Abstract
The utility of continuous glucose monitoring devices remains limited by an obstinate foreign body response (FBR) that degrades the analytical performance of the in vivo sensor. A number of novel materials that resist or delay the FBR have been proposed as outer, tissue-contacting glucose sensor membranes as a strategy to improve sensor accuracy. Traditionally, researchers have examined the ability of a material to minimize the host response by assessing adsorbed cell morphology and tissue histology. However, these techniques do not adequately predict in vivo glucose sensor function, necessitating sensor performance evaluation in a relevant animal model prior to human testing. Herein, the effects of critical experimental parameters, including the animal model and data processing methods, on the reliability and usefulness of preclinical sensor performance data are considered.
Keywords: animal model, biocompatibility, blood glucose, blood glucose meter, foreign body response, in vivo glucose sensor
Diabetes mellitus remains a costly and serious threat to patient health due in part to inadequate blood glucose (BG) regulation and persistent hyperglycemia.1 Investigations by both the Diabetes Control and Complications Trial Group2 and the United Kingdom Prospective Diabetes Study3-4 independently confirmed reductions in long-term health complications for type 1 and type 2 diabetes with increased frequency of BG measurement. While portable glucometers provide instantaneous snapshots of BG levels for home monitoring and diabetes management, frequent sampling using these meters is impractical and leads to inconsistent patient compliance and poor BG control. The implantable glucose biosensor that enables continuous monitoring represents the ultimate technology in diabetes management. Indeed, the use of continuous glucose monitors (CGMs) reduces both hypoglycemia and persistent hyperglycemia.2-4 Unfortunately, the maximum useful lifetime of implanted glucose biosensors remains limited to 3-6 days due to poor analytical performance, both acutely and after several days as a result of the foreign body response (FBR) to such devices.5-6
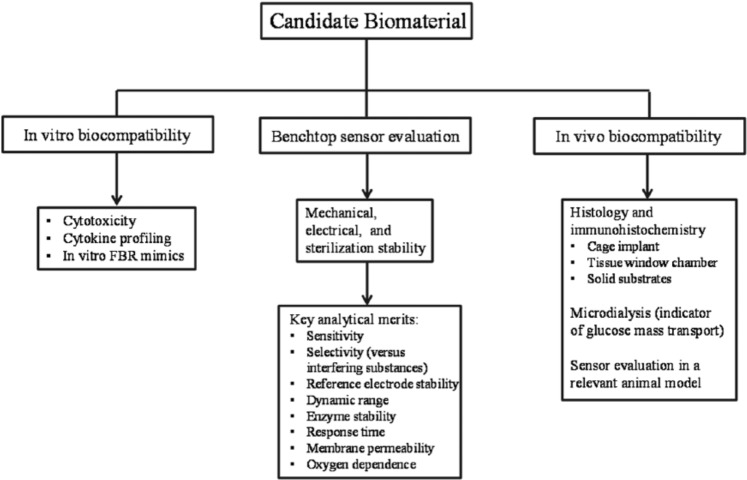
Chemical or physical modifications to the tissue-contacting sensor surface have been investigated to delay or avoid key events in the FBR (eg, inflammatory response, foreign body capsule formation) and improve sensor performance. In addition to sufficient stability and the ability to resist the FBR, these external coatings must not diminish the analytical performance of the sensor. Benchtop sensor-material compatibility studies,7-9 FBR models,10-11 and in vivo histological and immunohistochemical analysis12-13 are all used to evaluate the potential benefits of new sensor materials to device performance (Figure 1). Despite the utility of these tests for examining biocompatibility, a distinct correlation between in vitro or histological observations and actual sensor function is lacking in biomaterials literature. The aim of this commentary article is to discuss important experimental considerations in the preclinical evaluation of the analytical performance of continuous glucose biosensors. We focus specifically on the percutaneous electrochemical glucose biosensor system and discuss methods for assessing key analytical merits including sensitivity, accuracy, and lag time. Furthermore, we highlight usefulness of collected data as a function of animal model, BG dynamics, and data processing.
Figure 1.
