Abstract
Background:
Implantable glucose sensors demonstrate a rapid decline in function that is likely due to biofouling of the sensor. Previous efforts directed at overcoming this issue has generally focused on the use of synthetic polymer coatings, with little apparent effect in vivo, clearly a novel approach is required. We believe that the key to extending sensor life span in vivo is the development of biocompatible basement membrane (BM) based bio-hydrogels as coatings for glucose sensors.
Method:
BM based bio-hydrogel sensor coatings were developed using purified BM preparations (ie, Cultrex from Trevigen Inc). Modified Abbott sensors were coated with Cultrex BM extracts. Sensor performance was evaluated for the impact of these coatings in vitro and in vivo in a continuous glucose monitoring (CGM) mouse model. In vivo sensor function was assessed over a 28-day time period expressed as mean absolute relative difference (MARD) values. Tissue reactivity of both Cultrex coated and uncoated glucose sensors was evaluated at 7, 14, 21 and 28 days post–sensor implantation with standard histological techniques.
Results:
The data demonstrate that Cultrex-based sensor coatings had no effect on glucose sensor function in vitro. In vivo glucose sensor performance was enhanced following BM coating as determined by MARD analysis, particularly in weeks 2 and 3. In vivo studies also demonstrated that Cultrex coatings significantly decreased sensor-induced tissue reactions at the sensor implantation sites.
Conclusion:
Basement-membrane-based sensor coatings enhance glucose sensor function in vivo, by minimizing or preventing sensor-induced tissues reactions.
Keywords: extracellular matrices, basement membrane, biocompatibility, glucose sensor coatings, Trevigen, implantable glucose sensor, continuous glucose monitoring
Implantable glucose sensor monitoring of blood glucose levels in diabetic patients has been available for over 40 years.1 However, few sensors function optimally for more than a few days.2-7 Generally, sensor malfunction has been attributed to the triad of inflammation, fibrosis, and vessel regression. Previous efforts to overcome biofouling have generally focused on the uses of synthetic polymer coatings with limited success.8-13 However synthetic sensor coatings often experience poor biocompatibility, thus making them less than ideal to overcome tissue reactions to the foreign sensor object.” Therefore, materials with greater degrees of biocompatibility are in order. Our approach to overcome these biocompatibility issues by employing protein based tissue engineering. We hypothesize that extracellular matrices (ECM) are particularly well suited as sensor coatings as they are biologic hydrogels that permit rapid diffusion of glucose to the sensor. Moreover, autologous ECM are very biocompatible materials, as they are derived from tissues and cells rather than synthetic chemicals. For example autologous BM preparations are highly biocompatible and do not induce significant tissue reactions, such as inflammation or fibrosis, thus we hypothesized that these ECM would be highly effective coatings on glucose sensors. The importance of the usage of autologous ECM is underscored by previous ECM-based sensor coating studies when employing xenogenic collagen. Unfortunately, these studies are very limited in number and scope, which demonstrated marginal effects on sensor function in vivo.14 Tissue reactions that were induced by the cross-linked collagens, that is, inflammation and fibrosis, were likely causative.14 To our knowledge, basement membrane (BM) preparations (eg, Cultrex or Matrigel) have not been used as coating for glucose sensors or any other implantable devices. Autologous BM preparations are usually highly biocompatible and as such do not generally induce significant tissue reactions, such as inflammation or fibrosis when compared to synthetic preparations. Therefore we hypothesized that autologous ECM would be highly effective coatings on glucose sensors. We investigated 2 different sources of ECM (Cultrex and Matrigel) and we found that Cultrex experienced very little to no tissue reactions compared to Matrigel. As such, Cultrex was used for all studies
Cultrex Basement Membrane Extract components include the soluble ECM proteins of primarily composed to ECM including laminin (60%), type IV collagen (30%), as well as entactin, heparin sulfate, metalloproteinases, and a variety of growth factors such as PDGF, EGF, and TGFB, which are purified from the murine Engelbreth-Holm-Swarm tumor. In vivo, these ECM proteins create continuous sheets of specialized matrices. In addition to supporting cells and providing a supporting interface between cell layers and their adjacent stroma, they serve a critical role in wound healing and tissue regeneration. We believe that the use of ECM coatings (eg, Cultrex BM), around glucose sensor implantation sites may attenuate the unwanted induction of tissue reactions, such as inflammation, which results in biofouling and reduced glucose sensor function.
Our present studies demonstrate that Cultrex-based BM coatings of glucose sensors accomplished the dual goals of (1) decreased tissue reactivity of the glucose sensors in vivo as well as (2) extending the life span and function of glucose sensors in our murine model of continuous glucose monitoring (CGM). To our knowledge, these are the first studies that demonstrate the effectiveness of BM-based coatings in reducing sensor-induced tissue reactions as well as enhancing and extending glucose sensor function in vivo.
