Abstract
Brain derived neurotrophic factor (BDNF) acting through the tropomyosin-related kinase receptor B (TrkB) enhances neuromuscular transmission in the diaphragm muscle of adult mice, reflecting presynaptic effects. With aging, BDNF enhancement of neuromuscular transmission is lost. We hypothesize that disrupting BDNF/TrkB signaling in early old age will reveal a period of susceptibility evident by morphological changes at neuromuscular junctions (NMJ). Adult, male TrkBF616A mice (n=25) at 6 and 18 months of age, were used to examine the structural properties of diaphragm muscle NMJs (n=1097). Confocal microscopy was used to compare pre- and post-synaptic morphology and denervation following a 7 day treatment with the phosphoprotein phosphatase-1 derivative 1NMPP1, which inhibits TrkB kinase activity in TrkBF616A mice vs. vehicle treatment. In early old age (18 months), presynaptic terminal volume decreased compared to 6 month old diaphragm NMJs (~20 %). Inhibition of TrkB kinase activity significantly decreased the presynaptic terminal volume (~20 %) and motor end-plate 2D planar area (~10 %), independent of age group. Inhibition of TrkB kinase activity in early old age significantly reduced overlap of pre- and post-synaptic structures and increased the proportion of denervated NMJs (to ~20 %). Collectively these results support a period of susceptibility in early old age when BDNF/TrkB signaling at diaphragm NMJs supports the maintenance of NMJs structure and muscle innervation.
Keywords: Brain derived neurotrophic factor, Denervation, Innervation, Motor unit, Tropomyosin-related kinase receptor subtype B
Introduction
Neurotrophic signaling plays an important role in structural and functional properties of motor units. Brain derived neurotrophic factor (BDNF) acting through the high affinity tropomyosin-related kinase receptor B (TrkB) regulates synaptic transmission at the neuromuscular junction (NMJ) in adult (Funakoshi et al., 1995; Mantilla and Ermilov, 2012; Mantilla et al., 2014b; Mantilla et al., 2004b) and aging rodents (Greising et al., 2015a).
In the diaphragm muscle, measurable reductions in force and muscle fiber cross-sectional area are evident only by 24 months of age, at ~75 % survival (Greising et al., 2015a; Greising et al., 2013; Greising et al., 2015c). At 18 months of age (representing early old age; ~90 % survival), there is evidence of impaired neuromuscular transmission using a global measure that reveals the contribution of neuromuscular transmission failure to reduced force generation during repetitive electrical stimulation (Greising et al., 2015a). Importantly, in vitro application of BDNF enhanced neuromuscular transmission in 18 month old mice, similar to BDNF effects in 6 month old mice. However, BDNF exerted no effect on neuromuscular transmission in 24 month old mice. Selective inhibition of TrkB kinase activity in TrkBF616A mice (by using the phosphoprotein phosphatase-1 derivative 1NMPP1) impairs neuromuscular transmission only in 6 month old mice (Greising et al., 2015a; Mantilla et al., 2014b), suggesting that there are age-related disruptions in BDNF/TrkB signaling at diaphragm NMJs. In addition, age-related neuromuscular dysfunction in the diaphragm muscle is present at a time prior to frank sarcopenia suggesting that altered neurotrophic interactions at the NMJ may contribute to neuromuscular dysfunction in old age.
Previous studies have examined morphological changes at NMJs in old age at a time when frank sarcopenia is present. Retraction of presynaptic terminals, disaggregation and fragmentation of the motor end-plate all have been well documented in old age, especially in NMJs of limb muscles (Deschenes et al., 2010; Fahim et al., 1983; Fahim and Robbins, 1982; Valdez et al., 2010) and even in the diaphragm muscle (Prakash and Sieck, 1998; Valdez et al., 2012). However, morphological changes at the NMJ and the effect of disrupted BDNF/TrkB signaling in early old age, prior to the onset of frank sarcopenia, are not known. We hypothesize that disrupting BDNF/TrkB signaling in early old age will reveal a period of susceptibility evident by morphological changes at the NMJ of 18 month old, but not 6 month old TrkBF616A mice. Understanding changes in early old age may reveal both targets and a time frame to mitigate neuromuscular dysfunction and subsequent sarcopenia.
Methods
Animals
Adult male TrkBF616A mice (n=25) on a C57BL/6 x 129 background were examined at 6 (n=13) and 18 (n=12) months of age; representing survival rates of 100 % and 90% based on data from our colony and published estimates (Greising et al., 2015a; Greising et al., 2013). Mice were bred and maintained in colonies at the Mayo Clinic with genotype confirmed by PCR analysis of DNA isolated from tail snips (Greising et al., 2015a; Mantilla et al., 2014a; Mantilla et al., 2014b). Mice were group housed until randomization into treatment groups following which they were housed individually. All mice were maintained on a 12 hour light-dark schedule in specific pathogen-free rooms with free access to food and water through their lifespan. All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with National Institute of Health Guidelines.
The TrkBF616A mice have a phenylalanine-to-alanine mutation in the ATP binding domain of the TrkB receptor (Chen et al., 2005). This genetic mutation allows for rapid and selective chemical inhibition of TrkB kinase activity with oral treatment using 1NMPP1 at an IC50 ~3 nM (Chen et al., 2005; Mantilla et al., 2014a; Mantilla et al., 2014b). Mice were randomized to receive oral 1NMPP1 (25 µM in drinking water; Calbiochem #529581) or vehicle treatment (0.3 % DMSO in drinking water) for 7 days. Previously, the inhibition of TrkB kinase activity by 1NMPP1 was confirmed in vivo (Greising et al., 2015a; Mantilla et al., 2014a). At the terminal experiment, all mice were anesthetized with an i.p. injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and euthanized by exsanguination. The entire diaphragm muscle was carefully dissected and quickly excised for further histologic staining.