Noninclusive flow diagram for initial testing of biocompatible glucose sensor coatings. In vitro biocompatibility assays may be carried out to test for material toxicity, surface biofouling, and cell phenotype.7 Results from such assays generally help identify promising candidate materials for further study. Benchtop sensor evaluation should assess the stability of the final device and analytical performance in both physiological buffer and complex media (eg, serum, whole blood). In vivo analysis should only be pursued after in vitro studies and should examine several tissue characteristics (histology, tissue glucose transport, etc). Sensor evaluation in a relevant animal model represents the ultimate step in this process and may be used to examine any potential benefits to CGM performance identified in prior studies.
Animal Model Selection
Selection of an appropriate animal model is crucial for obtaining relevant and accurate sensor performance data. The size of the animal, cost, disease state (ie, healthy vs diabetic), and physiological relevance to humans are each important parameters. Murine14 and rodent15,16 models have often been used for in vivo sensor performance evaluation due to low cost and handling ease. Furthermore, the availability of genetic murine variants provides increased versatility over other, larger animal species (eg, dogs, swine). For example, Klueh et al used a transgenic mouse diphtheria toxin receptor knock-in model to examine the impact of short-term macrophage depletion on the numerical accuracy of percutaneous glucose biosensors.17 Importantly, long-term studies using mice and rats are not feasible due to their limited blood volume and incompatibility for serial glucose measurement. As a cost-effective alternative to mice and rats, rabbits have also been employed due to their increased size (2-6 kg) and available blood volume, which permits the evaluation of multiple sensors for short periods (1-2 days).18,19 Still, the ability to accommodate multiple implanted sensors in a single animal represents a significant advantage in situations where the performance of one sensor type is compared to that of another, as heterogeneity between individual animals is eliminated.
Of note, a concern for the smaller animal models relates to physical tethering of the sensor electronics and potentiostat. Indeed, most animals physically remove the sensors, thus limiting the practical implant duration to less than 2 days. Design improvements to both the geometry of the exposed sensor components and electrical connection with potentiostat leads should be considered to reduce shear and pulling forces imposed on the sensor. These engineering controls should include minimizing the length of connecting wires and providing protective casing for electronic hardware. Securing external sensor components using sutures or adhesives must be considered to minimize physical motion, but should be distant from the subcutaneous portion of the sensor to avoid interfering with the local wound healing process. With these modifications in place, longer studies would likely be feasible, although low blood volume would remain a limiting experimental parameter for the rodent and murine models.
While useful for sensor performance evaluation over short periods, the physiological relevance of the smaller animal models (eg, mice, rats, rabbits) to human tissue is questionable.20 Indeed, significant differences in subcutaneous tissue physiology exist between humans and mice/rats. Wisniewski et al quantified the concentrations of glucose, lactate, pyruvate, glycerol, and urea in both human and rat tissue for 8 days using microdialysis and found that absolute analyte concentrations and temporal variations were markedly different between species.21 In humans, microdialysis glucose recovery increased and eventually stabilized after 4 days, while the glucose recovery for probes implanted in rats steadily decreased for the entire 8-day implantation period. This data indicates a dramatic difference in tissue glucose transport dynamics that the authors attributed in part to the greater proportion of adipose tissue present in human subcutaneous space; more adipose tissue would inherently lead to a less severe FBR. Larger animal models that more accurately represent human tissue physiology should thus be used to obtain translatable sensor performance data. Whereas the rat subcutis is more collagenous,21 swine in particular possess a tendency to develop significant amounts of adipose tissue.22 The cutaneous blood supply, dermal thickness, and timeline of wound healing biochemical events in pigs are also more comparable to humans, suggesting excellent utility as a model for preclinical sensor testing.22 While significantly more expensive than the small animal models, the larger animal size of pigs permits evaluation of individual sensors over extended implant periods (weeks) with the added benefit of increased throughput (ie, multiple test devices per animal), making these models ideally suited for sensor investigations.
