Abstract
Background:
The purpose of this study was to evaluate the accuracy and efficacy of Dexcom G4 Platinum CGM System.
Methods:
Seventy-two subjects enrolled at 4 US centers; 61% were male; 83% had T1DM and17% had T2DM. Subjects wore at least 1 system for up to 7 days. Subjects participated in a total of 36 hours in the clinic to contribute YSI reference glucose measurements with venous blood draws every 15 minutes on study Day 1, Day 4, and Day 7.
Results:
The overall mean absolute relative difference (ARD) versus YSI was 13% with a median of 10%. Precision ARD was 9% ± 4% between 2 sensors with a 7% coefficient of variation. The mean ARD versus SMBG was 14% with a median of 11%. One hundred two (94%) sensors lasted 7 days and the systems displayed 97% of their expected glucose readings in average. The time spent in low CGM readings during nighttime hours decreased from the first night use to the 6th night (P < .001) with a small difference in average CGM glucose from 147 ± 40 mg/dL to 166 ± 62 mg/dL. There were no serious adverse events or infectious complications reported.
Conclusions:
The study showed the Dexcom G4 Platinum CGM System is one of the most accurate CGMs. The significant reduction in nocturnal time spent in a hypoglycemic state observed during this study suggests that a longer term study of CGM use, especially nocturnal use, could be beneficial for patients with hypoglycemia unawareness.
Keywords: continuous glucose monitor, hypoglycemia, accuracy, efficacy
Several real-time continuous glucose monitoring (CGM) systems have been used for diabetes management. Many people have identified patient specific benefits from using a CGM system. Whether their objective is an improved A1C, reduction in hypoglycemia, optimized therapy, or use in an artificial pancreas system, CGM is a tool helping people reach their diabetes management goals. Those goals are easier to reach due to significant advances in CGM such as better accuracy, more reliability, improved connectivity, and smaller form factors.
Continuous glucose monitors have long been associated with improved patient outcomes as a result of patients’ increased cognizance of their glucose levels. It was previously reported that the use of a 3-day, subcutaneous, real-time, continuous glucose sensor was well-tolerated and resulted in an improvement in glycemic excursions.1 There is abundant clinical evidence suggesting that patients who use their CGM data to improve their treatment decisions experience a reduction of A1c values2-4 and glycemic excursions.5,6 A recent JDRF CGM trial showed that using CGM is associated with improved glycemic control in adults.7 Also, CGM glucose readings provide a unique perspective from which to view diurnal glucose patterns without time and frequency biases.8 It has also been shown that viewing both continuous glucose readings and trend information help patients identify and prevent unwanted periods of hypo- and hyperglycemia.9 We have previously reported a continuous improvement in CGM performance that compares favorably to an earlier 7-day CGM product.10 Reports have shown that patients using a modified CGM system in conjunction with an insulin pump can significantly reduce their nocturnal hypoglycemia.11,12 Patients using CGM have better information to make better diabetes treatment decisions than ever before.13,14
This clinical study assessed the accuracy, safety and clinical benefit of the Dexcom G4 Platinum system, commercialized late 2013.
Materials and Methods
Study Population
Seventy-two subjects with diabetes mellitus were enrolled at 4 centers within the United States between November 2011 and February 2012. Forty-four subjects were male (61%), and 28 subjects were female (39%). The mean ± SD age of those who enrolled was 42.2 ± 14.0 years old with the youngest being 18 and the oldest being 74. The majority of subjects (94%) were White, 1 subject was Asian (1%), and 3 subjects were African American. Sixty-four subjects were not of a Hispanic or Latino ethnicity origin and 8 subjects were Hispanic or Latino. Sixty subjects were persons with type 1 diabetes (83%), and 12 subjects were persons with type 2 diabetes (17%). Fifty-nine subjects used insulin by multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) therapy (82%), while 7 other subjects used oral agents or non-insulin-injectable hypoglycemic agent treatment (10%), and 6 subjects used a combination treatment (8%).