Neuromuscular Junction Morphology
Immediately following dissection, the diaphragm muscle was stained as a whole tissue for morphologic assessment of pre- and post-synaptic structures of the NMJ, as previously described and validated (Mantilla et al., 2004a; Mantilla et al., 2007; Mantilla et al., 2014b; Prakash et al., 1996; Sieck et al., 2012). Briefly, the diaphragm muscle was incubated with α-bungarotoxin conjugated to Alexa Fluor 488 to stain the postsynaptic motor end-plate (0.1 µg/ml; B13422, Invitrogen Corp., Carlsbad CA), followed by fixation in 4 % paraformaldehyde and incubation with anti-synaptophysin antibody to stain the presynaptic terminals (1 mg/ml; SC-9116, Santa Cruz Biotechnology Inc., Santa Cruz, CA). A Cy-3 conjugated donkey anti-rabbit IgG secondary antibody was used (1:200; 711-165-152; Jackson Immuno Research Laboratories Inc., Baltimore, PA). All diaphragm muscle samples were stored in Tris-buffered saline until confocal imaging, which occurred no more than 3 days following the staining procedure.
The specificity of fluorescently-conjugated α-bungarotoxin and anti-synaptophysin labeling has been previously confirmed and validated (Issa et al., 2010; Prakash and Sieck, 1998). The immunoreactivity pattern observed was identical to that in previous reports (Mantilla et al., 2004a; Mantilla et al., 2007; Prakash et al., 1999; Rowley et al., 2007). Briefly, there was an absence of α-bungarotoxin staining outside of motor end-plates in the diaphragm and other skeletal muscles in mice and rats, as previously reported (Mantilla et al., 2004a; Mantilla et al., 2007; Prakash et al., 1996; Prakash et al., 1999; Rowley et al., 2007; Sieck et al., 2012). The synaptophysin antibody recognizes a region between amino acids 269 and 289 (Knaus and Betz, 1990) in the cytoplasmic domain of this integral membrane glycoprotein (~40 kD) (Wiedenmann and Franke, 1985) and only a single band of 38 kD molecular weight is detectable by Western blot. Synaptophysin staining was evident in presynaptic structures opposing motor end-plates in the diaphragm muscle.
Confocal Imaging
Imaging of the diaphragm muscle NMJs in whole mounts using confocal microscopy has been previously described (Mantilla et al., 2004a; Mantilla et al., 2007; Mantilla et al., 2014b; Prakash et al., 1993, 1994; Rowley et al., 2007; Sieck et al., 2012; Sieck et al., 1999; Sieck and Prakash, 1997). During all imaging and analysis, investigators were blinded to the age and treatment group. At least 40 ‘en face’ NMJs per animal were identified by α-bungarotoxin staining and imaged. Selected NMJs were no more than 60 µm deep on the thoracic side of the diaphragm muscle to minimize possible effects of antibody penetration and optical distortion. Imaging was conducted with an Olympus FluoView 300 laser scanning confocal microscope (Olympus America Inc., Melville, NY) mounted on an upright Olympus BX50WI microscope and equipped with Argon (488 nm) and HeNe (543 nm) lasers using an Olympus LUMPlanFl 40x/0.80 N.A. water immersion lens. Images were acquired in a 800 x 600 array with pixel dimensions (0.5 x 0.5 µm) above the optical resolution for this lens (thus avoiding oversampling). Step size was set to match the empirically-determined Z-axis resolution (0.8 µm), as determined previously (Mantilla et al., 2004a; Mantilla et al., 2007; Prakash et al., 1994; Sieck et al., 2012).
All analysis of pre- and post-synaptic structures of individual diaphragm muscle NMJs were conducted with confocal image stacks consisting of 12–16 optical slices (each a two-channel 12-bit multi-TIFF file) using MetaMorph (Molecular Devices, LLC, Sunnyvale, CA), as previously described (Mantilla et al., 2014b; Prakash et al., 1996; Prakash et al., 1999; Sieck et al., 2012). The volume of the pre- and post-synaptic structures was determined after manual thresholding using a customized algorithm in MetaMorph, as previously reported (Sieck et al., 2012 Mantilla, 2014 #14167), and the volume of apposition between pre- and post-synaptic structures was determined from the intersection of the two binarized volumes. The volume of apposition was expressed as the percentage of the presynaptic terminal volume opposing the motor end-plate (i.e., presynaptic apposition). In addition, a postsynaptic 2D planar area of the motor end-plate was obtained from a maximum intensity projection image. For each motor end-plate, a relative planar area was calculated by dividing the 2D planar area by the area defined by the main orthogonal axes of the end-plate; this measure reflects the complexity of the motor end-plate (i.e., branching and fragmentation). Denervation was determined as present at an individual diaphragm NMJ if there was partial overlap of pre- and post-synaptic structures based on visualization of the maximal intensity projections, and thus individual NMJs were classified as partially or totally denervated vs. intact.
For the purpose of publication, images were produced in Adobe Photoshop (Adobe Systems Inc.; San Jose, CA) by down-converting multi-TIFF files to 8-bit single-channel images without introducing any changes in brightness or contrast.
Statistical Analyses
All data was analyzed using JMP (version 10.0 SAS Institute, Inc., North Carolina). Data was analyzed by two-way repeated measures ANOVA for body weight (age x treatment x time – repeated). A mixed linear model (age x treatment), with animal as a random effect, was used for NMJ morphology data including pre- and post-synaptic volume, 2D planar area, relative planar area, and apposition. A two-way ANOVA (age x treatment) was used for the percent of total denervated diaphragm NMJs. Chi-squared was used to analyze the distribution of pre- and post-synaptic volumes. A receiver operating characteristic (ROC) curve was generated for the ratio of pre- to post-synaptic volume and denervation classification (partially or totally denervated vs. intact). Data are reported as least squares mean ± SE, unless otherwise specified, significance was accepted at P<0.05.
Results
Oral Inhibitor Treatment
In order to examine NMJ morphology, adult male TrkBF616A mice were randomized to receive vehicle or 1NMPP1 (inhibiting TrkB kinase activity) for 7 days at 6 and 18 months of age. As expected there were no adverse effects of inhibitor treatment in either age group. The 18 month old mice were slightly larger than the 6 month old mice, which was expected (P=0.025; Table 1). There was no significant difference in the body mass following 7 days of treatment (P=0.351).
Table 1.
