Abstract
Background: The present study aimed to determine the effect of lentil sprouts [LS] on lipid profiles in overweight and obese patients with type 2 diabetes.
Methods: Forty- eight overweight and obese type 2 diabetic patients, September and November2013, 30-65 years, participated in this clinical trial and randomly divided into two groups; LS group and controls. Patients in control group received conventional drug therapy, while patients in LS group received 60 g LS daily during 8 weeks along with routine medication. Significant differences among and between the groups were determined by independent t-test and paired sample t-test using SPSS software. The patients were blinded for the treatment. In this trial the effect of LS on serum lipid profiles were inves-tigated.
Results: Thirty-nine patients completed the study. After 8 weeks, serum levels of HDL-C was higher in the LS group compared to control group (48.3 ±1.9 vs. 42.8±1.7, P<0.03). TG and ox-LDL were lower in the LS group compared to controls [(127±13.4 vs. 170± 12.4.P<0.01) and (83.3±29.1 vs. 98.7±28.2.P<0.6)].
Conclusions: LS consumption could have favorable effect on serum lipid profiles.
Keywords: Lentil sprouts, Triglyceride, Oxidized LDL cholesterol, Type 2 diabetes
Introduction
Type 2 diabetes is a metabolic disorder characterized by absolute or relative insulin deficiency, hyperglycemia, and disorder in carbohydrate as well as lipid metabolism.1“The development of chronic hyperglycemia in diabetes leads to severe damage in bodily tissues, organ dysfunctions and finally the irreversible failure of some critical organs of the body, especially eyes, kidneys, nerves and cardiovascular system”.2 Due to side effects of drugs used to treat diabetes, using complementary therapies and modification of the dietary pattern are good ways to improve the condition of disease. Legumes consumption has many effects in health improving, control and protection against metabolic diseases such as type 2 diabetes and cardiovascular diseases (CVDs).3 According to food ingredient table data, the amount of lentil seed fiber is 3.7 gr/100 and has a low glycemic index (21.1). After germination of seeds the amount of fiber and protein are increased.4 After consumption of lentils in diabetic patients TC and glucose decreasedsignificantly5, and after consumption of lentil sprouts (LS) glucose decreased.6 Besides, oxidized low density lipoprotein [ox-LDL] has a major role at the beginning and the development of atherosclerosis.7 HDL-C has a major role in inhibiting LDL-C oxidation particles.8
There are limited data regarding the potential properties of lentil sprouts on metabolic disorders; we therefore conducted this trial to investigate the effect of LS on serum TG, TC, LDL-C, HDL-C levels and atherogenic lipid parameters including AIP (atherogenic index of plasma), the ratios of ox-LDL/TC, ox-LDL/HDL-C and ox-LDL/LDL-C, TG/HDL and LDL/HDL were measured in overweight and obese patients with type 2 diabetes.
Materials and Methods
Study population
Patients [male and female] with type 2 diabetes, referred to the Iran Diabetes Society and Endocrine Clinic of Taleghani Medical Center, Tehran, Iran were recruited for this study. Diagnosis of type 2 diabetes was according to American Diabetes Association.9 This trial was conducted between September and November 2013. The patients aged 30-65yr, with body mass index ranged 25-40 kg/m2 were included. Subjects with renal, hepatic disorders, gestation or lactation and patients who used insulin injection, contraceptive pills, glucocorticoid drugs or LS and multivitamin as well as supplements with antioxidant in the last 3 months were excluded from the study. Finally, forty-eight subjects [31 men and 17 women] were included and divided into two groups, LS=19 and control=20, randomly.
Intervention
Lentil sprouts preparation
The lentil seeds were placed in water for 30 hours. The seeds were placed on a wet cotton tissue. After 24 hours, lentil sprouts were packed and sent to patients. In these stages, temperature of environment was 25-30°C, but after the preparation, they were kept in a refrigerator.
