Abstract
The development of acute respiratory distress syndrome (ARDS) is associated with dys-regulated inflammation. Since corticosteroids are potent anti-inflammatory drugs, they are thought to be beneficial for ARDS patients. The study aimed to investigate the effectiveness of corticosteroids on mortality outcome in ARDS patients. The study was a secondary analysis of a prospective randomized controlled trial (NCT00979121). ARDS patients with invasive mechanical ventilation were enrolled. Corticosteroids use was defined as IV or PO administration of corticosteroids totaling more than 20 mg methylprednisolone equivalents during one calendar day. Missing data were handled using multiple imputation technique. Multivariable model was built to adjust for confounding covariates. A total of 745 patients were enrolled, including 540 survivors and 205 non-survivors. Patients in the non-survivor group were more likely to use corticosteroids (38% vs. 29.8%; p = 0.032). After adjustment for other potential confounders, corticosteroids showed no statistically significant effect on mortality outcome (OR: 1.18; 95% CI: 0.81–1.71). Furthermore, we investigated the interaction between corticosteroid use and variables of vasopressor and PaO2. The result showed that there was no significant interaction. In conclusion, the study failed to identify any beneficial effects of corticosteroids on mortality outcome in patients with ARDS.
Acute respiratory distress syndrome (ARDS) is commonly seen in the intensive care unit (ICU), with an estimated incidence around 20% to 50% depending on different study populations1,2. Development of ARDS or its less severe form acute lung injury (ALI) has been associated with adverse outcome. Therefore, strenuous effort has been done to investigate the treatment of ARDS. Although varieties of interventions such as protective ventilation, negative fluid balance, activated protein C and statin has been thought to be clinically useful for outcome improvement, none of them was supported by strong evidence.
Pathophysiologically, the development of ARDS is associated with dys-regulated inflammation, interstitial and alveolar edema, infiltration of cells into alveolar space and endothelial injury3,4,5. Corticosteroids are potent anti-inflammatory drugs that act primarily by down-regulating proinflammatory cytokines such as interleukins 1a, 1b, 2 and 3. Thus corticosteroids are thought to be effective in improving clinical outcomes of ARDS patients6.
Several studies have been conducted to investigate the effectiveness of corticosteroids in ALI and/or ARDS. These results are conflicting7,8,9, and the sample sizes are usually small. For example, Meduri GU and coworkers8 reported that methylprednisolone was able to ameliorate systemic inflammation response, resulting in significant improvement in pulmonary and extrapulmonary organ dysfunction and reduction in duration of mechanical ventilation and ICU length of stay. However, the study enrolled less than 100 subjects, which was subject to sampling error. In some studies, the effectiveness of corticosteroids in ARDS was only addressed in subgroup analysis. Several meta-analyses reviewed these studies and concluded that there were significant heterogeneity in component trials and the benefits of corticosteroid needs further investigations10,11,12. The present study aimed to investigate the effectiveness of low-dose corticosteroids on mortality outcome in ARDS patients.
Methods
The study was a secondary analysis of a prospective randomized controlled trial (NCT00979121). The dataset was collected from 44 enrolling hospitals in the national heart, lung and blood institute ARDS clinical trial network. The original study was approved by the institutional review board at each participating center13. The secondary data analysis was approved by the institutional review board of Jinhua municipal central hospital. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects in the original study. Patient records/information was anonymized and de-identified prior to analysis.
Study population
Patients were eligible if they fulfilled following criteria: (1) invasive mechanical ventilation; (2) a partial pressure of arterial oxygenation to fraction of inspired oxygen ratio of less than 300 mmHg; (3) bilateral infiltrates on chest radiography; (4) without evidence of left atrial hypertension. All these criteria must be fulfilled within 24 hours after randomization. Exclusion criteria included: (1) presence of ARDS for more than 48 hours; (2) chronic conditions that impair weaning from mechanical ventilation, or compromise adherence to study protocol; (3) inability to obtain consent13.
Corticosteroid use
Corticosteroids use was defined as IV or PO administration of corticosteroids totaling more than 20 mg methylprednisolone equivalents during one calendar day. 20 mg methylprednisolone equals to 3.75 mg dexamethasone, 25 mg prednisone and 100 mg hydrocortisone. Corticosteroids were recorded during day 1 to day 7. For patients who died or discharged before day 7, this was recorded as missing values.
Study endpoint
In the original study, patients were followed up until death or day 90 after enrollment. The study endpoint was categorized into three conditions: (1) Home with unassisted breathing (UAB): the patient is discharged home with unassisted breathing. The home here is defined as the place the patient lived prior to this episode of hospital admission; (2) death: the patient died prior to home discharge or died prior to achieving unassisted breathing at home for 48 hours; (3) Other: neither of the above condition was met. For example, if a patient went home on assisted breathing and has not achieved unassisted breathing for 48 hours, continues on assisted breathing, or has been transferred to another facility, other than home, on unassisted breathing. Conditions (1) and (3) were combined as survivors and condition (2) was regarded as non-survivors.