Materials and Methods
Trevigen Cultrex BM Preparations
Cultrex Basement Membrane Extract (Type 2) Clearpath was purchased from Trevigen Inc (Gaithersburg, MD). Cultrex Basement Membrane Extract is a soluble form of BM purified from murine Engelbreth-Holm-Swarm tumor. The BM is stored in Dulbecco’s Modified Eagle’s medium without phenol red, with 10-ug/ml gentamicin sulfate, at a storage and working concentration of approximately 15 mg protein/ml (Table 1). Generally the Cultrex preparations are kept frozen at −80C, thawed in ice water, and maintained on ice for general use.
Table 1.
Composition of Dulbecco’s Modified Eagle’s Medium Used in Cultrex Basement Membrane Preparations.
| Component | gm/L |
|---|---|
| Inorganic salts | 10.97 |
| Amino acids | 2.25 |
| Vitamins | 0.03 |
| Glucose | 4.5 |
| Others | 0.7 |
| Total | 18.45 |
Dialysis of Cultrex BM Preparations
To eliminate salts, vitamins, amino acids, and glucose present in the Cultrex preparations, the Cultrex was dialyzed against sterile deionized water with 3 changes of water using Thermo Scientific Slide-A-Lyzer Mini Dialysis devices. Generally 2 ml of Cultrex is dialyzed against 48 ml of water/exchange, for a total of 3 dialysis exchanges.
Cultrex BM Coating of Glucose Sensors
The modified Abbott Navigator glucose sensors used in the in vitro and in vivo studies were obtained from Abbott Diabetes Care (Alameda, CA). Sensors were sterilized by exposure to UV light overnight prior to administering the sensor coating. Aseptic techniques were utilized during the coating process and prior to implantation. To coat the glucose sensors with BM, the glucose sensor was placed on a sterile polytetrafluoroethylene (PTFE) liner, 50 uL of dialyzed Cultrex (15 mg protein /ml) was applied on 1 side of the sensor and placed in 37°C incubator for 2 hours. The glucose sensor was then turned over and an additional 50 uL of dialyzed Cultrex was applied on the opposite side of the sensor prior to placing it in 37°C incubator for 2 hours. This process was repeated for additional coats of BM for up to 2 mg of Cultrex for each sensor. Generally 0.5 to 2 mg of Cultrex was added to an individual sensor. Our coating technique allowed for a simple and consistent coating process. The Cultrex coated glucose sensors are stored dehydrated in a tissue culture hood until in vitro testing or implantation in mice.
In Vitro Glucose Sensor Testing
To determine if Cultrex coating negatively impacted sensor performance, sensor sensitivity of uncoated glucose sensors (controls), were evaluated pre– and post–Cultrex coating in vitro using our standard CGM system.15 Sensor sensitivity was characterized in tissue culture medium with an initial glucose concentration of 50 mg/dL at 200 mV. Background current was allowed to stabilize for about 15 minutes before sensors were subjected to increased glucose concentration in the culture medium. Sensors were then rinsed in saline and left in a tissue culture hood to dry. After the sensors were dry they were coated with various amount of Cultrex as described above and then retested in vitro using the same protocol as described above. Sensor sensitivity for both pre– and post–Cultrex coatings was determined as described below.14,16
Glucose Sensor Implantation and CGM in a Murine Model
Once it was established that sensor coating did not negatively impact sensor performance in vitro, we next evaluated the performance of the Cultrex coated sensors versus uncoated sensors in our CGM mouse model.17 Coated and uncoated sensors were implanted in CD-1 mice (Jackson Laboratory, Bar Harbor, ME) and CGM was undertaken for a period up to 28 days as described previously.15,17 Blood glucose reference measurements from the tail vein were obtained periodically over the 28-day implantation period using FreeStyle blood glucose monitors. All murine studies were approved by thee Institutional Animal Care and Use Committee of the University of Connecticut Health Center (Farmington, CT). The sensors were not recalibrated such that their readings represent raw sensor output in nano-Amperes (nA).