Treatment
Patients in LS group received 60 g LS daily, and the patients in control group continued regular diet. Patients in the LS group consumed LS alone or with salads fresh. According to Dietary Reference Intake, each patient consumed 60 g LS in LS group.10 In order to assess dietary changes during the study period three-day dietary recalls, including 2 weekends and 1 weekend day, was collected at baseline and again after 8 weeks from the subjects. Subjects provided with written informed consent. The method of consumption was written for the participants. They were asked to maintain their habitual life style, physical activity and dietary pattern during the eight weeks of study. Patients were excluded from the analysis if they consumed <85% of the packets11 or changed their medication or reported severe side effects. For being confident that the patients consume LS, the researcher called them weekly.
Anthropometric and biochemical measurements
Anthropometric measurements including weight, height and waist circumference were measured at baseline and eight weeks later. Weight was measured with digital scale (Seca 707, Hamburg, Germany, nearest to 100g) while the subjects were minimally clothed without shoes. Height was measured to the nearest0.5 cm, in a standing position without shoes, using a tape meter. Body mass index [BMI] was calculated as follows: weight/ square of height [kg/m2]. Venous blood samples were drawn after an overnight fasting at baseline and again after intervention [Days 0 and 56] and were centrifuged at 4°C and 500 g for 10 min to separate plasma. Venous blood sample volume was 7 ml. Serum concentrations of TC, triglyceride [TG] and HDL-C were evaluated by using the standard enzymatic methods with commercially available Parsazmun kits (Tehran, Iran). LDL-C was calculated from serum TC, TG and HDL-C according to the friedewalde equation.12 TC was assayed with the cholesterol esterase and cholesterol oxidize method and TG was assayed by using glycerol phosphate oxidize. The monoclonal antibody was used by ELISA kit to qualify the concentration of serum oxidized LDL (Mercodia Company, Uppsala, Sweden). AIP as defined logarithm of the TG/HDL-C ratio, directly related to lipoprotein particle size and the risk of atherosclerosis [AS].13,14 Inter- Intra-assay coefficient of variations of all assays were <5%.
Statistical analysis
According to statistical methods for triglyceride variable15 with 95% confidence and 80% power, the sample size with regard to possible loss of the samples was calculated 20 patients in each group. The experimental data were analyzed by SPSS software, version16 (SPSS Inc., Chicago, IL, USA). The normality of the distribution of variables was determined by the Kolmogorov– Smirnov test. Analysis of covariance was used to identify any differences between two groups after intervention, adjusted for baseline measurements and covariates including lipid lowering and anti- diabetic drugs. The changes in anthropometric measurements, lipid profile and ox-LDL parameters of the participants between the beginning and end of the trial were compared by paired sample t-tests. The percent change for each variable was also calculated by formula [(8- weeks values- baseline values)/baseline values×100]. Also, P- value <0.05 was considered significant. Main researcher generated the random, enrolled participants and assigned participants to interventions.
Ethical approval
Ethics approval was obtained from Ethical Committee of Tabriz University of Medical Science. The trial has been registered in the Iranian Registry of Clinical trial at http://www.irct.ir with the following identification IRCT201305251640N9.
Results
Forty-eight subjects were enrolled and divided into two groups randomly, but only 39 were available for data analysis because some of them could not complete the entire protocol or come for secondary evaluation. Final analysis was per protocol. Randomization of the patients was simply enveloped. Patients were blinded. These 39 patients were composed of 12 women and 27 men (Figure 1). Subjects’ demographics are shown in Table 1.
Fig. 1.