Data extraction
The original study examined the effectiveness of rosuvastatin on mortality outcome. However, the rosuvastatin showed neutral effect and we did not consider the effect of rosuvastatin on mortality. Demographics such as gender, age and ethnics were reported. The type of ICU including medical intensive care unit (MICU), surgical intensive care unit (SICU), cardiac SICU, coronary care unit (CCU), Neuro ICU, burn care unit, trauma ICU and mixed MICU/SICU were obtained. Other included variables were the number of quadrants with infiltrates on chest X-ray; suspected or documented infection site; vasopressor use, urine output, partial pressure of arterial oxygen (PaO2), central venous pressure (CVP), creatinine kinase (CK), alanine aminotransferase (ALT), C-reactive protein (CRP) and APACHE III score. All these variables were recorded within 24 hours after enrollment.
Statistical analysis
Univariate analysis
Variables were expressed as mean (SD) or the frequency as appropriate. Comparisons between survivors and non-survivors were performed by using student t test for continuous variables, or Chi-square test for categorical variables.
Multiple imputation
Because missing values were common in the dataset, we employed multiple imputation (IM) to address the problem of information loss due to listwise deletion of observations in estimation14,15. The main appealing features of MI included (1) the ability to perform varieties of completed-data analyses using existing statistical methods; and (2) separation of the imputation step from the analysis step. To reduce the sampling error due to imputations, we set the number of imputations to be 20 as recommended by some authors16.
Variables to be incorporated in the logistic regression model for completed-data analysis were gender, type of ICU, ethnic, source of infection, APACHE III, vasopressor use on day 0, CVP, the number of quadrats of infiltrates, CRP, CK, ALT, urine output. These variables were empirically proven or thought to be associated with mortality outcome17,18,19,20,21,22. Variables included in APACHE III as components were not used in multivariable model to avoid the potential problem of multicollinearity. We examined these variables with STATA command codebook, which showed that APACHE III, CVP, the number of quadrats of infiltrates, CRP, CK and urine output contained missing values.
We followed several steps to perform the MI procedure: (1) the dataset was declared as marginal long style, because it was a memory-efficient style. (2) All variables with missing values were registered as imputed variable. (3) multivariate normal regression model was used for the imputation procedure. Variables employed for imputation were those obtained within 24 hours after initiation of the study including mortality outcome, age, gender, source of admission, type of patients, chronic dialysis, vasopressor use, temperature, blood pressure, heart rate, respiratory rate and infection site. There were no missing values for these variables. We created 20 imputations to reduce the simulation (Monte Carlo) error. The seed was arbitrarily set to be 29390 for reproducibility. (4) We fitted the logistic regression using the mi estimate prefix command.
Model building strategy
Because the purpose of the study was to adjust for the effectiveness of corticosteroid, we included as much covariate as possible. Variables to be incorporated in the logistic regression model for completed-data analysis were gender, type of ICU, ethnic, source of infection, APACHE III, vasopressor use on day 0, CVP, the number of quadrats of infiltrates, CRP, CK, ALT, urine output and PaO2. Because patients on shock requiring vasopressors and/or severe hypoxia may benefit from the use of corticosteroids, we explored interactions between them. Because the aim of the study was to investigate the effectiveness of corticosteroids on ARDS patients (e.g. the predictive value of the model was not so important), we included all covariates that were thought to be associated with mortality outcome. Model discrimination and calibration were assessed by graphical presentation of observed and predicted outcomes, as well as the receiver operating characteristic curve (ROC). Also we reported the Homser-Lemeshow goodness-of-fit statistic for assessment of model fit23.
All statistical analyses were performed by using STATA 13.1 (College Station, TX 77845, USA). Statistical significance was considered at p < 0.05.
Results
A total of 745 patients were enrolled, including 540 survivors and 205 non-survivors. Patients in the non-survivor group were more likely to use corticosteroids (38% vs. 29.8%; p = 0.032). As expected, more patients in the non-survivors required vasopressor than survivors (63.4% vs. 51.5%; p = 0.003). Other variables such as gender, ethnic, ICU location, the number of quadrants with infiltrates and infection site were not significantly different between survivors and non-survivors (Table 1). Survivors were significantly younger (52.00 ± 15.92 vs. 59.71 ± 16.17 years, p < 0.001) and had lower values of APACHE III (88.42 ± 26.86 vs. 106.72 ± 27.30; p < 0.001) than non-survivors. CK value was higher in survivors than in non-survivors (244.88 ± 430.80 vs. 151.34 ± 327.31 U/l; p = 0.01). Survivors had significantly greater volume of 24-hour urine output than non-survivors (1668.75 ± 1235.44 vs. 1437.33 ± 1226.21 ml; p = 0.02). Other continuous variables such as CVP, ALT, CRP and PaO2 were not statistically different (Table 2). Missing values in corticosteroid use increased with time (Fig. 1). There were 10% missing values on day 1 and this figure monotonously increased to 40% on day 7.
