Abstract
Green tea polyphenols (GTP) are highly effective in inhibiting a variety of tumorigenic effects induced by carcinogens. In this study we assessed GTP mitigation on biomarkers of fumonisin B1 (FB1), a class 2B carcinogen, in blood and urine samples collected from an intervention trial. A total of 124 exposed people were recruited and randomly assigned to low-dose (GTP 500 mg, n = 42), high-dose (GTP 1,000 mg, n = 41) or placebo (n = 41) for 3 months. After one-month of intervention, urinary FB1 was significantly decreased in high-dose group compared to that of placebo group (p = 0.045), with reduction rates of 18.95% in the low-dose group and 33.62% in the high-dose group. After three-month intervention, urinary FB1 showed significant decrease in both low-dose (p = 0.016) and the high-dose (p = 0.0005) groups compared to that of both placebo group and baseline levels, with reduction rates of 40.18% in the low-dose group and 52.6% in the high-dose group. GTP treatment also significantly reduced urinary excretion of sphinganine (Sa), sphingosine (So), and Sa/So ratio, but had no effect on serum Sa, So, and Sa/So ratio. Analysis with mixed-effect model revealed significant interactions between time and treatment effects of GTP on both urinary free FB1 levels and Sa/So ratios.
Fumonisins (FNs) are a family of naturally occurring mycotoxins mainly produced by Fusarium verticilioides1. They are ubiquitous contaminants of cereal grains worldwide, with at least 15 variants isolated, including A, B, C and P variants2. Fumonisin B1 (FB1), the representative mycotoxin, causes several fatal animal diseases, including leukoencephalomalacia in horses, pulmonary oedema in swine, neural tube defects in mice, nephrotoxicity in rodents, and hepatotoxicity in horses, swine and rats3,4,5,6,7. In addition, FB1 is a carcinogen and strong tumor promoter in animal and human cell models8,9,10,11,12.
Human populations in certain areas of the world are exposed to higher levels of FB1 in their daily diets, mainly through ingestion of contaminated corn and corn-based foods2,13,14,15,16. Aetiologically, FB1 exposure has been linked to a broad spectrum of human diseases, such as hepatocellular carcinoma (HCC) and oesophageal cancer (EC) in South Africa, China and the Islamic Republic of Iran12,13,14,15,16. The International Agency for Research on Cancer (IARC) has classified FB1 as a group 2B carcinogen12. In addition to the carcinogenic property, FB1 exposure played a role in the occurrence of a cluster of neural tube defects along the Texas–Mexico border17.
Several studies have pinpointed the possible mechanism of action of FB1 by means of ceramide synthase inhibition and global disruption of lipid metabolism18,19,20. FB1 disrupts sphingolipid metabolism by inhibiting ceramide synthases, due to its structural similarity to long-chain sphingoid base backbones18. The inhibition of ceramide synthases causes an increase in intracellular free sphinganine (Sa) and, to a lesser extent, sphingosine (So), which precedes the depletion of complex bioactive lipids19,20. FB1-induced biochemical alterations, particularly the elevated Sa levels, Sa to So ratio (Sa/So) or Sa 1-phosphate to So 1-phosphate (SaP/SoP) ratio in tissues, urine and blood, have been proposed as potential biomarkers in various animal species, including foals, pigs, mink, rodents, vervet monkeys, and ducks15,21,22. However, the correlation between these biomarkers and human exposure levels remain conflicted in different reported population studies in different areas20,21,22.
Toxicokinetic data showed that orally dosed FB1 was eliminated rapidly from circulation15,21,22,23. This rapid elimination and low bioavailability, as well as the lack of a major metabolite, indicate that FB1 in biological fluids may be measured directly as a possible biomarker of FB1 exposure21,22. Efforts have been made to monitor free FB1 in human urine24,25,26, plasma26, faeces27 and hair28,29. Currently available data demonstrated that free FB1, as an exposure biomarker, has been widely applied in many studies to track FB1 e in humans24,25,26,30,31,32,33. Furthermore, this biomarker has been applied to evaluate intervention strategies that could reduce exposure to FB134,35.
The intervention strategies for FNs mainly focus on pre- and post-harvest control. However, the greater challenge now is to reduce the risk in individuals who have already been exposed to FNs for decades via diets. Chemoprevention was proposed as a promising strategy to help these high-risk individuals36. Green tea polyphenols (GTP), major components in green tea leaves, have been shown to be highly effective in inhibiting a variety of carcinogen-induced tumorigenesis in animal models at different target organ sites36,37,38,39,40. Previous studies from our laboratory found that GTP effectively modulated aflatoxin B1 (AFB1) metabolism as well as metabolic activation, as demonstrated by the decreased serum levels of AFB1-albumin adducts and urinary levels of AFM1 in high-risk individuals of liver cancer41. Further, GTP supplement effectively reduced levels of 8-hydroxydeoxyguanosine, the oxidative DNA damage biomarker, in populations exposed to high levels of AFB142. Given the co-contamination of FB1 with AFB1 found in food samples from several study populations, as well as the synergistic toxicity found in animal models14,43,44, chemopreventive measures such as the supplement of GTP may also potentially modulate the FB1-induced toxic effects, and thus mitigate the biomarker levels of FB1. This hypothesis has been tested in the current study via measurement of FB1 biomarkers, i.e. urinary-free FB1, urinary and serum Sa, So, Sa/Sa ratio in samples collected from an intervention study participants with GTP in Fusui county, a high-risk area for liver cancer in China, where high levels of co-contamination of AFB1 and FB1 in food staples were recorded13,14.
Results
Background survey of FB1 exposure
Before recruitment, a total of 90 corn and corn meal samples were collected from 45 households in two study villages. FB1 was detectable in 95.6% (86/90) of samples with average level of 1,270.23 ± 1,490.59 μg/kg (mean ± SD), as shown in Table 1, and median level of 402.35 μg/kg. Based on the recorded food consumption in these households and detected FB1 levels in food, the average daily FB1 intake was estimated to be 750.71 ± 1,983.57 (mean ± SD) μg/kg body weight, and the median was estimated to be 138.6 (93.9–495.3) μg/kg body weight. Background levels of FB1 biomarkers were obtained via analyzing portions of the screening samples (n = 250). The detectable rate of urinary free FB1 was 91.2% (228/250), with average level of 797.66 ± 716.76 (mean ± SD) pg/mg creatinine, and median level of 560.73 pg/mg creatinine. Sphingolipids were detectable in 100% (250/250) of screening samples, and the average levels, median, lower quartile, and upper quartile of Sa, So and Sa/So ratio in the serum and urine samples were shown in Table 1.