Flow chart of study participants
Table 1. Demographic characteristics of diabetic patients in Control and lentil sprout groups .
| Variables | Control (n=20) | LS (n=19) | P value |
| Age (yr) | 54±7.4 | 52±7.6 | 0.9 |
| Women/men(n) | 7/13 | 5/14 | 0.5 |
| Duration of diabetes(y) | 7.4±6.6 | 11.7±19.2 | 0.3 |
| Weight (kg) | 78.5±10.5 | 78.3±9.8 | 0.7 |
| Height (m) | 1.63±0.09 | 1.66±0.08 | 0.6 |
| BMI(kg/m2) | 28.2±2.6 | 29.4±3.6 | 0.3 |
| Glucoselowering drugs (n), (%) | (13), (65%) | (19), (100%) | 0.01 |
| Lipid lowering drugs (n) | 2 | 9 | 0.03 |
| Blood pressure lowering drugs(n) | 3 | 7 | 0.1 |
| FBS(mg/dl) | 162.5±55 | 159±44 | 0.8 |
a All values are mean±SD (unless stated otherwise).
No significant differences between groups were seen for mean age, duration of diabetes, weight, body mass index, blood lowering pressure drugs; nor were there any significant differences between the pretreatment serum values of total cholesterol, low density LDL-C, high density lipoprotein cholesterol [HDL-C], triglycerides, atherogenic index of plasma [AIP], TC/HDL-C ratio, LDL-C/HDL-C ratio, ox-LDL, ox-LDL/LDL-C ratio, ox-LDL/HDL-C ratio and ox-LDL/TC ratio in the 2 groups at baseline (Table2). Some patients used glucose lowering drugs including metformin and glibenclamide. Some participants used lipid-lowering drugs such as statins. Some of them used blood pressure lowering pressure such as nifdidin. Attention to disease severity differed amount of drugs for each patient.
Table 2. Biochemical values of diabetic patients in LS and control groups .
| Variables | Control (n=20) | LS (n=19) | P value |
| TC (mg/dl) | 167.9±0.3 | 158.8±0.4 | 0.162 |
| TG (mg/dl) | 130±55.5 | 132±68.2 | 0.234 |
| LDL-C (mg/dl) | 97.5±27.8 | 88.9±34.5 | 0.383 |
| HDL-C (mg/dl) | 44.2±10.8 | 43.4± 11.8 | 0.935 |
| AIP | 0.05±0.015 | 0.051±0.016 | 0.451 |
| TG/HDL-C ratio | 3.2±2 | 3.3± 2.3 | 0.253 |
| LDL-C/HDL-C ratio | 2.3±0.8 | 2.2± 0.9 | 0.484 |
| ox-LDL (mU/ml) | 111±38.1 | 102.7± 60.5 | 0.215 |
| ox-LDL/LDL-C ratio | 1.3±0.7 | 1.5± 1.6 | 0.244 |
| ox-LDL/HDL-C ratio | 2.6±0.9 | 2.6± 1.8 | 0.862 |
| ox-LDL/TC ratio | 0.7±0.33 | 0.69± 0.5 | 0.402 |
TC, total cholesterol; TG, triglyceride; LDL-C, low density; HDL-C, high density lipoprotein; ox-LDL, oxidized low density lipoprotein; AIP; atherogenic index of plasma
a All values are mean±SD.
There were significant differences in lipid lowering and anti-diabetic drugs between two groups, therefore these variables were adjusted in the analysis. The mean dietary intakes of participants at baseline and after 8-week of intervention a presented in Table 3.
Table 3. Dietary intakes of the study participants at baseline and after 8 weeks of intervention in the two groups .
