Abstract
Background:
Implantable biosensors for continuous glucose monitoring can greatly improve diabetes management. However, their applications are still associated with some challenges and one of these is the gradual functionality loss postimplantation as a consequence of the foreign body response (FBR). Sensor miniaturization in combination with drug-eluting biocompatible coatings is a promising strategy to enhance in vivo performance. However, limited study has been performed to understand the effect of initial trauma and implant size on foreign body reaction as well as in vivo performance of implantable glucose sensors.
Methods:
Different initial trauma was induced by implanting composite coated dummy sensors into rats using various sized needles and 3 different-sized dummy sensors were implanted to examine the size effect. Histological evaluation was performed to relate the inflammatory cell counts and foreign body capsule thickness with the implantation needle size and sensor size respectively. The effect of biocompatible coating on the performance of implantable glucose sensors was determined using both coated amperometric glucose sensors and microdialysis probes.
Results:
The results revealed that the degree of acute inflammation was mainly controlled by the extent of the initial trauma: the greater the trauma, the greater the acute inflammatory response. Implant size did not affect the acute inflammatory phase. However, the extent of chronic inflammation and fibrous encapsulation were affected by sensor size: the smaller the size the less the extent of chronic inflammation and fibrous encapsulation. Glucose sensors implanted using 14 gauge needles showed significantly lower initial in vivo response compared to those implanted using 16 gauge needles. This was not observed for sensors with dexamethasone-eluting biocompatible coatings since inflammation was suppressed.
Conclusions:
The results of the current study indicate that the extent of the inflammatory response post–sensor implantation varies as a function of the initial tissue trauma as well as the sensor size. Accordingly, miniaturization of implantable biosensors together with the utilization of a drug-eluting biocompatible composite coating may be a promising strategy to achieve long-term reliable continuous glucose monitoring.
Keywords: foreign body response, composite coatings, and implantable glucose biosensors
Control of blood glucose levels is critical to the overall treatment and care of diabetic patients. The typically large fluctuations in glucose levels associated with diabetes results in the many debilitating complications as well as the risk of life threatening hypo- and hyperglycemic events. Glucose meters, which are the most commonly used devices for diabetes management, require repeated blood sampling from the capillary vasculature through finger pricking. In addition, these devices are unable to provide continuous monitoring, which is very important to achieve accurate insulin dosing to minimize fluctuations in glucose levels. Several approaches have been investigated to achieve continuous monitoring in a reliable fashion using electrochemical based implantable glucose sensors.1-5 Glucose-oxidase (GOD)-based sensors are the most frequently studied class of glucose biosensors. GOD offers specificity and accuracy for glucose measurement and the fabrication of these sensors is relatively simple.3,4 The sensing principle employed is based on the reaction of D-glucose and oxygen catalyzed by the GOD enzyme to form D-gluconolactone and hydrogen peroxide (H2O2).2-4,6 The generated hydrogen peroxide is then oxidized at the sensing electrodes under an appropriate operating voltage to produce current response, which in turn is used to quantify the glucose concentration. At present, several glucose-sensing devices for continuous monitoring are on the market. Despite the improved accuracy, these systems are not approved for long-term use (more than 1 week) as they still suffer functionality loss soon after implantation.
Impaired sensor functionality in vivo can be largely attributed to biofouling (nonspecific protein/cell adsorption), and negative biological reactions in the vicinity of the sensors.3,4 The negative tissue response, known as the foreign body response (FBR), involves several phases including acute and chronic inflammation as well as fibrous encapsulation. The accumulated protein/cell layer, together with the fibrous capsule, impedes glucose diffusion to the sensing element causing degradation of sensor functionality. To overcome the FBR, hydrogels alone or in combination with drug delivery systems have been used as coating materials for implantable biosensors. As previously reported, a dexamethasone-loaded PLGA microsphere/PVA hydrogel composite has been developed for this purpose.7-11 The results have shown that local delivery of dexamethasone is effective in preventing the FBR at the implantation sites for prolonged periods of time. In addition, coatings that codeliver growth factors and dexamethasone to control FBR and promote blood vessel growth simultaneously have also been reported.10,12 Accordingly, miniaturization of the implantable biosensor in combination with the utilization of a drug-eluting biocompatible coatings would be promising to enhance the sensor in vivo performance and functional lifetime.
The FBR generally occurs in response to all implanted objects, but its extent can differ substantially. Factors affecting the extent of the FBR include but may not be limited to the initial trauma caused by the implantation process, sensor surface chemistry, structure and size, as well as the location within the host tissue.3,13 Several studies have been conducted to investigate the impact of surface chemistry and sensor structure on the FBR. The influence of polymer fiber diameter and surface charge on the fibrous capsule and vessel density in the surrounding tissue, has been investigated for single-fiber implants, with diameters in the micrometer range.14 However, there are no reports describing a detailed understanding of the effect of the initial tissue trauma and the sensor size (in millimeter range) on the FBR. As previously mentioned, local delivery of dexamethasone has been successfully utilized to prevent the FBR at sensor implantation sites. However, it is anticipated that the dexamethasone dosing may need to be adjusted to account for changes in the FBR associated with change in the initial trauma and in the sensor size. In addition to gaining a better understanding of the FBR to facilitate the development of drug-eluting biocompatible coatings for implantable biosensors, it is equally important to investigate the impact of initial tissue trauma on the in vivo sensitivity of glucose sensors. This information will assist in the development of miniaturized implantable biosensors.
The current work focuses on (1) investigation of the influence of initial trauma and sensor size on the extent of the FBR, (2) the dexamethasone dose required to control the FBR to difference sized sensors, and (3) the effect of insertion needle diameter on the initial in vivo performance of implantable glucose biosensors. To determine the impact of the initial trauma on the level of the inflammatory response, dummy sensors (silicon chips) of the same size (0.5 × 0.5 × 5 mm) as the actual sensors were implanted subcutaneously into male Sprague-Dawley rats using different insertion needles: 18, 16, and 14 gauge. To examine the effect of sensor size on the FBR, 3 different-sized dummy sensors (0.3 × 0.3 × 3 mm, 0.5 × 0.5 × 5 mm, and 0.75 × 0.75 × 9 mm) were implanted using the same needle size (16 gauge). At predetermined days, tissue samples in the vicinity of the implanted dummy sensors were excised and histological evaluation was conducted. Glucose sensors with and without the PLGA/PVA composite coatings were subcutaneously implanted into rats using different-sized needles (14 and 16 gauge) to determine any effect of initial trauma on in vivo sensor performance. To the best of our knowledge, the current work represents the first report describing the contribution of initial trauma as well as sensor size on the FBR, dexamethasone dose, and glucose sensor performance. Information obtained here will be helpful in developing adequate strategies to improve in vivo performance of implantable biosensors.
