Abstract
For implantable sensors to become a more viable option for continuous glucose monitoring strategies, they must be able to persist in vivo for periods longer than the 3- to 7-day window that is the current industry standard. Recent studies have attributed such limited performance to tissue reactions resulting from implantation. While in vivo biocompatibility studies have provided much in the way of understanding histology surrounding an implanted sensor, little is known about how each constituent of the foreign body response affects sensor function. Due to the ordered composition and geometry of implant-associated tissue reactions, their effects on sensor function may be computationally modeled and analyzed in a way that would be prohibitive using in vivo studies. This review both explains how physiologically accurate computational models of implant-associated tissue reaction can be designed and shows how they have been utilized thus far. Going forward, these in silico models of implanted sensor behavior may soon complement in vivo studies to provide valuable information for improved sensor designs.
Keywords: biomaterials, biosensors, modeling, biocompatibility
In an effort to improve the management and treatment of diabetes, implantable sensors that continuously monitor glucose levels have become popular alternatives to patient-administered finger prick measurements of blood glucose. However, following implantation, the performance of these implants suffers from inaccurate and erratic readings that compromise their useful lives. As a result, implantable glucose sensors remain limited as a platform for the reliable management of diabetes. While the interaction between the sensor and its surrounding tissue has been posited as a culprit for erroneous in vivo sensor performance, there remains little evidence to support that theory.
Computational modeling, though ubiquitous in other fields of biomedical research, remains a largely untapped resource for understanding glucose sensor biocompatibility. Indeed, even within the field of glucose physiology and glucose sensor design, computational modeling has served as an important research technique. Within glucose physiology, computational modeling has helped explain the mechanisms of cellular glucose uptake and metabolism as well as glucose transport through the interstitium.1-3 Computational methods have also been essential in the design of more effective sensors and closed loop insulin delivery systems. Computational models from the laboratory of David Gough have sought to describe new, more promising analytical chemistry techniques for glucose detection.4-7 Beyond glucose detection, control theory has been used to create predictive models for restoring normoglycemia in diabetics with a closed loop insulin delivery system.8-10
This review describes the effects that implant-associated tissue reactions have on implantable sensor function and how they can be examined using computational modeling techniques. While the biological factors affecting sensor behavior are numerous and well-documented, the issue of in vivo sensor performance is at its core one of analyte transport. An ideal sensor would be one that could sample the interstitial milieu inertly without any sort of impedance from outside agents. In reality though, no sensor will ever behave inertly in the body as the resultant effects of implantation vis-à-vis the formation of a foreign body capsule create barriers that hinder the transport of glucose to the sensor surface. As the cascade of events in foreign body capsule formation is well understood, it can be incorporated into computational models to examine its role in limiting analyte transport to a sensor surface. Indeed, recent in vivo studies from both Klueh et al as well as Gough et al have demonstrated that decreased mass transfer due to the presence of encapsulated tissue around the sensor limited functionality of the indwelling sensor.11-14 The recasting of this biocompatibility problem as an engineering problem of diffusion and convection presents a novel perspective on the issue and will allow researchers the ability to mechanistically understand how each aspect of the foreign body response contributes to decreased sensor response while creating predictive models of in vivo performance. With improved iterations, these models can become an essential part of the design process, both informing new sensor designs as well as supplementing experimental studies to allow for more focused understanding of glucose sensor performance.
Foreign Body Capsule Formation
To accurately model tissue response to sensor implantation, the chain of events following the implantation should be well understood and characterized. Since tissue response to an implant changes over time, the overall effect of these tissue reactions is broken into two temporal phases: (1) the phase of days to weeks immediately following sensor implantation when a provisional matrix of proteins and inflammatory cells envelops the sensor and (2) the phase of weeks to months following implantation when a mature foreign body capsule is present around the sensor.