Table 1. Comparison between survivors and non-survivors for categorical variables.
| Survivors | Non-survivors | Total | ||||
|---|---|---|---|---|---|---|
| No. | percentage | No. | percentage | No. | percentage | |
| Corticosteroids use§ | ||||||
| No | 379 | 70.2% | 127 | 62.0% | 506 | 67.9% |
| Yes | 161 | 29.8% | 78 | 38.0% | 239 | 32.1% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(1) = 4.6235 Pr = 0.032 | ||||||
| Gender | ||||||
| Male | 260 | 48.1% | 105 | 51.2% | 365 | 49.0% |
| Female | 280 | 51.9% | 100 | 48.8% | 380 | 51.0% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(1) = 0.5609 Pr = 0.454 | ||||||
| Ethnic | ||||||
| Hispanic or Latino | 66 | 12.2% | 20 | 9.8% | 86 | 11.5% |
| Others | 474 | 87.8% | 185 | 90.2% | 659 | 88.5% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(1) = 0.8850 Pr = 0.347 | ||||||
| Location | ||||||
| MICU | 337 | 62.4% | 131 | 63.9% | 468 | 62.8% |
| SICU | 27 | 5.0% | 6 | 2.9% | 33 | 4.4% |
| Cardiac SICU | 3 | 0.6% | 2 | 1.0% | 5 | 0.7% |
| CCU | 5 | 0.9% | 2 | 1.0% | 7 | 0.9% |
| Neuro ICU | 15 | 2.8% | 2 | 1.0% | 17 | 2.3% |
| Burn | 6 | 1.1% | 3 | 1.5% | 9 | 1.2% |
| Trauma | 16 | 3.0% | 3 | 1.5% | 19 | 2.6% |
| MICU/SICU | 126 | 23.3% | 53 | 25.9% | 179 | 24.0% |
| Others | 5 | 0.9% | 3 | 1.5% | 8 | 1.1% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(8) = 6.2595 Pr = 0.618 | ||||||
| Infection site | ||||||
| Thorax | 386 | 71.5% | 147 | 71.7% | 533 | 71.5% |
| Abdomen | 47 | 8.7% | 18 | 8.8% | 65 | 8.7% |
| Skin or soft tissue | 24 | 4.4% | 5 | 2.4% | 29 | 3.9% |
| Bacterial meningitis | 2 | 0.4% | 2 | 1.0% | 4 | 0.5% |
| Urinary tract | 38 | 7.0% | 13 | 6.3% | 51 | 6.8% |
| Central line | 1 | 0.2% | 1 | 0.5% | 2 | 0.3% |
| Osteomyelitis | 2 | 0.4% | 2 | 1.0% | 4 | 0.5% |
| Confirmed Swine Influenza A | 1 | 0.2% | 0 | 0.0% | 1 | 0.1% |
| Others | 38 | 7.0% | 16 | 7.8% | 54 | 7.2% |
| Suspected infection | 1 | 0.2% | 1 | 0.5% | 2 | 0.3% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(9) = 5.1841 Pr = 0.818 | ||||||
| Vasopressor use | ||||||
| No | 262 | 48.5% | 75 | 36.6% | 337 | 45.2% |
| Yes | 278 | 51.5% | 130 | 63.4% | 408 | 54.8% |
| Total | 540 | 100.0% | 205 | 100.0% | 745 | 100.0% |
| Pearson chi2(1) = 8.5413 Pr = 0.003 | ||||||
§Patients were considered to have corticosteroid use when they received IV or PO corticosteroids totaling >20 mg methylprednisolone equivalents on one calendar day during first 7 days.
Table 2. Comparison between survivors and non-survivors for continuous variables.
| Survivors | Non-Survivors | P value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age | 53 | 41–63 | 60 | 50–70 | <0.001 |
| APACHE III | 86.5 | 69–106 | 103 | 89–124 | <0.001 |
| CVP | 11 | 8.5–11.0 | 11 | 8–15 | 0.63 |
| CK | 103 | 39–265 | 66 | 31–138 | 0.01 |
| ALT | 27 | 17–44 | 25 | 17–38 | 0.91 |
| CRP | 22.5 | 12.6–30.8 | 21.4 | 12.9–31.6 | 0.90 |
| Urine output (24 hours) | 1388 | 799–2217 | 1062 | 579–2050 | 0.02 |
| Number of quadrants with infiltrates | 4 | 3–4 | 4 | 3–4 | 0.018 |
| PaO2 | 84 | 70–106 | 82 | 69–104 | 0.62 |
Abbreviations: SD: standard deviation; APACHE: acute physiology and chronic health evaluation; CVP: central venous pressure; CK: creatine kinase; CRP: C-reactive protein; ALT: alanine aminotransferase; PaO2: partial pressure of arterial oxygen; IQR: interquartile range.
Figure 1. Graphical presentation of patients receiving corticosteroids, those without receiving corticosteroids and those with missing data.
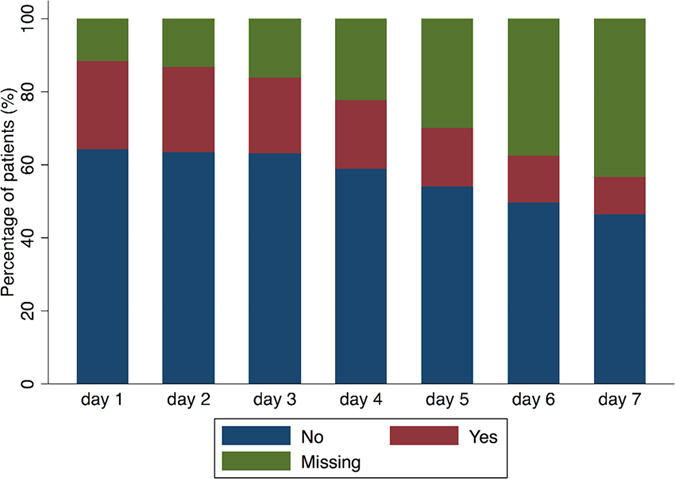
The proportion of patients with missing data increased from day 1 to day 7, which was attributable to ICU discharge or death (the end of follow up).
