or First Heart Failure Hospitalization According to Median BNP32 Level
| BNP32 Level | N | Unadjusted HR per 2-fold increase in proBNP1-108 (95% CI) | Interaction p-value | Adjusted HR per 2-fold increase in proBNP1-108 (95% CI)* | Interaction p-value |
|---|---|---|---|---|---|
| BNP32 < 125pg/ml | 378 | 1.4 (1.3–1.5) | <0.001 | 1.3 (1.2–1.4) | <0.001 |
| BNP32 > 125pg/ml | 377 | 1.1 (0.9–1.2) | 1.0 (0.9–1.1) |
Adjusted for all covariates in Table 4, Model 3
We also examined the predictive value of the residual values, derived from the linear regression of BNP32 on proBNP1-108. Here, a positive residual was indicative of more proBNP1-108 than predicted by BNP32. Interestingly, we found that an increased residual was also associated with a significant risk of all-cause death and cardiac transplantation (Supplementary Table 1). There were some significant, albeit much weaker associations between the residuals and the composite risk of death, transplantation, and first hospitalization (Supplementary Table 2).
Joint Effects of ProBNP1-108 and BNP32 Assessment on Risk of Adverse Cardiovascular Outcomes
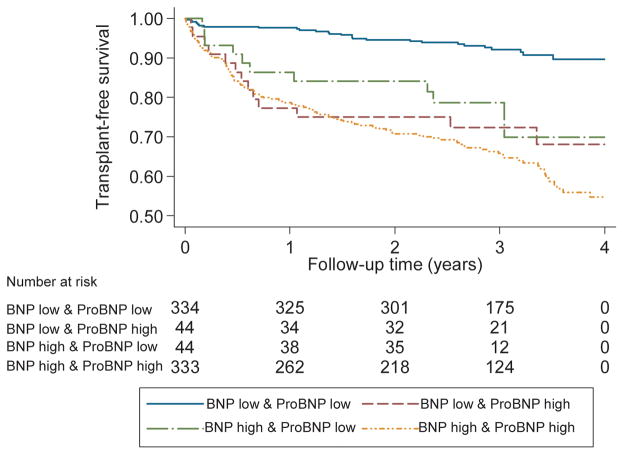
We then tested the combined influence of ProBNP1-108 and BNP32 assessment, to further determine the joint effects of proBNP1-108 and BNP32. Patients were divided into four subgroups based upon median proBNP1-108 and BNP32 levels. A number of patients (11.6%) demonstrated discordance between the two biomarkers, with 44 having a proBNP1-108 level less than the median and BNP32 above the median, and 44 with a proBNP1-108 level above the median and BNP32 less than the median. As demonstrated in Table 7, for the composite endpoint of all-cause death or cardiac transplantation, patients with a proBNP1-108 above the median, but BNP32 less than the median, had an unadjusted risk of death or transplantation of 4.1 (2.1–7.9, p<0.001) as compared to the group with low levels of both biomarkers. This association remained significant in multivariable-adjusted analyses. As demonstrated in Kaplan-Meier analyses, patients with an elevation in either BNP32 or proBNP1-108 had a significantly increased risk of adverse events compared to patients with low levels of both markers (Figure 4). These findings were similar for the combined endpoint of death, transplantation, or incident heart failure hospitalization (Table 8).
Table 7.
Joint Effects of BNP32 and ProBNP1-108 Assessment and Risk of All-Cause Death or Cardiac Transplantation
| BNP32 | ProBNP1-108 | N | Unadjusted HR (95% CI)† | N | Adjusted HR† |
|---|---|---|---|---|---|
| Low | Low | 334 | 1 | 327 | 1 |
| High | Low | 44 | 3.4 (1.6–6.9) | 43 | 3.0 (1.4–6.4) |
| Low | High | 44 | 4.1 (2.1–7.9) | 43 | 2.3 (1.1–4.6) |
| High | High | 333 | 5.5 (3.6–8.4) | 329 | 2.9 (1.8–4.7) |
Low/High BNP32 defined as below/above median (125 pg/ml); Low/High ProBNP1-108 defined as below/above median (271 pg/ml)
Adjusted for all covariates in Table 2, Model 3
Figure 4.
