Abstract
Background:
This 4-week, phase 3b, multicenter, open-label, single-arm, outpatient study demonstrated the safe and effective use of the dulaglutide single-dose pen containing 0.5 mL of placebo for subcutaneous injection in injection-naïve adult patients with type 2 diabetes (T2D), with A1C ≤ 8.5% (69 mmol/mol), BMI ≥ 23 kg/m2 and ≤ 45 kg/m2.
Method:
Patients completed a modified self-injecting subscale of the Diabetes Fear of Injecting and Self-Testing Questionnaire (mD-FISQ) and were trained to self-inject with the single-dose pen. Patients completed the initial self-injection at the site, injected at home for 2 subsequent weeks, and returned to the site for the final injection. The initial and final self-injections were evaluated for success; the final (initial) self-injection success rate was the primary (secondary) outcome measure, and the primary (secondary) objective was to demonstrate this success rate as being significantly greater than 80%. Patients recorded their level of pain after each injection. After the final injection, patients completed the mD-FISQ and the Medication Delivery Device Assessment Battery (MDDAB) to assess their perceptions of the single-dose pen, including ease of use and experience with the device.
Results:
Among 211 patients (mean age: 61 years), the primary objective was met, with a final injection success rate of 99.1% (95% CI: 96.6% to 99.7%). Among 214 patients, the initial injection success rate was 97.2% (95% CI: 94.0% to 98.7%), meeting the key secondary objective. Overall, most patients (>96%) found the device easy to use, were satisfied with the device, and would be willing to continue to use the single-dose pen after the study. There was a significant reduction (P < .001) from baseline to study end in patients’ fear of self-injecting, as measured by the mD-FISQ.
Conclusions:
The dulaglutide single-dose pen was found to be a safe and effective device for use by patients with T2D who were injection-naïve. A positive injection experience is an important factor for patients and providers when initiating injectable therapy.
Keywords: dulaglutide, self-injection, single-dose pen, type 2 diabetes
Many patients with type 2 diabetes (T2D) fail to achieve adequate glycemic control with oral antihyperglycemic therapy alone, but patients and clinicians often delay initiating injectable therapy1-6 due to patients’ fear of self-injecting and concerns with pain and device complexity.3,6 In addition, nonadherence and poor persistence with therapy are problems in T2D,7 particularly in patients requiring injectable therapy. Medication complexity and burden of administration have been associated with reduced adherence,8 with poor injection adherence associated with more daily injections, interference with daily activities, and injection pain.9 Reducing the burden, through improving patient experience with initiating and managing an injection regimen, may improve adherence to T2D therapy and patient outcomes. Studies have shown that improvements in injection delivery systems improve patient-reported outcomes such as treatment acceptability, treatment satisfaction, ease of use, convenience, injection pain, and decreased social stigma.10-14
The dulaglutide single-dose pen (Figure 1) is a disposable injection device that contains a prefilled syringe and is designed for subcutaneous delivery of a single 0.5 mL dose of once weekly, long-acting glucagon-like peptide-1 (GLP-1) receptor agonist, dulaglutide. The injection is user-initiated; however, needle insertion, dose delivery, and needle retraction are automated via a spring-loaded mechanism following initiation. The single-dose pen is a small, ready-to-use device (steps to use: uncap, place and unlock, and press to inject) with a hidden 29 gauge needle (5 mm injection depth) that enables a quick injection process (5-10 seconds) and provides dose confirmation. The flat base of the single-dose pen allows it to be held firmly against the skin at the injection site.
Figure 1.
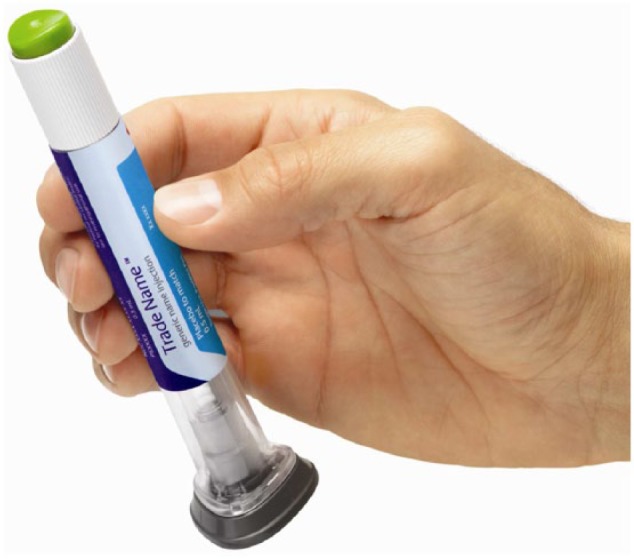
The single-dose pen.
