Abstract
Study Objectives:
Quantify the homeostatic and circadian effects on sleepiness and performance of adolescents. Examine age-related changes in homeostatic and circadian regulation of sleepiness and performance by comparing younger and older adolescent groups.
Design:
Three-week laboratory study including 12 cycles of a 28-h forced desynchrony protocol.
Setting:
Controlled laboratory environment with individual sleep and performance testing rooms and shared common areas.
Participants:
Twenty-seven healthy adolescents including 16 females. Ages ranged from 9.6–15.2 years and participants were split into younger (n = 14 ages 9–12) and older (n = 13 ages 13–15) groups based on median age split of 13.0 years.
Interventions:
N/A
Measurements and Results:
Testing occurred every 2 h during scheduled wake periods. Measures included sleep latency during repeated nap opportunities and scores from a computerized neurobehavioral assessment battery including a 10-min psychomotor vigilance task, a digit symbol substitution task, and the Karolinska Sleepiness Scale. Significant main effects of circadian and homeostatic factors were observed, as well as several circadian and homeostatic interaction effects. Age group did not have a significant main effect on sleep and performance data. A significant interaction of circadian phase and age group was found for sleep latency, with younger adolescents showing greater circadian modulation than older teens during the circadian night.
Conclusions:
Adolescents demonstrated a similar pattern of response to forced desynchrony as reported for adults. Sleepiness and performance were affected by homeostatic and circadian factors, and age group did not interact with homoeostatic and circadian factors for subjective sleepiness and most performance metrics. Younger adolescents had a shorter latency to sleep onset than older during the circadian bin spanning 4 to 8 h after the onset of melatonin secretion.
Citation:
Wu LJ, Acebo C, Seifer R, Carskadon MA. Sleepiness and cognitive performance among younger and older adolescents across a 28-hour forced desynchrony protocol. SLEEP 2015;38(12):1965–1972.
Keywords: forced desynchrony, adolescents, cognitive performance, sleepiness
INTRODUCTION
Homeostatic and circadian factors influence sleep/wake cycles, sleep structure, and waking behavior.1 Laboratory protocols can be used to parse the contributions of homeostatic and circadian factors by forcing the internal circadian system to run free from environmental schedules. In human experiments, the most common of such protocols schedule sleep and wake to occur at various circadian phases, and circadian phases to occur at varying times awake by enforcing a sleep/ wake schedule to which rhythms are unable to synchronize. These protocols are appropriately named forced desynchrony, and they allow for the separation, and thus independent quantification, of the influence of the homeostatic sleep-dependent (Process S) and circadian (Process C) processes on measures acquired during the sleeping and waking episodes.2
Research using forced desynchrony protocols has demonstrated that the two processes contribute differentially to slow wave activity during sleep, REM sleep, and sleep spindles. Process C and Process S work together to consolidate wakefulness into one bout, with Process C increasingly promoting wakefulness as core body temperature rises and peaking in influence at the entrained evening “wake maintenance zone” (about 240° with the circadian trough in core body temperature at 0°).3 The two processes also affect waking mood4,5 and cognitive performance, effects repeatedly observed in laboratory studies.6–14 Even when individuals were awake for fewer hours than the typical 16, declines in cognitive performance have been observed with increased sleep pressure across wake episodes.7,8,12 Similar to the wake maintenance zone, peaks in performance have also been demonstrated at about 240° with the trough in cognitive performance between 0° and 60°.8,9,12,15
Much of our understanding of circadian and homeostatic processes underlying sleep/wake behavior derives from studies of healthy young adults, though some forced desynchrony studies have demonstrated age-related differences in sleep structure16 and cognitive performance between younger (20s) and older (60s) adults.11 Much less is known about these processes in adolescents, especially as they mature.17 We have previously reported data from adolescents under a forced desynchrony protocol in small samples with a focus on period length18 and on sleep propensity alone.19 Evidence from other types of studies indicate that as adolescents mature, both homeostatic and circadian processes change. For example, older adolescents have shown a slower accumulation of slow wave delta power relative to younger,20 though adolescents have shown similar rates of homeostatic pressure decay during sleep even into young adulthood.21,22 Under conditions of extended wakefulness, postpubertal adolescents have demonstrated longer sleep latencies than prepubertal.23 Older adolescents have demonstrated a later circadian phase and also a preference for a later circadian phase, which translate to later preferred bed and wake times.24–28 Finally, lower amplitude in melatonin rhythm has been found in more mature adolescents than less mature.29
Given these developmental changes during adolescence, we propose that homeostatic load and circadian phase may affect sleepiness and performance differently in younger versus older adolescents. If, for example, younger adolescents accumulate homeostatic pressure for sleep faster than older, they may show greater sleep propensity than older adolescents with increasing time awake. In regard to psychomotor performance, faster reaction times are expected for older adolescents due to motor development30–33; it is also plausible that younger adolescents' performance deteriorates more rapidly with increased time awake or is more affected by circadian processes than older.