Materials and Methods
Materials
Dexamethasone, poly(vinyl alcohol) (PVA, MW 30-70 kDa), sodium chloride (ACS grade) and sodium azide were purchased from Sigma-Aldrich (St. Louis, MO). PVA (99% hydrolyzed, MW 133 kDa) was purchased from Polysciences, Inc (Warrington, PA). PLGA Resomer® RG503H (inherent viscosity 0.32-0.44 dl/g) was a gift from Boehringer-Ingelheim. Methylene chloride, sodium mono-hydrogen phosphate (ACS grade), acetonitrile (ACN, HPLC grade), dimethylformamide (DMF), and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). Disodium hydrogen phosphate (ACS grade) was purchased from VWR International. Nanopure quality water (Barnstead, Dubuque, IA) was used for all studies. Glucose oxidase enzyme (GOx) (E.C. 1.1.3.4, 157,500 units/g, Aspergillus Niger), glutaraldehyde (25% w/v solution in water), phenol, bovine serum albumin (BSA), glutaraldehyde (50% w/v), and D-glucose (reagent grade) were purchased from Sigma. Platinum and silver wires were purchased from World Precision Instruments. Selectophore (cat. # 81367; Tecoflex™) polyurethane (PU) was purchased from Fluka. Microdialysis probes (CMA 20) with membrane length of 10 mm were purchased from Harvard Apparatus (Hollinston, MA).
Methods
Preparation of Dummy Sensors
The dummy sensors were silicon chips that were cut from p-type Si using a dicer and 3 different sizes were prepared: 0.3 × 0.3 × 3 mm, 0.5 × 0.5 × 5 mm, and 0.75 × 0.75 × 9 mm. Silicon was chosen as it is an ideal substrate for fabrication of a totally implantable CGM platform which contains several micro/optoelectronic components that can be easily assembled on a Silicon wafer by advanced manufacturing processes (such as flip-chip bonding).15
Preparation of PLGA Microsphere/PVA Hydrogel Composite Coated Dummy Sensors
The PLGA microspheres were prepared using an oil/water emulsion based solvent evaporation/extraction as described before.16-18 A mold fabrication process was used to coat the dummy sensors with the PLGA microsphere/PVA hydrogel composite as reported before.7 Briefly, an appropriate amount of PLGA microspheres (the dexamethasone loading was approximately 7.3% w/w) was dispersed in PVA (MW 133 kDa) solution (5% w/v). The grooved mold was filled with this dispersion and the dummy sensors were placed into the mold. These were then subjected to 3 freeze-thaw cycles. Each freeze-thaw cycle comprised 2 hours freezing at −20°C followed by 1 hour thawing at 24°C. All the glassware and instrument parts used to prepare the microspheres and hydrogel composite coatings were autoclaved prior to the experiments. Formulations were prepared in a laminar airflow hood which was exposed to UV light overnight.
Fabrication of Glucose Biosensors
The method of sensor fabrication has been reported previously.2 Briefly, the miniaturized working electrodes were fabricated by coiling a 50 µm platinum (Pt) wire on a thicker (100 µm diameter) Pt wire, which served as the backbone. The sensors were electrochemically cleaned in a 0.5 M H2SO4 solution via cycling the potential between −0.21 and 1.25 V, until a stable background was achieved. A film of polyphenol was electropolymerized on the Pt working electrode from a 40 mM phenol solution in aqueous acetate buffer by scanning the applied potential between 0 and 1 V vs SCE 51 times at a scan rate of 0.05 V/s. The GOx enzyme was subsequently immobilized by dip coating the Pt/PPD electrode in a solution of 140 mg/ml glucose oxidase (GOx), 56 mg/ml BSA, and 25% w/v glutaraldehyde. After incubation overnight, these sensors were soaked in PBS buffer to allow any un-cross-linked enzyme to leach out. The sensors were subsequently coated with a polyurethane (PU) layer by dip coating the working electrodes with a 3% (w/w) polyurethane solution in 98% THF/2% DMF (w/w).
Preparation of Composite Coated Glucose Biosensors and Composite Coated Microdialysis Probes
The fabricated glucose sensors or the microdialysis probes were coated with the PVA solution containing dexamethasone-loaded PLGA microspheres and were immediately gelled via 3 freeze-thaw cycles to physically crosslink the PVA.
Animal Studies
All animal studies were conducted at the University of Connecticut in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines using an approved protocol (#A11-010).
In vivo pharmacodynamic study
Autoclaved sterile dummy sensors were implanted into the interscapular subcutaneous tissue of male Sprague-Dawley rats (weighing ~200 g) using a thin wall needle. To investigate the effect of initial trauma on the extent of FBR, each animal received 3 dummy sensors with size of 0.5 × 0.5 × 5 mm that were implanted using 3 different-sized thin-wall hypodermic needles: 18, 16, and 14 gauges. To investigate the effect of implant size on the extent of FBR, each animal received 3 different-sized dummy sensors (0.3 × 0.3 × 3, 0.5 × 0.5 × 5, and 0.75 × 0.75 × 9 mm) that were implanted using the same-sized needles (16 gauge). To determine dexamethasone dose for different-sized dummy sensors, each animal received 3 composite coated dummy sensors: 1 small dummy sensor (0.3 × 0.3 × 3 mm) and 2 large ones (0.75 × 0.75 × 9 mm). Microsphere concentrations used to prepare the composite coatings for the dummy sensors with dimensions of 0.3 × 0.3 × 3 and 0.75 × 0.75 × 9 mm were 75 mg per ml hydrogel. This concentration has previously been shown to be sufficient to prevent the FBR at the implantation site over a 1-month period for the 0.5 × 0.5 × 5 mm dummy sensors.7 An additional higher concentration (150 mg microspheres per ml hydrogel) was investigated for the largest dummy sensors (0.75 × 0.75 × 9 mm).