When a foreign body is implanted, there will be injury to vascularized tissue. With this injury comes the exudation of blood borne proteins, fluid and cells to the site of implantation. The convection of blood borne proteins induces an initial coagulation cascade where fibrin is formed from the cleavage of fibrinogen by thrombin. This fibrin network will then coalesce around the implant to form a provisional matrix or biofouling layer. In addition to the fibrin skeleton, the provisional matrix contains a number of different factors that contribute to the formation of an inflammatory response. First, it contains adhesive molecules such as fibronectin and thrombospondin that will allow for inflammatory cell attachment and migration. Second, the provisional matrix contains a number of cytokines and growth factors that will coordinate the extent and pace of immune reaction. These glycoproteins establish chemotactic gradients within the matrix that attract inflammatory cells to the site of injury.15
Once the biofouling layer has been established, inflammatory cells, guided by chemotactic gradients, changes in vascular flow, and the presence of adhesion molecules, will begin to infiltrate the site of inflammation. Neutrophils will initially interrogate the injured tissue and attempt to phagocytose the implanted sensor. However, given the size of implants relative to cells, neutrophils will recruit macrophages to the site of injury to attempt phagocytosis. In addition, macrophages will release cytokines, chemokines and growth promoting factors to encourage the recruitment of even more cell types. If neutrophils and macrophages cannot dispose of the foreign body, fibroblasts will migrate to the injury via the release of cytokines by macrophages.15 Fibroblasts generate collagen and proteoglycans to deposit around the site of injury. These cell types exhibit proinflammatory phenotypes and as such, they have an increased capacity for glucose consumption, which can be modeled as a sink in a computational algorithm. The extracellular matrix constituents produced by these cells forms the basis for the foreign body capsule. For a more complete description of these immune processes, Anderson provides an excellent review.15 This capsule will grow in both size and density as the chronic inflammatory process persists, creating an avascular diffusive barrier to glucose transport to the sensor surface.16-18 This foreign body capsule has been shown in various in vivo models to negatively impact sensor function, as seen through both a loss of sensitivity as well as an increased time lag between blood glucose and sensor glucose readings.11,18-20 As this causal link between capsule formation and sensor response continues to be defined, it is becoming more apparent that implant-associated changes in the tissue surrounding sensors are limiting their in vivo utility.
Modeling the Effects of a Fully Formed Capsule
As implanted sensors sample interstitial glucose, sensor readings inherently lag behind blood glucose readings in terms of both time and concentration. However, once a capsule has fully formed around a sensor, these instances of time lag and signal attenuation become exacerbated to the point where the sensor loses clinical utility. Though no two encapsulation tissues exhibit the exact same histology, three hallmarks of fully formed capsular tissue exist: (1) a dense porous network dominated by the extracellular matrix network, (2) little to no blood vessels, and (3) the presence of metabolically active cells.17,18 Each of these characteristics has been posited to negatively impact sensor function, through either limited diffusion of analyte (extracellular matrix presence), limited source of analyte (avascular tissue), or the consumption of analyte by inflammatory cells. As the long-term tissue effects to sensor implantation have a stable number of components and geometry, they can be numerically modeled.
Sharkawy et al were among the first to model the transport of glucose through fully formed encapsulation tissue.21 In their initial study, Fick’s second law of diffusion was used to examine how the presence of a foreign body capsule would affect the concentration of glucose at the sensor surface following a step increase in plasma glucose levels. Both the foreign body capsule and the subcutaneous tissue were modeled as homogenous, passive compartments through which glucose would diffuse before reaching a sensor. Differences between the subcutaneous space and the capsule were defined by different compartmental diffusion coefficients and thicknesses. Using this model, the authors were able to predict how the presence of avascular capsular tissue around a sensor would promote a lag between blood glucose values and sensor glucose values.