Table 1. Background information on FB1 exposure in screening study subjects.
| Mean ±SE | Median (Lower Quartile, Upper Quartile) | |
|---|---|---|
| FB1 Levels in household corn (mg /kg) | 1.27 ± 14.91 | 0.4 (0.1–1.2) |
| Estimated daily FB1 intake (μg /day bw) | 750.71 ± 1983.57 | 138.6 (93.9–495.3) |
| Urinary Free FB1 (pg/mg Crea.) | 797.66 ± 716.76 | 560.73 (313.45, 1051.67) |
| Serum Sphingolipids (nmol/L) | ||
| Sa | 16.07 ± 14.30 | 10.34 (5.57, 23.01) |
| So | 21.05 ± 14.07 | 17.95 (9.52, 31.53) |
| Sa/So | 0.81 ± 0.48 | 0.71 (0.45, 1.02) |
| Urinary Sphingolipids (nmol/L) | ||
| Sa | 10.82 ± 18.76 | 3.47 (1.69, 12.19) |
| So | 15.18 ± 20.48 | 5.68 (3.74, 21.19) |
| Sa/So | 0.65 ± 0.36 | 0.64 (0.42, 0.84) |
Baseline levels of FB1 biomarkers in different treatment arms
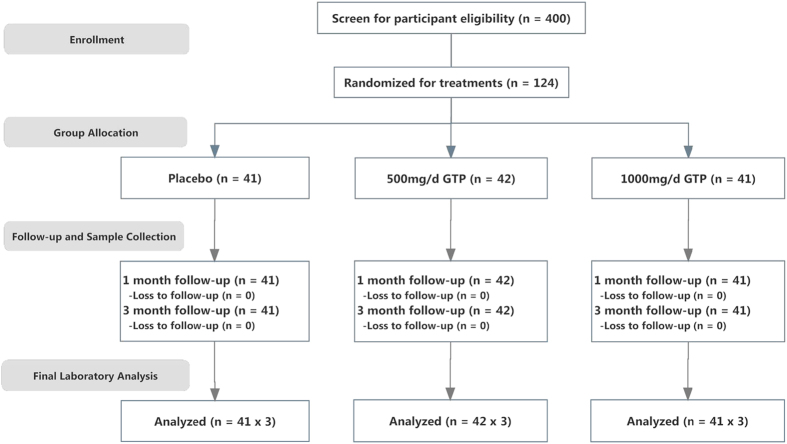
As demonstrated in Fig. 1, a total of 124 subjects were recruited and randomized into three different treatment arms. Blood and urine samples were collected before the treatment for determining the baseline levels of FB1 biomarkers, including urinary free FB1, urinary and serum Sa, So and Sa/So, which were shown in Table 2. Geometric means and 95% confident intervals were also given in Table 2. Because data was not normally distributed, log-transformation was performed for statistical evaluation. Levels for each FB1 biomarker in three treatment arms were comparable, with no significant differences among placebo, low-dose and high-dose groups (p = 0.673, p = 0.887 and p = 0.787, respectively).
Figure 1. Overall study design and study outline.
Table 2. Baseline Levels of FB1 biomarkers in treatment groups; GM: Geometric means.
| Placebo (GM; 95% CI) | GTP 500 mg (GM; 95% CI) | GTP 1000 mg (GM; 95% CI) | p | |
|---|---|---|---|---|
| Number | 41 | 42 | 41 | |
| Free Urinary FB1(pg/mg Crea.) | 575.44(436.52, 776.25) | 630.96(489.78, 812.83) | 512.86(380.19, 707.95) | 0.673 |
| Serum Sa(nmol/L)a | 11.02(8.50, 14.30) | 11.36(8.76, 14.88) | 11.36(8.50, 15.18) | |
| Serum So(nmol/L) | 16.12(12.55, 20.70) | 16.28(12.81, 20.70) | 15.8(12.06, 20.91) | |
| Serum Sa/So | 0.68(0.58, 0.81) | 0.69(0.59, 0.83) | 0.72(0.59, 0.87) | 0.887 |
| Urinary Sa(nmol/L) | 4.81(3.25, 7.10) | 4.26(2.66, 6.75) | 3.86(2.46, 6.11) | |
| Urinary So(nmol/L) | 7.69(5.31, 11.13) | 8.94(6.30, 12.81) | 7.61(5.42, 10.70) | |
| Urinary Sa/So | 0.63(0.56, 0.69) | 0.47(0.35, 0.65) | 0.5(0.39, 0.66) | 0.787 |
Modulation of GTP on urinary free FB1 levels
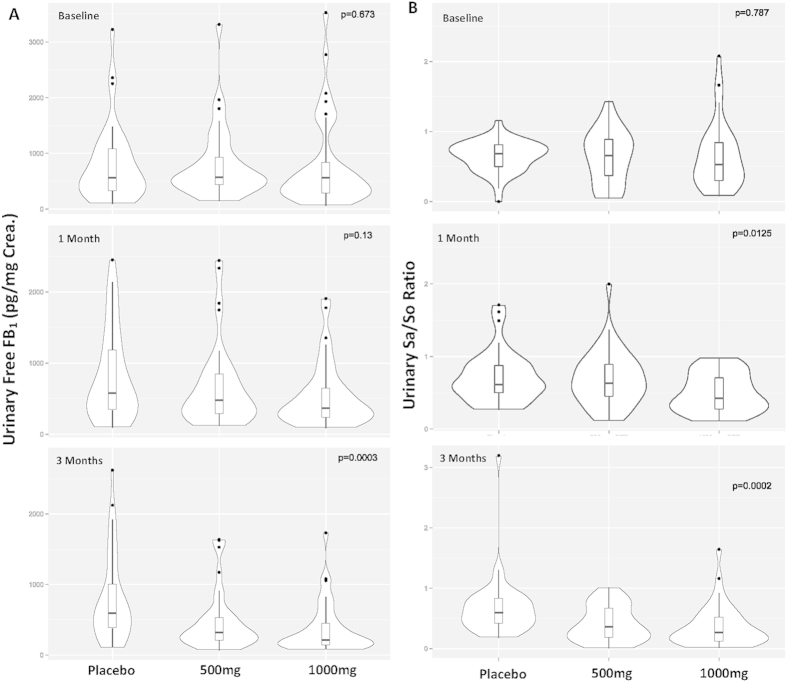
After one month of GTP intervention, the median levels of urinary free FB1 were 575.25, 477.79 and 364.94 pg /mg creatinine in placebo, low-dose and high-dose GTP groups, respectively. The reduction rate was 18.95% in the low-dose group and 33.62% in the high-dose group as compared to the placebo group (Fig. 2A). The urinary free FB1 level in the high-dose group was significantly lower than that in the placebo group (p = 0.045).