We measured sleepiness and performance across the waking periods of a 28-h forced desynchrony protocol to assess performance under different levels of homeostatic load and at different circadian phases. Our chief hypotheses were that younger adolescents would demonstrate worse overall reaction time performance, show an accelerated performance decline with time awake, and show a greater amplitude of circadian signal on performance relative to older adolescents.
METHODS
The project was approved by the Institutional Review Boards for the Protection of Human Subjects of Bradley and Lifespan Hospitals. All participants' parent or legal guardian provided written informed consent and participants provided assent. Participants were paid for participation. Data collection occurred during the summer months (June, July, and August) of 1997–2000.
Participants
Twenty-seven adolescents ages 9.6–15.2 years old (mean = 12.8, SD = 1.6 years; 16 females) completed the study. Participants were in good emotional and physical health and had no history of sleep disorders. Because we were interested in potential age-related differences in relation to circadian and homeostatic factors, participants were divided into younger (n = 14 ages 9–12; 9 females) and older (n = 13 ages 13–15; 7 females) age groups based on median age split at 13.0 years (Figure 1).
Figure 1.
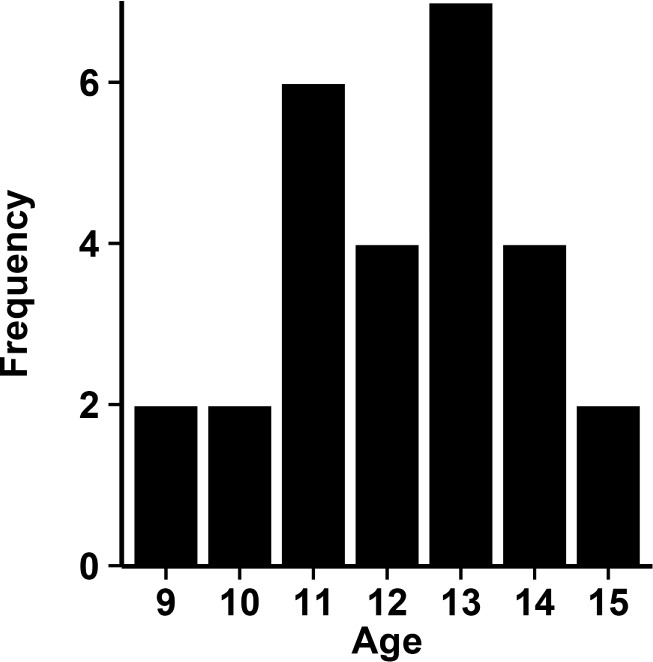
Histogram of the distribution of participant ages. Median age was 13 years and participants were split into younger (9–12) and older (13–15) age groups.
Procedures
Participants kept a 22:00–08:00 time in bed schedule for at least 10 days before completing a 3-week laboratory study. At-home sleep was monitored with written sleep diaries, calls to a time-stamped answering machine every night and morning, and wrist actigraphy (Mini Motionlogger, Ambulatory Monitoring Inc., Ardsley, NY). Participants were instructed to wear eye shades during scheduled sleep.
Upon arrival to the laboratory, participants completed an adaptation night, a 36-h constant routine, a recovery night, 12 cycles of a 28-h forced desynchrony protocol, a second 36-h constant routine, and a final recovery night (Figure 2).
Figure 2.
