Abstract
Background:
Elevated fasting intact proinsulin is a biomarker of late-stage ß-cell-dysfunction associated with clinically relevant insulin resistance. In this pilot investigation, we explored the potential value of measuring intact proinsulin as a functional predictor of ß-cell exhaustion during an oral glucose tolerance test (OGTT).
Methods:
The study was performed with 31 participants, 11 of whom were healthy subjects (7 female, age: 59 ± 20 years), 10 had impaired glucose tolerance (IGT, 6 female, 62 ± 10 years), and 10 had known type 2 diabetes (T2DM, 5 female, 53 ± 11 years, HbA1c: 7.0 ± 0.6%, disease duration: 8 ± 5 years). During OGTT, blood was drawn after 0 hours, 1 hour, and 2 hours for determination of glucose and intact proinsulin. Five years later, patients were again contacted to assess their diabetes status and the association to the previous OGTT results was analyzed.
Results:
The OGTT (0 hours/1 hour/2 hours) results were as follows: healthy subjects: glucose: 94 ± 8 mg/dL/140 ± 29 mg/dL/90 ± 24 mg/dL, intact proinsulin: 3 ± 2 pmol/L/10 ± 7 pmol/L/10 ± 5 pmol/L); IGT: glucose: 102 ± 9 mg/dL/158 ± 57 mg/dL/149 ± 34 mg/dL, intact proinsulin: 7 ± 4 pmol/L/23 ± 8 pmol/L/28 ± 6 pmol/L; T2DM: glucose: 121 ± 20 mg/dL/230 ± 51 mg/dL/213 ± 34 mg/dL; intact proinsulin: 7 ± 7 pmol/L/26 ± 9 pmol/L/27 ± 10 pmol/L). Five years later, all of the IGT and 2 of the healthy subjects had developed T2DM and one had devloped IGT. All of them had elevated 2-hour proinsulin values in the initial OGTT, while patients with normal intact proinsulin results did not develop diabetes.
Conclusions:
Elevated 2-hour intact proinsulin levels during OGTT were predictive for later type 2 diabetes development. Further studies need to confirm our findings in larger populations.
Keywords: intact proinsulin, ß-cell dysfunction, insulin resistance, oral glucose challenge, diabetes prediction
To date, about 40-50% of the US and EU population is overweight and is presenting with a measurable insulin resistance. A significant minority of this group will further proceed into development of type 2 diabetes,1 with increased risk of macrovascular complications.2,3 Type 2 diabetes has a genetic background but the age of disease manifestation is substantially influenced by lifestyle and culture. Family history and waist circumference are cheap and easy measures to identify patients at risk of diabetes development.4 However, daily practice shows that many patients are reluctant to modify their lifestyle, for example, by performing more exercise, based on the knowledge of a theoretical risk. When finally glucose metabolism starts to deteriorate into impaired glucose tolerance, macrovascular disease is frequently already present, and the patients are at increased risk of cardiovascular death.5 It might therefore be helpful to identify an early biomarker of diabetes development that indicates the onset of diabetes development prior to glucose deterioration. Such a biomarkers should be representative for the underlying disease causes, and—in a best case scenario—should also indicate an improvement of the metabolic condition, if appropriate lifestyle measures are taken by the patient.
Type 2 diabetes is characterized by a genetically driven dysfunction of the pancreatic ß-cells, insulin resistance, and a high endocrine activity of the visceral lipid tissue. Adipokines are therefore among the biomarker candidates. However, they are also deteriorated in obese patients that do not develop diabetes. Genetic biomarkers might be helpful to identify the disease candidates, but do not provide information regarding the age of disease manifestation. We were therefore investigating functional proteins associated with ß-cell function and insulin resistance with respect to their capability to indicate disease onset.