The disease state of the selected animal is an equally important consideration for in vivo sensor performance evaluation. Indeed, the choice of a diabetic over healthy animal is motivated by the clear deficiencies of diabetic wound healing, which include altered wound repair23-25 and disrupted blood flow.26 Several mouse and rat models exist for both type 1 and type 2 diabetes, produced either by selective inbreeding or gene targeting techniques.27 Diabetes can also be induced through administration of several small doses of alloxan or streptozotocin in rats, rabbits, and pigs.28-30 Delayed and impaired wound healing has been reported for chemically induced diabetic animal models, which is a primary intention in recreating the implant environment in diabetic humans.30 Diabetic swine are particularly useful for mimicking both the cardiovascular complications and insulin resistance associated with diabetes.28 Despite the greater care required to maintain diabetic animals, such models more accurately recapitulate the challenges (eg, deficient FBR and wound healing) that implantable glucose biosensors encounter, thereby increasing the usefulness of data collected in functional sensor evaluations.
Experimental Considerations and Data Collection
To accurately assess glucose biosensor performance, particularly in healthy animals, BG levels must be artificially altered to achieve glucose concentrations outside of the euglycemic range. Simply feeding the animal is often not reproducible enough to accomplish this goal. Direct administration of glucose via intraperitoneal (IP) injections16 or through an intravascular (IV) catheter18-19,31 results in BG changes of appreciable magnitude. Glucose delivery via an IV catheter, either as a bolus18,31 (0.7 g kg-1 in swine) or constant-rate infusion19 (20 mg kg-1 min-1 in rabbits) is the most reliable method for achieving hyperglycemia, enabling the determination of sensor lag time and accuracy over a range of clinically relevant BG concentrations. However, IV administration can be physiologically demanding, with greater than normal excursion rates (0-2 mg dL-1 min-1) and the risk of inflated sensor error (Figure 2).32 Intraperitoneal glucose injections are routinely used to manipulate BG levels in smaller animals 16,33,34 to circumvent difficulties in intravenous administration. While larger glucose doses must administered (≥1.5 g kg-1) to achieve hyperglycemia, the slower rate of glucose absorption may better approximate normal, gradual BG fluctuations in diabetic patients, thereby increasing the relevance of sensor accuracy data at the cost of reproducibility.35 As an additional experimental parameter for consideration, the required frequency of glucose dosing is dependent on the animal model and route of administration (ie, IP or IV). This parameter should be experimentally determined by the investigator so that adequate amounts of data are obtained in both the euglycemic and hyperglycemic regime for proper sensor evaluation. Excursions into the hypoglycemic BG range via insulin administration would also provide valuable sensor performance data. However, the administration of insulin to test animals to achieve such a goal presents animal welfare concerns and thus is generally outside the scope of preclinical sensor testing.36
Figure 2.

Reference glucose measurements and corresponding implanted CGM trace after IV glucose administration in a swine model. While the direct measurement of glucose using a handheld glucometer indicated an increase in BG, the sensor was unable to accurately track rapid fluctuations in BG. The BG maximum detected by the continuous sensor was thus both delayed in time and attenuated in magnitude. These shortcomings on the part of the sensor are further worsened by foreign body reactions.38
Numerical Analysis, Calibration, and Data Interpretation
The influences of physical (eg, animal motion) and electrical interferences require that sensor signals be filtered prior to analysis. In addition to analog filters that are constituents of the potentiostat hardware, digital filtering of sensor data is required to achieve a stable glucose response. Algorithms for use in CGM for minimally invasive sensors have been reviewed by Bequette.37 The use of predictive over retrospective data processing techniques, including median, Kalman, and finite impulse response filters, should be considered to ensure compatibility with real-time glucose sensing. In addition to filtering, a lag-time correction is often applied to the collected data to compensate for the intrinsic delay of the sensor signal relative to changes in BG. This lag incorporates both a physiological component, the result of blood–interstitial fluid glucose dynamics and foreign body reactions that reduce the local glucose supply,38-40 and a sensor component that originates from sensor external coatings impeding glucose diffusion to the sensing element. Lag-time characterization is most easily accomplished by the method of Poincaré (Figure 3A), which shifts the reference- and sensor-derived signals in time relative to one another.41 The signal overlap for each time delay value is using a statistical agreement criterion (AC). Frequency-based approaches for determining sensor lag time (ie, Fourier transform analysis; Figure 3B) also represent viable options, and may be less sensitive than time-shifting methods to inconsistencies in data processing (eg, noise filtering, sensor calibration).31
Figure 3.