Continuous Glucose Monitoring Data Analysis
Reference blood measurements were used to calculate the mean absolute relative difference (MARD) over a 4-week experiment for the 2 groups of mice with and without BM-coated sensors. Equations 1 to 3 describe the MARD calculation in detail. Sensitivity (S; mg/dl/nA) is calculated for each mouse based on the reference blood glucose and the sensor output (I; nA) measurements in an initial reference stage of the experiment, that is, k in equation 2 is approximately 5, for the first initial 5 measurements across 2 days.
| (1) |
| (2) |
| (3) |
Histopathological Analysis of Tissue Reactions at Glucose Sensor Implantation Sites
To evaluate tissue responses to Cultrex and non–Cultrex coated glucose sensors tissue samples were extracted from mice concluding CGM evaluation. Mice were euthanized and the full thickness of the skin and sensors were removed end bloc in approximately 3 × 3 cm2 sections and immediately placed in tissue fixative. Tissues were fixed in formalin for 24 to 48 hours followed by standard procedure, embedded in paraffin, and sectioned. The resulting 5-μm sections were then stained using standard protocols for hematoxylin and eosin stain and Masson trichrome for the evaluation of fibrosis. Histopathological evaluation of tissue reactions at sites of sensor implantation was performed on mouse specimens obtained at 1-28 days post–sensor implantation. The tissue section slides were viewed and assessed by a blinded experienced histopathologist (DLK) using a modified histologic scale.14-16,18 Histologic parameters included inflammatory response, foreign body reaction, fibrotic response, collagen organization, and neovascularization. After an initial review of all slides to gain a baseline measure of histologic parameters, each sample was reevaluated and scored against each other to obtain a semiquantitative measure of tissue responses to the implanted sensors. For the inflammatory response, the degree of infiltration of chronic inflammatory cells, principally lymphocytes and macrophages, surrounding the sensor was noted. Foreign body reaction was determined by the relative quantity of foreign body giant cells surrounding each sensor or adjoining tissue of sensor. Fibrotic change was a function of relative abundance of new collagen deposition at sites of sensor implantation, while collagen organization was determined by factors such as connective tissue density (loose vs dense) and arrangement of collagen bundles (parallel vs haphazard pattern). Neovascularization was a reflection of the number of new blood vessels per high power field.18
Statistical Analysis
The mean MARD values for each group, together or separated by week, were evaluated statistically, including tests to determine if the group MARD values were normally distributed. In cases where the mean MARD values were nonnormal in distribution, Mann–Whitney U tests were then conducted to determine the statistical differences between the 2 groups of average mean MARD values, as nonparametric equivalents to Student t tests. Microsoft Excel for Mac 2011 (version 14.1.4) and IBM SPSS Statistics 20 (release 20.0.0) were the software packages used for the calculations/graphing and statistical analyses, respectively.
Results
Development and Validation of Cultrex BM Extracts as Effective Protein Coatings for Glucose Sensors
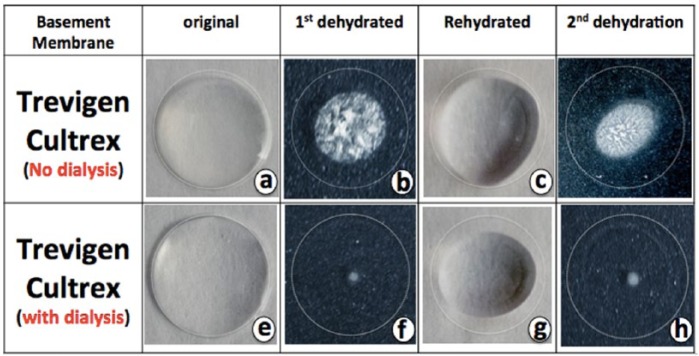
Our initial efforts in coating glucose sensors utilizing commercial Cultrex BM resulted in the significant presence of crystals at the sensor surface, which precluded usage of Cultrex as a coating agent. Commercial Cultrex preparations in Modified Eagle’s Media (MEM) contain salts, vitamins, amino acids, and glucose. To eliminate components of the MEM and other crystals, Cultrex BM was dialyzed against sterile deionized water (1/25 ratio of Cultrex/water with 3 water changes). Comparison of the dialyzed Cultrex BM to the starting nondialyzed Cultrex showed that the crystals completely disappeared in the dialyzed Cultrex (Figures 1a, 1b, 1e, 1f). We further found that the dehydrated Cultrex could be successfully rehydrated, resulting in the formation of clear protein coatings (Figures 1c, 1d, 1g, 1h). Figure 2 demonstrated that the dried Cultrex could be dehydrated into thin protein films and rehydrated into large hydrogels. Therefore, we developed a Cultrex coating protocol that provided a simple and consistent coating of glucose sensors with varying layers for in vitro and in vivo applications.
Figure 1.
Crystal formation in predialyzed and postdialyzed Trevigen Cultrex BM preparations. Commercial Cultrex BM preparations were dialyzed against water, using a 3500 MW cutoff “slid-A-lizer” dialysis system from Life Technologies (Thermo scientific). Trevigen’s Cultrex BM preparations were evaluated directly or after dialysis against water dehydration and rehydration patterns in vitro on glass microscope slides. A total of 50 ul of Cultrex (a) or dialyzed Cultrex (e) was spotted on glass slides and allowed to dry at 37°C. Nondialyzed Cultrex dried with significant crystal formation (b), but no significant amount crystals formation was seen on the dialyzed Cultrex (f). Rehydration of both of the dehydrated Cultrex preparation resulted in formation of “gelatin” drops similar to the original Cultrex drops seen prior to dehydration (c and g). Dehydration of the rehydrated Cultrex preparations resulted in identical crystal formation as was seen after the first dehydration (b and f).