At baseline, subjects performed SMBG 5.1 ± 2.7 times daily, and had an A1C of 7.7 ± 1.3% and Hematocrit of 44.1 ± 4.2%. Subject BMI was 28.7 ± 5.8 kg/m2, ranging from 19.6 to 49.4 kg/m2. The study protocol was approved by the institutional review boards of all participating centers, and all subjects provided witnessed, written informed consent prior to enrollment.
Study Procedures and Data Collection
This study was prospective, open-labeled, nonrandomized, and enrolled subjects from November 2011 to February 2012 (clinicaltrials.gov ID: NCT01514292). All subjects participated in 1 G4 Platinum sensor session that lasted up to 7 days (168 hours). For the purpose of assessing sensor precision, 36 subjects wore 2 systems simultaneously, 1 system was blinded and the other was unblinded during home use, and both systems were blinded during in-clinic hours.
All subjects were provided with a SMBG meter (OneTouch® Ultra2®, LifeScan, Inc, Milpitas, CA) and test strips. This meter was used to collect blood glucose measurements performed throughout the study for receiver calibration and diabetes self-management purposes; capillary samples were obtained from fingersticks (alternative-site testing was not allowed).
Subjects were asked to participate in between 36 to 39 hours of blood draws through an intravenous catheter spanning 3 in-clinic sessions. Subjects contributed fingersticks using their provided meter approximately once every 30 minutes (and as indicated for diabetes management or clinical safety purpose) for the clinic session duration, as well as undergoing peripheral intravenous (IV) catheterization of the dorsal hand, lower arm, or antecubital region to obtain blood samples for YSI blood glucose determination. Carbohydrate consumption, insulin dosing, and meal timing were manipulated to obtain a wide range of glucose values during the clinic session.
During home use 1 CGM system was set to display (prospective) mode. Subjects were asked to use the blood glucose meter and test strips provided to them to take a minimum of 7 fingersticks per day (for calibration, diabetes management, and confirming high and low CGM glucose alerts).
Adverse event screening and sensor insertion site assessments were performed at each clinic visit. Digital data from CGM receivers and SMBG meters were downloaded via personal computer for analysis. At all times, subjects were instructed to use SMBG values in conjunction with sequential CGM readings over time to guide diabetes management decisions.
Statistical Methods and Data Analyses
The system performance was evaluated in terms of the proportion of the CGM system values that are within ± 20% of relative difference of reference value at glucose levels >80 mg/dL and ± 20 mg/dL of absolute difference at glucose level ≤ 80 mg/dL (hereafter referred to as %20/20 mg/dL). The %20/20 metric measures the closeness of the CGM system to a reference standard. Performance of the system was also evaluated according to length of time from sensor insertion and system accuracy was assessed by the difference in the glucose measurements from the CGM system real-time display to subjects when compared to the laboratory standard results from YSI. Pearson correlation coefficients were used to evaluate the relationships between CGM, YSI, and SMBG measurements. Also, Clarke error grid (CEG) Analysis and continuous glucose error grid analysis (CG-EGA)15 were used to quantify the clinical accuracy of CGM in reference to the laboratory standard of YSI.
Diagnostic features of the CGM were assessed for both hypoglycemia and hyperglycemia. The features tested were detection rates, and true CGM alert rate. The true alert rate shows a percentage of how often the CGM alert is correct or incorrect; and the detection rate shows a percentage of how often the CGM recognizes and alerts the user to a hypoglycemia threshold event or how often it misses an event.
The frequency and time of CGM hypoglycemia (extreme low, hypoglycemia) events during nocturnal use (8 pm to 8 am) was also summarized. CGM extreme low, and CGM hypoglycemia were defined as a CGM reading ≤ 55 mg/dL, and ≤ 70 mg/dL respectively, and time of CGM hypoglycemia is estimated as the cumulated CGM readings. A CGM reading is accounted for 5 minutes interval of time.