The primary objective of this study was to determine if injection-naïve patients with T2D could safely and effectively use the dulaglutide single-dose pen, as demonstrated by the final injection success rate (the proportion of patients who successfully complete injection) being significantly greater than 80%. A key secondary objective was to demonstrate the initial injection success rate as being significantly greater than 80%. This will support use of the Demonstrator Device as an additional training tool in combination with the Instructions for Use (IFU) as effective in training patients. Secondary objectives included assessment of patient perceptions regarding pain, ease of use, experience, device features, and fear of self-injecting. Ease of training was assessed by site trainers and length of time to train patients was recorded. Patient-reported adverse events (AEs) were also recorded.
Methods
Research Design and Methodology
This was a phase 3b, multicenter, open-label, single-arm outpatient study that evaluated the safe and effective use of the single-dose pen in patients with T2D who were naïve to self-injection and injecting others (protocol H9X-MC-GBDZ, Eli Lilly and Company).
The dulaglutide single-dose pen was used by patients to self-inject 0.5 mL of placebo subcutaneously once weekly for a total of 4 injections, of which 2 (first and last injections) were observed and evaluated by the investigator or designee for success or failure. Before beginning the study, patients provided informed consent and medical history, underwent a physical exam, and verified that they were naïve to self-injection and injecting others. For the baseline assessment, the Diabetes Fear of Injecting and Self-Testing Questionnaire (modified self-injecting subscale only; hereafter referred to as the mD-FISQ)15,16 was administered prior to training on the device.
At baseline (week 0), site personnel trained patients with the Demonstrator Device—designed to simulate the look, feel, sound, and motion of the single-dose pen but without medication, placebo, or a needle—and IFU on self-injection with the single-dose pen. Patients then simulated an injection with the Demonstrator Device on an appropriate injection site (the abdomen or thigh). After a delay period of ≥1 hour, each patient was provided with a package of 4 single-dose pens containing placebo and the IFU. The delay simulated a clinic environment where a patient would receive training, receive a prescription to fill at a pharmacy, and then self-inject for the first time at home. Patients were allowed to refer to the IFU, but no additional instruction was provided by site personnel. A site trainer observed the patient self-inject and recorded the result as success or failure based on predetermined criteria. If the self-injection was a failure, the trainer provided additional instruction and observed the patient self-inject again. If the second self-injection was successful, the patient continued in the study. If the second attempt at baseline was not successful, the patient was discontinued from the study. Each patient completed an injection diary and rated the pain of needle insertion with an 11-point numeric rating scale, between 0 (“no pain”) and 10 (“as bad as you can imagine”), from the Medication Delivery Device Assessment Battery (MDDAB).
Patients performed self-injection at home during weeks 1 and 2 and completed the injection diary and pain scale after each self-injection. A site clinician contacted the patient by telephone to review self-injection completion, concomitant medications, AEs, and product complaints. Patients were reminded of the date and time of upcoming telephone contacts and/or the final site visit.
At week 3, patients performed the final self-injection at the investigative site. No additional instructions were provided. After the final self-injection, patients completed the mD-FISQ self-injecting subscale, as well as the MDDAB, containing individual modules evaluating ease of use attributes, experience using the device, and device features, and a final evaluation of pain. The site personnel reviewed concomitant medications, AEs, and product complaints.
Primary and Secondary Objectives—Injection Success Rates
The primary objective of this study was to achieve a final injection success rate (proportion of patients who successfully complete injection) significantly greater than 80%. To perform a successful injection with the dulaglutide single-dose pen, the patient must have completed the following steps: (1) remove the gray base cap, (2) place the clear base of the single-dose pen flat and firmly against the skin at the injection site, (3) unlock by turning the lock ring, (4) press and hold the green injection button, and (5) hold the clear base of the single-dose pen firmly against the skin until a second click occurs (within 5-10 seconds). Sequential order of these actions was not required for injection success, except in the event of an error that would result in a failed injection; patients were allowed to proceed unless intervention was required to prevent self-harm. The key secondary objective of the study was to achieve a baseline initial injection success rate significantly greater than 80%.
Additional Secondary Objectives—Patient-Reported Outcomes
Additional study objectives were to assess patient perceptions of the dulaglutide single-dose pen through completion of the MDDAB, consisting of 4 modules. Patients completed the MDDAB Pain scale after each weekly injection. After the final self-injection (or upon study discontinuation), patients completed modules for Ease of Use (12 items), Experience (8 points), and Device Features (13 items). The mD-FISQ was administered prior to training at baseline and following the final self-injection (or upon study discontinuation).