The tissue response to the implants was determined through serial sacrifice to investigate both the acute and chronic inflammatory phases (n = 6 at each time point). The acute phase starts right after implantation. For the chronic phase, although it is generally recognized that the chronic inflammatory phase begins 48 to 72 hours postimplantation, this is dependent on various factors, such as the implant type, location, and local delivery of drugs. Accordingly, the chronic phase may be delayed in some cases. Avula et al reported that local delivery of mistinib, a tyrosine kinase inhibitor, significantly delayed the development of chronic phase.19 In the current study, the acute inflammation phase was considered to be the 3 first days following implantation. The onset of the chronic phase was determined through characterization of the cells present.
A histologic evaluation of the excised tissue samples from the site of implantation was performed after staining with hematoxylin and eosin (H&E). Tissue samples were fixed in 10% neutral buffered formalin processed by dipping in alcohol followed by toluene and embedding in paraffin. Tissue sections of 4 μm thickness were cut and stained with H&E. The dummy sensors (silicon implants) were removed prior to sectioning. Formaldehyde caused a marginal shrinkage of the surrounding tissue, which generated separation between the tissue and the implants. Visual inspection was performed to determine any delamination or tissue loss during removal of the implants, and none was observed. Therefore, there was no justification for additional animal experiments to determine any biologic remnants attached to the removed sensors post–formalin fixation. The inflammatory cells stain basophilic (purple) and the connective tissue surrounding the implants stain eosinophilic (pink). Tissue samples were observed and digitally stored using an Evos microscope (XL Core, AMG America, Bothell, WA). Photomicrographs at a magnification of 200X were used to count the number of inflammatory cells in the vicinity of the implants. Ten boxes (1 × 1 inch) were randomly distributed in the vicinity of the implants and the inflammatory cells were counted manually. Collagen accumulation was assessed visually on day 14 followed by quantitative determination of the final fibrous capsules on day 28.
In vivo sensor testing and microdialysis studies
All animal studies were conducted at the University of Connecticut in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines using an approved protocol (#A11-010). In vivo sensor testing was performed on anesthetized male Sprague-Dawley (SD) rats (weight ~ 175 g). Each animal was subcutaneously implanted with 2 groups of glucose sensors (each group included 1 composite coated sensor and 1 uncoated sensor). Each group of sensors was implanted using either 14 or 16 gauge needles (n = 3 for each group). Sensor performance was determined using a CH Instruments (model CHI1010A) electrochemical analyzer that connected the sensors through the thin insulated wires that exited the skin. The sensors were stabilized for 1.5 hours to obtain a steady baseline signal. Two injections of sterile 30% (w/w) dextrose solution were given intraperitoneally to induce hyperglycemic events. The second injection was administered when the blood glucose level returned to normal levels following the first injection. The amperometric response corresponding to the glycemic events of the rats was recorded continuously, while the blood glucose levels were obtained periodically using a commercial glucose meter through tail vein pricking.
Microdialysis was used to study the glucose transport behavior at the implant site. Each animal received 2 microdialysis probes: 1 uncoated probe and 1 composite coated probe. These probes were implanted using either 14 or 16 gauge needles (n = 3 for each group). At each experiment, the implanted probes were connected to a syringe pump equipped with a 3-mL syringe filled with Ringer’s solution. The pumping rate was set at 5 µL/min. After a 15 min equilibration period, the perfusion fluid was collected every 6 minutes up to an hour. Meanwhile, the blood glucose concentration was determined periodically using a commercial glucose meter through tail vein pricking. The glucose concentrations in the collected perfusion samples were analyzed using a YSI 2300 STAT Plus™ Glucose & Lactate Analyzer. The glucose diffusion through the probes was determined as recovery, which was calculated using the following formula: recovery = glucose concentration in the perfusion fluid/blood glucose concentration.
Statistical Analysis
Paired Student’s t test was performed to determine whether there were statistically significant differences among the results of the pharmacodynamics studies as well as the results of in vivo sensor testing and microdialysis studies.
Results
The Effect of Implant Size on the Extent of the Foreign Body Reaction
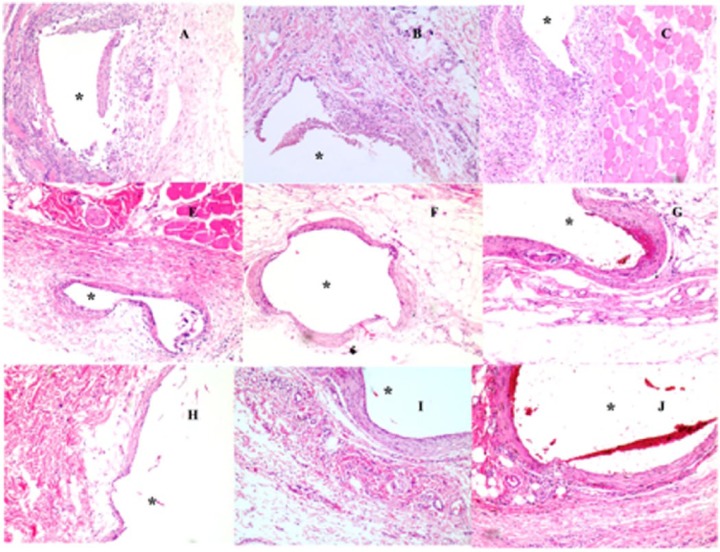
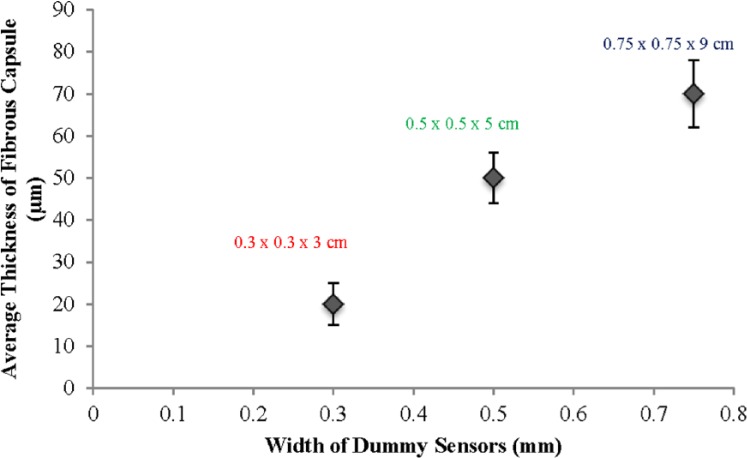