Figure 2.
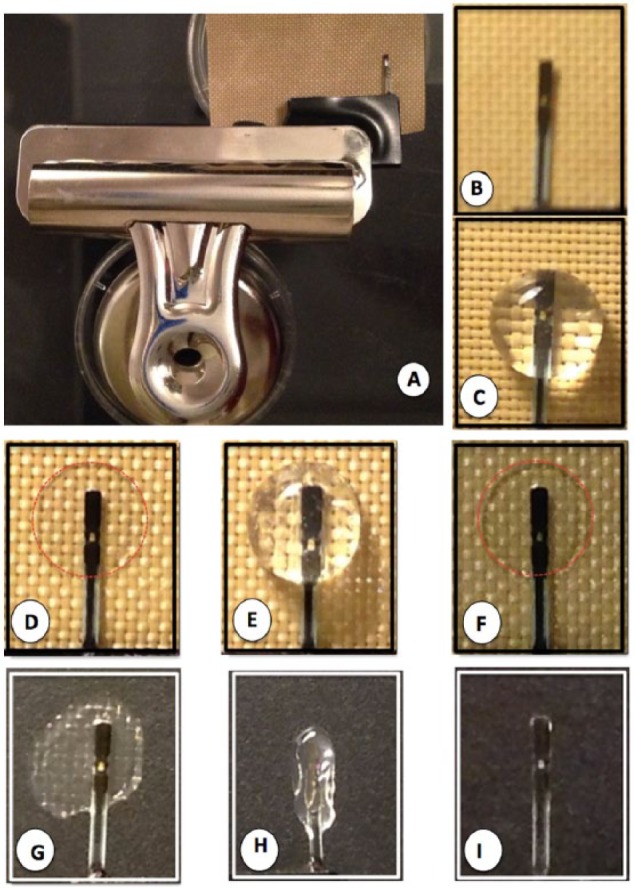
Coating of glucose sensors in vitro with Cultex BM preparations. To allow simple and reproducible coating of glucose sensors with Cultrex for in vitro and in vivo studies a clamp based system which utilizes a standard magnetic office clamp (OC), a modified Abbott glucose sensor (GS), and a polytetrafluoroethylene sheet (PTFE) was utilized (A). The Abbott sensor is centered on top of the PTFE sheet (B), and 50 ul of dialyzed Cultrex is added on top of the sensor (C) and allowed to dry at 37°C, resulting in a thin protein layer on the sensor and associated PTFE sheet (D, red dotted line). The sensor is then removed from the PTFE sheet, flipped over and an additional 50 ul of dialyzed Cultrex is added (E) and allowed to dry (F). This process can be repeated as needed to form a Cultrex coating on the sensor. Finally the sensor with a dry Cultrex coating is removed from the PTFE sheet (g), dipped in sterile water (h) and then allow to dry at 37°C until dry (i). The resulting Cultrex sensor is then utilized for in vitro studies or implanted subcutaneously for in vivo studies.
Impact of Cultrex Coatings on Glucose Sensor Function in Vitro and in Vivo
Following the successful BM sensor coating, the next question was whether Cultrex itself or the coating process compromised sensor function. To begin to answer this question we initially evaluated the impact of varying coatings of Cultrex on glucose sensor performance in vitro. Sensor sensitivity remained unchanged with and without coating within the range of 0 to 2 mg Cultrex/sensor and was determined to be 45.9 ± 4.8 mg/dL/nA and 48.6 ± 5.1 mg/dL/nA, respectively.