| Variable |
Control,(20)
Mean (SD) |
LS,(n=19)
Mean(SD) |
Mean difference
(95% confidence interval)* |
P * |
| Energy (kcal) | ||||
| Baseline | 1894.4(464.3) | 1892.5(427.4) | 1.9(-95.14-229.13) | 0.191 |
| After intervention | 1882.5(442.5) | 1880.7(492.6) | 2.02(-87.33-187.66) | 0.163 |
| Mean difference (95% confidence interval)** |
-12.1(-142.5-111.3) | -11.1(-122-109) | - | - |
| P** | 0.68 | 0.43 | - | - |
| Carbohydrate (g/d) | ||||
| Baseline | 249.4(69.2) | 239.2(86.6) | 10.2(-65.3-87.5) | 0.357 |
| After intervention | 242.5(76.7) | 226.3(64.5) | 16.4(-77.2-43.6) | 0.253 |
| Mean difference (95% confidence interval)** |
-7.1(-28-74) | -13.1(-64-35) | - | - |
| P** | 0.54 | 0.63 | - | - |
| Protein(g/d) | ||||
| Baseline | 84(21.5) | 76.1(28.2) | 8(-3.1-17.3) | 0.448 |
| After intervention | 86(26.3) | 80.2(22.5) | 6(-2.4-21.2) | 0.363 |
| Mean difference (95% confidence interval)** |
2(-3-16) | 4(-2-20) | - | - |
| P** | 0.78 | 0.48 | - | - |
| Total fat (g/d) | ||||
| Baseline | 63.1(25.2) | 64.3(28.5) | -1.2(-3.4-19) | 0.287 |
| After intervention | 61.4(21.5) | 62.8(28.1) | -1.4(-5.3-23) | 0.334 |
| Mean difference (95% confidence interval)** |
-1.7(-4-21) | -1.5(-1.8-19) | - | - |
| P** | 0.46 | 0.65 | - | - |
| Saturated fat(g/d) | ||||
| Baseline | 17.9(5.4) | 18.3(6.2) | -0.4(-7.1-15.3) | 0.631 |
| After intervention | 18.5(5.1) | 19.4(6.2) | -0.9(-5.3-19 | 0.484 |
| Mean difference (95% confidence interval)** |
0.6(-4-23) | 1.1(-3.3-25.2) | - | - |
| P** | 0.55 | 0.43 | - | - |
| Mono-unsaturated fat(g/d) | ||||
| Baseline | 17.2(6.2) | 18.4(7.5) | -1.2(-3.4-26.1) | 0.458 |
| After intervention | 16.7(6.6) | 17.8(8.1) | -1.1(-2.6-24.3) | 0.563 |
| Mean difference (95% confidence interval)** |
-0.5(-4.1-22.3) | -04(-3.2-21.4) | - | - |
| P** | 0.62 | 0.78 | - | - |
| Poly-unsaturated fat (g/d) | ||||
| Baseline | 15.7(7.4) | 16.4(6.8) | -0.7(-4.6-22.4) | 0.608 |
| After intervention | 15.1(7.1) | 15.9(6.2) | -0.8(-2.6-18.5) | 0.767 |
| Mean difference (95% confidence interval)** |
-0.6 (-3.2-17.4) | -0.5(-1.8-19.2) | - | - |
| P** | 0.34 | 0.48 | - | - |
| Vitamin C(mg/d) | ||||
| Baseline | 57.8(30.8) | 63.5(40.2) | -5.7(-4.2-25.1) | 0.254 |
| After intervention | 67.8(36.8) | 72.1(37.3) | -4.3(-2.8-28.4) | 0.342 |
| Mean difference (95% confidence interval)** |
10(-1.9-23.4) | 8.4 (-3.1-15.3) | - | - |
| P** | 0.33 | 0.52 | - | - |
| Vitamin E(mg/d) | ||||
| Baseline | 4.1(6.6) | 2.3(1.8) | 1.8(-5.2-17.3) | 0.47 |
| After intervention | 3.8(5.8) | 2.5(2.3) | 1.3(-4.2-18.1) | 0.526 |
| Mean difference (95% confidence interval)** |
-0.3(-3.4-16.8) | -0.2(-3.7-18.2) | - | - |
| P** | 0.44 | 0.35 | - | - |
| Total fiber (g/d) | ||||
| Baseline | 23.6(8.8) | 23.7(6.5) | -0.1(-3-24) | 0.454 |
| After intervention | 25.4(4.3) | 24.2(7.7) | 0.2(2.127.3) | 0.378 |
| Mean difference (95% confidence interval)** |
1.8(-2.4-24.1) | 0.5(-2.1-14.2) | - | - |
| P** | 0.65 | 0.78 | - | - |
*Independent t-test, ** paired t-test
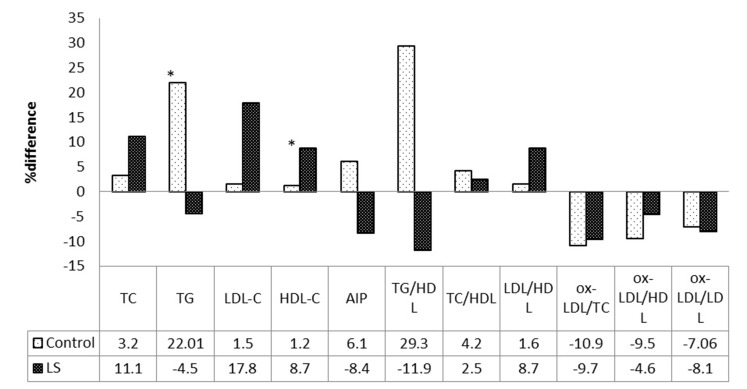
There was no significant difference between the groups in total energy and nutrient intakes, as estimated by 3-day dietary recalls. Eight-week biochemical values of participants in the two groups, and the treatment effects of LS on lipid profiles are presented in Figure 2.