The MDDAB and mD-FISQ were adapted from insulin-specific questionnaires15-17 and modified for use in an injection-naïve, non-insulin-requiring population. Prior to use in this study, content validity of these adapted questionnaires was established through a cognitive debriefing study in patients with T2D (n = 27). Results from this cognitive debriefing study confirmed the comprehensiveness, comprehensibility, and appropriateness of the measures for use in patients with T2D.
Trainer-Completed Measures
Site personnel evaluated ease of training for each patient with a single-item questionnaire and recorded the initial time to train each patient. Site personnel also recorded any AEs reported by the patients and indicated those possibly related to the study device or procedure.
Analysis Populations
The full analysis set (FAS) for this study included all enrolled patients who performed at least 1 self-injection at home with the single-dose pen and attempted the final injection, or who discontinued the study due to device (difficult to use or injection discomfort) or failure to perform a successful self-injection at baseline even after additional instruction prior to a second attempt. The primary analyses were conducted on the FAS. The initial injection success rate and AEs were evaluated in all enrolled patients.
Analysis Methods
For the final and initial injection success rates, descriptive statistics and a 95% 2-sided confidence interval (CI) were used to summarize the success rate, and the Wilson (score) CI was used.18 A paired t test assessed whether mean pre-to-post change in mD-FISQ self-injecting subscale total score was different from zero. All tests were conducted at a 2-sided alpha level of .05. For other outcomes, descriptive statistics were calculated. Patients who discontinued the study due to difficulty or dissatisfaction with the device or due to failure to perform a successful self-injection at baseline and, therefore, had missing data for the final injection, were considered failures in the primary analysis. There was no imputation for missing training data or patient-reported outcome data.
Results
Of 214 patients enrolled in the study, 210 completed the study and 4 were discontinued. Three patients were discontinued due to protocol violations, and 1 patient was discontinued due to failure to successfully perform the initial self-injection after 2 attempts. Thus, the FAS consisted of 211 patients; baseline characteristics are described in Table 1. Mean baseline patient demographics were aged 61 years, duration of diabetes 7.7 years, A1C 6.6% (49 mmol/mol), and body mass index 31.7 kg/m2. Of the patients, 36% had a high-school level of education or less, and 35% were aged 65 years or greater. Pre-existing conditions (Table 2) were representative of those expected in a population of patients with T2D not requiring insulin. Regarding concomitant pain medications, 9.3% of the patients reported having used hypnotics, sedatives, and/or barbiturates, and 13.6% of the patients reported having used analgesics and/or skeletal muscle relaxants.
Table 1.
Baseline Patient Characteristics, Full Analysis Set (N = 211).
| Variable | |
|---|---|
| Sex, % female | 50.2 |
| Mean age, years (SD) | 61 (10) |
| Age, % ≥65 years | 35.1 |
| Race, % white | 82.0 |
| Mean duration of diabetes, years (SD) | 7.7 (6.7) |
| Education, % high school or less | 36.0 |
| Mean weight, kg (SD) | 89.3 (18.8) |
| Mean height, cm (SD) | 167.4 (10.1) |
| Mean BMI, kg/m2 (SD) | 31.7 (5.4) |
| Mean A1C, % (SD) [mmol/mol] | 6.6 (0.8) [49] |
| Mean sitting heart rate, bpm (SD) | 71.9 (10.9) |
| Mean SBP, mm Hg (SD) | 127.2 (16.1) |
| Mean DBP, mm Hg (SD) | 76.5 (10.2) |
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Table 2.
Pre-existing Conditions in at Least 10% of Patients, All Enrolled Patients (N = 214).
| Condition | n (%) |
|---|---|
| Hypertension | 137 (64.0) |
| Hyperlipidemia | 73 (34.1) |
| Gastroesophageal reflux disease | 45 (21.0) |
| Hypercholesterolemia | 42 (19.6) |
| Hypothyroidism | 40 (18.7) |
| Depression | 38 (17.8) |
| Osteoarthritis | 38 (17.8) |
| Back pain | 31 (14.5) |
| Peripheral neuropathy | 30 (14.0) |
| Postmenopause | 27 (12.6) |
| Dyslipidemia | 26 (12.1) |
| Vitamin D deficiency | 26 (12.1) |
| Sleep apnea syndrome | 25 (11.7) |
| Anxiety | 23 (10.7) |
| Drug hypersensitivity | 23 (10.7) |
| Insomnia | 23 (10.7) |
Primary and Secondary Outcomes—Final and Initial Injection Success Rates
The primary and key secondary objectives of this study were achieved. Of 211 patients in the FAS, 209 (99.1% [95% CI: 96.6% to 99.7%]) achieved the primary outcome of final self-injection success at week 3—1 patient removed the single-dose pen from the skin before the needle retracted, and the patient discontinued due to failure to successfully perform the first self-injection after 2 attempts was counted as an injection failure (Table 3). Final injection success was achieved by 99.3% of patients aged <65 years and by 98.6% of patients aged ≥65 years.