Characteristics of TrkBF616A mice
| 6 Month | 18 Month | |||
|---|---|---|---|---|
| Vehicle | Inhibitor | Vehicle | Inhibitor | |
| Mice (n) | 7 | 6 | 6 | 6 |
| NMJs analyzed | 288 | 251 | 258 | 300 |
| Age (mo) | 6.0 ± 0.1 | 6.1 ± 0.1 | 17.9 ± 0.1 | 18.1 ± 0.1 |
| Pre-treatment body mass (g) | 32.5± 2.1 | 31.0 ± 0.6 | 34.0 ± 2.2 | 39.8 ± 1.5 |
| Post-treatment body mass (g) | 29.9 ± 4.9 | 29.6 ± 0.6 | 33.7 ± 2.2 | 39.5 ± 1.3 |
Mean±SE Body mass of TrkBF616A mice was measured at start and end of 7 day oral treatment period with either vehicle or 1NMPP1 inhibitor. Two-way repeated measures ANOVA, for body mass (age: P=0.025; treatment group: P=0.203; interaction: P=0.351). As expected, 18 month old mice are significantly larger than 6 month old mice.
Aging Effects on Diaphragm Neuromuscular Junction Volume
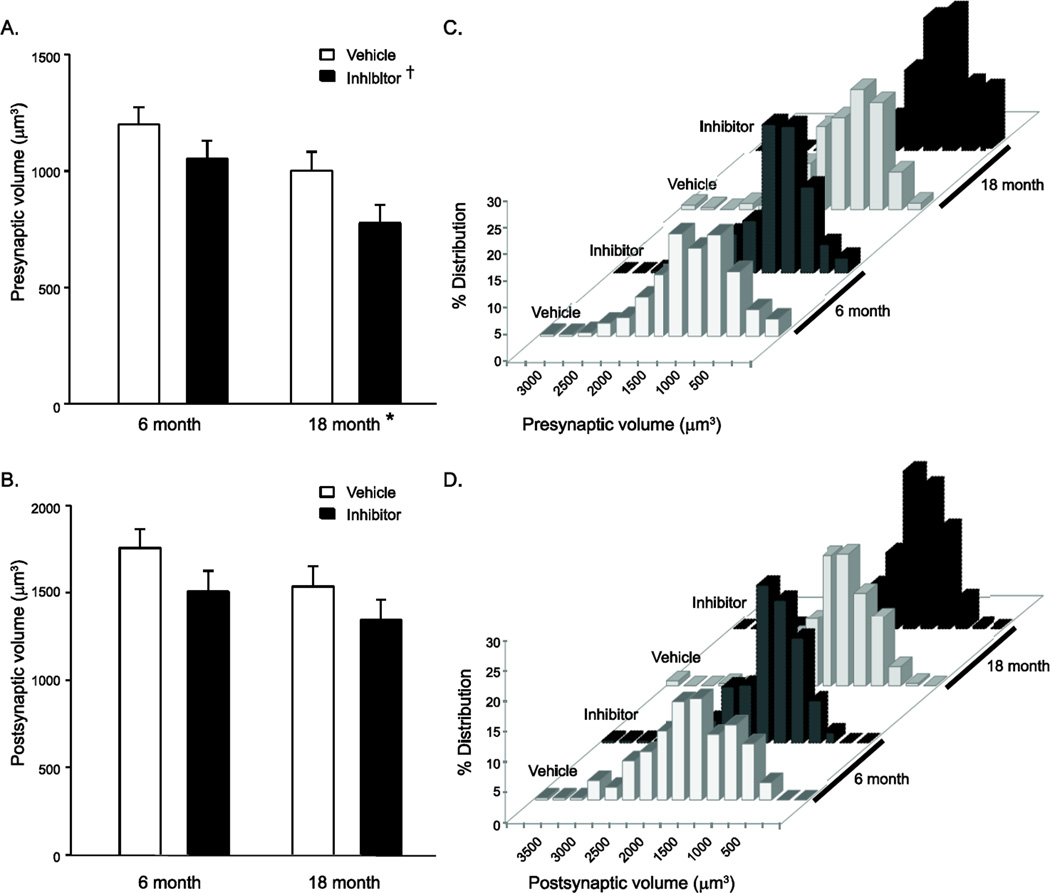
In total, 1097 individual diaphragm muscle NMJs were analyzed (Figure 1A and 1B). There was an overall decrease in the volume of the presynaptic terminal at 18 months of age (913 ± 56 µm3) compared to 6 months of age (1101 ± 54 µm3; main effect of age, P=0.025; Figure 2A). The least squares mean volume of the postsynaptic motor end-plate was slightly smaller at 18 months of age (1427 ± 81 µm3) compared to 6 months of age (1645 ± 79 µm3), although this difference was not statistically significant (main effect of age, P=0.068; Figure 2B). Evaluation of both pre- and postsynaptic volumes showed substantial variation across animals. Thus, we also analyzed the distribution of both the pre- and post-synaptic volumes of diaphragm muscle NMJs (Figure 2C and 2D). There was a significant shift in the distribution of both pre- and post-synaptic volumes due to age (P<0.001). Using the 6 month old vehicle treated mice as the reference distribution, with age there is a narrowing of the distribution.