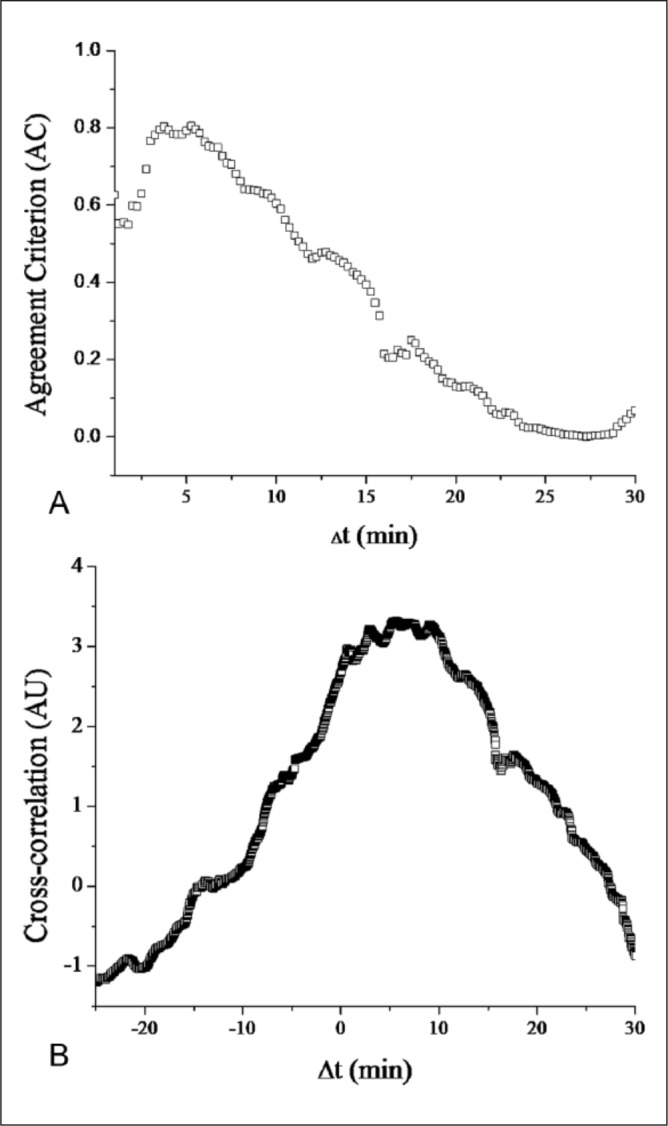
Digital analysis of CGM data presented in Figure 2. Prior to lag-time determination, the sensor and reference data sets were processed using a logarithmic transformation to yield approximately normal distributions.41 (A) Poincaré plot of CGM/reference data set using R2 (the coefficient of linear regression) as the agreement criterion. (B) Cross-correlation of CGM/reference set. The time delay at which local maxima (either the AC or cross-correlation) occur is the estimated sensor lag time.
Prior to any determination of sensor accuracy, the raw sensor signal (ie, current) must be converted to a glucose concentration response. Careful calibration of the sensor response is critical. Sensors calibrated using a 1-point calibration (equation 1), in which the background current (I0) is assumed to be negligible and the measured BG value is directly related to the measured current (I) and the sensor sensitivity (S), were demonstrated by Choleau et al to exhibit improved clinical accuracy over sensors calibrated using a 2-point calibration (equation 2).42,43 The inferiority of the 2-point calibration was attributed to greater error in the separate measurement of both the sensitivity and background current. However, in many cases a substantial background current (eg, due to endogenous electrochemical interferences) may require the use of a 2-point calibration.7,31 Regardless of the method, timing of the calibration is crucial and should be performed under conditions in which BG levels are approximately constant to limit error introduced by the sensor-blood lag. For the 2-point calibration, a substantial concentration difference (≥15 mg dL-1) between the two BG points is also required. In addition, the frequency of calibration must be carefully considered and kept constant across all data sets so that imprecision due to sensor drift is accounted for. Indeed, consistency in calibration conditions will improve the reliability of both sensor sensitivity and accuracy determinations.