Kaplan-Meier Survival Estimates for All-Cause Death or Cardiac Transplantation in Groups Stratified by Median BNP32 and ProBNP1-108 Values p<0.001 by log rank test
Table 8.
Joint Effects of BNP32 and ProBNP1-108 Assessment and Risk of All-Cause Death, Cardiac Transplantation, or First Heart Failure Hospitalization
| BNP32 | ProBNP1-108 | N | Unadjusted HR (95% CI)† | N | Adjusted HR† |
|---|---|---|---|---|---|
| Low | Low | 334 | 1 | 327 | 1 |
| High | Low | 44 | 2.0 (1.2–3.2) | 43 | 1.8 (1.1–2.9) |
| Low | High | 44 | 2.5 (1.6–3.9) | 43 | 1.6 (1.0–2.6) |
| High | High | 333 | 3.2 (2.5–4.1) | 329 | 1.9 (1.4–2.6) |
Low/High BNP32 defined as below/above median (125 pg/ml); Low/High ProBNP1-108 defined as below/above median (271 pg/ml)
Adjusted for all covariates in Table 4, Model 3
Discussion
The present study is the first to determine the utility of proBNP1-108 as an individual biomarker and in conjunction with BNP32 measurement in ambulatory patients with primarily chronic systolic heart failure. Our results demonstrate that a significant amount of unprocessed proBNP1-108 circulates in patients with systolic heart failure, and this amount is positively associated with risk of adverse outcomes. Furthermore, measurement of unprocessed proBNP1-108 in ambulatory heart failure patients adds value to BNP32 assessment. In particular, a low BNP32 value of less than 125 pg/ml, in conjunction with an elevated proBNP1-108, identifies a subset of patients with a significant risk of adverse outcomes that might otherwise be overlooked. Our findings thus suggest that simultaneous assessment of proBNP1-108 and BNP32 provides important additional information regarding risk for death, cardiac transplantation, or heart failure hospitalization, particularly for those patients with low BNP32 values and may be useful as a strategy to further guide the management of outpatients with chronic systolic heart failure.
We used a highly specific assay for proBNP1-108 which demonstrates no significant cross-reactivity for current commercial assays for BNP32 or NT-proBNP1-76. However, most of the commercially available BNP immunoassays (Architect, AxSYM, Centaur, Access, Triage) use antibodies aimed at epitopes in the ringed structure of the BNP molecule formed by disulfide bonds. This domain is also present in proBNP1-108 and, as expected, these immunoassays cross react to varying degrees with proBNP1-108. The degree of cross reactivity appears to depend on the degree of proBNP glycosylation. For example, the Architect assay (used in this analysis) demonstrates 38% cross-reactivity with glycosylated proBNP (expressed in CHO cells), but only 6% with non-glycosylated recombinant proBNP (expressed from E.Coli)14. The degree of glycosylation of circulating proBNP in heart failure has not been determined.
Several previous reports have highlighted the fact that unprocessed proBNP1-108 is a major circulating component of circulating BNP in humans with advanced heart failure. Utilizing mass spectrometry, Burnett and colleagues did not detect any processed BNP32 in the plasma of patients with advanced heart failure 18, but instead detected a number of higher molecular-weight forms likely representing unprocessed proBNP. This has since been verified using proBNP1-108 immunoassays 10 and is consistent with the findings in the present study. Although these studies have demonstrated that the absolute amount of proBNP1-108 correlates with the severity of heart failure, there is substantial inter-individual variability in the proportion of proBNP1-108 in patients with advanced heart failure 10. The basis for this variation remains unclear, but work by our own group suggests that in specific populations, inherited genetic variation in corin may confer variation in efficiency of natriuretic processing and thus lead to different proportions of proBNP1-108 and BNP32 19, 20. It may be that patients having low processed BNP32 levels but high proBNP1-108 levels are “impaired BNP processors.”