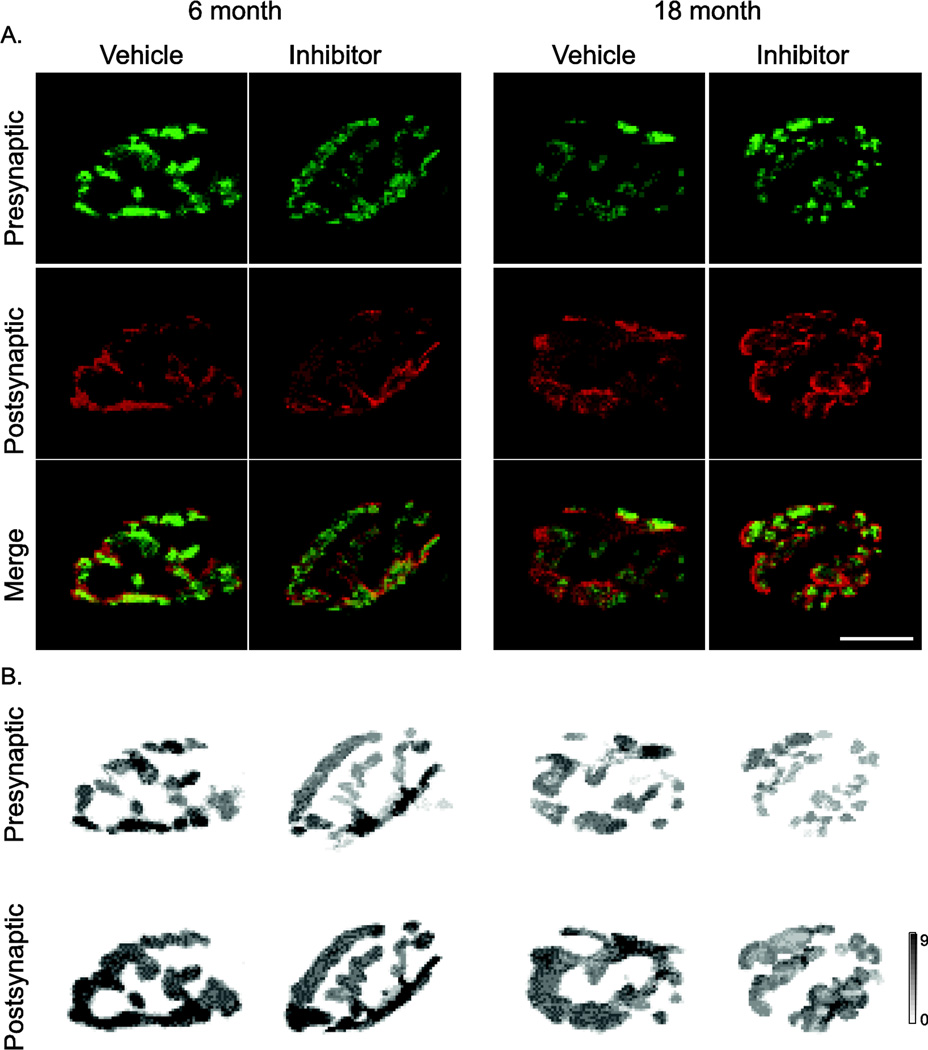
Figure 1.
Representative confocal micrographs of diaphragm muscle neuromuscular junctions (NMJ), labeled with synaptophysin (presynaptic terminal) and α-bungarotoxin (postsynaptic motor end-plate). A) Maximum-projection images; B) three-dimensional (3D) reconstructions showing varying depth by grayscale intensity in µm, scale bar at right. TrkBF616A mice at 6 and 18 months of age underwent oral treatment with vehicle or inhibitor treatment (1NMPP1, to inhibit TrkB kinase activity) for 7 days prior to morphological analyses. Bar, 10 µm.
Figure 2.
Presynaptic and motor end-plate volume for diaphragm muscle NMJs from 6 and 18 month old TrkBF616A mice after 7 day vehicle or inhibitor treatment. A) Least squares mean presynaptic volume was smaller with both age and inhibition of TrkB kinase activity. Data were analyzed with a mixed linear model with an individual animal as a random effect; main effect of age: *, P= 0.025, main effect of treatment: †, P=0.006, interaction: P=0.633. B) Least squares mean postsynaptic volume was not significantly affected by age or inhibition of TrkB kinase activity (main effect of age: P= 0.068, main effect of treatment: P=0.105, interaction: P=0.814). There was a significant shift in distributions of both C) pre- and D) post-synaptic volumes as analyzed by chi-squared analysis (P<0.001).
TrkB Kinase Inhibition Effects on Diaphragm Neuromuscular Junction Volume
There was a significant overall effect of inhibition of TrkB kinase activity, with a decrease in presynaptic terminal volume following 1NMPP1 treatment (888 ± 56 µm3) compared to vehicle treatment (1125 ± 54 µm3; main effect of treatment, P=0.006; Figure 2A). We previously found no effect of inhibition of TrkB kinase on presynaptic terminal volume in 6 month old mice (Mantilla et al., 2014b). In general agreement, in post hoc pairwise comparisons (Tukey’s HSD test), differences in mean presynaptic volume were only evident between the 6 month old vehicle-treated mice and the 18 month old 1NMPP1-treated mice. There was no effect of TrkB kinase inhibition on postsynaptic volume in either the 6 or the 18 month old mice (main effect of treatment, P=0.105; Figure 2B), also consistent with our previous result in 6 month old mice following inhibition of TrkB kinase activity (Mantilla et al., 2014b).
There was a significant shift in the distribution of both pre- and post-synaptic volumes following inhibition of TrkB kinase activity (P<0.001; Figure 2C and 2D). Using the 6 month old vehicle treated mice as the reference distribution, with inhibition of TrkB kinase activity there is a leftward shift in the distribution to smaller pre- and post-synaptic volumes, beyond the effects of age. Of note, the pattern of distribution of the 6 month old inhibitor treated and the 18 month old vehicle treated mice were similar. These data indicate that inhibition of TrkB kinase activity replicates the aging effect on the distribution of NMJ volumes. In addition, it is noteworthy that in the 1NMPP1-treated 18 month old group 11 % of diaphragm NMJs exhibit presynaptic volumes < 250 µm3, suggesting the presence of substantial denervation following only 7 days of inhibition of TrkB kinase activity.