Figure 2. Summaries of Urinary free FB1 levels and Sa/So ratios.
A: Urinary free FB1 levels (pg/mg creatinine) in each treatment arm at the baseline, 1-month, and 3-months of GTP intervention study. B: Urinary Sa/So ratio in each treatment arm at the baseline, 1-month, and 3-months of GTP intervention study. The violin plot represents the overall distribution of measured data and the overlapping box plot shows the median, 25th and 75th percentiles of the data.
After three months of intervention, urinary free FB1 levels continuously decreased in GTP intervention groups, with median levels of 319.45 and 215.83 pg/mg creatinine in the low- and high-dose groups, respectively, as compared to the placebo group (591.24 pg/mg creatinine). The reduction rate was 40.18% (p = 0.016) in the low-dose group and 52.6% (p = 0.0005) in the high-dose group, both of which had significant statistical differences compared to placebo group (Fig. 2A).
Effects of GTP on urinary sphingolipids levels
After one month of GTP intervention, the median levels of urinary Sa, So and Sa/So ratio were 3.04 nmol/L, 5.07 nmol/L and 0.63 in the low-dose group, which had no statistical significance compared to 3.85 nmol/L, 5.65 nmol/L and 0.62 in the placebo group (Fig. 2B). However, a 30.65% reduction (0.43) in Sa/So ratio was found in the high-dose group, which was statistically significant (p = 0.012) (Fig. 2B).
After three months of GTP intervention, the median levels of urinary Sa, So and Sa/So ratio were 2.32 nmol/L, 6.38 nmol/L and 0.36 in the low-dose group. Sa level and Sa/So ratio were significantly lower than those in the placebo group (3.61 nmol/L and 0.58), with reduction rates of 35.73% (p = 0.049) and 37.93% (p = 0.008), respectively, when compared to placebo group. No significant difference was found for So levels (5.56 vs 6.38 nmol/L; p > 0.05). The median levels of urinary Sa, So and Sa/So ratio were 1.56 nmol/L, 3.88 nmol/L and 0.26 in the high-dose group, with reduction rates of 56.79% (p = 0.0003), 30.22% (p = 0.23) and 55.17% (p = 0.0002), respectively, when compared to placebo group (Fig. 2B).
Effect of GTP on serum sphingolipid levels
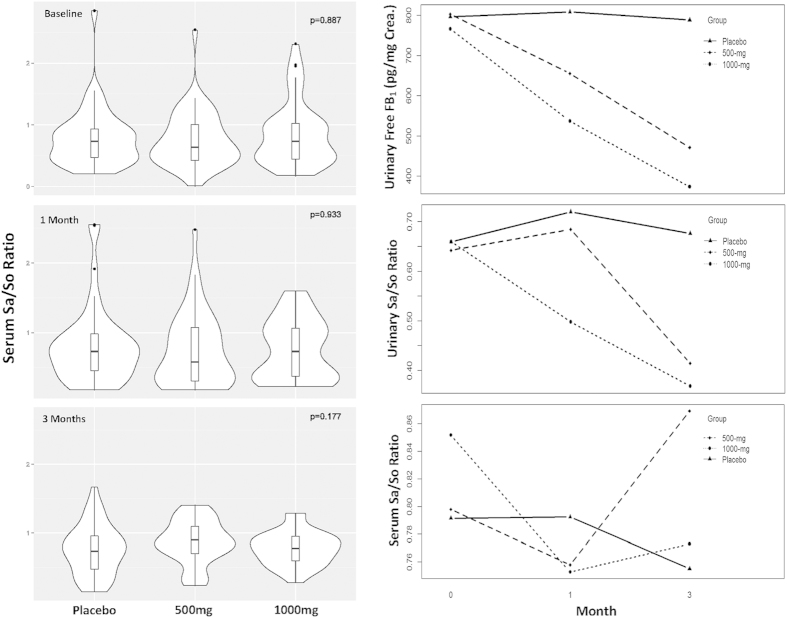
After one month of GTP intervention, the median levels of serum Sa, So and Sa/So ratio were 10.06 nmol/L, 16.42 nmol/L and 0.65 in the low-dose group, which had no statistical significances when compared to 14.11 nmol/L, 20.31 nmol/L and 0.73 in the placebo group. The median levels of serum Sa, So and Sa/So ratio were 13.1 nmol/L, 16.86 nmol/L and 0.73 in the high-dose group, which also had no significant differences with the placebo group (Fig. 3A).
Figure 3. Summaries of serum Sa/So ratios and treatment/time interaction.
A: Serum Sa/So ratio in each treatment arm at the baseline, 1-month, and 3-months of GTP intervention study. The violin plot represents the overall distribution of measured data and the overlapping box plot shows the median, 25th and 75th percentiles of the data. B: Plots of interaction between group and time generated by the mixed-effect model for three biomarkers, urinary free FB1 levels, urinary Sa/So ratio, and serum Sa/So ratio, in three treatment arms of GTP intervention study.
After three months of GTP intervention, the median levels of serum Sa, So and Sa/So ratio were 11.37 nmol/L, 13.79 nmol/L and 0.90 in the low-dose group, which had no statistical significances when compared to 11.32 nmol/L, 13.34 nmol/L and 0.73 in the placebo group. The median levels of serum Sa, So and Sa/So ratio were 7.5 nmol/L, 9.5/nmol/L and 0.76 in the high-dose group. The reduction rates were 33.75% (p = 0.038) for Sa levels and 28.79% (p = 0.045) for So levels, which were statistical significant when compared to the placebo group, but the Sa/So ratio (0.76 vs 0.73) was not significantly different (Fig. 3A).
