Abstract
von Willebrand factor is an adhesive glycoprotein critical to normal hemostasis. It is stored in the Weibel-Palade body of endothelial cells and upon release may mediate platelet adhesion. Herpesvirus-infected endothelium is known to be prothrombotic and to support enhanced platelet adherence. We previously identified P-selectin as a monocyte receptor that is translocated from the Weibel-Palade body to the endothelial cell surface in response to the local generation of thrombin on herpesvirus infected cells. In this study, we show that viral injury to vascular endothelial cells induces secretion of von Willebrand factor which mediates enhanced platelet adhesion to these cells.
Full text
PDF
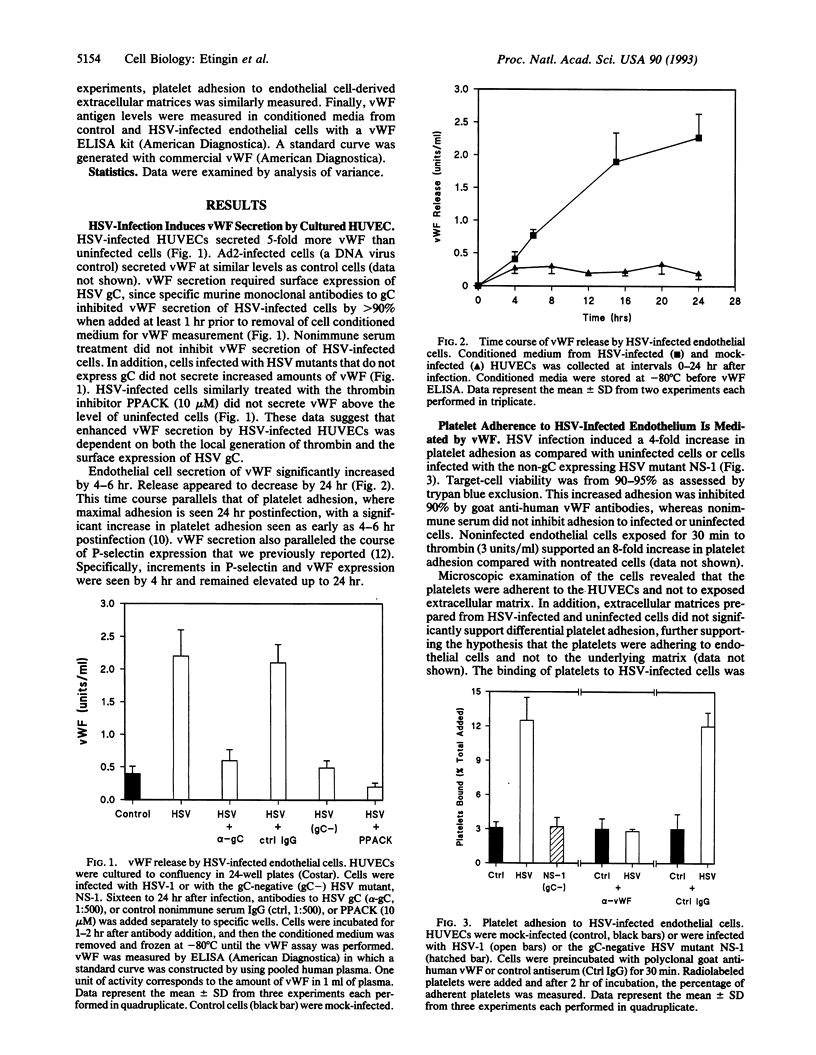
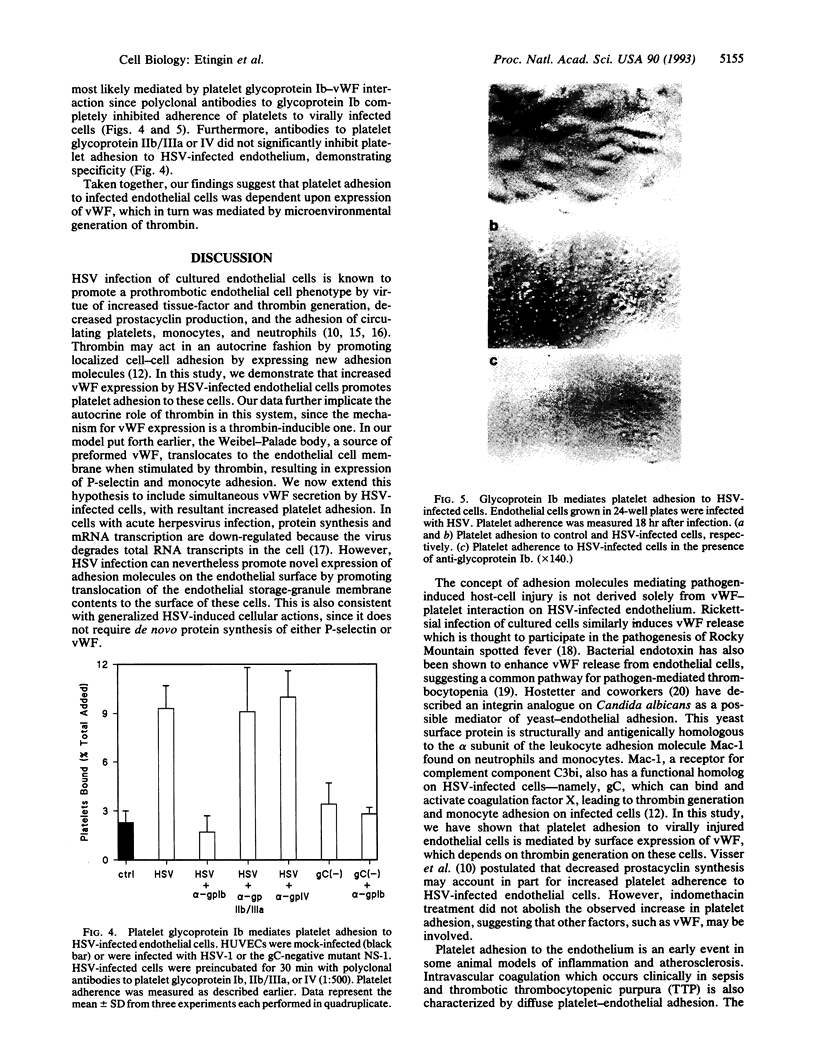

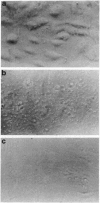
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7200–7203. doi: 10.1073/pnas.88.16.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici A. B., Bader R., Pagani S., Colibretti M. L., De Marco L., Mannucci P. M. Binding of von Willebrand factor to glycoproteins Ib and IIb/IIIa complex: affinity is related to multimeric size. Br J Haematol. 1989 Sep;73(1):93–99. doi: 10.1111/j.1365-2141.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R. von Willebrand factor, integrins, and platelets: their role in cancer. J Lab Clin Med. 1992 May;119(5):444–447. [PubMed] [Google Scholar]
- Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J Clin Invest. 1991 Jun;87(6):1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key N. S., Vercellotti G. M., Winkelmann J. C., Moldow C. F., Goodman J. L., Esmon N. L., Esmon C. T., Jacob H. S. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen B. S., Harpel P. C., Nachman R. L. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Invest. 1987 Oct;80(4):1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Friedman H. M., Macarak E. J., Kefalides N. A. Virus infection of endothelial cells increases granulocyte adherence. J Clin Invest. 1980 Jun;65(6):1469–1477. doi: 10.1172/JCI109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D., Piétu G., Fressinaud E., Girma J. P. von Willebrand factor: structure and function. Mayo Clin Proc. 1991 May;66(5):516–523. doi: 10.1016/s0025-6196(12)62394-5. [DOI] [PubMed] [Google Scholar]