We hypothesize that the clinical significance and prognostic import of impaired BNP processing relates to an attenuation of the compensatory actions of the endogenous natriuretic peptide system in heart failure. The natriuretic peptide system exerts a variety of beneficial actions in advanced heart failure that include increased lusitropy; reduced endothelin release; venous and arterial vasodilation; reduced activation of the renin-angiotensin-aldosterone and sympathetic neurohormonal systems; and the promotion of natriuresis even in advanced stages of heart failure 5. For example, the acute administration of HS-121, a competitive antagonist of the NPR-A receptor, to dogs with severe pacing-induced systolic heart failure resulted in an increase in adverse neurohormonal activation, increased systemic vascular resistance, an elevation of cardiac filling pressures and reduced fractional excretion of sodium. Conversely, the administration of recombinant, active BNP (nesiritide) to patients with NYHA Class II to IV heart failure reduces pulmonary capillary pressures acutely and improves dyspnea scores 21, 22. Therefore, it is not unexpected that impaired natriuretic peptide processing might be causally related to heart failure progression or the risk for decompensation leading to heart failure hospitalization. This paradigm is supported by in vitro models demonstrating a decreased ability of recombinant proBNP1-108 as compared to recombinant BNP32 to stimulate cGMP production in cardiomyocytes and fibroblasts 8, 9.
Alternatively, the presence of impaired proBNP processing might simply be a marker of an underlying “molecular signature” that is associated with the risk of heart failure progression. Although the molecular mechanisms that underlie BNP processing in human heart failure remain incompletely understood, recent data have provided important insights. Corin has been identified as a contributor to natriuretic peptide processing, although other enzymes such as furin are also postulated to play a role. Corin is expressed abundantly in cardiomyocytes, and it possesses an extracellular serine protease that has demonstrated the ability to process proANP and proBNP in vitro and in vivo 23, 24. Abnormalities in corin abundance or corin bioactivity might contribute to impaired BNP processing, but this remains speculative. Elucidating the underlying molecular mechanisms that account for impaired BNP processing in human heart failure may provide novel insights into heart failure progression and new targets for pharmacological intervention.
The clinical implications of our findings suggest that the use of both biomarkers might permit the identification of high-risk ambulatory heart failure patients that otherwise might be misclassified on the basis of a low BNP32 value. Although one might raise the issue that the number of patients with discordant BNP32 and proBNP1-108 values (11.6% of this cohort) is relatively small, we feel these findings are important from a mechanistic standpoint as well as clinically as biomarkers become increasingly applied as a multimarker strategy. The limitations of our study do include confirmation in additional cohorts. In addition, there exists a lack of ability to precisely define the cross-reactivity of the BNP32 isoform with proBNP1-108. Although we sought to adjust for an extensive list of confounders, there may be unknown covariates that we failed to adjust for in our multivariable models. Finally, this study population represents those recruited from a tertiary referral heart failure clinic and represent patients with primarily systolic heart failure, nonischemic heart failure, and a relatively young age. In accordance with a high-volume heart failure and transplant center, our outcomes assessment included a significant number of cardiac transplantations. As such, our results may not be generalizable to all populations with heart failure, particularly those with diastolic heart failure.
In conclusion, we have demonstrated that in ambulatory heart failure patients, elevated proBNP1-108 levels are associated with a significantly increased risk of adverse cardiovascular outcomes. In addition, joint assessment of both proBNP1-108 and BNP32 leads to improved risk stratification. In particular, in patients with low measured BNP32 levels, assessment of proBNP1-108 levels may permit the identification of a high risk cohort for death, cardiac transplantation, or heart failure hospitalization that cannot be adequately identified using BNP32 values alone. It also permits the identification of a group with a very low risk for these events. If confirmed in additional studies, we propose that the simultaneous assessment of BNP32 and proBNP1-108 may be useful for improved risk stratification in patients with systolic heart failure, especially patients with a BNP32 value < 125 pg/mL that might otherwise reassure the clinician.
Supplementary Material
CLINICAL PERSPECTIVE.