Earlier studies suggested that elevated proinsulin and disproportionate levels of the des31,32-proinsulin intermediate are viewed as symptoms of a functionally compromised ß-cell, most often arising from the over-stimulation of chronic hyperglycemia or in later disease stage from therapeutic intervention.6-9 Intact proinsulin, the uncleaved insulin precursor protein, has a half-life of only few minutes and can be used as an indicator of actual ß-cell function when measured with a highly specific test.10 We have been able to demonstrate that elevated fasting morning intact proinsulin is a highly specific indirect indicator for insulin resistance.11 We have also used intact proinsulin as a marker to describe the impact of different treatment interventions on the disease pathology to help to identify drugs that are not just lowering glucose, such as sulfonylurea drugs,12 but have additional beneficial effects on the underlying disease conditions, such as early insulin, glitazones, or GLP-1 based therapies.12-16
In this pilot study we determined the time course of plasma levels of glucose and intact proinsulin in the first 2 hours after an oral glucose tolerance test (OGTT) in healthy subjects, patients with impaired glucose tolerance, and patients with type 2 diabetes to investigate the functional capacity of the ß-cells. Approximately 5 years later, patients were contacted again to assess their diabetes state in light of the previous OGTT results.
Patients and Methods
This study was approved by the responsible Ethics Committee of the State of Rheinland-Pfalz, Germany, and was performed in accordance with the guidelines of good clinical practice. After giving written informed consent, healthy subject, patients with known impaired glucose tolerance and patients with type 2 diabetes on diet and/or metformin monotherapy were included into the study. All participants had to have a BMI > 25 kg/m2, and the diabetes patients had to have an HbA1c value below 8.0%. They arrived at the study site in the morning after an overnight fast. Blood was drawn for glucose, insulin and intact proinsulin determination and they ingested 75 g of glucose in a 250 mL drink. Further blood draws were performed after 1 hour and after 2 hours, and patients were discharged when the blood glucose levels had returned to values below 180 mg/dL. The samples were centrifuged at approximately 1500 × g for 10 minutes at 4°C and the supernatant was immediately stored at −20°C until measurement.
Laboratory Analyses
A standard glucose oxidase reference method was used for measurement of capillary blood glucose levels (YSI Stat 2300, Yellow Springs, OH, USA). Insulin was measured using a chemiluminescence immunoassay (Invitron, Cardiff, UK). Intact proinsulin was analyzed by means of a specific ELISA method (TecoMedical, Sissach, Switzerland). Next to assessment of insulin resistance by intact proinsulin secretion as published previously,11 homeostatic model assessment (HOMAIR) score calculation was applied as a second measure for insulin resistance analysis17 in patients with normal ß-cell function (ie, normal intact proinsulin values). The estimate of insulin resistance by HOMAIR score was calculated with the following formula: fasting serum insulin (µU/ml) × fasting plasma glucose (mmol/l) / 22.5. As described by Hedblad et al, patients with HOMAIR score values exceeding the 75th percentile of a nondiabetic population (ie, 2.0) were considered to have insulin resistance.18
Follow-Up Visit After 5 Years
The initial OGTT experiments were performed in the first quarter of 2008. In the first quarter of 2013, all nondiabetic and IGT patients were recontacted by the investigative site and invited for a personal interview. They were asked to bring their most recent laboratory results with them and the diabetes state of the patients was assessed.
Statistical Analysis
All analyses were performed in an exploratory sense with appropriate parametrical and nonparametrical methods. The difference between the 3 groups was assessed by using t-test statistics. All P values < .05 were interpreted as statistically significant.
Results
A total of 31 patients participated in the OGTT experiments. Eleven participants were healthy subjects (7 female, age: 59 ± 20 years, BMI: 28.8 ± 3.2 kg/m2, HbA1c: 5.3 ± 0.3%), 10 had an established impaired glucose tolerance (IGT, 6 female, age: 62 ± 10 years, BMI: 31.2 ± 6.1 kg/m2, HbA1c: 5.8 ± 0.3%) and 10 were known to have type 2 diabetes and were treated with diet or metformin monotherapy (5 female, age: 53 ± 11 years, BMI: 34.1 ± 5.4 kg/m2, HbA1c: 7.0 ± 0.6%).