- Moake J. L., Rudy C. K., Troll J. H., Schafer A. I., Weinstein M. J., Colannino N. M., Hong S. L. Therapy of chronic relapsing thrombotic thrombocytopenic purpura with prednisone and azathioprine. Am J Hematol. 1985 Sep;20(1):73–79. doi: 10.1002/ajh.2830200110. [DOI] [PubMed] [Google Scholar]
- Mohri H., Yoshioka A., Zimmerman T. S., Ruggeri Z. M. Isolation of the von Willebrand factor domain interacting with platelet glycoprotein Ib, heparin, and collagen and characterization of its three distinct functional sites. J Biol Chem. 1989 Oct 15;264(29):17361–17367. [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorer A. E., Moldow C. F., Rick M. E. Interleukin 1 or endotoxin increases the release of von Willebrand factor from human endothelial cells. Br J Haematol. 1987 Oct;67(2):193–197. doi: 10.1111/j.1365-2141.1987.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Chavin S. I., Marder V. J., Wagner D. D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985 Sep;76(3):1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Shi R. J., Lawrence S. O., Silverman D. J., Marder V. J. Rickettsia rickettsii infection of cultured endothelial cells induces release of large von Willebrand factor multimers from Weibel-Palade bodies. Blood. 1991 Nov 15;78(10):2595–2602. [PubMed] [Google Scholar]
- Visser M. R., Tracy P. B., Vercellotti G. M., Goodman J. L., White J. G., Jacob H. S. Enhanced thrombin generation and platelet binding on herpes simplex virus-infected endothelium. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8227–8230. doi: 10.1073/pnas.85.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells. Identification of a large precursor polypeptide chain. J Biol Chem. 1983 Feb 25;258(4):2065–2067. [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick T. M., Moake J. L., Udden M. M., Eskin S. G., Sears D. A., McIntire L. V. Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flow. J Clin Invest. 1987 Sep;80(3):905–910. doi: 10.1172/JCI113151. [DOI] [PMC free article] [PubMed] [Google Scholar]