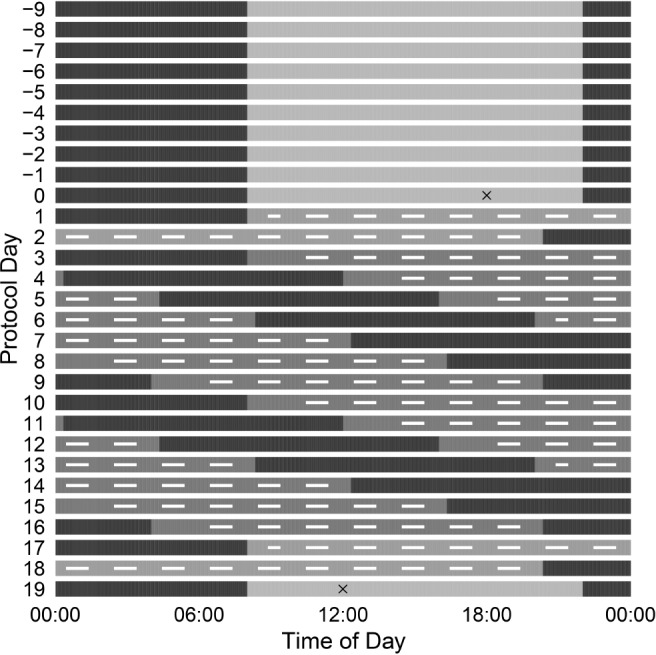
Schematic of the study protocol. Each row is a 24-h day and protocol day is shown on the y axis. Scheduled sleep is shown in black and wake in gray. Entry to and exit from the laboratory portion of the study are indicated with a black “X.” Participants completed 10 nights of fixed bed/wake time at home (days −9 to −1), an adaptation night (day 0), a 36-h constant routine, 12 cycles of a 28-h day forced desynchrony protocol (days 3 to 16), and a second 36-h constant routine. The data discussed here are from the 12 cycles of the 28-h forced desynchrony protocol and the first 15 h of wakefulness in the second constant routine. Testing times are indicated with white dashes.
The current analyses focused on the data collected during the 12 cycles of forced desynchrony and the initial 15 h of the waking period of the second constant routine. Each 28-h cycle offered a 10- to 24-h sleep to wake ratio and thus included 11 h 40 min of sleep opportunity and 16 h 20 min of scheduled wake time. Lights were maintained at < 1 lux during scheduled sleep and < 20 lux during scheduled wake. Participants completed the study in groups of 4. Sleep and testing took place in private bedrooms and free time was spent in common areas. During 2 of the 12 forced desynchrony cycles (numbers 4 and 10) participants completed a “mini constant routine,” where they remained semi-reclined in bed in order to collect circadian data. The testing protocol remained the same during these cycles except participants completed an additional battery of tests about 1 h after wake (described below).
Measures
Saliva samples were collected at 30- to 50-min intervals during waking phases of the study and frozen. Melatonin levels were later determined by radioimmunoassay (Alpco, Windham, NH). Dim light melatonin onset (DLMO) and offset phases were determined by linear interpolation from samples across the protocol using a 4 pg/mL threshold. Each participant's intrinsic circadian period (tau) was computed by linear regression of salivary DLMO measured across forced desynchrony cycles.18 Sleep propensity was measured during nap opportunities as per the multiple sleep latency test (MSLT),34 beginning 2.5 h after wake and administered thereafter every 2 h during the scheduled wake periods for a total of 7 tests per 28-h cycle. Participants were instructed to lie in a dark room with their eyes closed and to try to fall asleep while being measured with standard polysomnography. Each nap opportunity was terminated after unequivocal sleep onset (3 consecutive 30-sec epochs of stage 1 sleep or after the first k complex) or after 20 min if the sleep criteria were not met. Sleep latency of 0–20 min was used as the metric for each test.
A computerized neurobehavioral assessment battery (NAB, from the University of Pennsylvania laboratory of D. F. Dinges) was administered every 2 h during scheduled wake periods, beginning 3 h after scheduled wake time for the majority of forced desynchrony cycles. Two of the forced desynchrony cycles (numbers 4 and 10), and the second constant routine also included a NAB 1 h after scheduled wake time. All NAB included the Karolinska Sleepiness Scale (KSS) as a measure of subjective sleepiness35; response choices range from 1–9 (1 = “extremely alert” to 9 = “extremely sleepy, fighting sleep”). Reaction time was measured with a 10-min visual psychomotor vigilance task (PVT), a task sensitive to the effects of sleep loss.36 The task involves pressing a button as soon as a stimulus is detected with randomly varying presentation rates of 2–10 sec. Participants were introduced to and practiced the PVT-192 (Ambulatory Monitoring Inc., Ardsley, NY) before data were collected. PVT metrics included in this analysis are median reaction time, fastest 10% of reaction times, the slowest 10% of speed (1 / reaction time × 1000), and lapses. Lapses are defined as ≥ 500 ms latency between stimulus presentation and participant response. Participants also completed a processing speed task, the digit symbol substitution task (DSST), during each battery.37 In this task, participants were presented with a legend of 9 symbols paired with a number 1–9 and tasked with matching the symbols with their corresponding number from the legend as accurately and as fast as possible. The number of correct responses in 90 sec was the outcome variable for the analyses in this report.