Denervation of Diaphragm Neuromuscular Junctions
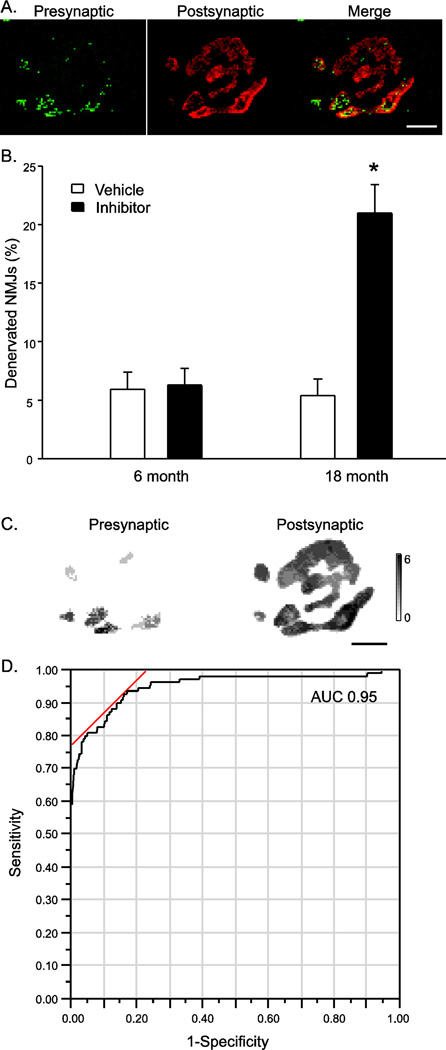
A qualitative assessment of innervation was completed on all diaphragm NMJs analyzed by assessing the degree of overlap of the pre- and post-synaptic structures on maximum intensity projections of en face diaphragm NMJs (Figure 3A). There was no main effect of age on denervation of diaphragm NMJs. Inhibition of TrkB kinase activity significantly increased diaphragm denervation in early old age. In 18 month old mice, there was a significant increase in NMJs displaying signs of denervation following inhibition of TrkB kinase activity (two-way ANOVA interaction, P<0.001; Figure 3B), overall ~21 % of diaphragm NMJs showed at least partial denervation (i.e., only partial overlap of pre- and post-synaptic structures) and 4 % of all motor end-plates that were assessed showed complete absence of presynaptic terminals. In contrast, in 6 month old mice, there was no effect of inhibiting TrkB kinase activity, with ~6 % of NMJs displaying signs of partial or complete denervation in both the vehicle and 1NMPP1-treated groups.
Figure 3.
Diaphragm NMJ denervation in 6 and 18 month old TrkBF616A mice after 7 day vehicle or inhibitor treatment. A) Representative confocal micrograph of a denervated diaphragm NMJ from an 18 month old TrkBF616A treated with 1NMPP1; bar 5 µm. Denervation was determined in diaphragm NMJs that displayed partial overlap of the pre- (red) and post-synaptic (green) structures based on maximum intensity projections of en face NMJs. B) Evidence of denervation was significantly greater at 18 months of age following inhibition of TrkB kinase activity. Data were analyzed with a mixed linear model with an individual animal as a random effect (age x treatment x animal); *, interaction P<0.001. C) Three- dimensional (3D) reconstruction for the same NMJ as in A showing varying depth by grayscale intensity in µm, scale bar at right. The ratio pre- to post-synaptic volume was used to generate a quantitative measure of NMJ denervation. D) Receiver Operating Characteristic (ROC) curve for the ratio of pre- to post-synaptic volume and denervation classification, where a value of 51 % or lower had a 93 % sensitivity and 84 % specificity for detecting denervated NMJs (P<0.001).
Morphological Assessment of Denervation
In an effort to quantitatively assess the extent of denervation, the relative volume of apposition between pre- and post-synaptic structures of the NMJ was also determined. There was no effect of age on the fraction of the presynaptic terminal directly opposing the motor end-plate (main effect of age, P=0.318); overall, in 6 month and 18 month old mice it was 82 ± 2 % and 80 ± 2 %, respectively. In addition, there was no effect of 1NMPP1 inhibitor treatment in TrkBF616A mice (main effect of treatment, P=0.798), with presynaptic apposition 81 ± 2 % and 80 ± 2 % in the vehicle and 1NMPP1 treated groups, respectively. A ROC curve for the ratio of pre- to post-synaptic volume and denervation classification (Figure 3C) revealed that a value of 51 % or lower had a 93 % sensitivity and 84 % specificity for detecting denervated NMJs (Area Under Curve: 0.95; P<0.001); a value of 32 % or lower had a 68 % sensitivity and 99 % specificity for detecting denervated NMJs. Using this latter quantitative criterion, generally similar results were obtained compared to the manual classification (in 6 month old mice, 4 ± 3 % of NMJs were classified as denervated in both treatment groups; in 18 month old mice, 3 ± 3 % vs. 16 ± 3 % of NMJs were denervated in the vehicle and inhibitor treatment groups (two-way ANOVA interaction, P=0.055).
Postsynaptic Remodeling of Diaphragm Neuromuscular Junctions
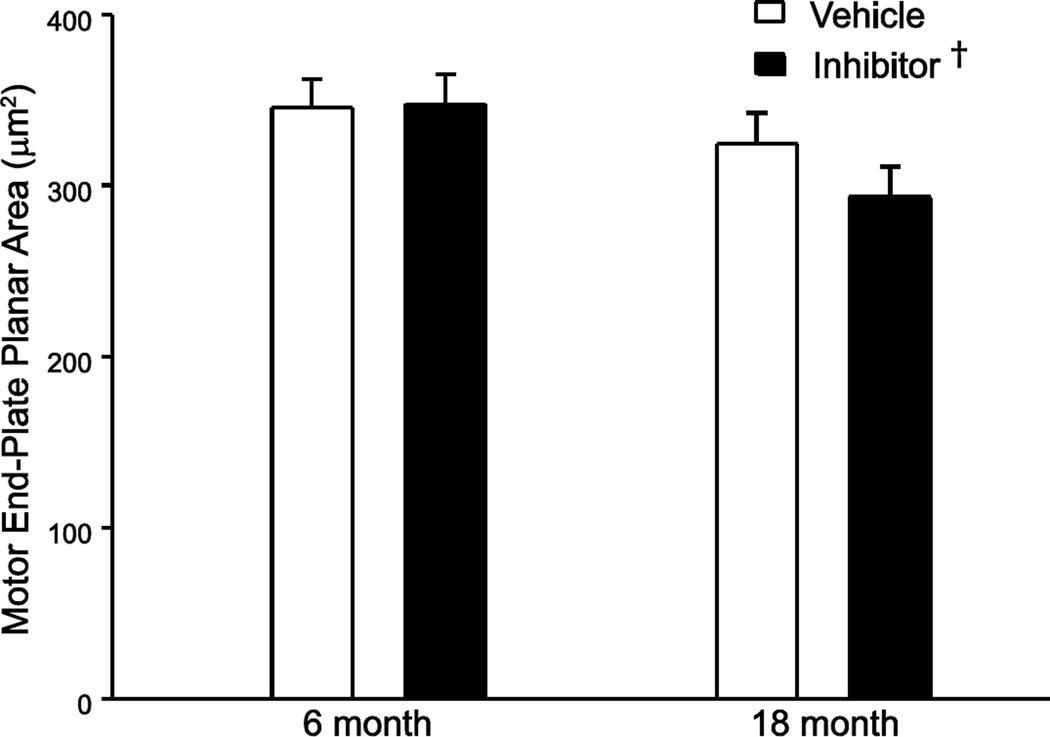
To measure postsynaptic remodeling across age groups and following inhibition of TrkB kinase activity, maximum-intensity projections for each NMJ were used to measure motor end-plate area (2D planar area) and an index of NMJ complexity given by the relative planar area (Sieck et al., 2012). There was no effect of age on the 2D planar area of the NMJ (main effect of age, P=0.416; Figure 4). There was no effect of age on the relative planar area of the NMJ (45.7 ± 1.3 % and 44.1 ± 1.3 % for the 6 and 18 month old mice, respectively; main effect of age, P=0.384).