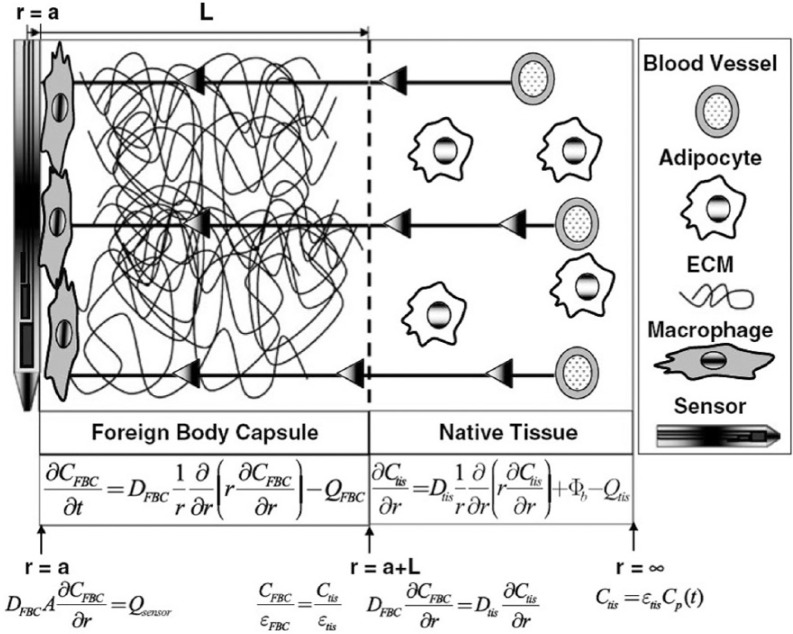
While the findings of Sharkawy et al represented a significant advancement by accurately describing in vivo sensor behavior with a model-based approach, the model was limited in its description of fully formed capsular tissue, describing only 1 of the 3 hallmark traits of capsular tissue. Though described as passive diffusive barriers, in reality the foreign body capsule and surrounding subcutis are perfused materials comprised of complex, heterogeneous porous networks that are both diffusive barriers and bioactive consumers of glucose. A recent study by the authors of this review sought to more completely model fully formed capsular tissue in such a way as to account for the three hallmarks listed above.22 Figure 1 shows a two-compartment model that more completely describes glucose transport through a mature foreign body capsule (adapted from Novak et al).22 Similar to Sharkawy et al, the model was treated as a compartmental system, but the makeups of the constituent compartments were more physiologically relevant than in previous studies. The foreign body capsule compartment was treated as a porous diffusive barrier to account for the presence of a dense collagen matrix. No intrinsic source of glucose from vessels was incorporated into this compartment, thus making it avascular. In addition, the compartment incorporated a consumptive term (QFBC) to represent the presence of metabolically active inflammatory macrophages and neutrophils around the sensor. Glucose transport in the native tissue surrounding the capsule was represented as diffusion through a porous space embedded with native adipocytes and blood vessels. Similar to macrophages in the capsular tissue, adipocytes in the native tissue are seen as consumptive barriers to glucose transport (Qtis), meaning that they are capable of removing glucose from the system. The presence of blood vessels may be seen as the source of glucose for this system (Φb) as the analyte enters the system by traversing across the vessel wall. In both compartments, the concentration of glucose was assumed to only spatially vary in the radial direction. As histology suggests that the components of capsular tissue are consistent along the length of the sensor as well as in the azimuthal angular direction of the cylindrical sensor, this assumption appears valid in described glucose transport to the sensor surface. Furthermore, the relevant changes in tissue composition due to implantation (biofouling, cell adhesion, extracellular matrix deposition) most apparently vary radially relative to the sensor surface.
Figure 1.
Computational description of glucose transport through a fully formed foreign body capsule.
Using this more physiologically accurate model, the authors were able to both recreate experimentally observed instances of sensor signal attenuation and lag as well as establish what aspects of a fully formed capsule contribute most to these effects. The thickness of the capsule was the greatest determinant of sensor signal lag whereas the vascularity of the subcutaneous space surrounding the capsule had the largest effect on sensor signal attenuation. In addition, a high subcutaneous vessel density was found to best promote the ideal scenario of low signal attenuation and lag times. Taken together, these modeling iterations have been able to provide a mechanistic description of how different constituents of a fully formed capsule contribute to limited sensor functionality.
Modeling Early Stage Sensor Response
As all commercially available glucose sensors are approved by the FDA for no more than 7 days in vivo, a small but growing body of literature is focusing on how tissue reactions in that time window occurring just days after implantation affect sensor function.23,24 Functionally speaking, sensor behavior during this period would best be considered as anomalous and erratic, further limiting sensor efficacy even during the FDA-approved time window.