Data Analysis
Scores were averaged within individuals during the first forced desynchrony day (7 tests) to compare initial raw performance between age groups. The first day was chosen because testing times occurred during normal waking hours and participants were not yet desynchronized. Raw scores were compared with t-tests. The Mann-Whitney U test was used to compare means between age groups on median PVT reaction time and the fastest 10% of reaction times, as these measures were not normally distributed.
Z-scores were computed for each variable for each participant based on raw performance data collected across the repeated forced desynchrony cycles. Homeostatic analyses were performed based on assigning variables to 8, 2-h homeostatic bins based on time elapsed from scheduled wake (homeostatic bins corresponded to approximately 1, 3, 5, 7, 9, 11, 13, and 15 h awake). Circadian analyses used 60° bins based on each participant's first measured DLMO and his or her intrinsic period (each bin = 4 “circadian hours;” melatonin onset = 0°).
Mixed effects modeling was used to examine sleep propensity, subjective sleepiness, and cognitive performance across homeostatic and circadian bins between younger and older adolescents. Models comparing the relative contribution of homeostatic and circadian factors were performed separately for each variable on the participant's transformed z-scores. Z-scored data were used in the models in order to compare individual changes in sleepiness and performance across the circadian and homeostatic time bins. Age group, homeostatic load, and circadian phase were fixed effects, and subject intercept was entered as a random effect to control for individual differences. Models also included interaction effects for homeostatic load × circadian phase, age group × homeostatic load, and age group × circadian phase. Three-way interactions between age group × homeostatic load × circadian phase were tested and none was statistically significant; thus, the final models presented here do not include any 3-way interaction terms. Where significant effects were observed, paired comparisons were conducted with the Bonferroni correction for multiple comparisons. All statistical procedures were carried out with IBM SPSS Statistics for Macintosh, version 19 release 19.0.0 (IBM Corp., Armonk, NY).
RESULTS
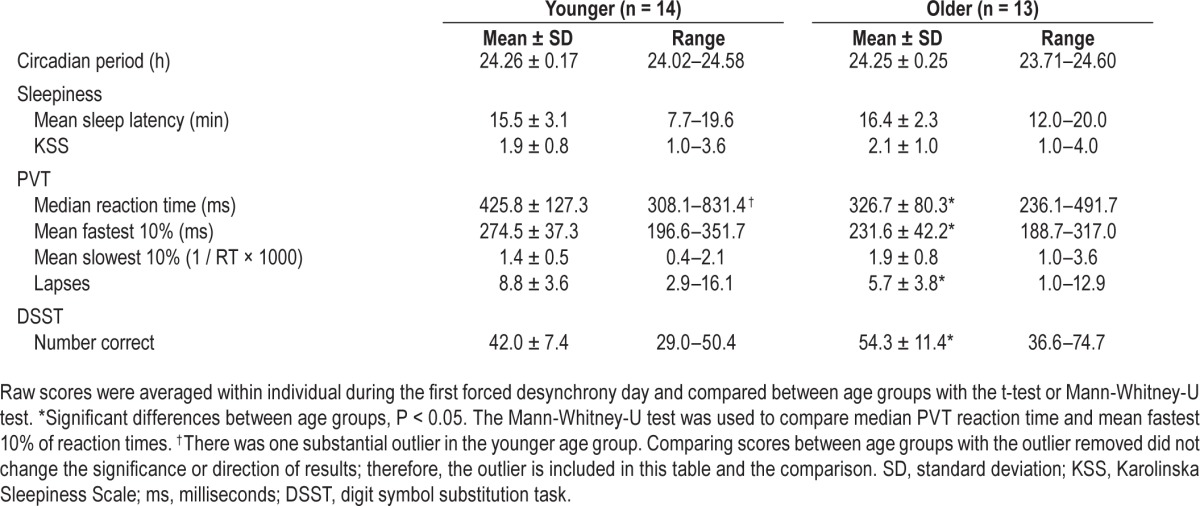
Descriptive and performance score statistics (first averaged within individual) for younger and older age groups on the first forced desynchrony day are presented in Table 1. Younger and older adolescents did not differ in mean sleep latency or subjective sleepiness. On the PVT, the older adolescents demonstrated faster median reaction times, fastest 10% of responses, and fewer lapses. The younger adolescents also had fewer number of correct responses on the DSST.
Table 1.
First day sleepiness and performance scores between younger and older adolescents.
Influence of Process S and Process C
Table 2 shows the results of the models and Figure 3 illustrates the results for sleepiness measures.
Table 2.
Model results.