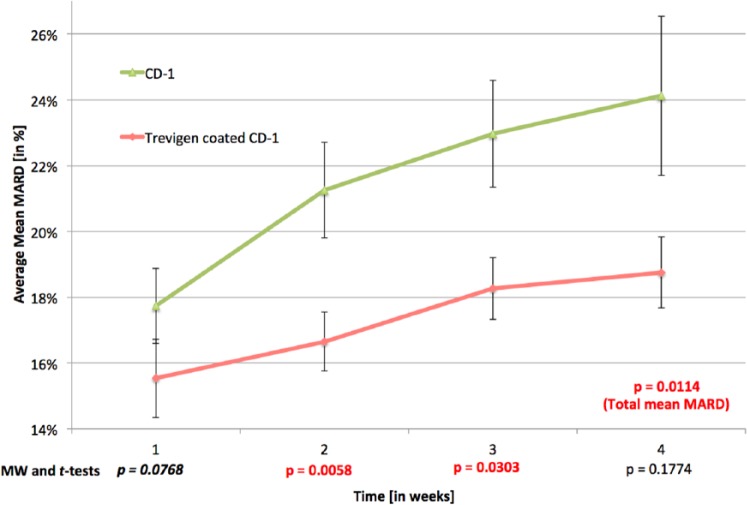
Once we demonstrated that Cultrex coatings ranging from 0.5 to 2 mg of Cultrex/sensor did not negatively affect sensor performance in vitro, we next determined the effect of Cultrex coatings on sensor performance in vivo for a period of up to 28 days. These in vivo studies were performed utilizing our established murine model of CGM.15,17 MARD values of BM coated and uncoated sensors in CD-1 mice as a measure of performance and error of the CGM sensors over time was determined; that is, the lower the MARD values, the lower the error, the better the performance. It is important to note that no sensor recalibration occurred for these studies. Thus MARD values used in these studies are for raw sensor output. As illustrated in Figure 3, by the second week, the average MARD value for the CD-1 mice with Cultrex coated sensors was 16.7% in a group of 34 mice, as compared to the CD-1 control mice with 20.52% on average, in a group of 39 mice. These sample sizes are relatively large for such investigations. A calculation of standard deviation of the MARD values for these groups of mice indicates that the CD-1 controls present a larger standard deviation of 6.58% while the Cultrex coated CD-1 MARD values present a smaller standard deviation of 6.14%.
Figure 3.
The Impact of Cultrex coating on continuous glucose monitoring (CGM) in vivo. To determine the impact of Cultrex coatings on sensor function we utilized our murine model of CGM.37 For these studies Cultrex coated Abbott sensors were compared with uncoated Abbott sensor implanted subcutaneously in mice over a 4-week time period. The resulting sensor output (nA) and actual blood glucose levels were evaluated using standard MARD analysis using Mann–Whitney U tests and Student t tests. Cultrex coated sensors preformed significantly better then uncoated sensors over the entire 4-week time period (total mean MARD P = .0114). Cultrex coated sensor performed statistically better then uncoated sensors at 2 weeks (P = .0058) and 3 weeks (P = .0303) post–sensor implantation. Although sensor performance was better in the Cultrex coated sensor at week 4, it was not statistically significant (P = .1774).
As illustrated in Figure 3, a trend analysis of the MARD values of the BM coated sensors in CD-1 mice and their CD-1 control mice, over the course of the 4-week experiment, illustrates (shows) a significant improvement in sensor performance, that is, lower MARD values, for the BM coated sensors, particularly in weeks 2 and 3. This difference in MARD values between the 2 groups was statistically evaluated by Student t tests and its nonparametric equivalent, Mann–Whitney U tests. The MARD values for weeks 1, 2, and 4 required the nonparametric Mann–Whitney U tests, while week 3 and the total mean MARD values were evaluated with Student t tests. Total mean MARD values are MARD values not separated by week, that is, each sensor/mouse has 1average or mean MARD value for the entire or total experiment. A statistical comparison between the 2 groups by total mean MARD revealed that they were significantly different, P = .0114. The statistical comparison between the 2 groups by week demonstrated that in the first and fourth weeks, there were no significant or trending significant difference, that is, P = .0768 and .01774, respectively. We believe that the lack of difference at week 1 was the result of established performance of sensors for 7 days postimplantation. Therefore, with or without sensor coatings one would expect good performance in the first week postimplantation. As expected the uncoated sensors displayed a decrease in sensor performance beyond 7-day implantation but the Cultrex coated sensor displayed significantly better performance over the same 28 days of the study (Figure 3). For example, in the second week, a Mann–Whitney U test, revealed a statistically significant difference between the MARD values for Cultrex coated sensors in CD-1 mice when compared to the uncoated sensor implanted in CD-1 control mice (P = .0058). In addition, in the third week, a Student t test revealed that the Cultrex coated sensors showed statistically significant better performance (P = .0303). By the fourth week, although the Cultrex coated sensors did appear to be out performing the uncoated sensors, the MARD between them was not statistically different (P = .1774). We hypothesized that the increased MARD values seen in the 3 and 4 weeks post–sensor implantation was likely the result of degradation of the Cultrex coatings, a result of the normal tissue remodeling. This hypothesis was supported by the histologic studies described below. This degradation ultimately exposes the underlying glucose sensor, triggering the regular tissue reactions including inflammation and tissue remodeling leading to further degradation of the Cultrex coating of the sensors.