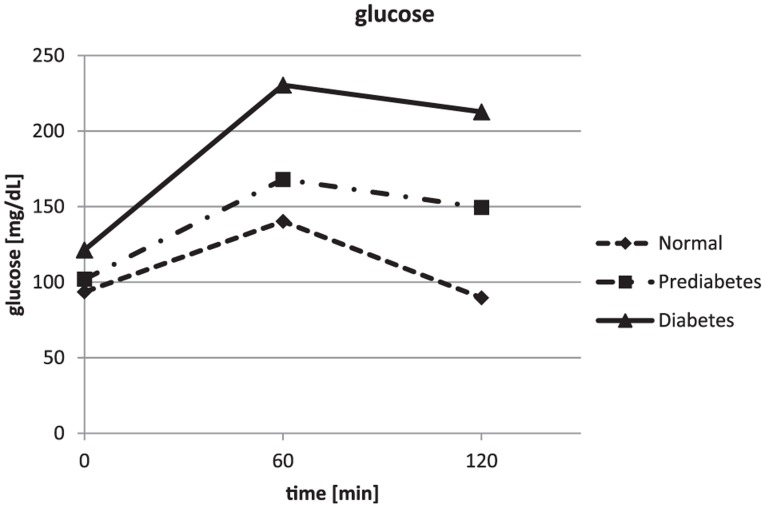
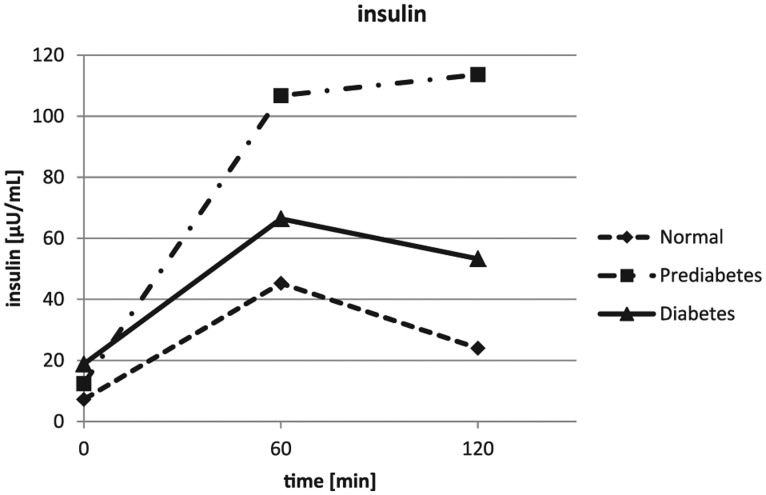
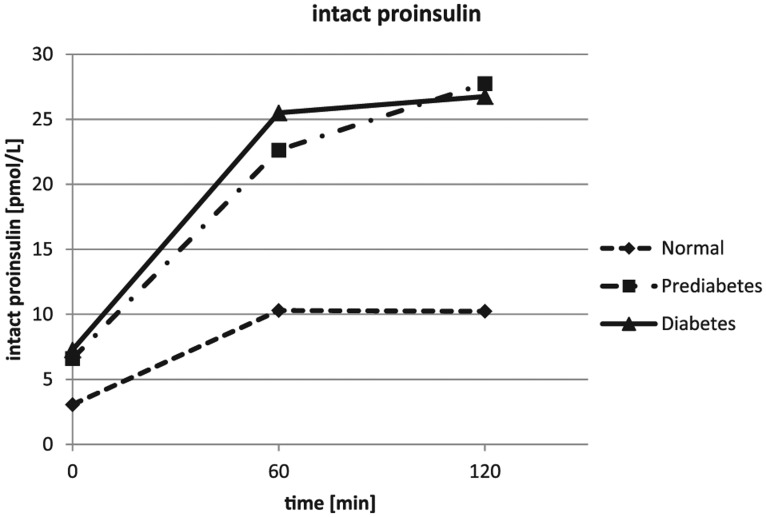
The glucose values obtained during the OGTT were confirming the clinical diagnosis obtained from the patients on inclusion into the study. The glucose results are depicted in Figure 1 and Table 1. The insulin results showed a lower insulin response for the healthy subjects. The highest insulin concentrations were seen in the IGT group after 1 hour and 2 hours, while insulin was only moderately increased in the diet and/or metformin treated diabetes patients (see Figure 2 and Table 1). The mean intact proinsulin levels stayed below the normal fasting reference value (11 pmmol/L) in the healthy subjects during the entire experiment but increased to 2.5-fold higher concentrations simultaneously after 1 hour and 2 hours in the IGT and the diabetes group (Figure 3 and Table 1). All diabetes patients and all IGT patients had an intact proinsulin level after 2 hours above 15 pmol/L. From the healthy subjects, 4 individuals presented with elevated proinsulin values after 2 hours (14.6-17.8 pmol/L). The HOMAIR score calculation revealed values of 1.7 ± 0.8 in the healthy subjects, 3.1 ± 1.4 in the IGT group (P < .01 vs control) and 5.8 ± 3.5 in the type 2 diabetes group (P < .005 vs control and P < .05 vs IGT).