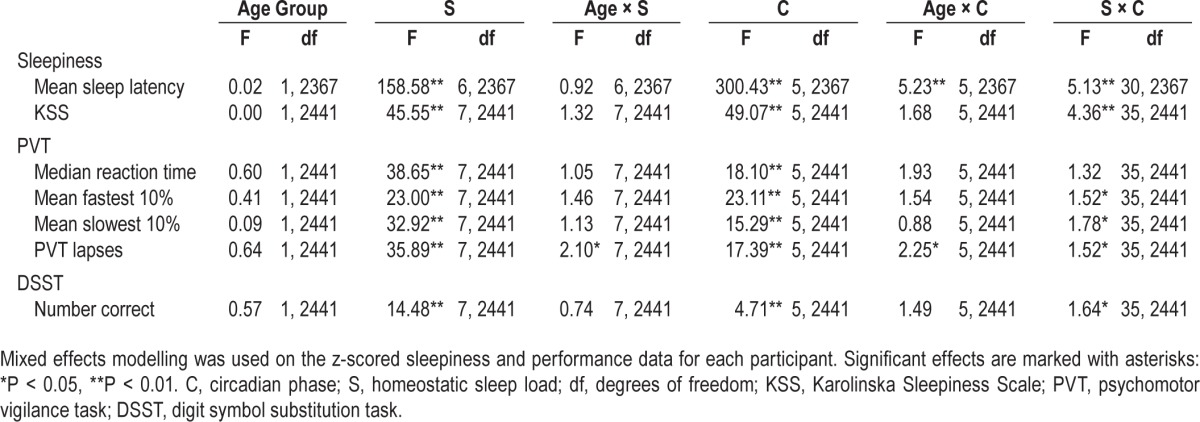
Figure 3.
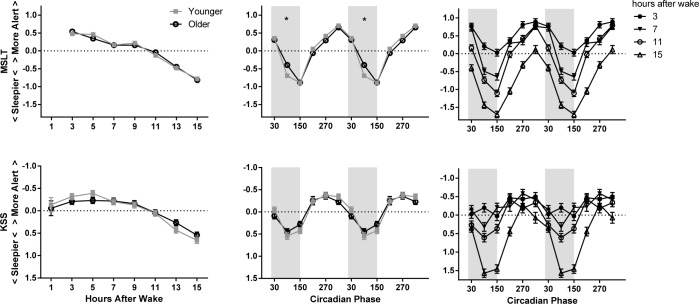
Model-estimated z scores (± standard error) as a factor of homeostatic load (left), circadian phase (middle), and the interaction of homeostatic load and circadian phase (right) on sleepiness measures. The younger adolescent age group is indicated with gray filled squares and the older with black open circles. The circadian data are double plotted to better show circadian variation. Melatonin onset = 0°. Circadian bins are marked with the midpoint (i.e., 30° is DLMO-60°). Shaded areas indicate the approximate at-home baseline sleep schedule. The y axis has been reversed for KSS; higher indicates higher levels of alertness. The significant difference between age groups on the MSLT is marked with asterisks. MSLT, multiple sleep latency test; KSS, Karolinska Sleepiness Scale.
Sleepiness: MSLT and KSS
Age group did not show a significant main effect on sleep propensity (MSLT) or subjective sleepiness ratings, nor were the interactions of age group and homeostatic load statistically significant for either measure. On the other hand, we observed a main effect of homeostatic load on sleep propensity and subjective sleepiness, with participants demonstrating greater sleep propensity and reporting increased sleepiness the longer they were awake (see left side of Figure 3). As expected, participants were also sleepier during the circadian night, as indicated by a significant main effect of circadian phase and shown in Figure 3 by greatest sleepiness late in the circadian night at about 150°. We also found a significant interaction of age group and circadian phase for sleep propensity; the younger group had increased sleep propensity on the MSLT relative to the older, during the early part of the circadian night (90°). A significant interaction between homoeostatic load and circadian phase revealed an increase in the effect of circadian phase on sleep propensity and subjective sleepiness ratings as homeostatic load increased. This effect of time awake was more pronounced during the circadian night and less evident during the circadian day.
Cognitive Performance: PVT and DSST
A significant main effect of time awake occurred for all PVT measures (median reaction time, fastest 10% of responses, slowest 10% of speed, and lapses), and correct responses on the DSST; all measures worsened as time awake increased (see left side of Figures 4 and 5). The rate of performance deterioration with time awake was not significantly different between younger and older adolescents on any measure except for PVT lapses, where younger adolescents showed a steeper overall increase in lapses across the waking period. After controlling for multiple comparisons, performance was significantly different between age groups only between 5 and 7 h awake.