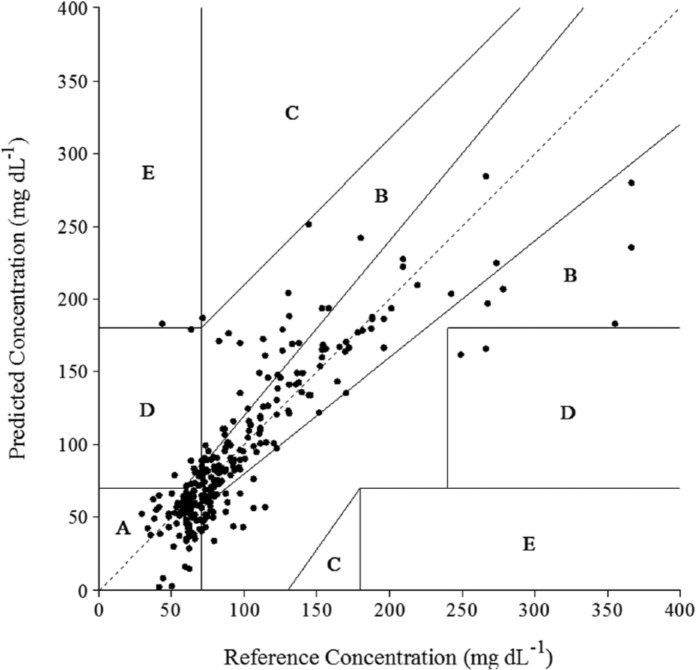
After converting the raw sensor signal to a usable glucose response, sensor accuracy is gauged using an appropriate metric. The most often reported statistic involves determining the mean average relative deviation (MARD).44 The MARD is typically presented as an aggregated statistic or stratified into separate, clinically relevant BG regimes (eg, hypoglycemic, hyperglycemic). The latter is the basis for the International Organization for Standardization (ISO) criteria for glucose sensor accuracy, an entity that separately assesses the percentage of BG determinations (1) within 15 mg dL-1 of the measurement derived from the reference method when BG is less than or equal to 75 mg dL-1 and (2) within 20% of the reference when BG is greater than 75 mg dL-1. In vivo biosensor performance evaluations should consider both descriptors of accuracy given the ease in which these calculations can be made. Other techniques for determining the clinical utility of CGMs have been suggested, including the use of the Clarke45 and consensus46 error grids, which assign pairs of sensor and reference-derived glucose measurements to zones (Figure 4) representing various clinical outcomes. More recently, a new accuracy determination method was reported by Kovatchev et al,47 which is based on BG rate of change and focuses on the predictive utility of CGMs for early hypoglycemia/hyperglycemia detection.
Figure 4.
Clarke error grid analysis for a glucose biosensor after implantation in a healthy swine model. The clinical accuracy of the CGM was determined by comparison to a One Touch Ultra handheld reference glucometer. Zones A and B represent clinically accurate measurements and clinically benign errors, respectively, while zones C, D, and E represent inaccurate and progressively worse glucose measurements.
In practice, the MARD, ISO criteria, and the original Clarke error grid analysis (C-EGA) are often simultaneously used to judge in vivo sensor performance, with the results of each analysis dependent on experimental parameters (eg, animal model, calibration). However, a limitation of clinical metrics (eg, C-EGA) is that accuracy data cannot be compared between animal models, or even between similar animals of different age, as the error grids impose unique accuracy requirements dependent on the magnitude of the BG measurement. To further exacerbate this issue of precision, the assumption of reference glucometer accuracy is often invalid as handheld glucometers usually serve the role as the reference method over benchtop glucose analyzers. While widely available and easy to use, the inherent error associated with BG measurement for most handheld glucometers (MARD 5-10%)48 approaches that of several commercially available CGMs (12-19%).49 This drawback has significant implications for the utility of nonstatistical clinical accuracy metrics (eg, C-EGA), as imprecision of the reference method will propagate into the EGA and suggest inadequate CGM performance, especially in the region of 50-100 mg dL-1 BG where accuracy requirements are most demanding. Statistical measures of CGM accuracy (ie, mean and median absolute and relative deviation) are thus more suitable for the initial evaluation of in vivo glucose sensor performance, as these descriptors are less sensitive to error in the reference method. Clinical accuracy analysis may be pursued in follow-up studies using more reliable reference methods (eg, benchtop analyzers).