Figure 1.
Results of the glucose assessments during the oral glucose tolerance tests. Error bars were left out for better readability (standard deviations are provided in Table 1).
Table 1.
Mean Plasma Glucose, Insulin, and Intact Proinsulin Concentrations During the Oral Glucose Challenge Tests.
| Parameter | Healthy subjects | Impaired glucose tolerance | Type 2 diabetes |
|---|---|---|---|
| N | 10 | 10 | 10 |
| Glucose (mg/dL) | |||
| 0 hours | 94 ± 8a,b | 102 ± 9b | 121 ± 20 |
| 1 hour | 140 ± 29b | 168 ± 57b | 230 ± 51 |
| 2 hours | 90 ± 24a,b | 150 ± 34b | 213 ± 34 |
| Insulin (µU/mL) | |||
| 0 hours | 7.2 ± 3.5a,b | 12.4 ± 5.5b | 18.8 ± 10.2 |
| 1 hour | 45.3 ± 33.5a | 106.8 ± 31.6b | 66.4 ± 29.6 |
| 2 hours | 24.0 ± 17.6a,b | 113.6 ± 114.7 | 53.3 ± 12.4 |
| Intact proinsulin (pmol/L) | |||
| 0 h | 3.1 ± 1.9a | 6.1 ± 4.1 | 7.3 ± 7.0 |
| 1 h | 10.3 ± 6.8a,b | 22.6 ± 8.1 | 25.5 ± 8.8 |
| 2 h | 10.2 ± 5.2a,b | 27.8 ± 5.9 | 26.7 ± 10.1 |
P < .05 vs IGT group. bP < .05 vs T2D group.
Figure 2.
Results of the insulin assessments during the oral glucose tolerance tests. Error bars were left out for better readability (standard deviations are provided in Table 1).
Figure 3.
Results of the intact proinsulin assessments during the oral glucose tolerance tests. Error bars were left out for better readability (standard deviations are provided in Table 1).
In the follow-up visit after 5 years, it was possible to obtain information from 9 IGT patients (90%) and 9 healthy subjects (82%). The other 2 patients were lost to follow-up. All former IGT patients had in the meantime developed overt type 2 diabetes and were on oral treatment. From the healthy subjects, 2 had developed type 2 diabetes and 1 patient was in the state of impaired fasting glucose tolerance. When comparing with the prior OGTT results, all 3 patients had presented with elevated 2 hours intact proinsulin in the test 5 years ago. In contrast, the individual insulin concentrations, the glucose concentrations, and the proinsulin/insulin ratio were not indicative for the diabetes development (data not shown). The fourth patient with prior elevated 2-hour intact proinsulin levels was 1 of the lost-to-follow-up patients and could not be analyzed.