Interactions between time and treatment
A log-transformed mixed–effect model was used to analyse interactions between time and treatment effects, and the outcomes were shown in Table 3 and Fig. 3B. The interaction between time and treatment effects on urinary free FB1 was linear and highly significant (p = 0.0027 in the low-dose group and p = 0.0006 in the high-dose group). Interaction between time and treatment effects on urinary Sa/So ratio was also significant, with p = 0.0387 in the low-dose group and p = 0.02 in the high-dose group, respectively. However, no interaction between time and treatment effects was found on serum Sa/So ratio, as demonstrated in both Table 3 and Fig. 3B.
Table 3. Significances on interactions (Pr > |t|) between time and treatment with the mixed-effect model.
| Effect | Groups | urinary FB1 | Urinary Sa/So | Serum Sa/So |
|---|---|---|---|---|
| Intercept | <0.0001 | <0.0001 | <0.0001 | |
| Groups | placebo | – | – | – |
| Groups | 500mg | 0.8587 | 0.9010 | 0.8137 |
| Groups | 1000mg | 0.7939 | 0.5078 | 0.8073 |
| Time | 0.8306 | 0.9494 | 0.6992 | |
| Time * Groups | placebo | – | – | – |
| Time * Groups | 500mg | 0.0027 | 0.0387 | 0.3785 |
| Time * Groups | 1000mg | 0.0006 | 0.0204 | 0.8576 |
Discussion
The key finding from this study was that urinary biomarkers, free FB1 and Sa/So ratio, decrease significantly in GTP treatment groups, and the effect was greater over time, which demonstrated the mitigative effect of GTP on FB1 exposure in this high-risk human population of HCC. The urinary free FB1 reflects the amount absorbed into circulating system, which was found to be the most sensitive biomarker among all FB1 biomarkers examined. Gong et al.30 showed the correlation of urinary FB1 with maize intake in a Mexican population. Similarly, van der Westhuizen et al.31 found urinary FB1 level reflected changes in FNs exposure in Centane, South Africa, when an intervention measure was applied to the population to reduce FN intake. Torres et al.33 have further confirmed that urinary free FB1 mirrored estimated FB1 intake, by assessing urinary and maize samples collected from high and low exposure communities in Guatemala. In addition, Shirima et al.32 have used urinary free FB1 level as biomarker to assess the exposure among Tanzanian children.
FB1 is an ubiquitous food contaminant, which acts as a competitive inhibitor to ceramide synthase, thus disrupting synthesis of complex sphingolipids, resulting in accumulation in the levels of free sphingolipids such as sphingosine and sphinganine15,18,19,20,21. Our laboratory had previously focused on validating various FB1 biomarkers, including FB1-induced changes in sphingolipid metabolism as well as frees FB122,26. Urinary Sa/So ratio was found to be more sensitive than serum Sa/So ratio in F344 rats in response to both acute and sub-chronic exposure to FB1. Free FB1 excretions in urine and faeces were strongly correlated with urinary sphingolipid metabolites in the repeat-dose study22. Findings from the cross-sectional study supported the notion that humans were exposed to higher levels of dietary FNs in the high-risk areas of oesophageal cancer and HCC in China, as compared to the low-risk area of both cancers13. Moreover, higher estimated FB1 daily intakes could be mirrored by elevations of urinary free FB1 and urinary Sa/So ratio among residents of those high-risk areas26. Longitudinal biomonitoring study also demonstrated temporal variations in urinary and serum Sa/So ratios, which reflected the seasonal changes of FB1 intake and food contamination in the high-risk areas of oesophageal cancer and HCC26.
As demonstrated by various animal studies20,21, the alterations in the sphingolipid levels as well as ratios were direct consequences of lipid profile changes induced by FB1. The decrease in the levels of urinary free FB1 and Sa/So ratio pinpoint to a decreased level of FB1 absorption, as well as reduced toxicity, when treated with GTP. It should be noted that, while the urinary Sa/So ratio displayed statistically significant decrease over time in treated groups, the serum Sa/So ratios did not. Levels of Sa, So and Sa/So ratio in blood had previously been proposed as potential biomarkers due to their association with treatment in animal models as well as the mechanistic implication; however, the reliability of these biomarkers in human populations remains questionable, mainly due to the influence of other factors and inconsistency in human studies21. For example, Solfrizzo et al.45 found that Sa/So ratio in South Brazil population was significantly higher than that of North Argentina, despite of similar maize and FNs intake in the two areas. Similar phenomenon was found by van der Westhuizen et al.46, in assessing Sa and So levels in different areas of former Transkei, South Africa. They found no association between levels of Sa, So and/or Sa/So ratio and dietary levels of FB1 in both urine and plasma samples in area of low and high oesophageal cancer incidences in Transkei region of South Africa47. Others, as well as our previous studies, also found neither between FB1 exposure and serum Sa/So ratio26, nor association between serums Sa/So ratio and oesophageal cancer risk48. One hypothesis for this lack of correlation may be due to role of blood stream in buffering sphingolipids. Because new sphingolipids were continually synthesized de novo and released into bloodstream, this would in turn result in high variation in the sphingolipid levels. Moreover, the two sphingolipids were affected differently by the salvage pathway of sphingolipid metabolism, which may also cause discrepancies in serum ratio.
There have been great concerns in recent years on co-contamination of AFB1 and FB1 in cereal grains and potential health risks of dietary co-exposure to these two mycotoxins14. Co-contamination of AFB1 and FB1 in dietary components, especially in corn and corn products, has been reported worldwide14,49,50,51,52. US corn and corn products were found with detectable levels of AFB1 in 0.5 to 200 ppb and FB1 in 0.5 to 150 ppm53. Synergistic interactions on toxicity and co-carcinogenicity were found in various animal models co-exposed to AFB1 and FB1, including in trout, rats, chicks, and mosquito fish43,44,54,55,56. Co-exposure to these two mycotoxins has also been suggested to be linked with the aetiology of acute mycotoxicosis, reproductive and developmental disorders, as well as primary liver cancer and oesophageal cancer14. It has been well known that AFB1 is potent genotoxic agent, and genetic alterations played important roles in its mode of actions, whereas FB1 is not genotoxic, and its tumorigenic effect is believed to be mainly via epigenetic mechanisms, including disruptions of apoptosis, cell cycle, and sphingolipids signalling pathways.