Impact of Cultrex Coatings on Sensor-Induced Tissue Reactions at Sites of Glucose Sensor Implantations
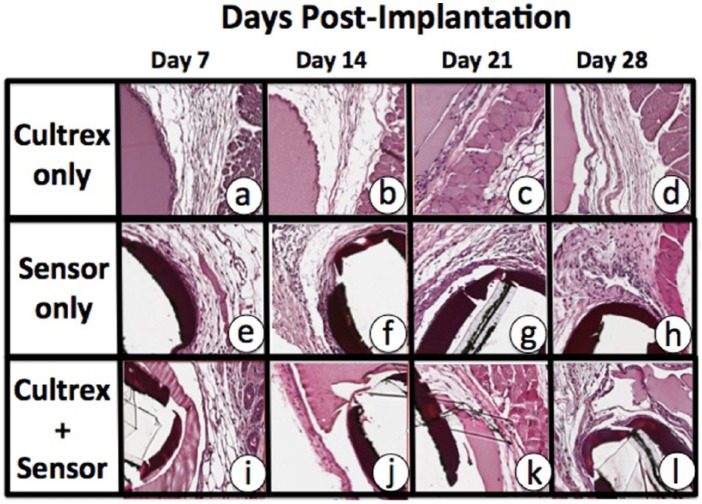
Previous studies in our laboratories have demonstrated that Trevigen’s Cultrex BM preparations (gel form) can be implanted in mouse subcutaneous tissue for extended periods of time without inducing significant tissue reactions (personal observations). As such, the biocompatibility of the Cultrex in vivo suggested that it could be a strong candidate for biocompatibility coating for implanted devices, such as glucose sensors. Developing a dehydrated version of the Cultrex would be important both for ease of implantation, as well as shelf life. Unfortunately we had not previously tested the tissue reactivity of dehydrated Cultrex in mice. As such, we evaluated the tissue reactivity of dehydrated Cultrex BM when implanted subcutaneously for up to 28 days postimplantation. As can be seen in Figure 4, histologic evaluation of the tissue reactivity of dehydrated Cultrex demonstrated that this BM preparation was very biocompatible for the tested time period of 7-28 days (see Figures 4a-4d). When we evaluated the tissue reactivity of Cultrex coated sensors, as we hypothesized, the Cultrex coating enhanced sensor function in vivo. The coating also prevented the sensors from inducing significant tissue reactivity (Figures 4e-4h) at the implantation site when compared to uncoated sensors (Figures 4i-4l). As suspected, during the first 14 days post–senor implantation, the Cultrex appeared intact on the sensor surface and significant less tissue reaction at the implantation site was observed (Figures 4i and 4j). However by 21 and 28 days there was significant loss of the Cultrex coating on the sensor surface and early indication of tissue reactions being induced at the sensor implantation site (Figures 4k and 4l). These histologic studies are consistent with the sensor function study present in Figure 3.
Figure 4.
Tissue reactions induced in murine models of CGM by Cultrex only, glucose sensors only, and Cultrex coated glucose sensors. Cultrex and Abbott sensors (with and without Cultrex coatings) were obtained as implantation sites at 7, 14, 21, and 28 days postimplantation, processed for standard histopathology using paraffin embedding and H&E staining. Tissue reactions to Cultrex only implantations demonstrated that Cultrex (smooth pink staining) was very biocompatible and it did not induces any significant tissue reactions over the 28-day test period (a-d). As expected sensor only (black bands are remnants of sensor) implantation sites displayed significant tissue reaction characterized by implantation and fibrosis (e-h). Tissue reactions to Cultrex coated sensors (i-l) demonstrated that the Cultrex coating minimized tissue reactions to the glucose sensors, particularly in the first 3 weeks (i-k), but by the 4 weeks post–sensor implantation there was significant degradation of the Cultrex, exposure of the glucose sensor and induction of tissue reactions (l). Glucose sensor performance generally followed the same pattern as was seen with the Cultrex coatings, that is, early time points (1-3 weeks) have intact Cultrex coatings and good sensor performance, but as the Cultrex degraded exposing the glucose sensor, resulting in increase tissue reactions and sensor performance decreased.