Figure 4.
Two-dimensional (2D) planar area of diaphragm muscle NMJs in 6 and 18 month old TrkBF616A mice after 7 day vehicle or inhibitor treatment. Least squares mean 2D planar areas were significantly smaller following inhibition of TrkB kinase activity. Data were analyzed with a mixed linear model with an individual animal as a random effect; main effect of age: P=0.416, main effect of treatment: †, P=0.046, interaction: P=0.361.
Inhibition of TrkB kinase activity in TrkBF616A mice resulted in a slight decrease in 2D planar area of diaphragm NMJs compared to vehicle treated mice (main effect of treatment, P=0.046). We previously reported no effect of inhibiting TrkB kinase on the 2D planar area in the 6 month old mice (Mantilla et al., 2014b). However, there were no differences across age or treatment groups in gutter depth estimated by the ratio of postsynaptic volume to 2D planar area (interaction of age and treatment, P=0.172), indicating that the small changes observed in 2D planar area in the present study do not reflect substantial postsynaptic remodeling. In agreement, there was no effect of inhibitor treatment on NMJ complexity, with relative planar areas of 44.8 ± 1.3 % and 45.1 ± 1.3 % for the vehicle and inhibitor treated mice, respectively (main effect of treatment, P=0.875), with no significant interaction of age and treatment on relative planar area (interaction, P=0.090).
Discussion
Using a chemical-genetic approach in adult male TrkBF616A mice, we examined diaphragm NMJs following inhibition of TrkB kinase activity at 6 and 18 months of age in order to evaluate a period of susceptibility to the effects of disrupting neuromuscular BDNF/TrkB signaling. Morphological changes were evident at NMJs of 18 month old compared to 6 month old TrkBF616A mice, consistent with previously reported neuromuscular dysfunction in the diaphragm muscle occurring well before measurable sarcopenia which is evident only by 24 months of age (Greising et al., 2015a; Greising et al., 2015b; Greising et al., 2015c). The pre- and post-synaptic NMJ volumes of diaphragm NMJs exhibit an age-related reduction in the numbers of larger NMJs (narrowed distribution). Additionally, inhibition of TrkB kinase activity resulted in an increased proportion of denervated NMJs, and a further decrease in presynaptic volume. Small changes were evident post-synaptically with inhibition of TrkB kinase activity, with a slight reduction in NMJ area and no effect on gutter depth. Collectively, these results support early old age as a period of neuromuscular susceptibility to disruption of BDNF/TrkB signaling. Indeed, 7-day inhibition of BDNF/TrkB signaling in young mice replicates a number of features evident at older NMJs.
During the aging progression, several morphological changes are evident at NMJs, including denervation and decreased pre- and post-synaptic volumes (c.f.Gonzalez-Freire et al., 2014). Previous reports examining NMJ morphology usually examine old age groups with reduced survival rates. For example, Valdez and colleagues recently found in 24–28 month old mice with expected survival ~75 to 40% that diaphragm NMJs displayed axonal swelling, motor end-plate fragmentation, and denervation (Valdez et al., 2012). These results are consistent with our previous findings in very old rats (~50 % survival) in which diaphragm NMJs showed increased motor end-plate fragmentation (Prakash and Sieck, 1998). To date very-few studies have examined changes occurring in NMJs during early old age. In rats between 10 and 21 months of age (~100% and 80% survival, respectively), NMJ remodeling in limb muscles was consistent with early signs of denervation, particularly in the plantaris muscle (Deschenes et al., 2010). In rat diaphragm NMJs, examined across various ages between 1 – 26 months of age (down to ~50% survival), there were minimal changes in early old age (~12 to 20 months), which were primarily related to irregular NMJs suggestive of ongoing remodeling and denervation (Cardasis and LaFontaine, 1987). These changes were evident prior to significant motor end-plate fragmentation and denervation which occurred in old age (>23 months of age). In general agreement, the present study reports relative minor morphological changes at mouse diaphragm NMJs in early old age (18 month old; 90% survival) compared to mature adult mice (6 month old; 100% survival). Indeed, although there was a decrease in the presynaptic terminal volume in combination with a leftward shift in both pre- and post-synaptic volume (i.e., fewer large NMJs), there was no measurable change in the percentage of denervated NMJs in this age group.
In the present study, there was significant denervation evident at diaphragm NMJs following inhibition of TrkB kinase activity in the early old age mice but not in the mature adult mice at 6 months of age. Indeed, more than 20 % of the NMJs examined showed partial or complete signs of denervation following inhibition of TrkB kinase activity. The mechanisms driving age-related denervation are not understood. The present results indicate that alterations or disruptions in neurotrophic signaling, specifically in BDNF/TrkB kinase activity, may contribute to denervation in old age. In general agreement, BDNF/TrkB signaling mitigates the contribution of neuromuscular transmission failure to reduced force generation during repetitive electrical stimulation into early old age (Greising et al., 2015a; Mantilla and Ermilov, 2012; Mantilla et al., 2004b), but not in older age groups (24 months of age). Furthermore, inhibition of TrkB kinase activity in 6 month old TrkBF616A mice replicates the changes in neuromuscular transmission evident in 18 and 24 month old mice. This global measure of neuromuscular transmission incorporates all components of neural excitation and synaptic transmission, and thus reflects morphological and structural changes at the NMJ in addition to frank denervation.