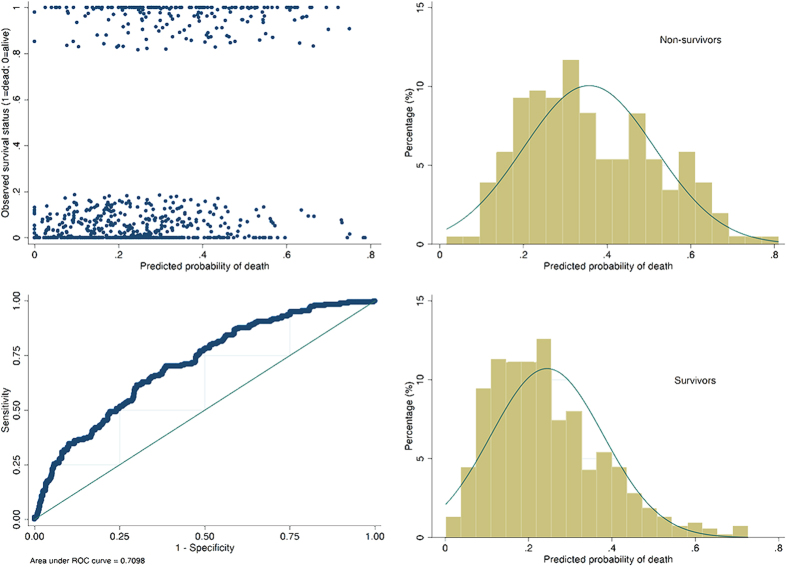
After careful examination of all variables, we found that APACHE III, CVP, the number of quadrats with infiltrates, CK, CRP, PaO2 and urine output had missing values (Table 3). MI was performed to impute missing values and all missing values were imputed. After adjustment for other potential confounders, corticosteroids showed no statistically significant effect on mortality outcome (OR: 1.18; 95% CI: 0.81–1.71). As expected, APACHE III was a significant predictor of mortality (OR: 1.02; 95% CI: 1.02–1.03). In multivariable model, CK continued to be an important protector of mortality outcome (OR: 0.999; 95% CI: 0.998–0.9998), but the effect size was marginal and of limited clinical relevance (Table 4). Furthermore, we investigated the interaction between corticosteroid use and variables of vasopressor and PaO2. The result showed that there was no significant interaction (Table 5). Discrimination power of the model was moderate in predicting mortality outcome (area under curve was 0.71, Fig. 2).
Table 3. The result of multivariate imputation by using multivariate normal regression model.
| Variables | Observations per m | |||
|---|---|---|---|---|
| Complete | Incomplete | Imputed | Total | |
| APACHE III | 707 | 38 | 38 | 745 |
| CVP | 456 | 289 | 289 | 745 |
| Number of quadrats with infiltrates | 560 | 185 | 185 | 745 |
| CK | 743 | 2 | 2 | 745 |
| CRP | 699 | 46 | 46 | 745 |
| PaO2 | 733 | 12 | 12 | 745 |
| Urine output | 741 | 4 | 4 | 745 |
Abbreviations: APACHE: acute physiology and chronic health evaluation; CVP: central venous pressure; CK: creatine kinase; CRP: C-reactive protein; PaO2: partial pressure of arterial oxygen.
Note: right-hand-side variables (variables used for multiple imputation) have missing values; model parameters estimated using listwise deletion. Complete + incomplete = total; imputed is the minimum across m of the number of filled-in observations.
Table 4. Adjustment of confounding factors with multivariate regression model.