While molecular mechanisms related to the synergistic adverse health outcomes induced by co-exposure are under investigation, several intervention strategies targeting co-exposure to AFB1 and FB1 have been devised and studied. Given the source of mycotoxin production, sorting and cleaning up has shown to effectively reducing FB1 and AFB1 exposure31. Alternatively, conjugation of AFB1 and FB1 with enterosorbents may prevent absorption, thereby reducing internal dose and toxic effects. Calcium montmorillonite clay in diet was shown to effectively bind to AFB1, and to some extent FB1, reducing the absorption of both, as well as urinary excretion of the biomarkers34,55. Another intervention involves chemically modifying these two mycotoxins in food source via alkaline treatment (nixtamalization), which greatly reduced the amount of AFB1 and FB1 in food and significantly reduced toxicity observed4,56. Effectiveness of this strategy in reducing human co-exposure and toxicity is in active investigation.
By comparison, we previously found chemopreventive effect of GTP in reducing AFB1 biomarkers41 in the same population, and this study demonstrated a mitigation of FB1 exposure via reducing FB1 biomarkers, rather than by a physical means of intervention. While the direct binding of GTP to AFB1 and FB1 seems unlikely, GTP modulated the expression of key enzymes involved in Phase 2 detoxifying enzymes or ceramide synthesis pathway, both of which played significant roles in AFB1 and FB1 toxicity and carcinogenicity36,37,38,39,40,41,42. GTP modulated apoptosis and cell cycle regulating genes in animals and human cells27,28,29,30. As potent antioxidants, GTP may also play a role in maintaining the integrity of cell membrane and preventing lipid oxidation, which may mitigate the toxic effects of FB1.
The toxicokenetics of FB1 were characterized by low absorption, rapid distribution and elimination, and a lack of phase 1 metabolism in animal models in vitro or in vivo21. Because of the absence of measurable metabolites, free FB1 itself was proposed as a biomarker20,21,22. Kinetics study showed that urinary excreted FB1 consists of average 0.5–1% of dietary intake, and would be eliminated from the system rapidly22. The enteric track plays a major role in limiting FB1 absorption, as it is the primary entry into bloodstream for both AFB1 and FB1. In cell model, enteric cells are shown to limit FB1 absorption, in particular, the P-glycoprotein and multidrug resistance associated proteins, which, when inhibited, significantly increases the passage of FB1 through the cells57. Co-exposure to AFB1 and FB1 may potentially affect the enteric cellular structure, resulting in increased absorption of both AFB1 and FB158. Treatment with GTP, on the other hand, reduces the oxidative effect, allowing preservation of the integrity of enteric barrier, therefore mitigating the exposure to both toxins. Given the potential role of the enteric system involved in absorption and metabolism of AFB1 and FB1, the enteric system can be a potential target for chemopreventive measures, both in terms of structural integrity and the gut micro-environment. GTP can promote the growth and maintenance of gut microbiota59, potentially modulate the absorption and metabolism of AFB1 and FB1.
In summary, GTP can effectively mitigate urinary FB1 biomarkers in a high risk population via several mechanisms as discussed above, and supplement of GTP can potentially be a useful chemopreventive strategy for reducing co-exposure to AFB1 and FB1.
Materials and Methods
Chemicals
GTP used for the study was obtained from the US-China joint venture Shili Natural Product Company, Inc. (Guilin, Guangxi, China) and the purity of GTP is higher than 98.5% according to the analysis by Guangxi Standard Bureau. Boric acid, D-erythro-sphingosine, D-erythro-sphinganine, FB1 from F. verticilioides (approximately 98% by HPLC), formic acid, hydrochloric acid, 2-mercaptoethanol, O-phthaldialdehyde (OPA), 10 x phosphate-buffered saline (PBS), and sodium phosphate monobasic were purchased from Sigma-Aldrich (St. Louis, MO, USA). D-erythro-C20-sphingosine was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Triethylammonium formate HPLC buffer (pH 3.0) was purchased from Regis Technologies, Inc. (Morton Grove, IL, USA). Other HPLC-grade solvents including acetonitrile, ethyl acetate, methanol, 2-propanol, and water were from Honeywell Burdick & Jackson (Muskegon, MI, USA). OPA reagents were prepared by dissolving 10 mg of OPA and 30 μl of 2-mercaptoethanol in 250 μl of methanol and mixing with 4.75 ml of 3% boric acid buffer (pH 10.5).
Study sites, subjects and protocols
The study site included two villages (Sanhe and Zhuqing), located 45 km southwest of Fusui county, Guangxi Zhuang Autonomous Region, China. The site is a rural farming community with 7500 residents, and it belongs to the Qujiu Township, one of the three townships with the highest incidence and mortality of liver cancer (approximately 100/100 000) in Fusui county. The overall study design was previously described in detail36 and outlined in Fig. 1. The study was approved by the Institutional Review Boards of University of Georgia and Guangxi Cancer Institute for human subject protection, and was carried out in accordance with the corresponding guidelines.
Participant inclusion criteria included urinary and serum FB1 biomarker-positive adults aged 20–55, with normal liver and kidney function tests, alpha-fetoprotein negative, no personal history of cancer, no use of prescribed medication, and no pregnancy and lactation for female participants. Informed consent was obtained from all study participants. Four-hundred adults were screened via survey with a short questionnaire, physical exam, and clinical and biomarker analysis of their blood and urine samples. Those who didn’t meet criteria were excluded. A total of 124 healthy eligible adults aged 20–49 years were recruited, about one third (41/124) were females. The participants were randomized into three groups, each treated daily with placebo, 500 mg GTP (low-dose), or 1000 mg GTP (high-dose) capsules for 3 months. The low and high doses were chosen to be equivalent of consumption of two and four cups of green tea consumption, respectively, which are reflective of typical daily intake level in common people. The GTP treatments consist of 250 mg GTP capsules, each containing 126 mg epigallocatechin gallate (EGCG), 53 mg epicatechin gallate (ECG), 25 mg epicatechin (EC), 19 mg epigallocatechin (EGC), 14 mg gallocatechin gallate (GCG) and 11 mg catechin (C), according to HPLC-ECD and HPLC-UV analysis by Guangxi Standard Bureau and our laboratory. Participants were arranged to take capsules twice a day, both after meal.