Discussion
Increasing the in vivo biocompatibility of implantable glucose sensors is critical to extending the useful life span of these sensors in the future. In the present study we hypothesized that biocompatible BM-based bio-hydrogels as coatings for glucose sensors aid in extending sensor life span in vivo. In this study we utilized Cultrex Basement Membrane Extract as 1 possible BM sensor coating. In previous studies we also tested other BMs, such as Matrigel (BD) and PuraMatrix (Waltham, MA) (data not shown). Although PuraMatrix is a synthetic peptide hydrogel, we included this in our study because if successful PuraMatrix would allow a speedy transition into other animal models or humans. Nevertheless Matrigel and PuraMatrix coatings showed significant more tissue reactivity (ie, inflammation) when compared to Cultrex, we focused all of our subsequent sensor studies on this type of membrane. To move toward this goal, we demonstrated that BM coating of glucose sensors enhanced sensor biocompatibility and function in vivo. Specifically, our data demonstrate a superior performance of the Cultrex coated sensors versus noncoated sensors in the first 3 weeks. Nonetheless, there was a subsequent decline in sensor performance after first 3 weeks, which may be due to natural degradation processes related to extracellular turnover. We also hypothesize that this degradation process exposes the original sensor surface inducing the commonly observed foreign body tissue reaction. Thus, the bio-hydrogel delays the tissue reaction at the sensor implantation site, which is dependent on the decay rate of the outer sensor protected Cultrex coating. Future efforts aimed at slowing the degradation rate of the bio-hydrogel may well extend the functional life of the glucose sensor. Alternatively, extracellular Cultrex turnover could also induce binding of proteins and/or drugs. For example, naturally occurring matrices present numerous binding sites for growth factors and cytokines within their molecular structures.19-28 In vivo, these binding sites serve a critical role in configuring matrix bound factors and proteins for regulation of cell and tissue responses to injury, including inflammation, repair and regeneration (eg, neovascularization). For example, when angiogenic factors, such as VEGF and FGF are bound to bio-matrices, they are more stable and potent than free angiogenic factors.29-33 Commercial BM preparations such as Cultrex and Matrigel, have been used extensively to investigate cell attachment and growth in vitro and in vivo, as well as a matrix for supporting angiogenesis.34-36 Thus, these naturally occurring matrices with additional bioactive factors will likely have a profound positive influence on cell, tissue and sensor function in vivo. In the future we plan to extend these BM based sensor coatings into porcine models of CGM and ultimately humans using autologous BM. For example, Trevigen Inc already produces human BM from human placenta tissue and Trevigen Inc is currently developing porcine BM for the usage in pig models (personnel communication). Therefore, BM based sensor coatings appear to be an extremely useful sensor coatings, which will likely further control tissue reactions and enhance glucose sensor function in the future by the addition of bioactive factors.
Footnotes
Abbreviations: BM, basement membrane; CGM, continuous glucose monitoring; ECM, extracellular matrices; MARD, mean absolute relative difference; MEM, Modified Eagle’s Media; PTFE, polytetrafluoroethylene.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Leona M. and Harry B. Helmsley Charitable Trust and the National Institute of Health provided the funding for this study.
Reference
- 1. Gifford R. Continuous glucose monitoring: 40 years, what we’ve learned and what’s next. Chemphyschem. 2013;14(10):2032-2044. [DOI] [PubMed] [Google Scholar]
- 2. Buhling KJ, Kurzidim B, Wolf C, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp Clin Endocrinol Diabetes. 2004;112(10):556-560. [DOI] [PubMed] [Google Scholar]
- 3. Djakoure-Platonoff C, Radermercker R, Reach G, Slama G, Selam JI. Accuracy of the continuous glucose monitoring system in inpatient and outpatient conditions. Diabetes Metab. 2003;29(2 pt 1):159-162. [DOI] [PubMed] [Google Scholar]
- 4. Feldman B, Brazg R, Schwartz S, Weinstein R. A continuous glucose sensor based on wired enzyme technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5(5):769-779. [DOI] [PubMed] [Google Scholar]
- 5. Kapitza C, Lodwig V, Obermaier K, et al. Continuous glucose monitoring: reliable measurements for up to 4 days with the SCGM1 system. Diabetes Technol Ther. 2003;5(4):609-614. [DOI] [PubMed] [Google Scholar]
- 6. Maran A, Crepaldi C, Tiengo A, et al. Continuous subcutaneous glucose monitoring in diabetic patients: a multicenter analysis. Diabetes Care. 2002;25(2):347-352. [DOI] [PubMed] [Google Scholar]