| Mortality outcome | Odds Ratio | Lower limit of 95% CI | Upper limit of 95% CI | P > t |
|---|---|---|---|---|
| Corticosteroids | 1.18 | 0.81 | 1.71 | 0.396 |
| Female (male as reference) | 0.86 | 0.60 | 1.22 | 0.393 |
| Location | ||||
| SICU | 0.81 | 0.30 | 2.14 | 0.666 |
| Cardiac SICU | 1.13 | 0.15 | 8.72 | 0.908 |
| CCU | 0.89 | 0.15 | 5.16 | 0.898 |
| Neuro ICU | 0.75 | 0.16 | 3.65 | 0.726 |
| Burn | 2.05 | 0.36 | 11.55 | 0.418 |
| Trauma | 0.64 | 0.16 | 2.60 | 0.531 |
| MICU/SICU | 1.20 | 0.79 | 1.81 | 0.402 |
| Others | 1.42 | 0.30 | 6.70 | 0.656 |
| Other ethnic (Hispanic or Latino as reference) | 1.28 | 0.72 | 2.26 | 0.406 |
| Infection site | ||||
| Abdomen | 0.88 | 0.47 | 1.64 | 0.691 |
| Skin or soft tissue | 0.53 | 0.17 | 1.66 | 0.276 |
| Bacterial meningitis | 2.26 | 0.26 | 19.78 | 0.461 |
| Urinary tract | 0.83 | 0.41 | 1.69 | 0.613 |
| Central line | 1.79 | 0.11 | 29.82 | 0.685 |
| Osteomyelitis | 1.61 | 0.20 | 12.63 | 0.652 |
| Confirmed Swine Influenza A | 1 | |||
| Others | 0.89 | 0.45 | 1.77 | 0.744 |
| Suspected infection (no site specified) | 4.19 | 0.22 | 78.35 | 0.338 |
| APACHE III | 1.02 | 1.02 | 1.03 | <0.001 |
| Vasopressor use | 1.04 | 0.71 | 1.54 | 0.829 |
| CVP | .98 | 0.94 | 1.02 | 0.419 |
| Number of quadrats with infiltrates (with each one increase) | 1.22 | 0.93 | 1.60 | 0.142 |
| CK | 0.999 | 0.998 | 0.9998 | 0.009 |
| ALT | 1.001 | 0.996 | 1.006 | 0.816 |
| CRP | 1.00 | 0.99 | 1.005 | 0.713 |
| Urine output | 1.00 | 1.00 | 1.00 | 0.843 |
| PaO2 | 1.00 | 0.99 | 1.01 | 0.839 |
| Constant term | 0.03 | 0.01 | 0.12 | <0.001 |
Abbreviation: APACHE: acute physiology and chronic health evaluation; CVP: central venous pressure; CK: creatine kinase; CRP: C-reactive protein; PaO2: partial pressure of arterial oxygen.
Table 5. Interactions between corticosteroid use and arterial oxygen partial pressure and vasopressor use.
| Variables | Odds ratio | Lower limit of 95% CI | Upper limit of 95% CI | p |
|---|---|---|---|---|
| Interaction between Corticosteroid and Vasopressor | ||||
| Corticosteroid | 1.32 | 0.72 | 2.42 | 0.361 |
| Vasopressor | 1.11 | 0.70 | 1.76 | 0.654 |
| Corticosteroid × Vasopressor | 0.83 | 0.39 | 1.76 | 0.623 |
| Interaction between Corticosteroid and partial pressure of arterial oxygen | ||||
| Corticosteroid | 1.26 | 0.40 | 3.98 | 0.696 |
| PaO2 | 1.00 | 0.99 | 1.01 | 0.924 |
| Corticosteroid × PaO2 | 1.00 | 0.99 | 1.01 | 0.902 |
Note: interaction terms were assessed in independent models by adjusting for the same covariates. Corticosteroid and vasopressor were indicator variables, and PaO2 was continuous variable.
Abbreviations: PaO2: partial pressure of arterial oxygen; CI: confidence interval.
Figure 2. Four diagnostic plots to describe discrimination in a model fit with an area under operating characteristics curve of 0.71.
The plot of jittered outcome versus estimated probability of death showed that survivors were morel likely to appear below 0.4. However, non-survivors were normally distributed with the mean value at somewhere between 0.3 and 0.4. The Hosmer-Lemeshow chi2 statistic was 4.89 (p = 0.7689).
Propensity score matching
Table 6 shows the clinical characteristics of patients with and without corticosteroid treatment. The results showed that patients treated in MICU were more likely to receive corticosteroids (73.6% vs. 57.7%, p < 0.001). Patients on vasopressor on the first day were more likely to receive corticosteroids (62.3% vs. 51.2%, p = 0.005). Patients receiving corticosteroids were more critically ill with higher APACHE III scores (p < 0.001). There was no difference between patients with and without corticosteroids in gender, ethnics, infection site, CVP, CK, PaO2 and age. In multivariate model, the SICU appeared to be a factor against use of corticosteroids (OR: 0.22, 95% CI: 0.08–0.53). Use of vasopressor was associated with higher probability of corticosteroids use (OR: 1.91, 95% CI: 1.10–3.36). Urine output and CVP were not independently associated with corticosteroids use (Table 7). Overall the model had moderate discrimination in predicting corticosteroid use (AUC = 0.71, Fig. 3).
Table 6. Characteristics of patients with and without corticosteroids.