Table 3.
Final Self-Injection Success Rate, Full Analysis Set (N = 211).
| Final injection success/failure | |
|---|---|
| Success, n (%) | 209 (99.1) |
| 95% CI for final self-injection success rate | 96.6 to 99.7 |
| Failure, n (%) | 2 (0.9) |
| Reason injection was not successful, n (%) | |
| Failed to or was unable to remove base cap | 0 (0.0) |
| Unlocked single-dose pen and pressed button before removing base cap | 0 (0.0) |
| Unlocked single-dose pen and pressed button before placing on skin | 0 (0.0) |
| Replaced base cap (before injection) | 0 (0.0) |
| Placed single-dose pen upside down or inverted single-dose pen | 0 (0.0) |
| Failed to unlock single-dose pen | 0 (0.0) |
| Failed to or was unable to press button | 0 (0.0) |
| Performed incomplete button press | 0 (0.0) |
| Removed single-dose pen from skin before needle retracted | 1 (0.5) |
| Sponsor required discontinuationa | 1 (0.5) |
| Subject withdrawal due to device | 0 (0.0) |
| Site stopped injection due to possible subject injury, n (%) | 0 (0.0) |
CI, confidence interval.
Patient discontinued study due to failure to perform a successful self-injection at visit 2 even after additional training. Injection failure was imputed for a patient at visit 3 and counted in this table.
Of 214 enrolled patients, 208 (97.2% [95% CI: 94.0% to 98.7%]) achieved the key secondary outcome of initial self-injection success at baseline (Table 4). Initial self-injection success at baseline was achieved by 98.6% of patients aged <65 years and by 94.7% of patients aged ≥65 years. Reasons for injection failure are summarized in Table 4.
Table 4.
Initial Self-Injection Success Rate, All Enrolled Patients (N = 214).
| Initial injection success/failure | |
|---|---|
| Success, n (%) | 208 (97.2) |
| 95% CI for initial self-injection success rate | 94.0 to 98.7 |
| Failure, n (%) | 6 (2.8) |
| Reason injection was not successful, n (%) | |
| Failed to or was unable to remove base cap | 1 (0.5) |
| Unlocked single-dose pen and pressed button before removing base cap | 0 (0.0) |
| Unlocked single-dose pen and pressed button before placing on skin | 0 (0.0) |
| Replaced base cap (before injection) | 0 (0.0) |
| Placed single-dose pen upside down or inverted single-dose pen | 2 (0.9) |
| Failed to unlock single-dose pen | 0 (0.0) |
| Failed to or was unable to press button | 0 (0.0) |
| Performed incomplete button press | 1 (0.5) |
| Removed single-dose pen from skin before needle retracted | 2 (0.9) |
| Site stopped injection due to possible subject injury, n (%) | 2 (0.9)a |
CI, confidence interval.
Two patients stopped injections when the single-dose pen was placed upside down or inverted.
Injection Pain Scores
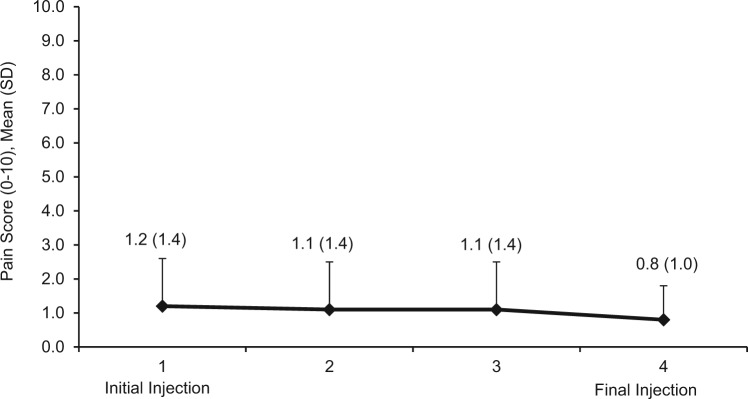
In the FAS, the overall pain score (mean ± standard deviation [SD]) across all injections was 1.0 ± 1.09 (median score, 0.8). Of the 840 pain scores recorded, no pain (pain score of 0) was reported with 45.7% (384) of injections, and a pain score of 1 (suggesting minimal pain) was reported with 28.9% (243) of injections. For the initial injection, 39.5% (83) of patients reported no pain, and 53.3% (112) of patients reported no pain with the final injection. The mean ± SD overall pain score was 1.2 ± 1.18 among patients <65 years and 0.7 ± 0.85 among those aged ≥65 years (Figure 2).