Though tissue reaction to sensor implantation during this phase is highly dynamic, it is dominated by two processes: (1) the adsorption of a biofouling layer on the sensor surface and (2) the migration of inflammatory cells to the site of injury. Computationally speaking, the biofouling layer represents a porous diffusive barrier to glucose transport while the presence of inflammatory cells, such as macrophages, is modeled as both a diffusive and consumptive term. This consumptive term is analogous to a sink that removes glucose from the system. The consumption of glucose by inflammatory macrophages is mediated by glucose surface receptors and has been well characterized in numerous studies.25,26 Those studies have shown that cellular glucose uptake agrees well with the Michaelis-Menten formalism for enzymatic activity. As a result, consumption can be modeled using Michaelis-Menten kinetics with values specific to glucose uptake by macrophages.
The authors have modeled this early stage case of glucose transport through a porous biofouling layer embedded with inflammatory macrophages.23 Similar to the fully formed capsule case, it was represented as a two-compartment model. However, the presence of dense extracellular matrix in the foreign body tissue was not included, as this time stage focused on times before that particular reaction. Model results were able to recreate early stage in vivo declines in sensor signal. Moreover, signal declines were shown to be a result of glucose consumption by macrophages adhered on the sensor surface and not the diffusion barrier posed by the biofouling layer. The presence of these consumptive macrophages were also shown to create a “depletion zone” of glucose with respect to distance from the sensor, further contributing to the position that instead of sensors “failing” in vivo, that tissue reaction to the sensor creates an environment that is not conducive for sensing. These findings have been supported by the work of Klueh et al who have shown through multiple in vivo studies that the consumptive capacity of activated macrophages proximal to the sensor surface can induce a pronounced decline in sensor signal.12,14
Future Considerations for Improved Modeling
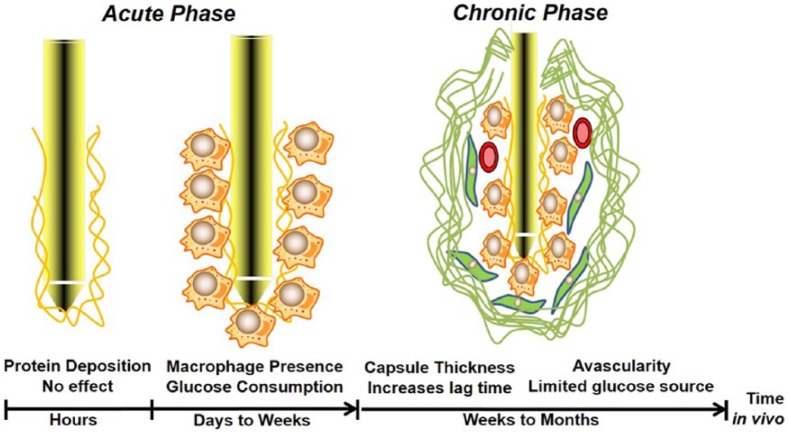
Figure 2 represents our current findings using computational modeling to study glucose sensor biocompatibility. While the current models of early and late stage tissue reaction to sensor implantation represent a novel approach to addressing the problem of biocompatibility, they are by no means complete in their descriptions of the surrounding milieu. Indeed, a full understanding of their predictive power involves a full understanding of their caveats. In fact, these techniques should be viewed as a springboard for more comprehensive in silico models for implant/tissue interaction. By incorporating the following considerations, model-based techniques will become more physiologically relevant and, hopefully, more representative of in vivo tissue reactions.
Figure 2.
Time course of tissue effects on implanted glucose sensor function.
Incorporating Oxygen Transport
A large class of commercially available glucose sensors relies on the enzymatic activity of glucose oxidase for the detection of glucose. As oxygen is a necessary input for glucose oxidase to produce a signal, a thorough examination of a depleted sensing environment should also include the interaction between tissue and oxygen proximal to the sensor. Inflammatory cells have elevated affinity for oxygen to facilitate antimicrobial defense mechanisms like respiratory burst. To further complicate matters, the tissue wounded from implantation is shown to have decreased oxygen tension relative to untreated tissue.27-30 Such an increased affinity for oxygen is exacerbated by the decreased amount of oxygen present in wounded tissue. Gough et al have demonstrated that oxygen permeability within tissue surrounding an implant decreases over time, with the steepest drop coming in the first week.11,31 In addition, the concentration of unbound oxygen in tissue is much lower than that of glucose, meaning that oxygen limits the glucose oxidase reaction in the body.4,5 This situation of both increased demand and limited supply of oxygen could negatively impact sensor function where the sensor cannot produce hydrogen peroxide despite the presence of glucose. In effect, the sensor would become more sensitive to oxygen than glucose.