Figure 1 shows tissue samples taken from the vicinity of the different-sized dummy sensors (0.3 × 0.3 × 3, 0.5 × 0.5 × 5, 0.75 × 0.75 × 9 mm) on predetermined days covering both the acute and chronic inflammatory phases. All the dummy sensors were implanted using 16 gauge hypodermic needles. Endotoxin levels were not directly measured and therefore the possibility of the presence of endotoxin, although unlikely, cannot be excluded. However, although the presence of endotoxin may affect the tissue response to subcutaneous implants, all implant groups underwent the same sterilization process, and accordingly the endotoxin levels, if any, would be equivalent for all samples tested. Therefore, the differences in tissue response are normalized for any potential endotoxin contribution. On day 3, acute inflammation was observed in all the tissue samples (Figure 1 (A, B, C represent tissue samples taken from the vicinity of the small, medium, and large dummy sensors, respectively). The average number of inflammatory cells per unit area in the vicinity of the small, medium, and large dummy sensors was approximately 79 ± 18, 74 ± 23, and 81 ± 14, respectively. The result of a paired Student’s t test did not show any statistical difference among the 3 groups, which indicated that the extent of the acute inflammation is similar among the 3 dummy sensor sizes investigated. Following the acute inflammatory phase, chronic inflammatory reactions were observed in the tissue sections surrounding the implanted dummy sensors as characterized by monocytes, lymphocytes, macrophages, fibroblasts, and multinucleated giant cells (30-50 µm in diameter). This is the usual reaction of the body to the continuous presence of any nonbiodegradable foreign material. Figure 1 (E, F, G represent tissue samples excised on day 14 postimplantation containing small, medium, and large dummy sensors, respectively). Based on the types of cells present on day 14, such as monocytes, lymphocytes, macrophages, fibroblasts, and multinucleated giant cells, it was concluded that the tissue reaction moved from the acute inflammatory phase into the chronic inflammatory phase by day 14. It was observed that the 3 dummy sensors were surrounded by collagen producing activated fibroblasts. The smallest sensor had the least collagen accumulation, whereas the largest dummy sensor had the greatest collagen accumulation. By day 28, fibrous capsules were formed at the sites of the 3 sensors investigated (Figure 1 [H, I, and J represent tissue samples from the vicinity of the small, medium, and large dummy sensors, respectively]). As shown in Figure 2, the thickness of the fibrous capsule increased with the sensor size. The result of a paired Student’s t test indicated that the differences among the thickness of the fibrous capsule were statistically significant (P < .05).
Figure 1.
Histologic changes in representative tissue sections taken from the subcutaneous tissue of rats implanted with different-sized dummy sensors (0.3 × 0.3 × 3 mm [small], 0.5 × 0.5 × 5 mm [medium], 0.75 × 0.75 × 9 mm [large]) on day 3 (A, B, and C represent small, medium, and large dummy sensors, respectively); day 14 (E, F, and G represent small, medium, and large dummy sensors, respectively); and day 28 (H, I, and J represent small, medium, and large dummy sensors, respectively). Hematoxylin and eosin (H&E) stains inflammation mediating cells basophilic (purple) and subcutaneous connective tissue eosinophilic (pink). The asterisk indicates the position of the dummy sensors in the tissue.
Figure 2.
Effect of dummy sensor size on the thickness of the fibrous capsules formed in the vicinity of the implantation site. Three different-sized dummy sensors were investigated 28 days postimplantation: 1: 0.3 × 0.3 × 3 mm; 2: 0.5 × 0.5 × 5 mm; and 3: 0.75 × 0.75 × 9 mm.
The Effect of Insertion Needle Diameter on the Extent of Foreign Body Response
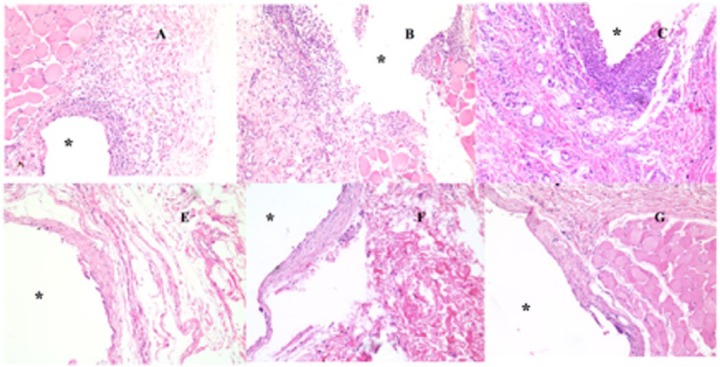
Each rat received 3 same-sized dummy sensors (0.5 × 0.5 × 5 mm), which were implanted using 3 different-sized hypodermic needles (18, 16, and 14 gauge). Figure 3 shows tissue sections taken from the vicinity of the dummy sensors on the predetermined days covering both the acute and chronic inflammatory phases. Acute inflammation characterized by accumulation of neutrophils (polymorphonuclear leukocytes, PMNs) was observed in all the tissue samples collected 3 days postimplantation (Figure 3 [A, B, C represent tissue samples containing dummy sensors implanted using 18, 16, and 14 gauge needles, respectively]). Tissue samples containing dummy sensors implanted using 14 gauge needles showed the highest density of neutrophils in the vicinity of the implants. Following the acute inflammatory phase, a network of fibrous tissue together with lymphocytes, macrophages, and multinucleated giant cells gradually developed in the vicinity of the dummy sensors. Fibrous capsules were formed around the dummy sensors by day 30 postimplantation (Figure 3 [E, F, and G represent tissue samples containing dummy sensors implanted using 18, 16, and 14 gauge needles, respectively]).
Figure 3.
Histologic changes in the representative tissue sections taken from the subcutaneous tissue of rats implanted with the same-sized dummy sensors (0.5 × 0.5 × 5 mm using different needles) on day 3 (A, B, and C represent dummy sensors implanted using 18 gauge, 16 gauge, and 14 gauge, respectively) and day 28 (E, F, and G represent dummy sensors implanted using 18 gauge, 16 gauge, and 14 gauge, respectively). Hematoxylin and eosin (H&E) stains inflammation mediating cells basophilic (purple) and subcutaneous connective tissue eosinophilic (pink). The asterisk indicates the position of the dummy sensors in the tissue.