Conclusions
Relevant and translational animal models of diabetes should be used to assess the utility of new biomaterials on in vivo CGM performance. Data obtained from such experiments must be meticulously analyzed with respect to the animal model, BG levels and excursion rates, filtering and lag-time correction techniques, and calibration methods. Careful attention to these parameters is critical for ensuring both data reliability and appropriate rigor in evaluating any perceived benefits from other testing methods (eg, cytotoxicity, surface biofouling assays, histology).
Footnotes
Abbreviations: AC, agreement criterion; BG, blood glucose; C-EGA, Clarke error grid analysis; CGM, continuous glucose monitor; FBR, foreign body response; IP, intraperitoneal; IV, intravascular; ISO, International Organization for Standardization; MARD, mean average relative deviation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. World health statistics 2012. Geneva, Switzerland; 2012. [Google Scholar]
- 2. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 3. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 4. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. [PubMed] [Google Scholar]
- 5. Wilson GS, Zhang Y. Introduction to the glucose sensing problem. In In Vivo Glucose Sensing. New York, NY: John Wiley; 2009:1-27. [Google Scholar]
- 6. Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH. Biocompatible materials for continuous glucose monitoring devices. Chem Rev. 2013;113(4):2528-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bindra DS, Zhang Y, Wilson GS, et al. Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal Chem. 1991;63(17):1692-1696. [DOI] [PubMed] [Google Scholar]
- 9. Vaddiraju S, Wang Y, Qiang L, Burgess DJ, Papadimitrakopoulos F. Microsphere erosion in outer hydrogel membranes creating macroscopic porosity to counter biofouling-induced sensor degradation. Anal Chem. 2012;84(20):8837-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novak MT, Yuan F, Reichert WM. Macrophage embedded fibrin gels: An in vitro platform for assessing inflammation effects on implantable glucose sensors. Biomaterials. 2014;35(36):9563-9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: Macrophages and continuous glucose monitoring. Biomaterials. 2014;35(10):3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Cao Z, Bai T, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotech. 2013;31(6):553-556. [DOI] [PubMed] [Google Scholar]
- 13. Sussman E, Halpin M, Muster J, Moon R, Ratner B. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42(7):1508-1516. [DOI] [PubMed] [Google Scholar]
- 14. Klueh U, Kreutzer DL. Murine model of implantable glucose sensors: a novel model for glucose sensor development. Diabetes Technol Ther. 2005;7(5):727-737. [DOI] [PubMed] [Google Scholar]
- 15. Mang A, Pill J, Gretz N, et al. Biocompatibility of an electrochemical sensor for continuous glucose monitoring in subcutaneous tissue. Diabetes Technol Ther. 2005;7(1):163-173. [DOI] [PubMed] [Google Scholar]
- 16. Gifford R, Batchelor MM, Lee Y, Gokulrangan G, Meyerhoff ME, Wilson GS. Mediation ofin vivo glucose sensor inflammatory response via nitric oxide release. J Biomed Mater Res A. 2005;75A(4):755-766. [DOI] [PubMed] [Google Scholar]
- 17. Klueh U, Qiao Y, Frailey JT, Kreutzer DL. Impact of macrophage deficiency and depletion on continuous glucose monitoring in vivo. Biomaterials. 2014;35(6):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mastrototaro JJ, Johnson KW, Morff RJ, Lipson D, Andrew CC, Allen DJ. An electroenzymatic glucose sensor fabricated on a flexible substrate. Sensors Actuators B Chem. 1991;5(1-4):139-144. [Google Scholar]
- 19. Johnson KW, Mastrototaro JJ, Howey DC, et al. In vivo evaluation of an electroenzymatic glucose sensor implanted in subcutaneous tissue. Biosens Bioelectron. 1992;7(10):709-714. [DOI] [PubMed] [Google Scholar]
- 20. Roy S, Biswas S, Khanna S, et al. Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics. 2009;37(3):211-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wisniewski N, Rajamand N, Adamsson U, et al. Analyte flux through chronically implanted subcutaneous polyamide membranes differs in humans and rats. Am J Physiol Endocrinol Metab. 2002;282(6):E1316-E1323. [DOI] [PubMed] [Google Scholar]