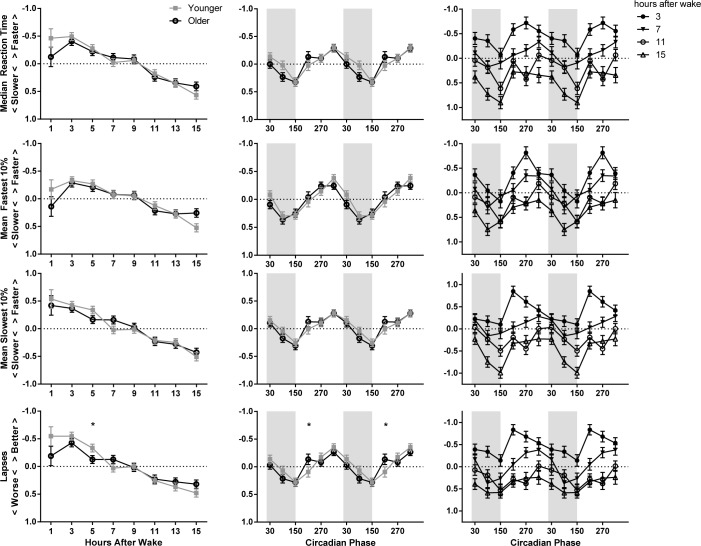
Figure 4.
Model-estimated z scores (± standard error) as a factor of homeostatic load (left), circadian phase (middle), and the interaction of homeostatic load and circadian phase (right) on PVT performance. The younger adolescent age group is indicated with gray filled squares and the older with black open circles. The circadian data are double plotted to better show circadian variation. Melatonin onset = 0°. Circadian bins are marked with the midpoint (i.e., 30° is DLMO-60°). Shaded areas indicate the approximate at-home baseline sleep schedule. The y axes have been reversed for median reaction time, the fastest 10% of reaction times, and lapses; higher indicates better PVT performance. The significant difference between age groups on PVT lapses are marked with asterisks. Post hoc comparisons were only performed on significant interaction terms.
Figure 5.
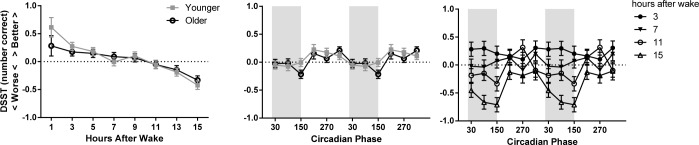
Model-estimated z scores (± standard error) as a factor of homeostatic load (left), circadian phase (middle), and the interaction of homeostatic load and circadian phase (right) on DSST performance. The younger adolescent age group is indicated with gray filled squares and the older with black open circles. The circadian data are double plotted to better show circadian variation. Melatonin onset = 0°. Circadian bins are marked with the midpoint (i.e., 30° is DLMO-60°). Shaded areas indicate the approximate at-home baseline sleep schedule. Higher scores indicate more correct responses. DSST, digit symbol substitution task
The middle panel of Figures 4 and 5 displays a significant main effect of circadian phase for all PVT measures and DSST correct responses, with the best performance occurring during the circadian afternoon and early evening (240–360°) and the worst during the circadian night (60–180°). Circadian phase also interacted with age group for PVT lapses: older adolescents showed a relative improvement in lapses during the 210° bin, whereas younger adolescents did not show this improvement. The interaction of circadian phase and age group was not significant for any other PVT metric.
Finally, we observed a significant interaction of homeostatic and circadian factors for most PVT measures and DSST performance, though the magnitude and timing of the effect differed among measures. In general, better scores were observed at all circadian phases with low homeostatic loads (e.g., 3–7 h since waking), and the impact of circadian phase was greater with a higher homeostatic load (see right panel of Figures 4 and 5).
DISCUSSION
In this study we aimed to better understand the effect of the homeostatic and circadian processes on sleepiness and performance in adolescents. Previous research has shown developmental changes in the accumulation of homeostatic sleep pressure and circadian physiology across adolescence. Using a 28-h forced desynchrony protocol, we observed sleepiness and performance in younger (< 13) and older (≥ 13) age groups. Our analyses focused on exploring differences in the effect of the homeostatic and circadian processes between the two age groups.
As expected, older adolescents' raw scores on measures of reaction time and cognitive performance were better than those of the younger before desynchrony (during protocol day 3). Older adolescents were faster than the younger on the PVT median reaction time and fastest 10% of reaction times, demonstrated fewer lapses, and answered more items correctly on the DSST. Once data were normalized with z-scores, however, we did not observe a main effect of age group on any performance metric.