In addition to regular epidemiological questionnaires, blood samples (7 ml) were drawn after overnight fast, before the treatment (as the baseline), and at 1 month and 3 months of the GTP supplement. Serum was separated via centrifugation at 2500 rpm for 15 min and immediately stored at −20 °C. Twenty-four hour urine samples were collected in the morning, noon and evening of the same day, and stored in amber bottles containing ascorbic acid (20 mg/mL) and EDTA (0.1 M). Aliquots of urine samples (50 ml) were treated with 500 mg ascorbic acid and 12.5 mg EDTA for GTP components and biomarker analysis. All samples were shipped frozen to Guangxi Cancer Institute at Naning City, and further shipped in frozen via air to US laboratory. Laboratory personnel who performed analysis were blinded to sample sources.
Follow-up visits to the participants’ houses were made every other day to record adverse-effects, as well as to assess adherence to treatment. Clinical tests were conducted during each sample collection, including blood counts, blood chemistry, urinary protein, glucose, and blood, which showed no severe adverse-effects36. Excellent person- time compliance (99.5%) was achieved, and no consumption of tea or tea products or broccoli outside of treatments assigned was reported for any participant in this trial. Sample collection, storage and shipment complied with guidelines of both Chinese and US governments.
Food samples collection and FB1 analysis
Corn or corn meal samples (500 g/item) were collected from individual households of the study villages before recruitment. The FB1 level in food samples was measured using the enzyme-linked immunosorbent assay (ELISA) kit from Neogen Corp. (Lansing, MI, USA) according to the manufacturer’s procedures. The limit of detection is 0.05 mg/kg.
Sample size determination
Data from previous studies26 and screening study was used for calculation of sample size. Equal sample size for each treatment group (placebo, low-dose and high-dose GTP) was assumed. The primary objective is to test if FB1 biomarker concentrations will be affected by GTP intervention. According to the mean and standard deviation (SD) values for the primary outcome of urinary FB1 (ng/mg creatinine) found in previous studies, the sample size was calculated based on detecting a 20% change in urinary FB1, as compared to the placebo group, with a power of 0.8 at α = 0.05. An assumed correlation coefficient of 0.80 between baseline and follow-up measurements within a treatment group was used. To meet the statistical power, the sample size for each group was estimated to be 35, which requires 105 participants at an attrition rate of 10–15%.
Urinary free FB1 extraction and analysis with HPLC and LC/MS confirmation
Frozen urine samples were extracted and purified according to the previously developed protocol with FumoniTest immunoafinity column from VICAM (Watertown, MA, USA)12,16. HPLC-fluorescence analysis was carried out on Agilent 1100 liquid chromatography system (Agilent Technologies, Wilmington, DE, USA) with the emission wavelength of 440 nm and excitation wavelength of 330 nm. An on-line automatic injector program performed the derivitization of FB1. Chromatographic separations were performed on a Luna C18 column (5μm particle size, 250 × 4.6 mm) from Phenomenex (Torrance, CA, USA). The mobile phase consisted of a linear gradient starting from 0.1 M sodium phosphate monobasic (pH 3.4)-methanol (35/65, v/v) to 0.1 M sodium phosphate monobasic (pH 3.4)-methanol (20/80, v/v) over 13 min, with flow rate of 1.0 ml/min and injection volume of 100 μl. The limit of quantitation for this method was 20 pg per injection. The mean recovery rate was 83.4% ± 0.8%. The relative standard deviation (RSD) was 4.42%. FB1 concentration was verified using LC/MS methods described in previous studies26. Urinary creatinine levels were analysed using the 96-well Creatinine Assay Kit from Cayman Chemical (Ann Arbor, MI, USA).
Extraction and analysis of serum and urine sphingolipids
Extraction procedure and analysis were slightly modified from protocols of previous reports20,26. The fluorescent derivatives of So, Sa and C20So standard were resolved using Agilent 1100 liquid chromatography system (Agilent Technologies, Wilmington, DE, USA) with the excitation and emission wavelengths of 340 and 455 nm, respectively. An on-line antomatic injector program performed the derivitization of sphingoid bases. Separations were performed using a Zorbax Eclipse XDB-C18 column (5μm particle size, 250 × 46 mm, Agilent Technologies). The mobile phase consist of a linear gradient starting from 5 mM triethylammonium formate (pH 4.3)-methanol-acetonitrile (15/45/40, v/v/v) to methanol-acetonitrile (60/40, v/v) over 20 minutes at flow rate of 1.0 ml/min. The limit of quantitation for this method was 0.03 pmol per injection.
Statistical analysis
The analyses comprised 3 components: (i) a description of urinary FB1, serum and urinary sphingolipids at the individual level, (ii) a comparison of levels of FB1 biomarkers by treatment arms at baseline, before the administration of the GTP and (iii) an evaluation the overall effects of GTP on FB1 biomarkers. The concentrations of FB1, serum and urinary sphingolipids (sa, so and sa/so ratio), were expressed as mean ± SD and median (interquartile range), unless otherwise stated. The levels of FB1 biomarker in urine were adjusted by the concentration of creatinine, and were summarized using descriptive statistics. For the baseline comparison between the treatment and placebo arms, a Wilcoxon rank-sum test for each biomarker was conducted to test if randomization achieved comparable groups. To evaluate the treatment effects, separate mixed-effect models for urinary FB1, Sa, So, and Sa/So ratio were constructed. The model included the intercept, indicators for treatment group, time, and a treatment by time interaction term as fixed effect terms. Then individual-level intercept and time variables were included as random effects. The model has the mathematical form as shown in equation (1):
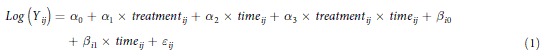 |
where Yij Yij is the response variable value for sample i measured at time j; i = 1, 2, …, N, with N being the total sample size;
j = month 0, 1 and 3;
α0, α1, α2, α3 α0, α1, α2, α3 are the fixed effect coefficients;
βί0 βi0′s and βί1 βi1′s are the random effect coefficients;
εij is the error term.
Response variables that were not normally distributed were transformed by logarithmic to improve normality. The final mixed model was fitted using PROC MIXED in SAS software. Parameters of the mixed model were estimated using Maximum Likelihood Estimation (MLE) method. The Akaike Information Criteria (AIC) and the Bayesian Information Criteria (BIC), where smaller values for both are considered more preferable, were used as measures of the relative qualities of particular models. Both AIC and BIC dealt with the trade-off between the goodness of fit of the model and the complexity of the model, and thus provided valid means for model selection60. Statistical hypothesis tests were two-tailed and assumed an alpha error of 0.05. All analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC, USA).