| Variables | No corticosteroids (n = 506) | Corticosteroids use (n = 239) | P |
|---|---|---|---|
| Gender (male, %) | 251 (49.6) | 114 (47.7) | 0.684 |
| Location (N, %) | <0.001 | ||
| Burn | 8 (1.6) | 1 (0.4) | |
| Cardiac SICU | 4 (0.8) | 1 (0.4) | |
| CCU | 4 (0.8) | 3 (1.3) | |
| MICU | 292 (57.7) | 176 (73.6) | |
| MICU/SICU | 137 (27.1) | 42 (17.6) | |
| Neuro ICU | 13 (2.6) | 4 (1.7) | |
| Others | 3 (0.6) | 5 (2.1) | |
| SICU | 29 (5.7) | 4 (1.7) | |
| Trauma | 26 (3.2) | 3 (1.3) | |
| Ethnic (N, %) | 1 | ||
| Hispanic or Latino | 58 (11.5) | 28 (11.7) | |
| Others | 448 (88.5) | 211 (88.3) | |
| Infection sites (N, %) | 0.713 | ||
| Abdomen | 49 (9.7) | 16 (6.7) | |
| Bacterial meningitis | 2 (0.4) | 2 (0.8) | |
| Central line | 1 (0.2) | 1 (0.4) | |
| Confirmed Swine Influenza A | 1 (0.2) | 0 | |
| Osteomyelitis | 2 (0.4) | 2 (0.8) | |
| Others | 41 (8.1) | 13 (5.4) | |
| Skin or soft tissue | 18 (3.6) | 11 (4.6) | |
| Suspected infection | 1 (0.2) | 1 (0.4) | |
| Thorax | 356 (70.4) | 177 (74.1) | |
| Urinary tract | 35 (6.9) | 16 (6.7) | |
| Vasopressor (N, %) | 259 (51.2) | 149 (62.3) | 0.005 |
| APACHE III (median, IQR) | 89 (70–109) | 96.5 (79–117.2) | <0.001 |
| CVP (mmHg) | 11 (8–14) | 11 (9–15) | 0.176 |
| The number of quadrants with infiltrates (median, range) | 4 (0–4) | 4 (2–4) | 0.037§ |
| CK (mmol/l) | 91 (40–233) | 75 (31–195) | 0.336 |
| ALT (mmol/l) | 26 (16–41) | 27 (17–44) | 0.298 |
| CRP (mg/dl) | 22.7 (13.5–31.35) | 21.2 (10.98–30.60) | 0.003 |
| Urine output (24 hours) | 1400 (785–2218) | 1170 (645.2–1968) | 0.063 |
| PaO2 (mmHg) | 84 (70–104.5) | 83 (68–105) | 0.700 |
| Mortality (N,%) | 127 (25.1) | 78 (32.6) | 0.039 |
| Age (years) | 55 (42–66) | 56 (43–65) | 0.866 |
Abbreviations: IQR: interquartile range; APACHE: acute physiology and chronic health evaluation; CVP: central venous pressure; CK: creatine kinase; CRP: C-reactive protein; PaO2: partial pressure of arterial oxygen.
§ statistical test was performed by using Cochran-Armitage trend test.
Table 7. Multivariate regression model to predict corticosteroid use.
| Variables | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Locate (MICU as reference) | |||
| Mixed ICU | 0.45 | 0.24–0.83 | 0.011 |
| SICU | 0.22 | 0.08–0.53 | 0.002 |
| Vasopressor | 1.91 | 1.10–3.36 | 0.024 |
| APACHE III (for each 10 points) | 1.10 | 0.998–1.212 | 0.056 |
| CVP | 1.01 | 0.97–1.07 | 0.575 |
| Number of quadrants with infiltrates | 1.36 | 0.98–1.92 | 0.073 |
| CRP | 0.98 | 0.96–0.99 | 0.036 |
| Urine output (for each 100ml) | 1.01 | 0.99–1.030 | 0.581 |
Abbreviations: MICU: medical intensive care unit; ICU: intensive care unit; SICU: surgical intensive care unit; PACHE: acute physiology and chronic health evaluation; CVP: central venous pressure; CRP: C-reactive protein.
Figure 3. Receiver operating characteristics curve showing the discrimination power of the logistic regression model in predicting corticosteroid use.
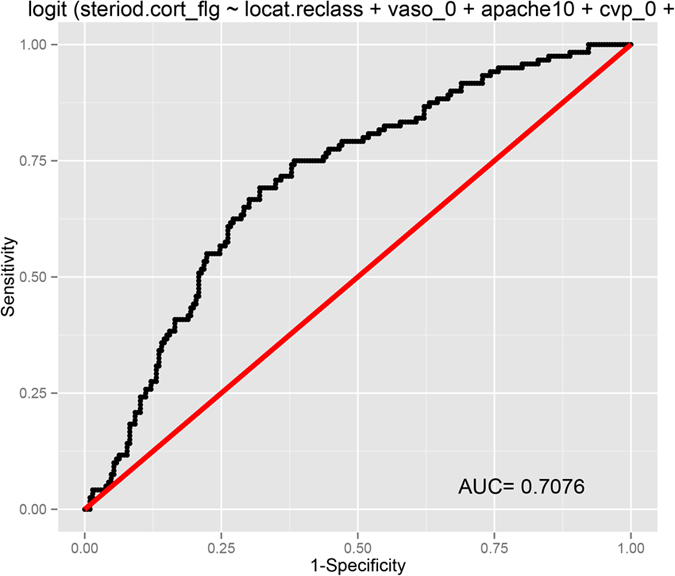
The area under curve was 0.71.
CVP and urine output were excluded from logistic regression model for generate propensity score. Number of quadrants with infiltrates was also excluded because this variable has too many missing values and it was only marginally significant. We used nearest matching strategy. The propensity scores of individual patients in all patients before and after matching were shown in Fig. 4. A total of 239 treated patients were matched to 239 control patients. The remaining 267 patients in the control group were not matched. In the matched cohort, the mortality risk in the corticosteroid group was not significantly different from that in the control group (48.1% vs. 54.5%, p = 0.231).
Figure 4. Distribution of propensity scores.