Discussion
With the introduction of new immunoassays that are specific for the uncleaved intact proinsulin molecule, a new diagnostic tool for ß-cell function assessment became available about a decade ago.10,19 Fasting intact proinsulin has been established as a highly specific marker for insulin resistance,11,20 and can be used in combination with the HOMA-score to stage ß-cell dysfunction independently from glycemic control.20,21 Intact proinsulin lowers blood glucose however with a lower efficacy than insulin and is known to stimulate adipokine secretion from the visceral adipose tissue.21 It, for example, induces PAI-I secretion, resulting in a higher risk for macrovascular events independently from glycemic control.22 Elevated levels of intact proinsulin in nondiabetic patients are associated with a 3-times-higher cardiovascular mortality.23 An attempt to develop proinsulin as an antidiabetic drug was abandoned in the 1980s, because of the unexpected appearance of several cardiovascular events in the proinsulin arm of a phase II comparator study versus insulin in patients with early stage type 1 and type 2 diabetes.24
In our study we included intact proinsulin into the protocol of a diagnostic OGTT. The concentrations increased in diabetes patients and subjects with impaired glucose tolerance within the first 2 hours as an indication of ß-cell exhaustion in the course of the glucose challenge, while only a minimal increase was seen in the control group of healthy subjects. These results may represent the dynamic situation of the compromised ß-cell especially in the state of impaired glucose tolerance. They show that patients with impaired glucose tolerance are not different from overt diabetes patients with respect to ß-cell dysfunction and put in our opinion a question mark to the current diagnosis of diabetes solely based on glucose and the surrogate marker HbA1c. This finding is also independent from the BMI, which was much higher in the diabetes patients than in the IGT group.
When we were able to reassess the diabetes patients 5 years later, it turned out that those normal individuals that had developed diabetes in the meantime were the ones with the elevated intact proinsulin levels in the prior OGTT. It appears logical that a pancreas that shows a dynamic indication of temporary exhaustion, when challenged by oral glucose uptake, represents a higher probability for diabetes development than a normally functioning organ. Our results are in line with previous studies performed almost 20 years ago with less specific assays, jointly measuring intact proinsulin and its specific and unspecific cleavage products that have a much longer plasma stability than the original molecule alone (further referred to as “total” proinsulin tests). In a study by Inoue et al with a design similar to our investigation with 51 patients, subjects with a higher 120-min total proinsulin response to glucose during the initial OGTT showed a significant correlation with increased fasting plasma glucose levels after a 2.5-year follow-up period and developed type 2 diabetes. The authors concluded that this finding suggest that the proinsulin response to glucose loading might be a useful indicator for predicting worsening to diabetes in subjects with impaired glucose tolerance.25 In another study, Kahn et al observed that diabetes development in 87 Japanese-American patients was predicted by elevated fasting total proinsulin levels 5 years earlier.26 A similar finding was reported from a study performed in Caucasian patients in the Netherlands. Nijpels et al followed 158 patients with impaired glucose tolerance for a period of 2 years. The cumulative incidence of type 2 diabetes was 28.5% The initial 2-hour postload plasma glucose levels and the fasting total proinsulin were significantly related to the incidence of diabetes. The authors concluded that beta-cell dysfunction rather than insulin resistance plays the most important role in the future development of diabetes in a high-risk Caucasian population.27
Despite these conclusive results, proinsulin was never really adopted as a diagnostic marker for diagnosis and monitoring of diabetes until today. The major reason may have been the diagnostic blurriness of the results induced by the older total proinsulin assays, which were not specific for the dynamic ß-cell secretion product. Other reasons may include availability of the tests in local laboratories, costs, and the additional burden to patients and health care professionals when measuring an additional marker in daily routine. Why may it be important to consider inclusion of intact proinsulin assessment into the regular OGTT procedure? First, intact proinsulin is capable to lower blood glucose and may be a reason for a potentially delayed diagnosis of diabetes. Elevated 2-hour values indicating dynamic ß-cell exhaustion may be helpful for an additional diabetes risk assessment not solely based on glucose levels, and to enable an earlier and thus more likely to be efficient prevention. Second, elevated intact proinsulin induces an elevated macrovascular risk. The 2-hour intact proinsulin value may also be helpful to identify patients at higher risk for cardiovascular disease in a more timely fashion, who may then better respond to preventive measures. Finally, a lowering or even normalization of intact proinsulin next to normalization of glycemic control may be a worthwhile second treatment target in the attempt to reduce the increased cardiovascular mortality in diabetes patients.