The results of the present study support BDNF/TrkB signaling exerting a primarily presynaptic effect, consistent with modulation of synaptic vesicle release evident at both developing and adult NMJs (Garcia et al., 2010; Greising et al., 2015a; Lohof et al., 1993; Lu, 2004; Mantilla et al., 2014b; Mantilla et al., 2004b) as well as other synapses (Boulanger and Poo, 1999; Dieni et al., 2012; Jovanovic et al., 2000; Tyler and Pozzo-Miller, 2001). In addition, TrkB receptor mRNA expression measured in putative motoneurons using in situ hybridization reportedly decreases in old age (Johnson et al., 1996, 1999). It is worth noting that inhibition of TrkB kinase activity with oral 1NMPP1 treatment in TrkBF616A mice may not exclusively disrupt neurotrophic signaling at the NMJ, but may also possibly change dendritic gene expression in motoneurons, which are evident in cultured cortical neurons (Cohen et al., 2011), or impair Schwann cell-dependent axonal myelination (Ng et al., 2007). Regardless, although the mechanisms responsible for the age-related changes in NMJ morphology and muscle denervation require further examination, the present study identifies a period of susceptibility in early old age in which the effects of disrupting neuromuscular BDNF/TrkB signaling contribute to NMJ dysfunction.
It is remarkable that neurotrophin-dependent effects at diaphragm muscle NMJs precede the development of diaphragm muscle sarcopenia. Indeed, impairments in diaphragm neuromuscular transmission are significant at early old age (18 months of age), when frank sarcopenia is not (Greising et al., 2015a). Accordingly, the results of the present study demonstrate an important role of BDNF/TrkB signaling in regulation of NMJ morphology and muscle innervation prior to the onset of frank sarcopenia. A key component of sarcopenia is the fiber type specific atrophy of skeletal muscles, specifically of the type IIx and/or IIb fibers (Greising et al., 2013; Greising et al., 2015c; Kung et al., 2014). Of note, the type IIx and/or IIb diaphragm muscle fibers are significantly larger and produce more force than type I and type IIa fibers (Gawel and Kostera-Pruszczyk, 2014; Geiger et al., 2000; Geiger et al., 1999; Greising et al., 2013; Zhan et al., 1997). It is possible that the fiber type specific atrophy evident in old age may be related to the denervation of NMJs at these muscle fibers. Various models such as unilateral phrenic denervation (which removes neurotrophic influences to that entire hemi-diaphragm) and tetrodotoxin blockade of phrenic nerve action potential propagation also result in type specific effects on type IIx and/or IIb fibers. Following 14 days of either unilateral phrenic denervation or tetrodotoxin blockade of phrenic nerve in adult rats, there is an ~40 % decrease in the cross-sectional areas of type IIx and IIb diaphragm muscle fibers (Zhan et al., 1997). This fiber type-specific atrophy is occurring in parallel to a 50 % force loss in the diaphragm muscle in the denervation and tetrotodoxin models of diaphragm inactivity (Miyata et al., 1995). Collectively, the similarities related to fiber type specific effects across models highlight possible shared mechanisms of NMJ dysfunction.
The present finding that more than 20 % of diaphragm NMJs displayed denervation following inhibition of TrkB kinase activity in early old age can be expected to result in a subsequent reduction in fiber cross-sectional area and impaired neuromuscular transmission. It is possible that NMJ denervation occurs at specific motor unit types, possibly those innervating type IIx and/or IIb muscle fibers, which are the largest and most susceptible to sarcopenia. Such motor unit type-specific effects are consistent with the loss of NMJs with the largest pre- and post-synaptic volumes due to both aging and inhibition of TrkB kinase activity as reported in the current study. Thus, it is possible that neurotrophic signaling is particularly important for a subgroup of motor units, especially into early old age. Future investigations should begin to examine the role of altered BDNF/TrkB signaling in fiber type specific atrophy, as changes in neurotrophic signaling throughout the motor unit are likely to have functional effects on muscle fiber properties.
In contrast to previous reports examining BDNF/TrkB signaling at the NMJ, the current study used a chemical genetic approach to inhibit TrkB kinase activity in a TrkBF616A mouse model that develops and ages normally (Chen et al., 2005; Mantilla and Ermilov, 2012; Mantilla et al., 2014a; Mantilla et al., 2014b). This model thus permits direct examination of the role of BDNF/TrkB signaling during the aging process that is not confounded by issues related to altered neurotrophic signaling during development. Furthermore, this chemical genetic approach does not disrupt BDNF binding to the TrkB receptor and downstream inhibition of TrkB kinase activity only occurs in the presence of phosphoprotein phosphatase-1 derivatives such as 1NMPP1 in very low concentrations (IC50 ~ 3 nM) (Chen et al., 2005). A previous report using mice heterozygous for TrkB (TrkB+/−), with low levels of TrkB receptor expression throughout the entire lifespan, documents morphological changes at the NMJ and impairments in neuromuscular transmission in the soleus muscle by 6 months of age similar to those of old mice at 24 months of age (Kulakowski et al., 2011). However, the effects of genetic reductions in full-length TrkB expression cannot help elucidate periods of susceptibility to reduced BDNF/TrkB signaling evident in early old age. In this regard, postsynaptic disassembly at the NMJ was previously reported in mice with viral-induced overexpression of truncated TrkB receptors (Gonzalez et al., 1999), consistent with reduced signaling via full-length TrkB receptors.