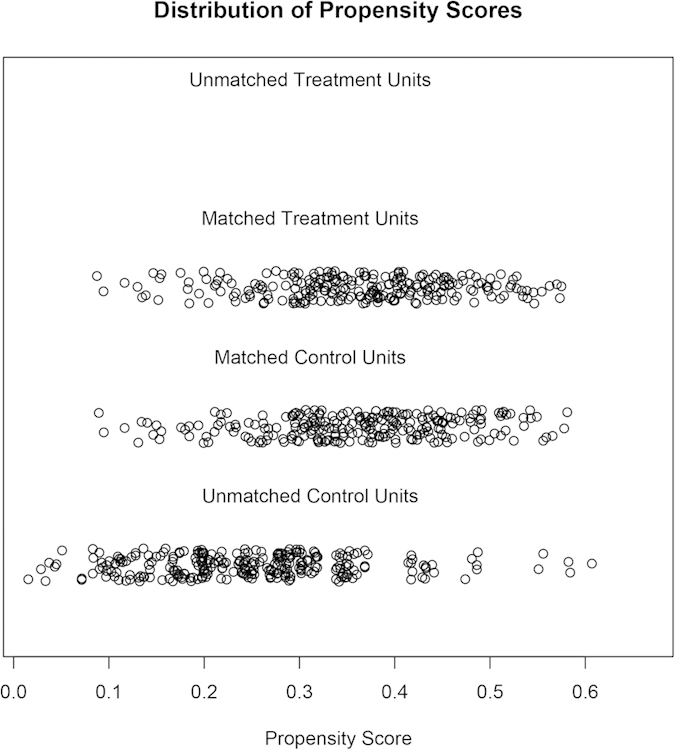
All treated patients were matched to the untreated patients.
Discussion
The study failed to identify any beneficial effects on mortality outcome in patients with ARDS. The study was a secondary analysis of a prospectively collected dataset. In this cohort, corticosteroids were more likely to be given to non-survivors. The most plausible causal relationship is that more critically ill patients were more likely to use corticosteroids. Although there was no strong evidence supporting the use of corticosteroids in ARDS patients, physicians are still prescribing corticosteroids for them as an alternative to conventional therapies in the hope that corticosteroids may ameliorate pulmonary edema. The American College of Critical Care Medicine issued a recommendation that glucocorticoids should be considered in the management strategy of patients with early severe ARDS24. In this background, the present study confirmed the futility of corticosteroids use in ARDS patients.
The use of corticosteroids in ARDS patients was not novel and several small studies have been conducted to address this issue. The first study conducted in early 1980s by Bernard and coworkers25. They investigated the high-dose corticosteroids on mortality outcome in ARDS patients. The study stopped early after enrollment of 99 patients because of the futility of the study drug. Because of the negative result of the study, the interests on this topic waned by the end of 1980s. However, the study by Annane and colleagues renewed the interests on corticosteroids, in which they found that corticosteroids were able to reduce the risk of death in patients with illness-related adrenal insufficiency (53% vs 63%; P = 0.04). Although the study population was sepsis, there was substantial number of patients with ARDS, accounting for 59% of the whole population7,26. However, the result could not be replicated in other studies9,10,12,27. Overall, the main findings in the literature were consistent with our result.
One limitation of the study was that there were some missing values in the dataset. We used MI to address the problem of information loss due to missing values. Missing data is common in publically available dataset and reflect the quality of the establishment of a dataset. In our dataset, the proportion of missing values can be as much as one third of a variable. If multivariable regression model was built by conventional method (listwise deletion), the number of observations remained in the model will be extremely small. There are other techniques for handling missing data, such as complete case analysis, overall mean imputation, and the missing-indicator method. However, these techniques are found to be less reliable than MI15,28. The other limitation of our study was that other clinically interesting outcomes were not investigated. These included ICU length of stay, organ failure free days and the duration of mechanical ventilation. Although these secondary study end points may not necessarily translate to mortality benefit, they are important from the perspective of cost-effectiveness. For example, if the duration of mechanical ventilation or ICU length of stay can be shortened, the medical cost can be substantially reduced. There are a few evidences supporting the beneficial effect of corticosteroids in improving these secondary outcomes. Meduri GU and coworkers reported that Methylprednisolone-induced down-regulation of systemic inflammation was associated with significant improvement in extrapulmonary and pulmonary organ failure, as well as the reduction in duration of mechanical ventilation and ICU length of stay8. The result was confirmed by subsequent systematic review10.
In conclusion, the study failed to identify any beneficial effect of corticosteroids on mortality outcome. Although non-survivors were more likely to use corticosteroids, the effect disappeared after adjustment by the severity of illness. The use of multiple imputation technique helped to improve the estimation of the effect size by preserving all useful information.
Additional Information
How to cite this article: ZHANG, Z. et al. The effectiveness of Corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci. Rep. 5, 17654; doi: 10.1038/srep17654 (2015).
Acknowledgments
This manuscript was prepared using Treatment of Acute Lung Injury (ALTA) research material obtained from the National Heart, Lung, Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the National Heart, Lung, Blood Institute.
Footnotes
Author Contributions Z.Z. conceived the idea and drafted the manuscript; L.C. helped analyze data and interpret the result; H.N. helped review the manuscript and interpret the result.