The major limitation of our study is the pilot character with only 10 or 11 patients per group. Follow-up after 5 years was only possible in 9 subjects without disturbed blood glucose regulation, and 9 patients with IGT. Elevated intact proinsulin levels 2 hours after the ingestion of 75 g glucose were predictive for the development of type 2 diabetes mellitus in the group of nondiabetic obese subjects (n = 9). It is not possible to draw conclusions on the predictive value of intact proinsulin in patients with IGT because all of the IGT subjects in the study developed type 2 diabetes mellitus. Despite these limitations, we obtained clear results in line with pathophysiological consideration and the respective literature. Another limitation is the selection of patients with elevated BMI only. Further studies in larger populations are now warranted to confirm our findings in larger populations.
In conclusion, measurement of intact proinsulin in addition to glucose during the OGTT provided additional insight into the state of ß-cell function of the patients and identified patients who developed type 2 diabetes in the 5-year follow-up period. Intact proinsulin response to glucose loading might indeed be a useful indicator for predicting worsening to diabetes in normal subjects or subjects with impaired glucose tolerance.
Acknowledgments
The authors would like to thank the laboratory and clinical staff of the former IKFE Institute in Mainz for their commitment when running this investigation.
Footnotes
Abbreviations: BMI, body mass index; ELISA, enzyme-linked immunosorbent assay; GLP-1, glucagon-like-peptide 1; HbA1c, hemoglobin A1c; HOMA, homeostatic model assessment; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Buchanan T. Pancreatic beta-cell loss and preservation in type 2 diabetes. Clin Ther. 2003;23(suppl B):B32-B46. [DOI] [PubMed] [Google Scholar]
- 2. Strutton DR, Stang PE, Erbey JR, Lydick E. Estimated coronary heart disease attributable to insulin resistance in populations with and without type 2 diabetes mellitus. Am J Manag Care. 2001;7:765-773. [PubMed] [Google Scholar]
- 3. Eschwege E, Richard JL, Thibult N, et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels: the Paris Prospective Study 10 years later. Horm Metab Res. 1985;15(suppl):41-46. [PubMed] [Google Scholar]
- 4. Cameron AJ, Magliano DJ, Söderberg S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes Rev. 2013;14:86-94. [DOI] [PubMed] [Google Scholar]
- 5. Mellbin LG, Anselmino M, Rydén L. Diabetes, prediabetes and cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17(suppl 1):S9-S14. [DOI] [PubMed] [Google Scholar]
- 6. Røder ME, Porte D, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired ß-cell secretory capacity in patients with non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:604-608. [DOI] [PubMed] [Google Scholar]
- 7. Hostens K, Ling Z, Van Schravendijk C, Pipeleers D. Prolonged exposure of human beta-cells to high glucose concentrations increases their release of proinsulin during acute stimulation with glucose or arginine. J Clin Endocrinol Metab. 1999;84:1386-1390. [DOI] [PubMed] [Google Scholar]
- 8. Seaquist ER, Kahn SE, Clark PM, Hales CN, Porte D, Jr, Robertson RP. Hyperproinsulinemia is associated with increased beta-cell demand after hemipancreatectomy in humans. J Clin Invest. 1996;97:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haffner SM, Mykkänen L, Stern MP, Valdez RA, Heisserman JA, Bowsher RR. Relationship of proinsulin and insulin to cardiovascular risk factors in nondiabetic subjects. Diabetes. 1993;42:1297-1302. [DOI] [PubMed] [Google Scholar]
- 10. Pfützner A, Pfützner AH, Kann P, et al. Clinical and laboratory evaluation of a new specific ELISA for intact proinsulin. Clin Lab. 2005;51:734-738. [PubMed] [Google Scholar]