Chi-squared tests were used for comparisons of categorical variables, and nonparametric tests were used for comparisons of continuous variables. All statistical comparisons were conducted at the α = .05 level of significance using 2-tailed tests. Analyses were performed using SAS® Software, version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Dexcom G4 Platinum Accuracy
Of the enrolled 72 subjects, 68 participated in all 3 clinic sessions including blood draws. A total of 9152 CGM-YSI temporally paired points that fell within a 40-400 mg/dL range of the CGM were analyzed. The Pearson correlation coefficient calculated between CGM and YSI measurements was 0.95 and is a statistically significant linear relationship (P < .0001); the overall median absolute relative difference (ARD) was 11% with a mean ± SD of 13 ± 11%; the total percentage points within 20 mg/dL or 20% of YSI reference values was 82%, the percentage within 30 mg/dL or 30% of YSI reference was 93%. CEG analysis showed 8934 (98%) points falling within clinically acceptable regions A or B, with 7363 (81%) in clinically accurate region A and 1571 (17%) in region B (errors leading to benign or no treatment); 25 (0.3%) points were in region C (errors resulting in overcorrection of acceptable glucose levels); 193 (2%) were in region D (errors representing failure to detect unacceptable glucose levels); and 0 (0.0%) were in region E (errors leading to erroneous treatment decisions). The CG-EGA results indicated that 80% of the System readings were accurate within the hypoglycemia range (BG < = 70 mg/dl), 98% were accurate within the euglycemia range (70 < BG < = 180 mg/dl), and 96% were accurate within hyperglycemia range (BG > = 180 mg/dl). Similar results were observed for the CGM-SMBG matched pair data. Overall, the accuracy results of CGM when compared to either laboratory standard YSI or SMBG meter were similar (Table 1).
Table 1.
Dexcom G4 Platinum System Overall Accuracy.
| Compared with YSI | Compared with SMBG | |
|---|---|---|
| N of samples | 9152 | 7508 |
| Mean ARD (%) | 13% | 14% |
| Median ARD (%) | 10% | 11% |
| %20/20/%30/30 | 82%/92% | 81%/94% |
| Mean ARD within days (Day 1/4/7) | 17%/11%/12% | 17%/13%/13% |
| Median ARD within days (Day 1/4/7) | 13%/8%/9% | 14%/10%/10% |
| CEG zone A (%)/ A+B (%) | 81%/ 98% | 79%/97% |
| CG-EGA accurate zone hypoglycemia/euglycemia/hyperglycemia | 80%/98%/96% | 80%/96%/94% |
| Mean AD (mg/dL) for CGM ≤ 100 mg/dL | 13 mg/dL | 12 mg/dL |
| Mean ARD (%) for CGM >100 mg/dL | 12 % | 13% |
| Sensor life (Up to 7 days) | 94% | |
| On time within days (288 per day) | 97% | |
Sensor Stability and Reliability
Performance of the system was evaluated according to length of time from sensor insertion. Sensor accuracy and stability were assessed by comparing mean ARD of the paired CGM-YSI values on days 1, 4, and 7 of sensor wear. The median ARD was observed to be statistically better on day 4 (8.2%) and on day 7 (8.9%) when compared with day 1 (13.2%) (P < .001); and the %20/20 was better on Day 4 and Day 7 (87%) when compared with day 1 (71%) (P < .001). The performance of G4 Platinum improved after the first day of use and was designed to be used for up to 7 days. Out of 108 evaluated sensors, 94% of the sensors lasted up to 7 days, and the vast majority of (93.5%) sensors provided at least 75% of expected readings during system use. During each day of sensor use, the average sensor provided glucose readings 97% ± 2% of the time.