Fig. 2.
Mean differences of variables compared with baseline values in two groups (*significant difference within the groups using paired t-test, P<0.05).TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein; HDL-C, high density lipoprotein; ox-LDL, oxidized- low density lipoprotein; AIP, atherogenic index of plasma
Data of this study were primary. In the LS group, the levels of HDL-C and LDL-C increased compared with baseline (P<0.03, P<0.06) but in the control group no significant change was seen. In the LS group the levels of TG, AIP and ox-LDL decreased compared with the baseline values (P<0.01 and P<0.07 and<0.6). Patients in the intervention group did not report any side- effects from LS consumption.
Discussion
In this trial, we investigated the effect of LS on lipid profiles in overweight and obese patients with type 2 diabetes. TG and ox-LDL decreased (P<0.01 and P<0.6) and HDL-C increased (P<0.03) in LS group compared to control group.
Lipid blood disorders play a major role in CVDs risk in diabetic patients.16 In an animal experiment, rats consumed whole cooked lentils, level of HDL-C was significantly increased.17 Low HDL-C is an independent cardiovascular risk factor and management of HDL-C may contribute to the optimization of cardiovascular risk in Type 2 diabetes.18 Changes in dietary pattern, physical activity and weight can convert HDL-C level.19 In this study, patients were asked not to change their dietary pattern and physical activity. Weight of patients did not differ significantly between 2 groups after 8 weeks intervention. In a study, a high-fiber diet improved glycemic control and reduced serum lipids and HDL-C level increased in LS group after 8 weeks of LS consumption and this effect could be due to some bioactive components in LS.20Lentil seeds are rich source of fiber and micronutrients. Picillium increased the level of serum HDL-C in patients with type 2 diabetes.21Hypertriglyceridemia is a common form of dyslipidemia frequently associated with coronary heart disease (CHD).22 Our results showed that TG level decreased in LS group after 8 weeks of LS consumption (P<0.01).Several mechanisms could explain favorable effects of lentil and lentil sprouts on lipid and lipoprotein metabolism; high content of fiber could decrease bile acid and cholesterol reabsorption from Ileum.23
Another important finding of the current study was decreased TG/HDL-C ratio following treatment with LS. Decreased TG /HDL ratio is related to large and non-atherogen LDL-C particles.24,25
Diet rich in fruits and vegetables due to plant fibers, carotenoids, anti-oxidants and phytosterols can prevent and control non-communicable diseases such as diabetes.26 Herbal antioxidants have insulin-like effects and increased glucose absorption in peripheral tissues.27 Lentil seeds are rich source of antioxidants as compared to other cereals and have high antioxidant capacity.28 Lentils had a higher oxygen radical absorbing capacity (ORCA)value than most of the common fruits and vegetables such as apples, plums, blackberries, cherries, figs, peaches, pears, orangs, garlic, cabbage and almonds.29Nutrient composition of lentil is shown in Table 4.30
Table 4. Nutrient composition of lentil .