Additional Information
How to cite this article: Xue, K. et al. Mitigation of Fumonisin Biomarkers by Green Tea Polyphenols in a High-Risk Population of Hepatocellular Carcinoma. Sci. Rep. 5, 17545; doi: 10.1038/srep17545 (2015).
Acknowledgments
This study is supported by research grants CA 94683 and CA90997 from National Cancer Institute, National Institutes of Health and a research contract, ECG-A00-13-00001-00, via Peanut CRSP from United States Agency for International Development. Authors thanks for the study team members from Guangxi Cancer Institute, Fusui Liver Cancer Institute, and the township hospital. Authors also thanks for voluntary participated study subjects.
Footnotes
Author Contributions Conception and design (J.-S.W. and J.S.); acquisition of data (K.X., L.T. and Q.C.), analysis and interpretation of data (L.T., Y.S. and J.S.W.), writing, review, and/or revision of the manuscript (K.X., L.T. and J.-S.W.); administrative, technical, or material support (K.X., L.T., Q.C. and J.S.), and study supervision (L.T., J.S. and J.-S.W.).
References
- Vesonder R., Peterson R., Plattner R. & Weisleder D. Fumonisin B1: Isolation from corn culture, and purification by high performance liquid chromatography. Mycotoxin Res 6, 85–8 (1990). [DOI] [PubMed] [Google Scholar]
- Bulder A. S. et al. Safety evaluation of certain food additives and contaminants. (ed. JECFA) (World Health Organization, Geneva, 2012). [Google Scholar]
- Colvin B. M. & Harrison L. R. Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia 117, 79–82 (1992). [DOI] [PubMed] [Google Scholar]
- Voss K. A., Riley R. T., Snook M. E. & Waes J. G. Reproductive and sphingolipid metabolic effects of fumonisin B(1) and its alkaline hydrolysis product in LM/Bc mice: hydrolyzed fumonisin B(1) did not cause neural tube defects. Toxicol Sci 112, 459–67 (2009). [DOI] [PubMed] [Google Scholar]
- Voss K. A., Plattner R. D., Riley R. T., Meredith F. I. & Norred W. P. In vivo effects of fumonisin B1-producing and fumonisin B1-nonproducing Fusarium moniliforme isolates are similar: fumonisins B2 and B3 cause hepato- and nephrotoxicity in rats. Mycopathologia 141, 45–58 (1998). [DOI] [PubMed] [Google Scholar]
- Marasas W. F. et al. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res 55, 197–203 (1988). [PubMed] [Google Scholar]
- Voss K. A., Plattner R. D., Bacon C. W. & Norred W. P. Comparative studies of hepatotoxicity and fumonisin B1 and B2 content of water and chloroform/methanol extracts of Fusarium moniliforme strain MRC 826 culture material. Mycopathologia 112, 81–92 (1990). [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C. et al. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 54, 1806–11 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom W. C. et al. Cancer initiation by fumonisin B(1) in rat liver--role of cell proliferation. Cancer Lett 169, 127–37 (2001). [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C., Marasas W. F., Lebepe-Mazur S., Swanevelder S. & Abel S. Cancer initiating properties of fumonisin B1 in a short-term rat liver carcinogenesis assay. Toxicology 250, 89–95 (2008). [DOI] [PubMed] [Google Scholar]
- Alizadeh A. M. et al. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac J Cancer Prev 13, 2625–8 (2012). [DOI] [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation fo Carcinogenic Risks to Humans, 489–521 (IARC Press, Lyon, France, 2002). [Google Scholar]
- Sun G. et al. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit Contam 24, 181–5 (2007). [DOI] [PubMed] [Google Scholar]
- Sun G. et al. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28, 461–70 (2011). [DOI] [PubMed] [Google Scholar]
- WHO. Fumonisin B1. in Environmental Health Criteria 219 1–134 (World Health Organization, Geneva, Switzerland, 2000). [Google Scholar]
- Marasas W. F. et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr 134, 711–6 (2004). [DOI] [PubMed] [Google Scholar]
- Missmer S. A. et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect 114, 237–41 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Norred W. P., Bacon C. W., Riley R. T. & Merrill A. H. Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem 266, 14486–90 (1991). [PubMed] [Google Scholar]
- Wang E., Ross P. F., Wilson T. M., Riley R. T. & Merrill A. H. Jr. Increases in serum sphingosine and sphinganine and decreases in complex sphingolipids in ponies given feed containing fumonisins, mycotoxins produced by Fusarium moniliforme. J Nutr 122, 1706–16 (1992). [DOI] [PubMed] [Google Scholar]
- Riley R. T. et al. Alteration of tissue and serum sphinganine to sphingosine ratio: an early biomarker of exposure to fumonisin-containing feeds in pigs. Toxicol Appl Pharmacol 118, 105–12 (1993). [DOI] [PubMed] [Google Scholar]
- Shephard G. S., Van Der Westhuizen L. & Sewram V. Biomarkers of exposure to fumonisin mycotoxins: a review. Food Addit Contam 24, 1196–201 (2007). [DOI] [PubMed] [Google Scholar]
- Cai Q., Tang L. & Wang J. S. Validation of fumonisin biomarkers in F344 rats. Toxicol Appl Pharmacol 225, 28–39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard G. S., Thiel P. G. & Sydenham E. W. Initial studies on the toxicokinetics of fumonisin B1 in rats. Food Chem Toxicol 30, 277–9 (1992). [DOI] [PubMed] [Google Scholar]
- Riley R. T. et al. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol Nutr Food Res 56, 1445–55 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L. J., Pena A., Lino C. M., Fernandez M. F. & Manes J. Fumonisins determination in urine by LC-MS-MS. Anal Bioanal Chem 396, 809–16 (2010). [DOI] [PubMed] [Google Scholar]
- Xu L. et al. Evaluation of fumonisin biomarkers in a cross-sectional study with two high-risk populations in China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27, 1161–9 (2010). [DOI] [PubMed] [Google Scholar]
- Chelule P. K., Gqaleni N., Chuturgoon A. A. & Dutton M. F. The determination of fumonisin B1 in human faeces: a short term marker for assessment of exposure. Biomarkers 5, 1–8 (2000). [DOI] [PubMed] [Google Scholar]