- 22. Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344-356. [DOI] [PubMed] [Google Scholar]
- 23. Fahey TJ, III, Sadaty A, Jones WG, II, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res. 1991;50(4):308-313. [DOI] [PubMed] [Google Scholar]
- 24. Schaper NC, Havekes B. Diabetes: impaired damage control. Diabetologia. 2012;55(1):18-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wheat LJ. Infection and diabetes mellitus. Diabetes Care. 1980;3(1):187-197. [DOI] [PubMed] [Google Scholar]
- 26. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553-1579. [DOI] [PubMed] [Google Scholar]
- 27. Le NN, Rose MB, Levinson H, Klitzman B. Implant healing in experimental animal models of diabetes. J Diabetes Sci Technol. 2011;5(3):605-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J. 2006;47(3):243-258. [DOI] [PubMed] [Google Scholar]
- 29. Luo J, Quan J, Tsai J, et al. Nongenetic mouse models of non—insulin-dependent diabetes mellitus. Metabolism. 1998;47(6):663-668. [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Wan R, Mo Y, Zhang Q, Sherwood LC, Chien S. Creating a long-term diabetic rabbit model. Exper Diabetes Res. 2010;2010:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soto RJ, Privett BJ, Schoenfisch MH. In vivo analytical performance of nitric oxide-releasing glucose biosensors. Anal Chem. 2014;86(14):7141-7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamath A, Mahalingam A, Brauker J. Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11(11):689-695. [DOI] [PubMed] [Google Scholar]
- 33. Klueh U, Kreutzer DL. Murine model of implantable glucose sensors: a novel model for glucose sensor development. Diabetes Technol Ther. 2005;7(5):727-737; discussion 738-740. [DOI] [PubMed] [Google Scholar]
- 34. Thome-Duret V, Gangnerau MN, Zhang Y, Wilson GS, Reach G. Modification of the sensitivity of glucose sensor implanted into subcutaneous tissue. Diabetes Metab. 1996;22(3):174-178. [PubMed] [Google Scholar]
- 35. Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. JAALAS. 2011;50(5):600-613. [PMC free article] [PubMed] [Google Scholar]
- 36. Committee on Dogs, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Laboratory animal management: Dogs. Washington, DC: National Academies Press; 1994. [Google Scholar]
- 37. Bequette BW. Continuous glucose monitoring: real-time algorithms for calibration, filtering, and alarms. J Diabetes Sci Technol. 2010;4(2):404-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Novak M, Yuan F, Reichert W. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398(4):1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401-412. [DOI] [PubMed] [Google Scholar]
- 40. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598-605. [DOI] [PubMed] [Google Scholar]
- 41. Kovatchev BP, Shields D, Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11(3):139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor: part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosens Bioelectron. 2002;17(8):641-646. [DOI] [PubMed] [Google Scholar]
- 43. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients: part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17(8):647-654. [DOI] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration, Center for Devices and Radiological Health. The content of investigational device exemption (IDE) and premarket approval (PMA) applications for artificial pancreas device systems. 2012. [Google Scholar]
- 45. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622-628. [DOI] [PubMed] [Google Scholar]
- 46. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148. [DOI] [PubMed] [Google Scholar]
- 47. Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922-1928. [DOI] [PubMed] [Google Scholar]
- 48. Tack C, Pohlmeier H, Behnke T, et al. Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: a European multicenter study with 453 subjects. Diabetes Technol Ther. 2012;14(4):330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gifford R. Continuous glucose monitoring: 40 years, what we’ve learned and what’s next. Chemphyschem. 2013;14(10):2032-2044. [DOI] [PubMed] [Google Scholar]