Oxygen consumption by inflammatory cells has been well-documented in previous studies and can provide the basis for a model-based description of the problem.29,32 With this knowledge, a more complete model of tissue reaction to sensor implantation could encompass the modes by which these numerous processes affect oxygen transport as well as glucose transport.
Incorporating Dynamic Changes in Tissue Properties
A major limitation of the most current models is that they present discrete snapshots of tissue morphology and makeup at generalized time points (early and late stage tissue reaction). In reality though, the process of tissue reaction is dynamic, not static. Most of the constituent aspects of the foreign body response, from cell type and concentration to capsule porosity and thickness, are changing and evolving over time. A more complete model could incorporate the time-dependent nature of many of these processes to have a model that “builds” a foreign body capsule over the length of the simulation in much the same way that the capsule is built in vivo. In addition, previous models presumed that different transport variables existed independent of one another. However, a number of transport parameters, such as porosity and the diffusion coefficient are interrelated. Changes in microcirculation patterns in the surrounding tissue during inflammation and wound healing should also be incorporated. Besides the obvious changes in vessel density, there is also a decrease in the length and diameter of vasculature in wound healing tissue as well as a decrease in red blood cell velocity.33 These changes in vessel geometry, as well as vessel density, will affect glucose delivery to the tissue and sensor.
Incorporating Sensor Material Properties
As sensor micromotion has been demonstrated to induce periods of aggravated inflammation in the surrounding tissue, the stiffness of the implant itself can be of importance in shaping the extent of the immune response that occurs following implantation.34 Previous studies in the field of implantable microelectrodes have demonstrated that materials with a pronounced mismatch in material properties from the native brain tissue caused increased trauma, which could lead to increased local inflammation.35 In addition, these properties can impact the degree of injury that stems from implantation, which could affect downstream reactions.36
Conclusions
For continuous glucose monitoring to become a more reliable standard of care in the management of diabetes, sensors must be able to reliably operate beyond the time scale of days to weeks that is the current state of the art. While past studies have attempted to describe and solve the problem of sensor biocompatibility, there is still a lack of mechanistic understanding for how sensors interact with their surrounding tissue. We believe that in silico computational models of inflammation and wound healing around a sensor present an effective means for studying these phenomena. By analyzing the different aspects of the immune response, these models will help identify processes that both do and do not affect sensor behavior, thereby allowing for more rational designs of implantable sensors in the future.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Medtronic Corporation and by NIH Grant DK 54932 (WMR and MTN).
References
- 1. Luni C, Marth JD, Doyle FJ., III Computational modeling of glucose transport in pancreatic β-cells identifies metabolic thresholds and therapeutic targets in diabetes. PLOS ONE. 2012;7:e53130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Solomon TP, Haus JM, Saidel GM, Cabrera ME, Kirwan JP. Computational model of cellular metabolic dynamics: effect of insulin on glucose disposal in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1198-E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King C, Anderson SM, Breton M, Clarke WL, Kovatchev BP. Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. J Diabetes Sci Technol. 2007;1:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gough DA, Lucisano JY, Tse PHS. Two-dimensional enzyme electrode sensor for glucose. Anal Chem. 1985;57:2351-2357. [DOI] [PubMed] [Google Scholar]
- 5. Leypoldt JK, Gough DA. Model of a two-substrate enzyme electrode for glucose. Anal Chem. 1984;56:2896-2904. [DOI] [PubMed] [Google Scholar]
- 6. Makale MT, Jablecki MC, Gough DA. Mass transfer and gas-phase calibration of implanted oxygen sensors. Anal Chem. 2004;76:1773-1777. [DOI] [PubMed] [Google Scholar]
- 7. Jablecki M, Gough DA. Simulations of the frequency response of implantable glucose sensors. Anal Chem. 2000;72:1853-1859. [DOI] [PubMed] [Google Scholar]