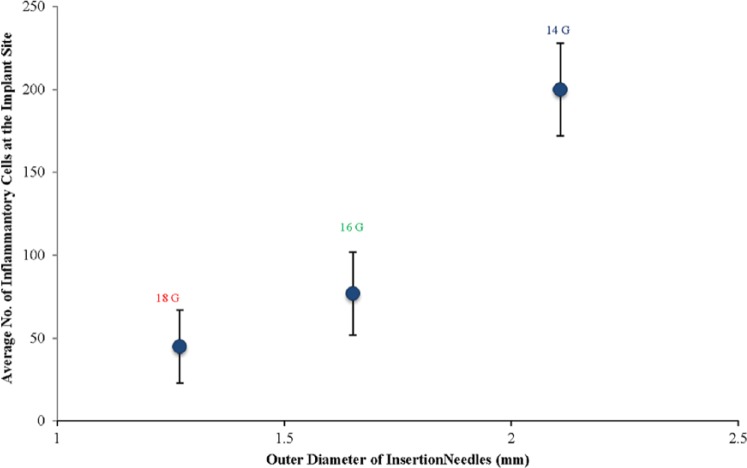
To quantity the extent of the acute inflammatory reaction induced by insertion needles with different diameters, the average number of inflammatory cells per unit area in the vicinity of the dummy sensors was counted and illustrated in Figure 4. The 18 and 16 gauge needles induced similar extents of acute inflammation, which were significantly less compared to the extent of acute inflammation induced by 14 gauge needles (P < .05). However, no significant difference was observed in terms of the chronic inflammatory phase, since the appearance and thickness of the fibrous capsules surrounding the dummy sensors implanted using the different diameter insertion needles were similar (data not shown).
Figure 4.
Effect of insertion needle diameter on the inflammatory-mediating cell population in the vicinity of the implantation site (3 days postimplantation).
Characterization of PLGA/PVA Composite Coated Dummy Sensors
The average drug loading of the PLGA microspheres used was 7.3 ± 0.18% (w/w) and the number based mean particle size was 7.5 µm. As mentioned above, microsphere concentrations used to prepare the composite coatings for the dummy sensors with dimensions of 0.3 × 0.3 × 3 and 0.75 × 0.75 × 9 mm were 75 mg per ml hydrogel. This concentration has previously been shown to be sufficient to prevent the FBR at the implantation site over a 1-month period for the 0.5 × 0.5 × 5 mm dummy sensors.7 An additional higher concentration (150 mg microspheres per ml hydrogel) was investigated for the largest dummy sensors since (1) these dummy sensors required the use of a 14 gauge needle for implantation (due to the increase in diameter resulting from the addition of the composite coating), which as shown above results in increased acute phase inflammation (Figure 4) and (2) the larger implant size may increase the extent of the chronic inflammatory phase as shown above (Figure 2). The average thickness of the composite coatings was approximately 150 µm. The same composite coating thickness was used irrespective of dummy sensor size and microsphere concentration.
In vivo Evaluation of Dummy Sensors Coated With Dexamethasone-Loaded PLGA Microsphere/PVA Hydrogel Composites
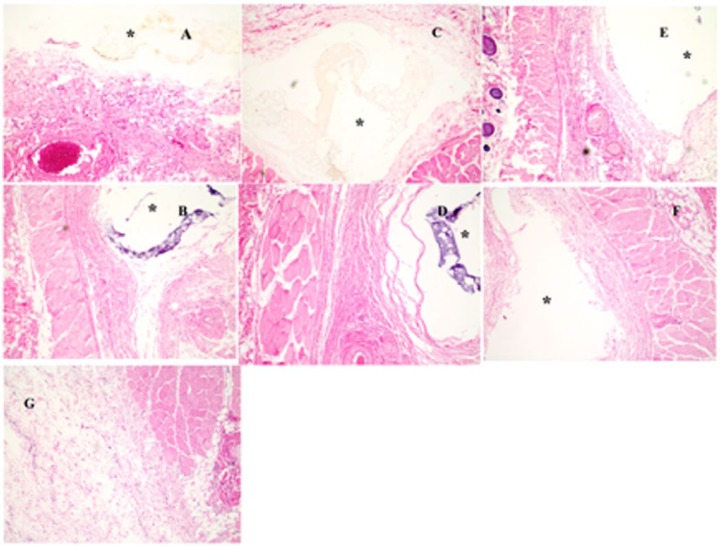
As shown in our previous studies, the dexamethasone-loaded PLGA microspheres used in the current study could continuously deliver dexamethasone locally up to approximately 28 days.20,21 PLGA based drug delivery systems may cause inflammation postadministration due to local acidity resulting from polymer degradation, as well as from the hydrophobic nature of this material. However, in the current study, PLGA microspheres were embedded in a hydrophilic PVA hydrogel, which significantly improves the tissue compatibility of the implant. Therefore, the impact of PLGA on the inflammatory response is considered negligible. Figure 5 shows H&E-stained histological sections of tissue samples taken from the vicinity of the PLGA/PVA composite coated dummy sensors. No acute or chronic inflammation was observed in the tissue samples containing the 0.3 × 0.3 × 3 mm dummy sensors postimplantation. Figure 5 (A and B represent tissue samples taken on days 3 and 28, respectively). Only a few lymphocytes and plasma cells were present in these tissue sections, which is comparable to normal tissue (Figure 5G). In the case of the larger sized dummy sensor (0.75 × 0.75 × 9 mm), no acute inflammation was observed in the tissue samples collected 3 days postimplantation. Both microsphere concentrations used were sufficient to prevent the acute inflammation as shown in Figures 5C and 5E. However, a thin fibrous capsule layer was formed around the dummy sensors coated with composites containing the lower amount of microspheres (75 mg microspheres per mL of hydrogel (Figure 5D). The higher microsphere concentration (150 mg microspheres/mL hydrogel) was sufficient to prevent chronic inflammation (Figure 5F). No fibrous capsule was observed and the tissue was comparable to normal untreated tissue (Figure 5G). This result indicates that larger size sensors require more drug to fully eliminate the inflammatory reaction, especially during the chronic inflammatory phase.