Sensor Precision
A subgroup of 36 subjects simultaneously wore 2 systems to evaluate the glucose precision of the system. Of the 63,078 total paired CGM data, the mean paired ARD (%) was 9%, with a mean coefficient of variation of 7%.
Hyperglycemia Detection and CGM Alert Accuracy
When the hypoglycemia alert was set at 70 mg/dL, the Dexcom G4 Platinum detected true hypoglycemia (in YSI blood glucose measurement ≤ 70 mg/dL) 83% of the time within 15 minutes, and alerted correctly 80% of the time within a 15 minute time window. When the hyperglycemia alert was set a 200 mg/dL, the G4 Platinum detected true hyperglycemia (in YSI blood glucose measurement ≥ 200 mg/dL) 97% of the time within 15 minutes, and alerted correctly 92% of the time within a 15 minute time window (Table 2).
Table 2.
Hypoglycemia and Hyperglycemia Detection and Alert Rates.
| Threshold level (mg/dL) | Evaluable events N | Subjects having event | Hypoglycemia detection rate (95% CI) | True alert rate (95% CI) |
|---|---|---|---|---|
| 70 | 1606 | 60 | 83% (81%, 85%) | 80% (79%, 81%) |
| 80 | 2357 | 65 | 86% (85%, 88%) | 88% (87%, 88%) |
| 90 | 3037 | 68 | 89% (88%, 90%) | 90% (89%, 90%) |
| 100 | 3803 | 71 | 90% (89%, 91%) | 93% (93%, 93%) |
| Threshold level (mg/dL) | Evaluable events N | Subjects having event | Hyperglycemia detection rate (95% CI) | True alert rate (95% CI) |
| 180 | 6250 | 70 | 97% (97%, 98%) | 92% (92%, 93%) |
| 200 | 5406 | 66 | 97% (96%, 97%) | 92% (91%, 92%) |
| 220 | 4736 | 62 | 95% (94%, 96%) | 91% (90%, 91%) |
| 240 | 4111 | 62 | 94% (93%, 95%) | 91% (91%, 92%) |
Nocturnal CGM Hypoglycemia Reduction
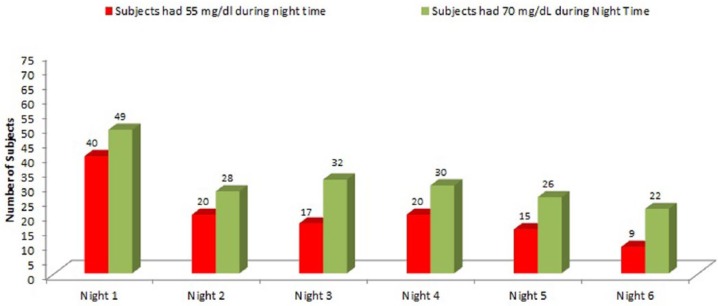
The number of hypoglycemia events that subjects experienced during the night decreased over the 7 days of system wear. Of subjects, 61% (44 out of 72) experienced CGM low events during their first night of CGM wear, which reduced to 28% (20 out of 72) of subjects for their 6th night of CGM wear (P = .002). Similarly, 56% (40 out of 72) of subjects experienced at least 1 CGM extreme low during their first night, which reduced to 13% (9 out of 68, 3 subjects’ sensors ended early) of their 6th night of CGM wear (P < .0001). The number of subjects experienced CGM hypoglycemia were also reduced (P = .0004) (Figure 1).
Figure 1.
Number of subjects experienced night time CGM extreme low (≤55 mg/dL) or hypoglycemia (≤70 mg/dL).