- Sewram V. et al. Assessing chronic exposure to fumonisin mycotoxins: the use of hair as a suitable noninvasive matrix. J Anal Toxicol 25, 450–5 (2001). [DOI] [PubMed] [Google Scholar]
- Sewram V., Mshicileli N., Shephard G. S. & Marasas W. F. Fumonisin mycotoxins in human hair. Biomarkers 8, 110–8 (2003). [DOI] [PubMed] [Google Scholar]
- Gong Y. Y. et al. Association between tortilla consumption and human urinary fumonisin B1 levels in a Mexican population. Cancer Epidemiol Biomarkers Prev 17, 688–94 (2008). [DOI] [PubMed] [Google Scholar]
- van der Westhuizen L. et al. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol Biomarkers Prev 20, 483–9 (2011). [DOI] [PubMed] [Google Scholar]
- Shirima C. P. et al. Dietary exposure to aflatoxin and fumonisin among Tanzanian children as determined using biomarkers of exposure. Mol Nutr Food Res 57, 1874–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. et al. Urinary fumonisin B1 and estimated fumonisin intake in women from high- and low-exposure communities in Guatemala. Mol Nutr Food Res 58, 973–83 (2014). [DOI] [PubMed] [Google Scholar]
- Robinson A. et al. Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B(1) exposure in rats and humans. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29, 809–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westhuizen L. et al. Simple intervention method to reduce fumonisin exposure in a subsistence maize-farming community in South Africa. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27, 1582–8 (2010). [DOI] [PubMed] [Google Scholar]
- Huang T., Yu J., Tang L., Luo h. & Wang J. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: I. design, clinical outcomes, and baseline biomarker data. Int. J. Cancer Prev 1, 269–280 (2004). [Google Scholar]
- Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys 557, 3–10 (2014). [DOI] [PubMed] [Google Scholar]
- Yamane T. et al. Inhibitory effects and toxicity of green tea polyphenols for gastrointestinal carcinogenesis. Cancer 77, 1662–7 (1996). [DOI] [PubMed] [Google Scholar]
- Narisawa T. & Fukaura Y. A very low dose of green tea polyphenols in drinking water prevents N-methyl-N-nitrosourea-induced colon carcinogenesis in F344 rats. Jpn J Cancer Res 84, 1007–9 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Agarwal R., Bickers D. R. & Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis 12, 1527–30 (1991). [DOI] [PubMed] [Google Scholar]
- Tang L. et al. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis 29, 411–7 (2008). [DOI] [PubMed] [Google Scholar]
- Luo H. et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis 27, 262–8 (2006). [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C. et al. Interaction of fumonisin B(1) and aflatoxin B(1) in a short-term carcinogenesis model in rat liver. Toxicology 171, 161–73 (2002). [DOI] [PubMed] [Google Scholar]
- Carlson D. B. et al. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N’-nitro-nitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol Appl Pharmacol 172, 29–36 (2001). [DOI] [PubMed] [Google Scholar]
- Solfrizzo M. et al. Comparison of urinary sphingolipids in human populations with high and low maize consumption as a possible biomarker of fumonisin dietary exposure. Food Addit Contam 21, 1090–5 (2004). [DOI] [PubMed] [Google Scholar]
- van der Westhuizen L., Shephard G. S., Rheeder J. P., Somdyala N. I. & Marasas W. F. Sphingoid base levels in humans consuming fumonisin-contaminated maize in rural areas of the former Transkei, South Africa: a cross-sectional study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25, 1385–91 (2008). [DOI] [PubMed] [Google Scholar]
- van der Westhuizen L., Shephard G. S., Rheeder J. P. & Burger H. M. Individual fumonisin exposure and sphingoid base levels in rural populations consuming maize in South Africa. Food Chem Toxicol 48, 1698–703 (2010). [DOI] [PubMed] [Google Scholar]
- Abnet C. C. et al. Sphingolipids as biomarkers of fumonisin exposure and risk of esophageal squamous cell carcinoma in china. Cancer Causes Control 12, 821–8 (2001). [DOI] [PubMed] [Google Scholar]
- Ali N., Sardjono, Yamashita A. & Yoshizawa T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit Contam 15, 377–84 (1998). [DOI] [PubMed] [Google Scholar]
- Siame B. A., Mpuchane S. F., Gashe B. A., Allotey J. & Teffera G. Occurrence of aflatoxins, fumonisin B1, and zearalenone in foods and feeds in Botswana. J Food Prot 61, 1670–3 (1998). [DOI] [PubMed] [Google Scholar]
- Wang D. S. et al. Natural co-occurrence of Fusarium toxins and aflatoxin B1 in corn for feed in north Vietnam. Nat Toxins 3, 445–9 (1995). [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Yamashita A. & Chokethaworn N. Occurrence of fumonisins and aflatoxins in corn from Thailand. Food Addit Contam 13, 163–8 (1996). [DOI] [PubMed] [Google Scholar]
- Chamberlain W. J., Bacon C. W., Norred W. P. & Voss K. A. Levels of fumonisin B1 in corn naturally contaminated with aflatoxins. Food Chem Toxicol 31, 995–8 (1993). [DOI] [PubMed] [Google Scholar]
- McKean C. et al. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem Toxicol 44, 868–76 (2006). [DOI] [PubMed] [Google Scholar]
- Mitchell N. J. et al. Calcium montmorillonite clay reduces AFB and FB biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. J Appl Toxicol (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Orozco R., Castellanos-Nava A., Gaytan-Martinez M., Figueroa-Cardenas J. D. & Loarca-Pina G. Comparison of nixtamalization and extrusion processes for a reduction in aflatoxin content. Food Addit Contam 19, 878–85 (2002). [DOI] [PubMed] [Google Scholar]
- Stockmann-Juvala H. & Savolainen K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol 27, 799–809 (2008). [DOI] [PubMed] [Google Scholar]
- De Angelis I. et al. Absorption of fumonisin B1 and aminopentol on an in vitro model of intestinal epithelium; the role of P-glycoprotein. Toxicon 45, 285–91 (2005). [DOI] [PubMed] [Google Scholar]
- Axling U. et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 9, 105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. & Prescott R. Applied Mixed Models in Medicine, 2nd Edition, 476 (John. Wiley & Sons, New York, 2006). [Google Scholar]