Figure 5.
Pharmacodynamic changes in representative tissue sections taken from the subcutaneous tissue of rats implanted with the dexamethasone-loaded composite coated dummy sensors: day 3 (A, C, and E represent small dummy sensors, large dummy sensors [75 mg microspheres/ml hydrogel], and large dummy sensors [150 mg microspheres/ml hydrogel], respectively) and day 28 (B, D, and F represent small dummy sensors, large dummy sensors [75 mg microspheres/ml hydrogel], and large dummy sensors [150 mg microspheres/ml hydrogel], respectively). Hematoxylin and eosin (H&E) stains inflammation mediating cells basophilic (purple) and subcutaneous connective tissue eosinophilic (pink). The asterisk indicates the position of the dummy sensors in the tissue.
Given that the extent of the acute inflammation was similar among the 3 dummy sensor sizes investigated, the dexamethasone concentration (75 mg/mL hydrogel) that was sufficient to prevent acute inflammation for 0.5 × 0.5 × 5 mg sensors was sufficient to prevent the acute inflammation for both the smaller (0.3 × 0.3 × 3 mm) and larger (0.75 × 0.75 × 9 mm) sensors. Whereas the higher extent of chronic inflammation caused by the larger (0.75 × 0.75 × 9 mm) sensors required a larger dexamethasone concentration compared to 0.5 × 0.5 × 5 mg sensors.
The Effect of Insertion Needle Diameter and the Presence of the PLGA/PVA Composite Coating on Glucose Sensor Performance
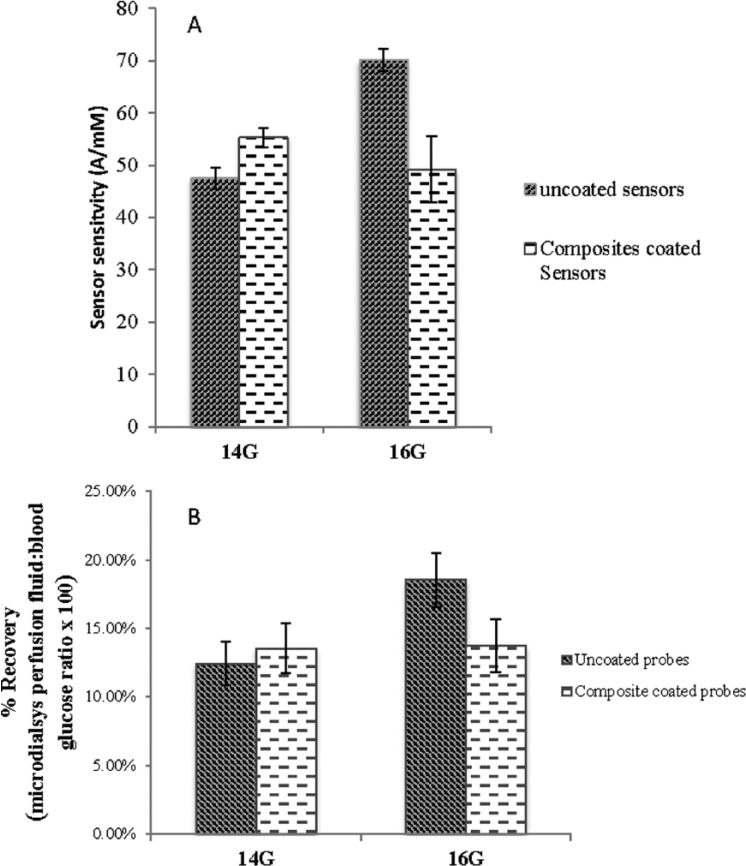
The in vivo sensitivity of the uncoated glucose sensors implanted using different needles was used to determine the effect of insertion needle diameter on sensor performance immediately following implantation. In vivo sensor sensitivity was determined from the average of I1/G1 and I2/G2 (S = (I1/G1+I2/G2)/2), wherein I1 and I2 represent the peak amperometric sensor response for the 2 glycemic events, while G1 and G2 represent the maximum glucose concentrations of the 2 glycemic peaks determined via a Bayer Contour® Blood Glucose Monitoring System.22,23 As shown in Figure 6A, the initial sensitivity of the uncoated glucose sensors implanted using 14 gauge needles was approximately 47 ± 2.0 nA/mM and the initial sensitivity of those implanted using 16 gauge needles was approximately 70 ± 2.2 nA/mM. The difference between these 2 groups was statistically significant as indicated by the result of paired Student’s t test (P < .05). Interestingly, the size of the insertion needle did not result in a significant difference in the initial sensitivity of the PLGA/PVA composite coated glucose sensors. The sensitivity of the composite coated glucose sensors implanted using 16 gauge needles was approximately 50 ± 6.31 nA/mM, which was comparable to the sensitivity of those implanted using 14 gauge needles (approximately 55 ± 1.92 nA/mM).
Figure 6.
(A) Effect of insertion needle diameter on the in vivo sensitivity of glucose sensors with and without the dexamethasone-loaded PLGA microsphere/PVA hydrogel composite coatings. (B) Effect of insertion needle diameter on the glucose diffusion through microdialysis probes with and without the dexamethasone-loaded PLGA microsphere/PVA hydrogel composite coatings. Both sensor testing and microdialysis probe study were conducted 1.5 hours postimplantation.
The initial sensitivity of glucose sensors with and without coatings was compared to determine the effect of the composite coatings on the performance of the glucose sensors (Figure 6A). The composite coated glucose sensors had lower sensitivity than the uncoated glucose sensors when 16 gauge needles were used for implantation. However, no significant difference was observed in the sensitivity of glucose sensors with and without the composite coatings when 14 gauge needles were used.