| Nutrient | Unit | Value per 100g | Cup 77g |
| Proximates | |||
| Energy | (kcal) | 106 | 82 |
| Protein | (g) | 9 | 7 |
| Total lipid | (g) | 0.6 | 0.4 |
| Carbohydrate, by difference | (g) | 22 | 17 |
| Minerals | |||
| Iron, Fe | (mg) | 3 | 2 |
| Potassium, K | (mg) | 322 | 248 |
| Sodium, Na | (mg) | 11 | 8 |
| Vitamins | |||
| Folat, DEF | (µg) | 100 | 77 |
| Vitamin C | (mg) | 17 | 13 |
| Niacin | (mg) | 1.2 | 0.8 |
Low HDL-c and high TG concentration is considered as a main risk factor for development of, CHD.31-33 Plasma ox-LDL increases in conditions such as diabetes,34central obesity35 and CAD. This may be considered as a marker of the AS.36 High level of ox-LDL may indicate the severity of pathological inflammatory response.37,38 Oxidation ratio of LDL had a stronger correlation with CVDs in comparison with plasma oxidized LDL.39 ox-LDL and oxidation ratio of LDL (ox-LDL/TC, ox-LDL/HDL-C and ox-LDL/LDL-C) were closely related with AS,39 and these indicators were better biomarkers for discriminating between patients with CVDs and healthy subjects than the usual tests.39 ox-LDL/TC, ox-LDL/LDL-C, ox-LDL/HDL-C were higher in CVDs group.40 Reduction of ox-LDL/LDL, ox-LDL/TC and ox-LDL/HDL-c could be related with key bioactive components in the LS.
The present study had a few limitations because the sample size was small and the study duration was 8 weeks. Further studies with longer duration and various doses may shed more light on importance of the therapeutic effects of LS in diabetic patients. Due to some inclusion and exclusion criteria such as specified BMI range defined for this study, our findings could not be generalized and interpreted to all type 2 diabetic patients.
Conclusion
Consumption of LS as supplementary treatment in type 2 diabetes could have favorable effects on lipid metabolism and atherogenic lipid parameters. Such findings imply that nutritional intervention and use of functional foods such as lentil sprouts along with common medical treatment may be an important strategy in management of cardiovascular risk factors and metabolic disorders in type 2 diabetic patients.
Competing Interests
There is no conflict of interests.
Acknowledgements
This study was funded by the Faculty of Nutrition, Tabriz University of Medical Sciences and Research Institute of Endocrine Sciences of Shahid Beheshti University of Medical Sciences. The authors express appreciation to the participants of this study. The authors wish to thank Mrs. N. Shiva for critical editing of English grammar and syntax of the manuscript.
Citation: Aslani Z, Mirmiran P, Alipour B, Bahadoran Z, Abbassalizade Farhangi M. Lentil Sprouts Effect On Serum Lipids of Overweight and Obese Patients with Type 2 Diabetes. Health Promot Perspect 2015; 5(3): 215-224. doi: 10.15171/hpp.2015.026
References
- 1.Akbar S, Bellary S, Griffiths HR. Dietary antioxidant interventions in type 2 diabetes patients: a meta-analysis. Br J Diabetes Vasc Dis. 2011;11:62–68. doi: 10.1177/1474651411407558. [DOI] [Google Scholar]
- 2.Gan MJ, Albanese-O’Neill A, Haller MJ. Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care. 2012;42:269–291. doi: 10.1016/j.cppeds.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Giacco R, Parillo M, Rivellese AA, Lasorella G, Giacco A, D'Episcopo L. et al. Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care. 2000;23:1461–1466. doi: 10.2337/diacare.23.10.1461. [DOI] [PubMed] [Google Scholar]
- 4. Asghari E, Rahmani K, Taslimi A. Evaluation of physical and chemicalproperties supplementary food prepared from germinated wheat and lentil. Journal of Food Science and Technology 2006;3:33-40.[In Persian].