Previously, we examined the inhibition of TrkB kinase activity and subsequent recovery to basal BDNF/TrkB signaling levels in mature adult mice using TrkBF616A mice given that this chemical-genetic approach permits rapid and reversible inhibition (Mantilla et al., 2014b). Seven days of TrkB kinase inhibition resulted in diaphragm NMJs that were more compact and had greater apposition between the presynaptic terminal and the motor end-plate. Consistent with this, the current study found minimal changes in diaphragm muscle NMJs following inhibition of TrkB kinase activity in mature adult mice (6 months of age), while more significant changes were found in mice at early old age (18 months of age). In TrkBF616A mice that were allowed to recover from TrkB kinase inhibitor-treatment, there was remodeling of both pre- and post-synaptic structures at diaphragm NMJs and restored neuromuscular transmission (Mantilla et al., 2014b). Taken together these finding suggest that neurotrophic signaling may be disrupted with the aging process, limiting ongoing remodelling at NMJs. Future studies should focus on the possible effects of BDNF/TrkB signaling in mitigating aging-, injury- or disease-related NMJ dysfunction. Notably, all components of NMJ dysfunction should be examined including both structural and functional changes throughout the neuromuscular system.
The combination of altered NMJ morphology at the diaphragm muscle (present study) and neuromuscular dysfunction (Greising et al., 2015a) which are evident in early old age support the role of trophic interactions at the NMJ in establishing the susceptibility (or resilience) to aging effects on the neuromuscular system. Specifically, the current study shows that susceptibility to age-related changes in NMJ morphology and denervation depends on trophic influences exerted by BDNF/TrkB signaling. Furthermore, neurotrophic interactions can determine the susceptibility to neuromuscular damage resulting from additional insults such as systemic inflammatory conditions (e.g., infection or critical illness), an important contributor to functional decline, increased morbidity and mortality in old age. Future studies will be important in determining whether age-related disruptions in neurotrophic signaling constitute an important mechanism underlying responsible for the development of sarcopenia, and whether such trophic signaling can contribute to alterations in autophagy and apoptosis throughout the motor unit.
Highlights.
Susceptibility to neuromuscular damage precedes diaphragm muscle sarcopenia
Increased susceptibility to NMJ dysfunction occurs in early old age
Inhibition of TrkB kinase activity increases the proportion of denervated NMJs
Acknowledgments
This research was supported by grants from National Institute of Health R01-AG044615 (CBM & GCS) and T32-HL105355 (SMG), and the Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
Conception and design of the experiments: SMG, GCS, CBM. Collection, analysis and interpretation of data: SMG, JMS, CBM. Drafting the article or revising it critically for intellectual content: SMG, GCS, CBM. All experiments were carried out at the Mayo Clinic. All authors have read and approved the final submission.
References
- Boulanger LM, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- Cardasis CA, LaFontaine DM. Aging rat neuromuscular junctions: a morphometric study of cholinesterase stained whole mounts and ultrastructure. Muscle Nerve. 1987;10:200–213. doi: 10.1002/mus.880100303. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Bas Orth C, Kim HJ, Jeon NL, Jaffrey SR. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc Natl Acad Sci U S A. 2011;108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Holley JA, Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. J Neurocytol. 1983;12:13–25. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenasa E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Garcia N, Tomas M, Santafe MM, Besalduch N, Lanuza MA, Tomas J. The Interaction between Tropomyosin-Related Kinase B Receptors and Presynaptic Muscarinic Receptors Modulates Transmitter Release in Adult Rodent Motor Nerve Terminals. J Neurosci. 2010;30:16514–16522. doi: 10.1523/JNEUROSCI.2676-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawel M, Kostera-Pruszczyk A. Effect of age and gender on the number of motor units in healthy subjects estimated by the multipoint incremental MUNE method. J Clin Neurophysiol. 2014;31:272–278. doi: 10.1097/WNP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol. 2015a;593:431–440. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Medina-Martinez JS, Stowe JM, Sieck GC. Functional Impact of Diaphragm Muscle Sarcopenia in both Male and Female Mice. Am J Physiol Lung Cell Mol Physiol. 2015b;309:L46–L52. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle & nerve. 2015c;52:76–82. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa AN, Zhan WZ, Sieck G, Mantilla CB. Neuregulin-1 at synapses on phrenic motoneurons. J Comp Neurol. 2010;518:4213–4225. doi: 10.1002/cne.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T, Ulfhake B. Decreased expression of TrkB and TrkC mRNAs in spinal motoneurons of aged rats. Eur J Neurosci. 1996;8:494–499. doi: 10.1111/j.1460-9568.1996.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T, Ulfhake B. Expression of p75(NTR), trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Brain Res Mol Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Knaus P, Betz H. Mapping of a dominant immunogenic region of synaptophysin, a major membrane protein of synaptic vesicles. FEBS Lett. 1990;261:358–360. doi: 10.1016/0014-5793(90)80591-6. [DOI] [PubMed] [Google Scholar]
- Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol. 2011;111:844–852. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- Kung TA, Cederna PS, van der Meulen JH, Urbanchek MG, Kuzon WM, Jr, Faulkner JA. Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. J Gerontol A Biol Sci Med Sci. 2014;69:657–665. doi: 10.1093/gerona/glt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–150. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve. 2004a;30:774–783. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004b;29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC. TrkB Kinase Activity is Critical for Recovery of Respiratory Function after Cervical Spinal Cord Hemisection. Exp Neurol. 2014a;261:190–195. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB Kinase Activity Maintains Synaptic Function and Structural Integrity at Adult Neuromuscular Junctions. J Appl Physiol. 2014b;117:910–920. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Ng BK, Chen L, Mandemakers W, Cosgaya JM, Chan JR. Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J Neurosci Methods. 2007;27:7597–7603. doi: 10.1523/JNEUROSCI.0563-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage. 1993;1:95–107. doi: 10.1006/nimg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Application of the Cavalieri principle in volume estimation using laser confocal microscopy. Neuroimage. 1994;1:325–333. doi: 10.1006/nimg.1994.1017. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miller SM, Huang M, Sieck GC. Morphology of diaphragm neuromuscular junctions on different fibre types. J Neurocytol. 1996;25:88–100. doi: 10.1007/BF02284788. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Morphological adaptations of neuromuscular junctions depend on fiber type. Can J Appl Physiol. 1997;22:197–230. doi: 10.1139/h97-014. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB, Prakash YS. Volume measurements in confocal microscopy. Methods Enzymol. 1999;307:296–315. doi: 10.1016/s0076-6879(99)07019-6. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]