Perfusion fluid samples obtained via microdialysis were used to determine the effect of initial trauma and the presence of the PLGA/PVA composite on local glucose transport. Similar to the sensor performance testing, both uncoated and PLGA/PVA composite coated microdialysis probes were used and these were implanted using either 14 or 16 gauge hypodermic needles. Figure 6B illustrates the results of the microdialysis studies. The mean glucose recovery of the uncoated microdialysis probes implanted using 14 gauge needles was approximately 10.84%, which was much lower than the mean recovery of those implanted using 16 gauge needles (approximately 18.54%). This difference is statistically significant as determined via a t test (P < .05). The larger needle resulted in reduced glucose transport, which could explain the difference observed in the initial sensitivity of the uncoated glucose sensor as shown in Figure 6A. The mean glucose recovery of the composite coated probes implanted using 14 gauge needles (approximately 15.64%) were very similar to the recovery of those implanted using 16 gauge needles (approximately 15.81%). No statistically significant difference was observed. The composite coated probes had a slightly lower mean glucose recovery than the uncoated probes when 16 gauge needles were used for implantation. However, this difference is not statistically significantly. On the other hand, the 14 gauge needles resulted in an opposite effect: the composite coated probes had a slightly higher (no statistical significance) mean glucose recovery compared to the uncoated ones. These observations are in agreement with the results of the sensor performance testing as shown in Figure 6A.
Discussion
The Effect of Insertion Needle Diameter and Sensor Size on the Extent of Inflammation and Foreign Body Reaction
Host reactions following implantation of biosensors include tissue trauma, acute inflammation, chronic inflammation, and fibrous capsule development. Acute inflammation is an initial, relatively nonspecific response to the tissue damage. Several factors can induce acute inflammation, including thermal, electrical, chemical, and irradiation injury; mechanical trauma; viral, bacterial, and fungal infections; as well as the presence of foreign objects.24 In the case of implantable biosensors, acute inflammation typically occurs immediately following implantation and usually lasts 3 to 5 days. The insertion needle and the presence of the implanted device will result in mechanical trauma, which will in turn contribute to the development of an acute inflammatory reaction. The results of the current studies indicate that the degree of the acute inflammatory response is mainly controlled by the extent of the initial trauma caused by the insertion needle during the implantation procedure. This is evident from Figures 3 and 4, which show that increasing the needle outer diameter by less than 1 mm resulted in a 4-fold increase in the inflammatory cells present in the vicinity of the implanted dummy sensors. The bigger insertion needles will cause more damage to the local tissue and capillaries, and therefore may result in a higher degree of blood protein deposition on the sensor surface. In addition, mast cells, which reside in connective tissue are activated upon tissue injury and undergo degranulation to release histamine, which leads to vessel dilation.25 Consequently, more inflammatory cells, such as neutrophils (polymorphonuclear leukocytes, PMNs), will migrate from the local capillaries and accumulate in the vicinity of the dummy sensors through adhering to the adsorbed blood proteins (mainly fibrinogen). On the other hand, the results indicate that the implant size does not play an important role in the development of acute inflammation, since all 3 dummy sensors investigated resulted in a similar degree of acute inflammation when implanted using the same-sized needles (Figure 1).
The acute inflammatory response to implanted devices usually resolves quickly, typically in less than 1 week. However, if an acute inflammatory response cannot be resolved, it becomes chronic. In comparison to the acute inflammatory phase, chronic inflammation is less uniform histologically. In the case of implantable sensors, the persistent presence of the nonabsorbable sensor alters the normal wound healing process and induces chronic inflammation and the FBR. The FBR is characterized by the presence of macrophages, the formation of giant cells (a collection of fused macrophages), and the development of a fibrous capsule. Macrophages play a critical role in the development of the FBR.26 Macrophages are responsible for detecting foreign materials and invaders. They engulf microbes and foreign particles and their lysosomes are capable of destroying the engulfed foreign material via reactive oxygen species, and enzymes. However, in case of implantable biosensors, the macrophages are incapable of engulfing the foreign object due to the large discrepancy between the size of the implant and the size of the macrophages. This induces the formation of foreign body giant cells, which together with the activated macrophages produce various cytokines. It was observed in the current study that the larger the implant size, the greater amount and rate of collagen production (Figure 2). This observation indicates that the discrepancy between the implant size and the macrophage size could play an important role in promoting the formation of fibrous capsule. The larger the implant, the greater the amount of macrophages recruited to the implant site, which subsequently generate collagen to form fibrous capsules. In addition, the cross section of the formed fibrous capsule revealed inconsistencies in the capsule thickness. The thinnest fibrous capsule was formed around the edge of the dummy sensors, which is consistent with the above rationale and is related to the contact surface area between the sensor and the local tissue. The surface area of the edge is small compared to the surface area of the side and therefore less proteins and cells can be adsorbed leading to less collagen production.
The Impact of the Initial Trauma and the PLGA/PVA Composite Coating on the In Vivo Performance of the Glucose Biosensors
Glucose diffusion toward the sensing electrode plays a critical role in the performance of implantable biosensors. Factors affecting glucose diffusion behavior could potentially cause a decline in sensor sensitivity, which include but are not limited to local glucose concentration at the implant site, biofouling (nonspecific adsorption of proteins, cells, and other biological components), and fibrous encapsulation. Most implantable glucose biosensors under development are designed for subcutaneous implantation. The glucose concentration in the interstitial fluid is generally considered to be equal to the blood glucose level, although a short lag phase is reported.27
Tissue injuries caused during the implantation process induce acute inflammation with associated edema. This large amount of fluid will change the local glucose concentration and hence affect the sensor response. The change in fluid mass surrounding the sensors has also been shown to affect the lag phase.28 The greater the extent of the acute inflammatory response, the greater the effect on the initial sensor response. In addition, the extent of the acute inflammatory response will affect the degree of biofouling at the biosensor surface. This accumulation of proteins and cells may impede analyte diffusion by acting as a diffusion barrier to glucose. It was observed in the current study that sensors implanted using 14 gauge needles had a significantly lower response compared to those implanted using 16 gauge needles (Figure 6A). Similarly, the mean glucose recovery from microdialysis probes inserted using 14 gauge needles was lower than those implanted using 16 gauge needles (Figure 6B). This in vivo microdialysis study indicates the decreased glucose transport is responsible for the reduced sensor response. It was revealed that the 14 gauge needles caused a greater extent of acute inflammation than the 16 gauge needles (Figures 3 and 4). Accordingly, it is reasonable to assume that the extent of edema is greater in the case of implantation using 14 gauge needles, and this is responsible for the lowered sensor response and reduced glucose transport through the microdialysis probes. When the glucose sensors and microdialysis probes were coated with the PLGA/PVA composites, the local release of dexamethasone prevented the development of edema and acute inflammation (Figure 5). Accordingly, sensor performance and glucose transport were unaffected regardless of implantation needle size. These data indicate that the initial adsorption of proteins, which occurs regardless of the presence of dexamethasone, does not impose significant diffusion resistance to glucose. The role of proteins and protein fragments penetrated into the sensor outer membranes is not taken into account in the current discussion since significant accumulation of proteins/protein fragments inside the sensor outer membranes takes longer than the time frame of current study (within 5 hours postimplantation). Biofouling is a dynamic and continuous process, and protein accumulation/penetration as well as protein conformational change over time may affect glucose transport to the biosensor. Future long-term studies will investigate the effect of these changes on sensor performance.