Clinicians managing ambulatory heart failure patients may utilize measurement of BNP as an additional tool to identify patients at risk for decompensation. In moderate to severe chronic heart failure patients, a substantial proportion of circulating BNP consists of the 108 amino acid proBNP pro-hormone. Usually proBNP1-108 is proteolytically cleaved by corin into an inactive amino-terminal fragment (NT-BNP) and a biologically active 32-amino acid carboxyl fragment (BNP-32). In vitro experiments have also demonstrated that proBNP1-108 has substantially reduced biological activity. Commercially available C-terminal BNP immunoassays incompletely cross-react with proBNP1-108, creating the potential to underestimate the total amount circulating BNP, inm particular in patients with increased ratios of unprocessed/processed BNP. Utilizing a novel immunoassay specific for unprocessed proBNP 1-108, we demonstrate that in patients with low BNP-32 levels(< 100 pg/ul) , the additional measurement of proBNP1-108 can separate patients into a low and high risk groups for heart failure progression based on the amount of Unprocessed proBNP1-108 ; patients with low BNP-32 levels but elevated proBNP1-108 levels were at increased risk for heart failure progression. We hypothesize that this might reflect the fact that the total sum of processed and unprocessed BNP more accurately reflects the aggregate physiological demands on BNP gene transcription and release from cardiomyocytes, an attenuation of the compensatory actions of the natriuretic peptide system, since proBNP1-108 is substantially less biologically active, or a combination of the two. In summary, we propose that the simultaneous measurement of processed and unprocessed BNP1-108 , in particular in patients with low BNP values using commercially available immunoassays directed at the carboxyl domain of BNP, improves risk stratification in ambulatory heart failure patients.
Acknowledgments
Funding Sources
Dr. Dries was supported by NIH HL091663 and Dr Ky was supported by NIH/Clinical and Translational Science Award KL1RR024132 and Heart Failure Society of America Research Fellowship Award. This work was also supported by NIH HL088577 (Dr. Cappola) and by a National Scientist Development Grant from the American Heart Association 0730375N (Dr. Rame).
Footnotes
Disclosures
Dr. Cappola reports receiving research support from Abbott Diagnostics.
References
- 1.Lam CS, Burnett JC, Jr, Costello-Boerrigter L, Rodeheffer RJ, Redfield MM. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol. 2007;49:1193–1202. doi: 10.1016/j.jacc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751:82–94. doi: 10.1016/j.bbapap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 6.Burnett JC., Jr The atrial peptide system in cardiac disease. Am J Hypertens. 1988;1:410S– 420S. doi: 10.1093/ajh/1.4.410s. [DOI] [PubMed] [Google Scholar]
- 7.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 8.Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC., Jr Immunoreactivity and guanosine 3',5'-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–1119. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 9.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, Ferriere M, Saussine M, Cristol JP, Dupuy AM, Merigeon E, Merle D, Villard S. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52:1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 11.Vorovich E, Chuai S, Li M, Avena J, Marwin M, Wolfe D, Reilly MP, Cappola TP. Comparison of MMP-9 and BNP as clinical biomarkers in chronic heart failure. American Heart Journal. 2008;155:9920997. doi: 10.1016/j.ahj.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, Sawyer DB, Cappola TP. Neuregulin-1beta is Associated with Disease Severity and Adverse Outcomes in Chronic Heart Failure. Circulation. 2009;120:310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 14.Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Tate J, Wu AH, Ler R, Apple FS. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54:619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]
- 15.Astor BC, Yi S, Hiremath L, Corbin T, Pogue V, Wilkening B, Peterson G, Lewis J, Lash JP, Van Lente F, Gassman J, Wang X, Bakris G, Appel LJ, Contreras G. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: the African American Study of Kidney Disease and Hypertension (AASK) Circulation. 2008;117:1685–1692. doi: 10.1161/CIRCULATIONAHA.107.724187. [DOI] [PubMed] [Google Scholar]
- 16.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, Girod JP, Lee MJ, Starling RC, Young JB, Van Lente F, Francis GS. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 18.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 22.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 23.Antos LK, Abbey-Hosch SE, Flora DR, Potter LR. ATP-independent activation of natriuretic peptide receptors. J Biol Chem. 2005;280:26928–26932. doi: 10.1074/jbc.M505648200. [DOI] [PubMed] [Google Scholar]
- 24.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.