Homeostatic load affected both sleepiness and performance variables, with younger and older adolescents' sleepiness increasing and performance worsening as time awake increased. The increase in sleepiness was commensurate to our previous report of sleep latency in adolescents.19 The effect of the homeostatic drive was evident despite the limit of 16 h 20 min to the waking day, with the last sleep latency and performance bout scheduled to occur after about 15 h of wakefulness. This general decline in performance as time awake increased is similar to previous reports from adults during 20-h,8,11,38 28-h,6,7,9,12,15 and 43-h forced desynchrony protocols.14
The interaction of age group and homeostatic load showed no significant differences in the effect of the homeostatic process for sleep propensity and subjective sleepiness as a function of age. Thus, these results do not support the finding that younger adolescents accumulate homeostatic pressure faster than older,20 at least as homeostatic pressure manifests in sleep latencies across a 15-h waking episode. In a previous study, we showed a lower sleep tendency in post-pubertal versus pre-pubertal adolescents but only after homeostatic loads of 14.5 to 16.5 h.23 Thus, our current protocol may have included too short a waking day for this interaction to emerge with sleep latency. On the other hand, the significant interaction between age group and homeostatic load for PVT lapses could indicate a difference in the rate of accumulation of homeostatic-related performance decrement across time awake: younger adolescents showed more variation in performance and a steeper overall increase of PVT lapses across 1 to 15 h awake than older. However, the interaction of age group and homeostatic load was not significant for any other PVT performance measure, nor for the DSST. Overall, the evidence does not support the hypothesis that younger adolescents accumulate homeostatic pressure faster than older for these measures. Again, the final sleep latency and performance testing occurred after 15 h of wakefulness during each forced desynchrony cycle.
Similar to our previous report19 and findings from forced de-synchrony studies in adults, adolescents' sleep latencies were shortest during the circadian night (150°, approximately 8–12 h after DLMO) and longest during the circadian day (330°, approximately 0–4 h before DLMO).3,8,38,39 Subjective sleepiness also varied in a manner consistent with reports of subjective sleepiness and alertness in adult studies.7–11 Comparison of the raw scores for both subjective sleepiness and sleep latencies showed that the younger adolescents reported overall higher levels of subjective sleepiness and generally demonstrated greater sleep propensity than the older. We observed a significant interaction of age group and circadian phase for sleep propensity, though this interaction was not significant for subjective sleepiness. Younger adolescents showed a significantly greater sleep propensity around 90°, during the early-mid circadian night approximately 4–8 h after DLMO, expressing faster sleep onset. We previously reported that younger adolescents demonstrate greater amplitude of melatonin rhythm than older29; that circadian phase had a greater effect on sleep propensity in the younger adolescents supports this notion.
Both age groups also showed the expected circadian modulation of cognitive performance, with best performance during the circadian day and into the early night and worsening performance later during the circadian night and into the early morning. This finding is consistent with previous reports of circadian modulation of attentional performance stability in teenaged females40 and of reaction time and other cognitive task performance in adults.6–15
Older adolescents showed a reduced number of lapses relative to the younger adolescents immediately following the circadian trough. This finding may indicate that older adolescents are better able to maintain performance through the circadian night, which may correspond to their increased evening phase preference.24–28 This finding should be interpreted with caution, however, as the lapse criterion was set to 500 ms, and the younger adolescents' median raw reaction time was 450 ms. Thus, an age-adjusted lapse criterion may be more appropriate for younger children and adolescents.41
Our final set of analyses examined the interactions of homeostatic and circadian factors on our outcome measures. We observed statistically significant interactions for sleepiness and most performance metrics. Others have reported similar significant interactions for alertness ratings,8,42 and some previous reports noted an interaction on performance metrics,6,12,42,43 although not all performance measures exhibit such an interaction.8,15 Our data show increased circadian modulation of sleepiness and performance as time awake increases (see Figures 3–5). Of interest was our observation of a strong circadian effect on performance metrics after just 1 h awake, likely an effect of sleep inertia. Indeed, studies of adults show a similar effect of sleep inertia as a function of circadian phase.44,45 More in-depth examination of wake-time performance in adolescents at different circadian phases would be both informative and relevant to early morning school performance. Our finding of strong circadian modulation at wake time could also be an artifact of unstable estimates. Each participant completed a performance battery after 1 h awake on only 3 occasions (protocol days 6, 13, and 17). Thus, the estimates for the 1-h homeostatic bin are less precise than those for the other homoeostatic bins where participants contributed 13 measurements each. When the analyses were completed excluding the 1-h awake bin, the trends and significant results were unchanged. Therefore, we included data from all of the homoeostatic bins in our analytical models.