- 11. Pfützner A, Kunt T, Mondok A, et al. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27:682-687. [DOI] [PubMed] [Google Scholar]
- 12. Pfützner A, Marx N, Lübben G, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control—results from the pioneer study. J Am Coll Card. 2005;45:1925-1931. [DOI] [PubMed] [Google Scholar]
- 13. Pfützner A, Lorra B, Abdollhania M, et al. Preprandial short-acting insulin analogue substitution has an immediate and comprehensive ß-cell protective effect in patients with type 2 diabetes mellitus—results from a randomized comparator study vs. glimepiride. Diabetes Technol Ther. 2006;8:375-384. [DOI] [PubMed] [Google Scholar]
- 14. Pscherer S, Larbig M, von Stritsky B, Pfützner A, Forst T. In type 2 diabetes patients, insulin glargine is associated with lower postprandial release of intact proinsulin compared with sulfonylurea treatment. J Diabetes Sci Technol. 2012;6:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forst T, Dworak M, Berndt-Zipfel C, et al. Effect of vildagliptin compared to glimepiride on postprandial proinsulin processing in the β cell of patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:576-579. [DOI] [PubMed] [Google Scholar]
- 16. Forst T, Michelson G, Ratter F, et al. Addition of liraglutide in patients with type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29:1115-1118. [DOI] [PubMed] [Google Scholar]
- 17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [DOI] [PubMed] [Google Scholar]
- 18. Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17:299-307. [DOI] [PubMed] [Google Scholar]
- 19. Pfützner A, Kunt T, Löbig M, Knesovic M, Forst T. Clinical and laboratory evaluation characteristics of two new chemiluminescence assays for intact and total proinsulin. Clin Chem Lab Med. 2003;41:1234-1238. [DOI] [PubMed] [Google Scholar]
- 20. Pfützner A, Standl E, Hohberg C, et al. IRIS II study: intact proinsulin is confirmed as highly specific marker for insulin resistance in a cross-sectional study design. Diabetes Technol Ther. 2005;7:478-486. [DOI] [PubMed] [Google Scholar]
- 21. Pfützner A, Pfützner AH, Larbig M, Forst T. Role of intact proinsulin in diagnosis and treatment of type 2 diabetes mellitus. Diabetes Technol Ther. 2004;6:405-412. [DOI] [PubMed] [Google Scholar]
- 22. Nordt TK, Bode C, Sobel BE. Stimulation in vivo of expression of intra-abdominal adipose tissue plasminogen activator inhibitor type I by proinsulin. Diabetologia. 2001;44:1121-1124. [DOI] [PubMed] [Google Scholar]
- 23. Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation. 2002;105:2153-2158. [DOI] [PubMed] [Google Scholar]
- 24. Galloway JA, Hooper SA, Spradlin CT, et al. Biosynthetic human proinsulin. Review of chemistry, in vitro and in vivo receptor binding, animal and human pharmacology studies, and clinical trial experience. Diabetes Care. 1992;15:666-692. [DOI] [PubMed] [Google Scholar]
- 25. Inoue I, Takahashi K, Katayama S, et al. A higher proinsulin response to glucose loading predicts deteriorating fasting plasma glucose and worsening to diabetes in subjects with impaired glucose tolerance. Diabet Med. 1996;13:330-336. [DOI] [PubMed] [Google Scholar]
- 26. Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Berstrom RW, Fujimoto WY. Proinsulin as a marker for the development of type 2 diabetes in Japanese-American men. Diabetes. 1995;44:173-179. [DOI] [PubMed] [Google Scholar]
- 27. Nijpels G, Popp-Snijders C, Kostense PJ, Bouter LM, Heiner RJ. Fasting proinsulin and 2-h post-load glucose levels predict the conversion to type 2 diabetes in subjects with impaired glucose tolerance: the Hoorn study. Diabetologia. 1996;39:113-118. [DOI] [PubMed] [Google Scholar]