- 5. Shams H, Tahbaz F, Entezari MH, Abadi A. Effects of cooked lentils on glycemic control and blood lipids of patients with type 2 diabetes. ARYA Atherosclerosis 2010;4.
- 6.Aslani Z, Alipour B, Bahadoran Z, Bagherzadeh F, Mirmiran P. Effect of lentil sprouts on glycemic control in overweight and obese patients with type 2 diabetes. Int J Nutr Food Sci. 2015;4:10–14. doi: 10.11648/j.ijnfs.s.2015040201.13. [DOI] [Google Scholar]
- 7.Van Himbergen TM, Van Tits LJH, Roest M, Stalenhoef AFH. The story of PON1: how an organophosphate-hydrolysing enzyme is becoming a player in cardiovascular medicine. Neth J Med. 2006;64:34–38. [PubMed] [Google Scholar]
- 8.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV. et al. Distribution of apoA-I–containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2670–2676. doi: 10.1161/01.ATV.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 10.McCrory MA, Lovejoy JC, Palmer PA, Eichelsdoerfer PE, Gehrke MM, Kavanaugh IT. et al. Effectiveness of legume consumption for facilitat¬ing weight loss: a randomized trial. FASEB J. 2008;22:1084–1088. [Google Scholar]
- 11.Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabet Res Clin Pract. 2012;96:348–354. doi: 10.1016/j.diabres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Cordova CM, Schneider CR, Juttel ID, Cordova MM. Comparison of LDL-cholesterol direct measurement with the estimate using the Friedewald formula in a sample of 10,664 patients. Arq Bras Cardiol. 2004;83:476–481. doi: 10.1590/S0066-782X2004001800006. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 15.Afaghi A, Ziaee A, Afaghi M. Effect of low-glycemic load diet on changes in cardiovascular risk factors in poorly controlled diabetic patients. Indian J Endocrinol Metab. 2012;16:991–995. doi: 10.4103/2230-8210.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liese AD, Nichols M, Sun X, D'Agostino RB Jr, Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32:1434–1436. doi: 10.2337/dc09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslani Z, Alipour B, Mirmiran P, Bahadoran Z. Aslani Z, Alipour B, Mirmiran P, Bahadoran ZLentil’s (Lens culinaris L) functional properties in prevention and treatment of non-communicable chronic diseases: A review. Int J Nutr Food Sci. 2015;4:15–20. doi: 10.11648/j.ijnfs.s.2015040201.14. [DOI] [Google Scholar]
- 18.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R. et al. Primary prevention of cardio¬vascular diseases in people with diabetes melli¬tus: a scientific statement from the American Heart Association and the American Diabetes Associa¬tion. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-0463. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL. et al. Managing abnormal blood lipids: A collaborative approach: Cosponsored by the Councils on Cardiovascular Nursing; Arteriosclerosis, Thrombosis, and Vascular Biology; Basic Cardiovascular Sciences; Cardiovascular Disease in the Young; Clinical Cardiology; Epidemiology and Prevention; Nutrition, Physical Activity, and Metabolism; and Stroke; and the Preventive Cardiovascular Nurses Association. Circulation. 2005;112:3184–3209. doi: 10.1161/CIRCULATIONAHA.105.169180. [DOI] [PubMed] [Google Scholar]
- 20.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–1398. doi: 10.1056/nejm200005113421903. [DOI] [PubMed] [Google Scholar]
- 21. Ziaee SA, Ardeshir larijani MB, Fakhrzade H, Dast pak A, Bandarian F, Rezaee A, et al. Effect of Pecilium(Plantago ovata L.) in controlling blood fat of patients with type 2 diabetes. Journal of Medicinal Plants 2004;12:33-42.[In Persian].