The study design allowed for repeated measures over time with multiple observations per participant in each homeostatic and circadian bin. However, the small sample size in each group is a possible limitation to our study.
In summary, adolescents generally behave similarly to adults during forced desynchrony both in terms of sleepiness and cognitive performance. Sleepiness, measured subjectively and with a physiologic measure, and performance were affected by homeostatic and circadian factors. Age group did not interact with homoeostatic and circadian factors for subjective sleepiness or most performance metrics, though raw scores before desynchrony established overall greater sleepiness and poorer performance in the younger adolescents. We also found evidence that younger adolescents had a greater sleep propensity (measured by sleep latency) during the circadian night. Finally, we observed homeostatic and circadian interactions for sleepiness and most performance metrics, but this was not significantly affected by age group, and we found no significant interaction of homeostatic load, circadian bin, and age group on sleepiness or performance.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by the NIH National Institute of Mental Health MH52415, MH01358, and MH076969 awarded to Dr. Carskadon. Data were collected and analyzed at the E.P. Bradley Hospital Sleep and Chronobiology Research Laboratory. Dr. Acebo is employed by Jazz Pharmaceuticals. Her work on this study preceded this employment. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants, laboratory staff, and Dr. David Barker for statistical guidance.
REFERENCES
- 1.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Czeisler CA, Allan JS, Kronauer RE. A method for assaying the effects of therapeutic agents on the period of the endogenous circadian pacemaker in man. In: Montplaisir J, Godbout R, editors. Sleep and biological rhythms: basic mechanisms and applications to psychiatry. New York: Oxford University Press; 1990. pp. 87–98. [Google Scholar]
- 3.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 5.Murray G, Nicholas CL, Kleiman J, et al. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Ferguson SA, Matthews RW, et al. Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep. 2011;34:931–41. doi: 10.5665/SLEEP.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 9.Wright KP, Jr., Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 10.Hull JT, Wright KP, Jr., Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003;18:329–38. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- 11.Silva EJ, Wang W, Ronda JM, Wyatt JK, Duffy JF. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep. 2010;33:481–90. doi: 10.1093/sleep/33.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Wang W, Silva EJ, et al. Neurobehavioral performance in young adults living on a 28-h day for 6 weeks. Sleep. 2009;32:905–13. doi: 10.1093/sleep/32.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 14.Grady S, Aeschbach D, Wright KP, Jr., Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacol. 2010;35:1910–20. doi: 10.1038/npp.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Ferguson SA, Matthews RW, et al. Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep. 2011;34:57–63. doi: 10.1093/sleep/34.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–32. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- 19.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–14. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 20.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 21.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 22.Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–77. doi: 10.1016/j.neuroscience.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–44. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 25.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 26.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 27.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 28.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 29.Crowley SJ, Acebo C, Carskadon MA. Human puberty: salivary melatonin profiles in constant conditions. Dev Psychobiol. 2012;54:468–73. doi: 10.1002/dev.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Dev Med Child Neurol. 1973;15:635–45. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- 31.Largo RH, Caflisch JA, Hug F, et al. Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol. 2001;43:436–43. doi: 10.1017/s0012162201000810. [DOI] [PubMed] [Google Scholar]
- 32.Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114:1662–70. doi: 10.1016/s1388-2457(03)00130-5. [DOI] [PubMed] [Google Scholar]
- 33.Gasser T, Rousson V, Caflisch JON, Jenni OG. Development of motor speed and associated movements from 5 to 18 years. Dev Med Child Neurol. 2010;52:256–63. doi: 10.1111/j.1469-8749.2009.03391.x. [DOI] [PubMed] [Google Scholar]
- 34.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 35.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 36.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 37.Weschler D. Weschler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 38.Van Reen E, Rupp TL, Acebo C, Seifer R, Carskadon MA. Biphasic effects of alcohol as a function of circadian phase. Sleep. 2013;36:137–45. doi: 10.5665/sleep.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt JK, Dijk DJ, Ritz-de Cecco A, Ronda JM, Czeisler CA. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 40.Valdez P, Ramírez C, García A, Talamantes J, Cortez J. Circadian and homeostatic variation in sustained attention. Chronobiol Int. 2010;27:393–416. doi: 10.3109/07420521003765861. [DOI] [PubMed] [Google Scholar]
- 41.Fallone GP, Carskadon MA. PVT performance among children in comparison to adults. Sleep. 2002;25:A433. (Abstract Suppl) [Google Scholar]
- 42.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–81. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 43.Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva EJ, Duffy JF. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neurosci. 2008;122:928–35. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]