- 7. Tansey MJ, Beck RW, Buckingham BA, et al. Accuracy of the modified Continuous Glucose Monitoring System (CGMS) sensor in an outpatient setting: results from a diabetes research in children network (DirecNet) study. Diabetes Technol Ther. 2005;7(1):109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudo H, Sawada T, Kazawa E, Yoshida H, Iwasaki Y, Mitsubayashi K. A flexible and wearable glucose sensor based on functional polymers with soft-MEMS techniques. Biosens Bioelectron. 2006;22(4):558-562. [DOI] [PubMed] [Google Scholar]
- 9. Kvist PH, Iburg T, Aalbaek B, et al. Biocompatibility of an enzyme-based, electrochemical glucose sensor for short-term implantation in the subcutis. Diabetes Technol Ther. 2006;8(5):546-559. [DOI] [PubMed] [Google Scholar]
- 10. Kvist PH, Iburg T, Bielecki M, et al. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Technol Ther. 2006;8(4):463-475. [DOI] [PubMed] [Google Scholar]
- 11. Mang A, Pill J, Gretz N, et al. Biocompatibility of an electrochemical sensor for continuous glucose monitoring in subcutaneous tissue. Diabetes Technol Ther. 2005;7(1):163-173. [DOI] [PubMed] [Google Scholar]
- 12. Nishida K, Sakakida M, Ichinose K, et al. Development of a ferrocene-mediated needle-type glucose sensor covered with newly designed biocompatible membrane, 2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate. Med Prog Technol. 1995;21(2):91-103. [PubMed] [Google Scholar]
- 13. Poitout V, Moatti-Sirat D, Reach G. Development of a glucose sensor for glucose monitoring in man: the disposable implant concept. Clin Mater. 1994;15(4):241-246. [DOI] [PubMed] [Google Scholar]
- 14. Ju YM, Yu B, West L, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. II. Long-term in vitro/in vivo sensitivity characteristics of sensors with NDGA- or GA-crosslinked collagen scaffolds. J Biomed Mater Res A. 2010;92(2):650-658. [DOI] [PubMed] [Google Scholar]
- 15. Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35(10):3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A. 2008;87(1):136-146. [DOI] [PubMed] [Google Scholar]
- 17. Klueh U, Qiao Y, Frailey JT, Kreutzer DL. Impact of macrophage deficiency and depletion on continuous glucose monitoring in vivo. Biomaterials. 2014;35(6):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW. Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res. 2012;176:423-429. [DOI] [PubMed] [Google Scholar]
- 19. Hall H, Baechi T, Hubbell JA. Molecular properties of fibrin-based matrices for promotion of angiogenesis in vitro. Microvasc Res. 2001;62(3):315-326. [DOI] [PubMed] [Google Scholar]
- 20. Wagner WR, Hubbell JA. Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J Lab Clin Med. 1990;116(5):636-650. [PubMed] [Google Scholar]
- 21. Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13(15):2214-2224. [DOI] [PubMed] [Google Scholar]
- 22. Ye Q, Zund G, Benedikt P, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17(5):587-591. [DOI] [PubMed] [Google Scholar]
- 23. Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18(4):415-419. [DOI] [PubMed] [Google Scholar]
- 24. Herbert CB, Nagaswami C, Bittner GD, Hubbell JA, Weisel JW. Effects of fibrin micromorphology on neurite growth from dorsal root ganglia cultured in three-dimensional fibrin gels. J Biomed Mater Res. 1998;40(4):551-559. [DOI] [PubMed] [Google Scholar]
- 25. Herbert CB, Bittner GD, Hubbell JA. Effects of fibinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. J Comp Neurol. 1996;365(3):380-391. [DOI] [PubMed] [Google Scholar]
- 26. Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69(1):149-158. [DOI] [PubMed] [Google Scholar]
- 27. Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75-81. [DOI] [PubMed] [Google Scholar]
- 28. Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF—fibrin matrices for endothelialization. J Control Release. 2001;72(1-3):101-113. [DOI] [PubMed] [Google Scholar]
- 29. Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery. 2002;132(2):259-267. [DOI] [PubMed] [Google Scholar]
- 30. Sahni A, Baker CA, Sporn LA, Francis CW. Fibrinogen and fibrin protect fibroblast growth factor-2 from proteolytic degradation. Thromb Haemost. 2000;83(5):736-741. [PubMed] [Google Scholar]
- 31. Pandit AS, Wilson DJ, Feldman DS. Fibrin scaffold as an effective vehicle for the delivery of acidic fibroblast growth factor (FGF-1). J Biomater Appl. 2000;14(3):229-242. [DOI] [PubMed] [Google Scholar]
- 32. Quirinia A, Viidik A. The effect of recombinant basic fibroblast growth factor (bFGF) in fibrin adhesive vehicle on the healing of ischaemic and normal incisional skin wounds. Scand J Plast Reconstr Surg Hand Surg. 1998;32(1):9-18. [DOI] [PubMed] [Google Scholar]
- 33. Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96(12):3772-3778. [PubMed] [Google Scholar]
- 34. Albini A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol Oncol Res. 1998;4(3):230-241. [DOI] [PubMed] [Google Scholar]
- 35. Benelli R, Albini A. In vitro models of angiogenesis: the use of Matrigel. Int J Biol Markers. 1999;14(4):243-246. [DOI] [PubMed] [Google Scholar]
- 36. Yamamura K, Kibbey MC, Jun SH, Kleinman HK. Effect of Matrigel and laminin peptide YIGSR on tumor growth and metastasis. Semin Cancer Biol. 1993;4(4):259-265. [PubMed] [Google Scholar]
- 37. Klueh U, Liu Z, Cho B, et al. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):402-412. [DOI] [PubMed] [Google Scholar]