Figure 2.
Mean pain scores at each injection.
Ease of Use Module Scores
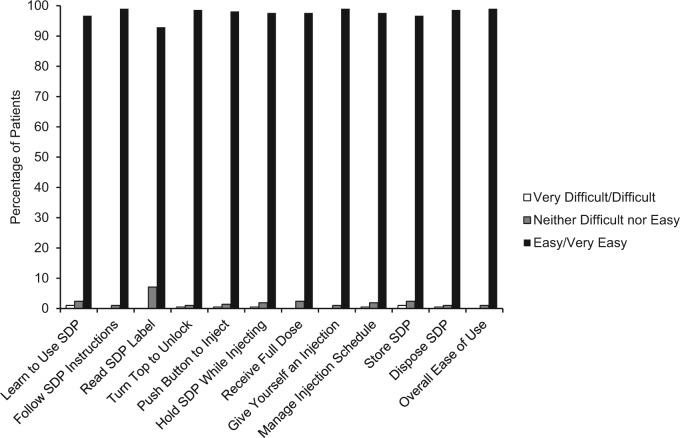
Among 210 patients, 208 (99.0%) reported that overall, the single-dose pen was “easy” or “very easy” to use. Also, 208 patients (99.0%) responded that it was “easy” or “very easy” to follow the instructions when using the device and to self-administer an injection. Most patients (96.7%) responded that it was “easy” or “very easy” to learn how to use the device (Supplemental Table 1 and Figure 3).
Figure 3.
Ease of Use module results. SDP, single-dose pen.
Experience Module Scores
A total of 206 patients (98.1%) found it convenient to self-inject with the single-dose pen. The majority of patients (79.0%) responded that they “disagreed” or “strongly disagreed” that it was painful to self-inject with the single-dose pen. Most patients were confident they could identify that the full dose was delivered (96.7%), in their overall ability to use the single-dose pen (99.0%), and in their ability to continue using the single-dose pen (100.0%). The majority of patients “agreed” or “strongly agreed” that they were satisfied with the overall injection experience (97.1%), were “mostly willing” or “definitely willing” to continue using the device (96.7%), and were “mostly willing” or “definitely willing” to recommend the device to others (97.1%) (Supplemental Table 2).
Device Features Module Scores
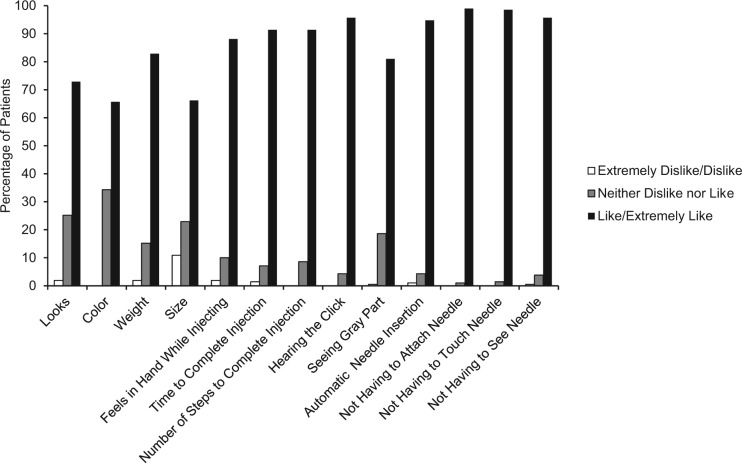
Device Features module scores showed the majority of patients liked the single-dose pen features, with the 5 highest rated items relating to needle features and being able to hear the click, which verified dose completion. Most patients favored not having to attach the needle (99.0%), touch the needle (98.6%), or see the needle (95.7%) (Supplemental Table 3 and Figure 4).
Figure 4.
Device Features module results.