- 22.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S. et al. Triglycerides and the risk of coronary heart disease 10 158 incident cases among 262-525 participants in 29 western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 23.Fontvieille AM, Rizkalla SW, Penfornis A, Acosta M, Bornet FR, Slama G. The use of low glycaemic index foods improves metabolic control of diabetic patients over five weeks. Diabet Med. 1992;9:444–450. doi: 10.1111/j.1464-5491.1992.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 24.Alhassan S, Kiazand A, Balise RR, King AC, Reaven GM, Gardner CD. Metabolic syndrome: do clinical criteria identify similar individuals among overweight premenopausal women? Metabolism. 2008;57:49–56. doi: 10.1016/j.metabol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama C, Imamura K, Teramoto T. Assessment of LDL particle size by triglyceride/HDL-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J Atheroscler Thromb. 2003;10:186–191. doi: 10.5551/jat.10.186. [DOI] [PubMed] [Google Scholar]
- 26.Urooj A, Puttaraj S. Glycaemic responses to cereal-based Indian food preparations in patients with non-insulin-dependent diabetes mellitus and normal subjects. Br J Nut. 2000;83:483–488. doi: 10.1017/S0007114505000611. [DOI] [PubMed] [Google Scholar]
- 27. Madani H. Ahmadi Mahmoodabadi N, Vahdati A. Effects of hydroalcoholic extract of Anethum graveolens (Dill) on plasma glucose and lipid levels in diabetes induced rats. Iranian Journal of Diabetes and Lipid2006;2:109-116.[In Persian].
- 28.Xu B, Chang SK. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 29. Haytowitz DB, Bhagwat S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. Maryland: U.S. Depart¬ment of Agriculture; 2010. Available from: http://www.orac-info-portal.de/download/ORAC_R2.pdf.
- 30. National Nutrient Database for Standard Reference Release 27.2015. Basic report 15232. Available from: http://ndb.nal.usda.gov/ndb/foods/show/4683.
- 31.Barzi F, Patel A, Woodward M, Lawes CM, Ohkubo T, Gu D. et al. A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol. 2005;15:405–413. doi: 10.1016/j.annepidem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 33.Rizos E, Mikhailidis D. Are high-density lipoprotein and triglyceride levels important in secondary prevention: impressions from the BIP and VA-HIT trials. Int J Cardiol. 2002;82:199–207. doi: 10.1016/S0167-5273(01)00625-8. [DOI] [PubMed] [Google Scholar]
- 34.Nakhjavani M, Khalilzadeh O, Khajeali L, Esteghamati A, Morteza A, Jamali A. et al. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321–327. doi: 10.1007/s11745-010-3401-8. [DOI] [PubMed] [Google Scholar]
- 35.Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB. et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimada K, Mokuno H, Matsunaga E, Miyazaki T, Sumiyoshi K, Miyauchi K. et al. Circulating oxidized low-density lipoprotein is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis. 2004;174:343–347. doi: 10.1016/j.atherosclerosis.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM. et al. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23:1444–1448. doi: 10.1161/01.ATV.0000080379.05071.22. [DOI] [PubMed] [Google Scholar]
- 38.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB. et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Ma R, Liu D, Liu C, Ma Y, Mai W. et al. Oxidized low-density lipoprotein cholesterol and the ratio in the diagnosis and evaluation of therapeutic effect in patients with coronary artery disease. Dis Markers. 2012;33:295–302. doi: 10.3233/DMA-2012-00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Mai W, Liu D, Hao Y, Tao J, Dong Y. The oxidation ratio of LDL: a predictor for coronary artery disease. Dis Markers. 2008;24:341–349. doi: 10.1155/2008/371314. [DOI] [PMC free article] [PubMed] [Google Scholar]