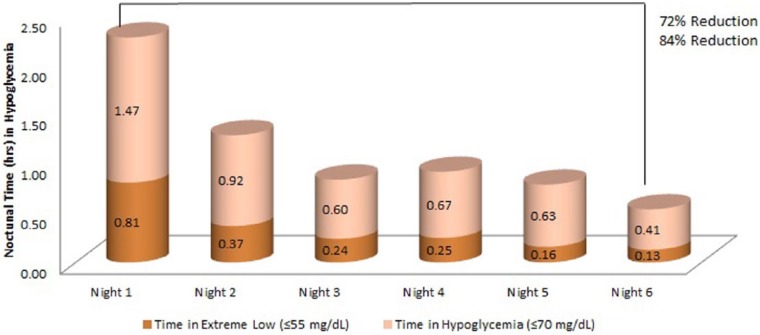
The time (hours) spent in hypoglycemia during the night also decreased over the 7-day use of the Dexcom G4 Platinum CGM. For CGM low, the average time decreased from 1.2 ± 1.4 hours on the first night to 0.2 ± 0.6 hours on the 6th night (P < .0001). Similarly, the average time spent at CGM extreme low reduced from 0.8 ± 1.0 hours on the first night to 0.1 ± 0.5 hours on the 6th night (P < .0001). The time spent in CGM hypoglycemia was also reduced (P < .0001) (Figure 2).
Figure 2.
Nocturnal time spend in CGM extreme low (≤55 mg/dL) or hypoglycemia (≤70 mg/dL).
Safety
No serious adverse events or serious device-related adverse events occurred during the study. Infrequent (< 10%) very mild skin irritation, such as erythema or edema, occurred around the sensor adhesive area.
Discussion
In this study, the overall mean ARD between CGM measurements of interstitial glucose levels and venous YSI reference blood glucose levels was small (13%). A strong linear relationship between CGM and YSI reference blood glucose levels was found (indicated by a statistically significant correlation coefficient of 0.95, P < .0001). A satisfactory clinical assessment for CGM was seen using CEG analysis through finding that 81% of paired sensor-YSI points falling within the A zone and 98% of paired points falling within both the A and B zones. These results are better than accuracy measures observed in a previous report of a 7-day use of a CGM system.10,11 Moreover, the present study found that G4 Platinum accuracy significantly improved after the first day of use (13% median ARD), with the median ARD on day 4 and day 7 being 8% and 9%, respectively. The significantly improved CGM performance, particularly the high correlation of CGM versus YSI that accompanies improved rate of change performance, suggests that a CGM device will be ready for independent diabetes management and is currently able to provide patients and caregivers real insights into carbohydrate intake, illness, exercise, and effect of insulin on metabolic control. This increased awareness and control suggest a lasting clinical efficacy for the use of CGM in the treatment of diabetes.
The study showed improved CGM reliability with a paired ARD between G4 Platinum systems of 9%, and 94% of the sensors lasted until day 7. A JDRF multicenter randomized study7 found that the adult cohort that used CGM more frequently (83% used at least 6 days per week) showed substantially greater improvement in glycemic control without a significant increase in hypoglycemia. The impressive sensor life of this new CGM device will help increase the adherence of CGM and facilitate subjects’ improvement in their glycemic control.
We report here that the Dexcom G4 Platinum showed better performance of its diagnostic features comparing previous generation of CGM system to supplement a previous report regarding the system.10 The true hyperglycemia alert rates of more than 90% suggests that the device should reduce patient alert fatigue and increase trust in CGM alerts since hyperglycemia would be correctly detected by the CGM at least 90% of the time. These findings are certainly encouraging as they convey the realistic possibility of recommending that patients dose insulin on the CGM readings. Having reliable nighttime alerts can also be a major advantage over traditional SMBG in terms of reducing time in hypoglycemia since the CGM remains active during sleep and can wake the patient when intervention is necessary. However, while CGM does offer these advantageous features over SMBG, we found approximately 20% false detection rates and also 20% false alert rates, which is 1 area that the diabetes community will welcome improvements.