Self-Injecting Subscale Scores
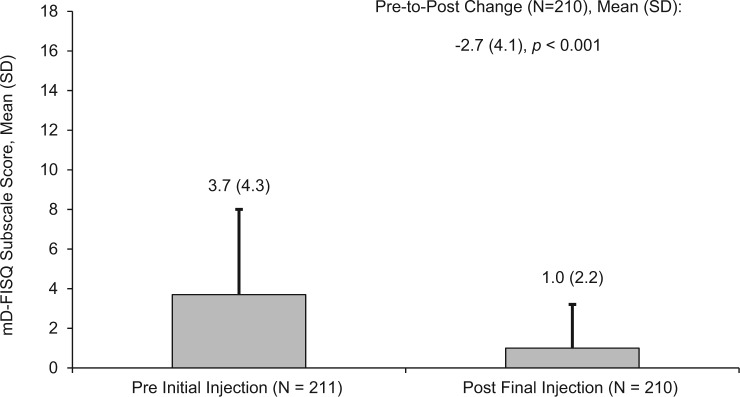
There was a significant (P < .001) mean reduction from baseline to end of study in patients’ fear of self-injecting, as measured by change in the mD-FISQ self-injecting subscale total score between the time points prior to the initial self-injection and following the final self-injection (Figure 5). Per-patient changes in self-injecting subscale scores are shown in Supplemental Figure 1.
Figure 5.
Change in patients’ fear of self-injecting scores between baseline and week 3. mD-FISQ, modified Diabetes Fear of Injecting and Self-Testing Questionnaire.
Ease of Training and Time to Train Questionnaire Results
Site personnel evaluated ease of training using a single-item question and recorded the initial time to train each patient. The designated trainers rated it “easy” or “very easy” to train most patients (92.5% overall, 94.2% of those aged <65 years, and 89.3% of those aged ≥65 years), and the average time (mean ± SD) to train was 10.4 ± 6.5 minutes (10.1 ± 5.2 minutes for those aged ≥65 years). Only 2.3% of patients required additional training due to injection failure on the first attempt.
Adverse Events
No deaths or serious AEs were reported and no reported AEs led to study withdrawal. There were 34 treatment-emergent adverse events (TEAEs) reported in 26 patients (Table 5). In the opinion of the investigators, there were no TEAEs related to the device. Eight events in 6 patients were coded as injection site reactions and/or reported by the investigator as related to study procedure. Of these 6 patients, 5 reported 6 injection site reactions: injection site bruising, injection site bleeding (reported by 2 patients), injection site mass, or injection site pain. One patient reported injection site bleeding on 2 occasions; these occurrences were not deemed related to study device or procedure in the investigator’s opinion. All injection site reactions were rated mild in severity; 4 of the 6 resolved on the same or next day. Two additional bruising events in 2 patients were reported as related to study procedure but not coded as injection site reactions.
Table 5.
Treatment-Emergent Adverse Events, All Enrolled Patients (N = 214).
| Treatment-emergent adverse event (TEAE)a | N (%) |
|---|---|
| Patients with ≥1 TEAE | 26 (12.1) |
| Injection site-related TEAEs | |
| Injection site hemorrhage | 2 (0.9) |
| Injection site bruising | 1 (0.5) |
| Injection site mass | 1 (0.5) |
| Injection site pain | 1 (0.5) |
| Most frequent other TEAEs | |
| Nasopharyngitis | 6 (2.8) |
| Headache | 3 (1.4) |
| Sinus congestion | 3 (1.4) |
| Upper respiratory tract infection | 3 (1.4) |
| Contusion | 2 (0.9) |
| Sinusitis | 2 (0.9) |
An adverse event was defined as treatment-emergent if it either occurred or worsened at any time after initial injection.
Discussion
The results of this study indicate that the once weekly dulaglutide single-dose pen can be safely and effectively used for self-injection by patients with T2D who are naïve to self-injecting or injecting others. This study’s primary and secondary objectives were met, with final injection success observed in 99.1% of patients (95% CI: 96.6% to 99.7%) and initial injection success observed in 97.2% (95% CI: 94.0% to 98.7%). Final self-injection success was 99.3% in patients aged <65 years and 98.6% in those aged ≥65 years, suggesting that age is not a factor in successful use of the dulaglutide single-dose pen.
At baseline, 6 patients failed to successfully inject. One patient failed to remove the base cap, 2 patients attempted to hold the single-dose pen upside down, 1 patient incompletely depressed the injection button, and 2 patients removed the single-dose pen before full needle retraction. At the final injection, 2 patients failed to successfully inject; 1 of these was an imputed failure from discontinuation at the initial injection, and the other patient removed the single-dose pen prior to injection completion. None of the observed errors would lead to acute clinical harm; however, these are use errors that health care providers may anticipate as they train patients to use the single-dose pen.