- 8. Dua P, Doyle FJ, Pistikopoulos EN. Model-based blood glucose control for type 1 diabetes via parametric programming. IEEE Trans Biomed Eng. 2006;53:1478-1491. [DOI] [PubMed] [Google Scholar]
- 9. Parker RS, Doyle FJ, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;20:65-73. [DOI] [PubMed] [Google Scholar]
- 10. Parker RS, Doyle FJ, Peppas NA. A model-based algorithm for blood glucose control in Type I diabetic patients. IEEE Trans Biomed Eng. 1999;46:148-157. [DOI] [PubMed] [Google Scholar]
- 11. Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci Transl Med. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35:3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klueh U, Liu Z, Feldman B, et al. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5:583-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klueh U, Qiao Y, Frailey JT, Kreutzer DL. Impact of macrophage deficiency and depletion on continuous glucose monitoring in vivo. Biomaterials. 2014;35:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson JM. Biological responses to materials. Ann Rev Mat Sci. 2001;31:81. [Google Scholar]
- 16.Economic costs of diabetes in the U.S. in 2007. Alexandria, VA: American Diabetes Association; 2008. [DOI] [PubMed] [Google Scholar]
- 17. Koschwanez HE, Yap FY, Klitzman B, Reichert WM. In vitro and in vivo characterization of porous poly-L-lactic acid coatings for subcutaneously implanted glucose sensors. J Biomed Mat Res. 2008;87A:792-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu B, Ju Y, West L, Moussy Y, Moussy F. An investigation of long-term performance of minimally invasive glucose biosensors. Diabetes Technol Ther. 2007;9:265-275. [DOI] [PubMed] [Google Scholar]
- 19. Gerritsen M, Jansen JA, Kros A, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mat Res. 2001;54:69-75. [DOI] [PubMed] [Google Scholar]
- 20. Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mat Res. 2007;85A:699-706. [DOI] [PubMed] [Google Scholar]
- 21. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mat Res. 1997;37:401-412. [DOI] [PubMed] [Google Scholar]
- 22. Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak MT, Yuan F, Reichert WM. Predicting glucose sensor behavior in blood using transport modeling: relative impacts of protein biofouling and cellular metabolic effects. J Diabetes Sci Technol. 2013;7:1547-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klueh U. Analysis: on the path to overcoming glucose-sensor-induced foreign body reactions. J Diabetes Sci Technol. 2013;7:452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed N, Kansara M, Berridge MV. Acute regulation of glucose transport in a monocyte-macrophage cell line: glut-3 affinity for glucose is enhanced during the respiratory burst. Biochem J. 1997;327:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan AS, Ahmed N, Berridge MV. Acute regulation of glucose transport after activation of human peripheral blood neutrophils by phorbol myristate acetate, fMLP, and granulocyte-macrophage colony-stimulating factor. Blood. 1998;91:649-655. [PubMed] [Google Scholar]
- 27. Kindt T, Osborne B, Goldsby R. Kuby Immunology. 6th ed. New York, NY: Freeman; 2006. [Google Scholar]
- 28. Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radical Biol Med. 2003;34:1507-1516. [DOI] [PubMed] [Google Scholar]
- 29. Babior BM. Oxygen-dependent microbial killing by phagocytes. N Engl J Med. 1978;298:659-668. [DOI] [PubMed] [Google Scholar]
- 30. Klegeris A, McGeer PL. Rat brain microglia and peritoneal macrophages show similar responses to respiratory burst stimulants. J Neuroimmunol. 1994;53:83-90. [DOI] [PubMed] [Google Scholar]
- 31. Kumosa LS, Routh TL, Lin JT, Lucisano JY, Gough DA. Permeability of subcutaneous tissues surrounding long-term implants to oxygen. Biomaterials. 2014;35:8287-8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257-6263. [DOI] [PubMed] [Google Scholar]
- 33. Dewhirst MW, Tso CY, Oliver R, Gustafson CS, Secomb TW, Gross JF. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int J Radiat Oncol Biol Phys. 1989;17:91-99. [DOI] [PubMed] [Google Scholar]
- 34. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface—effects of motion, pressure, and design on sensor performance and foreign body response—part ii: examples and application. J Diabetes Sci Technol. 2011;5:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J Neural Eng. 2005;2:103. [DOI] [PubMed] [Google Scholar]
- 36. Hilborn J, Bjursten LM. A new and evolving paradigm for biocompatibility. J Tissue Eng Regen Med. 2007;1:110-119. [DOI] [PubMed] [Google Scholar]