References
- Avecillas J. F., Freire A. X. & Arroliga A. C. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence, diagnosis, and outcomes. Clinics in chest medicine 27, 549–557; abstract vii, doi: 10.1016/j.ccm.2006.06.001 (2006). [DOI] [PubMed] [Google Scholar]
- Gu W. J., Wan Y. D., Tie H. T., Kan Q. C. & Sun T. W. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PloS one 9, e90426, doi: 10.1371/journal.pone.0090426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasarala S. et al. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PloS one 8, e57285, doi: 10.1371/journal.pone.0057285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm K. C. et al. Bacteria-specific neutrophil dysfunction associated with interferon-stimulated gene expression in the acute respiratory distress syndrome. PloS one 6, e21958, doi: 10.1371/journal.pone.0021958 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony D. S. et al. Inflammation and immune-related candidate gene associations with acute lung injury susceptibility and severity: a validation study. PloS one 7, e51104, doi: 10.1371/journal.pone.0051104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough C. L. Steroids for acute respiratory distress syndrome ? Clinics in chest medicine 35, 781–795, doi: 10.1016/j.ccm.2014.08.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D., Sebille V. & Bellissant E. & Ger-Inf-05 Study, G. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Critical care medicine 34, 22–30 (2006). [DOI] [PubMed] [Google Scholar]
- Meduri G. U. et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 131, 954–963, doi: 10.1378/chest.06-2100 (2007). [DOI] [PubMed] [Google Scholar]
- Steinberg K. P. et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England journal of medicine 354, 1671–1684, doi: 10.1056/NEJMoa051693 (2006). [DOI] [PubMed] [Google Scholar]
- Peter J. V. et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. Bmj 336, 1006–1009, doi: 10.1136/bmj.39537.939039.BE (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S. Y. et al. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Critical care 18, R63, doi: 10.1186/cc13819 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. M., Craig J. C., Eslick G. D., Seppelt I. & McLean A. S. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Critical care medicine 37, 1594–1603, doi: 10.1097/CCM.0b013e31819fb507 (2009). [DOI] [PubMed] [Google Scholar]
- National Heart L. et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. The New England journal of medicine 370, 2191–2200, doi: 10.1056/NEJMoa1401520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevret S., Seaman S. & Resche-Rigon M. Multiple imputation: a mature approach to dealing with missing data. Intensive care medicine, doi: 10.1007/s00134-014-3624-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders A. R., van der Heijden G. J., Stijnen T. & Moons K. G. Review: a gentle introduction to imputation of missing values. Journal of clinical epidemiology 59, 1087–1091, doi: 10.1016/j.jclinepi.2006.01.014 (2006). [DOI] [PubMed] [Google Scholar]
- White I. R., Royston P. & Wood A. M. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine 30, 377–399, doi: 10.1002/sim.4067 (2011). [DOI] [PubMed] [Google Scholar]
- Keegan M. T., Gajic O. & Afessa B. Comparison of APACHE III, APACHE IV, SAPS 3, and MPM0III and influence of resuscitation status on model performance. Chest 142, 851–858, doi: 10.1378/chest.11-2164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus W. A. et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100, 1619–1636 (1991). [DOI] [PubMed] [Google Scholar]
- Yeo C. D. et al. Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit. Journal of critical care 27, 739 e731–736, doi: 10.1016/j.jcrc.2012.07.014 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xu X., Ni H. & Deng H. Urine output on ICU entry is associated with hospital mortality in unselected critically ill patients. Journal of nephrology 27, 65–71, doi: 10.1007/s40620-013-0024-1 (2014). [DOI] [PubMed] [Google Scholar]
- Williams J. B. et al. Central venous pressure after coronary artery bypass surgery: does it predict postoperative mortality or renal failure ? Journal of critical care 29, 1006–1010, doi: 10.1016/j.jcrc.2014.05.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. & Ni H. C-reactive protein as a predictor of mortality in critically ill patients: a meta-analysis and systematic review. Anaesthesia and intensive care 39, 854–861 (2011). [DOI] [PubMed] [Google Scholar]
- Hosmer D. W., Hosmer T., Le Cessie S. & Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Statistics in medicine 16, 965–980 (1997). [DOI] [PubMed] [Google Scholar]
- Marik P. E. et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical care medicine 36, 1937–1949, doi: 10.1097/CCM.0b013e31817603ba (2008). [DOI] [PubMed] [Google Scholar]
- Bernard G. R. et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. The New England journal of medicine 317, 1565–1570, doi: 10.1056/NEJM198712173172504 (1987). [DOI] [PubMed] [Google Scholar]
- Annane D. et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. Jama 288, 862–871 (2002). [DOI] [PubMed] [Google Scholar]
- Sprung C. L. et al. Hydrocortisone therapy for patients with septic shock. The New England journal of medicine 358, 111–124, doi: 10.1056/NEJMoa071366 (2008). [DOI] [PubMed] [Google Scholar]
- Harel O. & Zhou X. H. Multiple imputation: review of theory, implementation and software. Statistics in medicine 26, 3057–3077, doi: 10.1002/sim.2787 (2007). [DOI] [PubMed] [Google Scholar]