Most patients indicated that they experienced little to no pain with self-injection, that the single-dose pen was “easy or “very easy” to use, and that they were satisfied with the overall injection experience. The majority of patients found the single-dose pen features favorable, particularly items relating to the hidden needle and being able to hear the click at dose completion. Most patients were willing to continue using the single-dose pen if it were available after the study and willing to recommend the device to others. Site personnel found it easy to teach injection-naïve patients how to successfully self-inject with the single-dose pen.
All patients who completed the home injections were able to successfully inject without contacting site personnel for additional instruction. This may foster health care provider confidence in patients’ ability to use the device independently, may minimize the frequency of patients calling for assistance, and may be beneficial regarding treatment adherence and persistence.
Fear of self-injection is a common barrier for patients with diabetes when progressing from oral therapy to injectable therapy6 and has been found to be associated with poor glycemic control, decreased general well-being, and other psychological comorbidities. The observed reduction in patients’ fear of self-injecting after using the single-dose pen for 4 weekly injections may give confidence to health care providers when prescribing dulaglutide for injection-naïve T2D patients.
Limitations of this study include the administration of placebo rather than active drug product and the willingness of injection-naïve patients to self-inject, which may not be entirely representative of an injection-naïve T2D patient population. The inclusion criterion of A1C ≤8.5% (69 mmol/mol) was established for this study to ensure patients enrolled were not in imminent need of treatment intensification. Thus, the patients in this study with fairly well-controlled T2D may not be entirely representative of an injection-naïve population progressing to injectable therapy. In addition, in clinical practice, patient training on self-injection with the single-dose pen may vary from the method provided by site personnel in this study.
In diabetes management, patients’ ability to initiate and continue therapy is related to the education and training they receive about their disease and treatments.19 Pen devices (eg, insulin pens) have been shown to be easier and more convenient for diabetes treatments than conventional vials/syringes.20 Pens that are simple to learn to use are more likely to give patients the confidence to self-inject, and thus more likely to lead to better adherence and glycemic control.21,22 In addition, less painful injection devices have the potential to improve adherence to diabetes treatments and improve glycemic control for many patients.23 Injection volume is related to injection pain, thus devices with lower drug volumes are likely to reduce this pain; however, injection speed has been shown to have no effect on perceived injection pain.24
Studies in other disease areas (eg, multiple sclerosis) have demonstrated the clinical benefits and favorable patient perceptions associated with easy-to-use and easy-to-train therapies that are self-injectable.25,26 A positive self-injection experience, including successful injection, minimal to no pain, ease of use, confidence in ability to use the device, and a once weekly injection schedule, is an important consideration for patients and providers when initiating injectable therapy for T2D.
Conclusions
This study demonstrated the dulaglutide single-dose pen could be used safely and effectively by injection-naïve patients with T2D to self-inject. Patient-reported outcome results indicated patient satisfaction with the single-dose pen injection experience.
Acknowledgments
The authors wish to thank Jeffrey Walter and Teri Tucker, of inVentiv Health Clinical, contracted by Eli Lilly and Company, for assistance writing and editing this manuscript, respectively; and Chrisanthi Karanikas of Eli Lilly and Company for assistance in reviewing the manuscript. The authors wish to acknowledge the clinical investigators who contributed to this study: Dr Opada Alzohaili, Dr Timothy S. Bailey, Dr Anna Chang, Dr Lisa Cohen, Dr Gildred Colon-Vega, Dr David DiCesar, Dr Steven Larry Duckor, Dr Valerie Espinosa, Dr David Fitz-Patrick, Dr Sam Griffin, Dr Leslie Klaff, Dr David Liljenquist, Dr Hiralal Maheshwari, Dr Glenn Matfin, Dr Francisco Miranda, Dr Samer Nakhle, Dr Paul Norwood, Dr Ramon Ortiz-Carrasquillo, Dr Kerem Ozer, Dr Jane Rohlf, Dr Julio Rosenstock, Dr John Rubino, Dr Michael Seidner, and Dr Alan Wynne.
Footnotes
Abbreviations: AE, adverse event; CI, confidence interval; FAS, full analysis set; GLP-1, glucagon-like peptide-1; IFU, Instructions for Use; MDDAB, Medication Delivery Device Assessment Battery; mD-FISQ, modified self-injecting subscale of the Diabetes Fear of Injecting and Self-Testing Questionnaire; SD, standard deviation; T2D, type 2 diabetes; TEAE, treatment-emergent adverse event.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kate Van Brunt, Alan G. Zimmermann, Rebecca Threlkeld, and Debra A. Ignaut are employees and/or stockholders of Eli Lilly and Company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Eli Lilly and Company.