Although very short term, this study showed statistically significant reductions both in number of subjects who experienced CGM hypoglycemia and time spent hypoglycemic during nighttime use. This could be due to a number of factors. For example, more accurate readings in hypoglycemia, better detection rates, or it could be basal insulin levels. While the answer is not clear from this study, it is clear that there is a phenomenon occurring in the short term, and the diabetes community would benefit from more focus on nighttime hypoglycemia reduction and prevention as well as the improvement of CGM accuracy. To fully explore this, a baseline period of blinded CGM is needed, but this study does raise the question and gives it cogency. Although we are not suggesting that these reductions were clinically meaningful for the long-term management of diabetes, the Dexcom G4 Platinum data is provided in the form of real-time glucose values, trend graphs, hyper-/hypoglycemia alerts and trend arrows that may enable users to reduce both high and low glucose excursions, which suggests better short-term management of their diabetes. Furthermore, the real-time data and trend features offer patients different options in terms of decision-making since they provide a more complete picture of glycemic control, in particularly during night time use.
The results for this CGM device are indeed encouraging and suggest that a long-term validation study of clinical CGM benefit should be pursued, as well as guidelines in how to best use CGM in different areas of diabetes management such as hypoglycemia unawareness, tight glycemic control, or the effects of insulin.
Acknowledgments
We are indebted to the dedicated research staff at all participating centers as well as the subjects who participated in this research project. We thank the study investigators and research staff, Timothy Bailey, MD, AMCR Institute, Escondido, CA; Mark Christiansen, MD, Diablo Clinical Research, Walnut Creek, CA; Elaine Watkins, MD, Profil, Chula Vista, CA; David Liljenquist, MD, Rocky Mountain Diabetes and Osteoporosis Center, Idaho Falls, ID. The authors thank Tyler Kent, Dexcom, Inc, for editorial supports.
Footnotes
Abbreviations: A1C, hemoglobin A1c; ARD, absolute relative difference; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; DCCT, Diabetes Control & Complications Trial; IQR, interquartile range; MDI, multiple daily injections; SMBG, self-monitored blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; YSI, Yellow Spring Instrument
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44-50. [DOI] [PubMed] [Google Scholar]
- 2. Chase HP, Kim LM, Owen SL, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107:222-226. [DOI] [PubMed] [Google Scholar]
- 3. Chase HP, Roberts MD, Wightman C, et al. Use of the GlucoWatch Biographer in children with type 1 diabetes. Pediatrics. 2003;111:790-794. [DOI] [PubMed] [Google Scholar]
- 4. Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111:933-938. [DOI] [PubMed] [Google Scholar]
- 5. Tanenberg R, Bode B, Lane W, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79:1521-1526. [DOI] [PubMed] [Google Scholar]
- 6. Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27:734-738. [DOI] [PubMed] [Google Scholar]
- 7. JDRF Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of Type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 8. Mazze R. Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): is cgm ready for real time? Diabetes Technol Ther. 2009;1:11-18. [DOI] [PubMed] [Google Scholar]
- 9. Bailey TS, Zisser H, Chang A. New features and performance of a next generation seven day continuous glucose monitoring system with short lag time. Diabetes Technol Ther. 2009;11:749-755. [DOI] [PubMed] [Google Scholar]
- 10. Christiansen M, Bailey TS, Watkins E, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous generation system. Diabetes Technol Ther. 2013;10:881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224-232. [DOI] [PubMed] [Google Scholar]
- 12. Grag SK, Braze RL, Bailey TS, et al. Hypoglycemia begets hypoglycemia: the order effect in the aspire in-clinic study. Diabetes Technol Ther. 2014,3:125-130. [DOI] [PubMed] [Google Scholar]
- 13. Mazze R, Strock E, Simonson G, Bergenstal R. Staged Diabetes Management: A Systematic Approach. 2nd ed., revised. Chichester, UK: Wiley; 2006. [Google Scholar]
- 14. Klonoff DC. Continous glucose monitoring roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231-1239. [DOI] [PubMed] [Google Scholar]
- 15. Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27:2922-2928. [DOI] [PubMed] [Google Scholar]