Conclusions
The current work indicates that the extent of the FBR varies as a function of the initial tissue trauma as well as the implant size. The results of the pharmacodynamic studies indicate that the degree of acute inflammation was mainly controlled by the extent of the initial tissue trauma caused during implantation, whereas the development of the fibrous capsule was mainly governed by the implant size. The acute inflammation significantly affected the initial in vivo response of the implanted glucose biosensors, which might be due to an accumulation of inflammatory cells (mainly PMN), edema, or protein deposition at the sensor location. The composite coated glucose biosensors had similar initial in vivo response regardless of the size of the implantation needles, since the local delivery of dexamethasone successfully prevented the inflammatory response. The dexamethasone concentration (75 mg microspheres/mL hydrogel) that was sufficient to prevent FBR for 0.5 × 0.5 × 5 mg sensors was sufficient for the smaller (0.3 × 0.3 × 3 mm) sensors for a 1-month period. A higher dexamethasone concentration (150 mg microspheres/mL) was required for the larger (0.75 × 0.75 × 9 mm) sensors. However, these doses may not be the minimum doses required for these sensor sizes and this will be a focus of future research. Overall, the results of the current study indicates that miniaturization of the implantable biosensor together with the utilization of a drug-eluting biocompatible composite coating may be a promising strategy to achieve long-term reliable continuous glucose monitoring.
Footnotes
Abbreviations: BSA, bovine serum albumin; DMF, dimethylformamide; FBR, foreign body response; GOD, glucose-oxidase; H&E, hematoxylin and eosin; HPLC, high performance liquid chromatography; H2O2, hydrogen peroxide; IACUC, Institutional Animal Care and Use Committee; PLGA, poly, lactic-co-glycolic acid; PMNs, polymorphonuclear leukocytes; PU, polyurethane; PVA, polyvinyl alcohol; SD, Sprague-Dawley; THF, tetrahydrofuran.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FP and SV declare a competing financial interest with Biorasis Inc. FP is 1 of its 2 founders, and SV was partially employed by this company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health Grants (-R21-HL090458-01, and R43 EB011886-01) and the US Army Medical Research Grants (#W81XWH-07-1-0688 and W81XWH-09-1-071).
References
- 1. Wilkins E, Atanasov P, Muggenburg BA. Integrated implantable device for long-term glucose monitoring. Biosens Bioelectron. 1995;10:485-494. [DOI] [PubMed] [Google Scholar]
- 2. Vaddiraju S, Legassey A, Wang Y, et al. Design and fabrication of a high-performance electrochemical glucose sensor. J Diabetes Sci Technol. 2011;5:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010;25:1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotanen CN, Moussy FG, Carrara S, Guiseppi-Elie A. Implantable enzyme amperometric biosensors. Biosens Bioelectron. 2012;35:14-26. [DOI] [PubMed] [Google Scholar]
- 5. David JAS, Cunningham D. In Vivo Glucose Sensing. Hoboken, NJ: John Wiley; 2009. [Google Scholar]
- 6. Vaddiraju S, Burgess DJ, Jain FC, Papadimitrakopoulos F. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosens Bioelectron. 2009;24:1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J Control Release. 2013. [DOI] [PubMed] [Google Scholar]
- 8. Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int J Pharmaceut. 2010;384:78-86. [DOI] [PubMed] [Google Scholar]
- 9. Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23: 1649-1656. [DOI] [PubMed] [Google Scholar]
- 10. Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68-79. [DOI] [PubMed] [Google Scholar]
- 11. Singarayar S, Kistler PM, De Winter C, Mond H. A comparative study of the action of dexamethasone sodium phosphate and dexamethasone acetate in steroid-eluting pacemaker leads. Pacing Clin Electrophysiol. 2005;28:311-315. [DOI] [PubMed] [Google Scholar]
- 12. Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81:858-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helton K, Ratner B, Wisniewski N. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J Diabetes Sci Technol. 2011;5:632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanders JE, Cassisi DV, Neumann T, et al. Relative influence of polymer fiber diameter and surface charge on fibrous capsule thickness and vessel density for single-fiber implants. J Biomed Mater Res A. 2003;65A:462-467. [DOI] [PubMed] [Google Scholar]
- 15. Croce R, Jr, Vaddiraju S, Kondo J, et al. A miniaturized transcutaneous system for continuous glucose monitoring. Biomed Microdevices. 2013;15:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawat A, Burgess DJ. Effect of ethanol as a processing co-solvent on the PLGA microsphere characteristics. Int J Pharmaceut. 2010;394:99-105. [DOI] [PubMed] [Google Scholar]
- 17. Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;122:338-344. [DOI] [PubMed] [Google Scholar]
- 18. Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. J Control Release. 2006;112:293-300. [DOI] [PubMed] [Google Scholar]
- 19. Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F. Modulation of the foreign body response to implanted sensor models through device-based delivery of the tyrosine kinase inhibitor, masitinib. Biomaterials. 2013;34:9737-9746. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J Control Release. 2013;169:341-347. [DOI] [PubMed] [Google Scholar]
- 21. Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. J Control Release. 2008;127:137-145. [DOI] [PubMed] [Google Scholar]
- 22. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients. Part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17:647-654. [DOI] [PubMed] [Google Scholar]
- 23. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor. Part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosens Bioelectron. 2002;17:641-646. [DOI] [PubMed] [Google Scholar]
- 24. Villarreal G, Zagorski J, Wahl SM. Inflammation: Acute. Hoboken, NJ: John Wiley; 2001. [Google Scholar]
- 25. Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochimica et Biophysica Acta. 2012;1822:21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward WK. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J Diabetes Sci Technol. 2008;2:768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11:S11-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. ASAIO J. 1999;45:555-561. [DOI] [PubMed] [Google Scholar]