References
- 1. Peyrot M, Matthews DR, Snoek FJ, et al. An international study of psychological resistance to insulin use among persons with diabetes. Diabetologia. 2003;46(suppl 2):A89. [Google Scholar]
- 2. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679. [DOI] [PubMed] [Google Scholar]
- 3. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(suppl 3):S18-S24. [DOI] [PubMed] [Google Scholar]
- 4. An J, Nichol MB. Multiple medication adherence and its effect on clinical outcomes among patients with comorbid type 2 diabetes and hypertension. Med Care. 2013;51:879-887. [DOI] [PubMed] [Google Scholar]
- 5. Hornsten A, Lundman B, Selstam EK, Sandstrom H. Patient satisfaction with diabetes care. J Adv Nurs. 2005;51:609-617. [DOI] [PubMed] [Google Scholar]
- 6. Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25:1413-1420. [DOI] [PubMed] [Google Scholar]
- 7. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19:31-41. [PubMed] [Google Scholar]
- 8. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126(9 suppl 1):S38-S48. [DOI] [PubMed] [Google Scholar]
- 11. Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27:S89-S100. [DOI] [PubMed] [Google Scholar]
- 12. Molife C, Lee LJ, Shi L, Sawhney M, Lenox SM. Assessment of patient-reported outcomes of insulin pen devices versus conventional vial and syringe. Diabetes Technol Ther. 2009;11:529-538. [DOI] [PubMed] [Google Scholar]
- 13. Rex J, Jensen KH, Lawton SA. A review of 20 years’ experience with the NovoPen family of insulin injection devices. Clin Drug Investig. 2006;26:367-401. [DOI] [PubMed] [Google Scholar]
- 14. Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27:2495-2497. [DOI] [PubMed] [Google Scholar]
- 15. Mollema ED, Snoek FJ, Pouwer F, Heine RJ, van der Ploeg HM. Diabetes Fear of Injecting and Self-Testing Questionnaire: a psychometric evaluation. Diabetes Care. 2000;23:765-769. [DOI] [PubMed] [Google Scholar]
- 16. Snoek FJ, Mollema ED, Heine RJ, Bouter LM, van der Ploeg HM. Development and validation of the Diabetes Fear of Injecting and Self-Testing Questionnaire (D-FISQ): first findings. Diabet Med. 1997;14:871-876. [DOI] [PubMed] [Google Scholar]
- 17. Szeinbach SL, Barnes JH, Summers KH, Lenox SM. Development of an instrument to assess expectations of and preference for an insulin injection pen compared with the vial and syringe. Clin Ther. 2004;26:590-597. [DOI] [PubMed] [Google Scholar]
- 18. Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101-133. [Google Scholar]
- 19. Lorenzi G, Schreiner B, Osther J, Boardman M. Application of adult-learning principles to patient instructions: a usability study for an exenatide once-weekly injection device. Clin Diabetes. 2010;28:157-162. [Google Scholar]
- 20. Davis EM, Bebee A, Crawford L, Destache C. Nurse satisfaction using insulin pens in hospitalized patients. Diabetes Educ. 2009;35:799-809. [DOI] [PubMed] [Google Scholar]
- 21. Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3:312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadeau DA, Campos C, Niemeyer M, Bailey T. Healthcare professional and patient assessment of a new prefilled insulin pen versus two widely available prefilled insulin pens for ease of use, teaching and learning. Curr Med Res Opin. 2012;28:3-13. [DOI] [PubMed] [Google Scholar]
- 23. McKay M, Compion G, Lytzen L. A comparison of insulin injection needles on patients’ perceptions of pain, handling, and acceptability: a randomized, open-label, crossover study in subjects with diabetes. Diabetes Technol Ther. 2009;11:195-201. [DOI] [PubMed] [Google Scholar]
- 24. Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16:971-976. [DOI] [PubMed] [Google Scholar]
- 25. Phillips JT, Fox E, Grainger W, Tuccillo D, Liu S, Deykin A. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011;11:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Arcy C, Thomas D, Stoneman D, Parkes L. Patient assessment of an electronic device for subcutaneous self-injection of interferon ß-1a for multiple sclerosis: an observational study in the UK and Ireland. Patient Prefer Adherence. 2012;6:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]