Abstract
A workshop was held at the National Institute for Diabetes and Digestive and Kidney Diseases with a focus on the impact of sleep and circadian disruption on energy balance and diabetes. The workshop identified a number of key principles for research in this area and a number of specific opportunities. Studies in this area would be facilitated by active collaboration between investigators in sleep/circadian research and investigators in metabolism/diabetes. There is a need to translate the elegant findings from basic research into improving the metabolic health of the American public. There is also a need for investigators studying the impact of sleep/circadian disruption in humans to move beyond measurements of insulin and glucose and conduct more in-depth phenotyping. There is also a need for the assessments of sleep and circadian rhythms as well as assessments for sleep-disordered breathing to be incorporated into all ongoing cohort studies related to diabetes risk. Studies in humans need to complement the elegant short-term laboratory-based human studies of simulated short sleep and shift work etc. with studies in subjects in the general population with these disorders. It is conceivable that chronic adaptations occur, and if so, the mechanisms by which they occur needs to be identified and understood. Particular areas of opportunity that are ready for translation are studies to address whether CPAP treatment of patients with pre-diabetes and obstructive sleep apnea (OSA) prevents or delays the onset of diabetes and whether temporal restricted feeding has the same impact on obesity rates in humans as it does in mice.
Citation:
Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, Hanlon EC, Keene AC, Joyner MJ, Karatsoreos I, Kern PA, Klein S, Morris CJ, Pack AI, Panda S, Ptacek LJ, Punjabi NM, Sassone-Corsi P, Scheer FA, Saxena R, Seaquest ER, Thimgan MS, Van Cauter E, Wright KP. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. SLEEP 2015;38(12):1849–1860.
Keywords: circadian disruption, circadian rhythm, diabetes, insulin resistance, metabolism, obesity, short sleep, sleep apnea, sleep disorders
INTRODUCTION
There is increasing evidence that inadequate sleep (for reviews, see1,2) and specific sleep disorders such as OSA (for reviews, see3,4) are independent risk factors for metabolic abnormalities such as insulin resistance and hyperglycemia. The sleep research community has also advanced our knowledge of possible mechanisms for the effects of inadequate sleep, obstructive sleep apnea, and other sleep disorders on metabolic function. In parallel to these advances, there has been substantial progress in studies of the role of central and peripheral clocks in metabolic control and regulation of metabolism in basic and clinical science.
Thus, it seemed timely to bring together experts in metabolism with experts in sleep and circadian research to assess our current knowledge and identify new opportunities for research. To accomplish this goal, the National Institute of Diabetes and Digestive and Kidney Diseases held a two-day workshop on “Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes.” The workshop was held at NIH in Bethesda on February 19–20, 2015. Invited participants gave presentations on their area of expertise. These presentations are summarized in this manuscript. This manuscript is not intended as a comprehensive review but rather a summary of the proceedings at the workshop. In addition, the Sleep Research Society organized a competitive process for travel awards for early-stage investigators to attend the workshop. The early-stage investigators participated in discussions, presented posters and did the initial draft of this white paper. These individuals represent the future for investigation in this area and it is critical that they are involved in identification of gaps and obstacles in this scientific area.
A particular goal of the workshop was to identify opportunities for translational research. Each invited participant discussed and presented their views on opportunities and needs for scientific development. These have been coalesced and are presented at the end of this document.
SESSION I: SLEEP DISRUPTION AND SLEEP DISORDERS: EFFECTS ON METABOLIC DISEASE
Dr. Eve Van Cauter began the workshop with an overview of the literature surrounding the associations between sleep disturbances, circadian dysfunction and diabetes risk, which has garnered both public and scientific interest. In a seminal study, by Spiegel and colleagues,5 the effect of short sleep on diabetes risk was examined utilizing an intravenous glucose tolerance test following either 5 nights of chronic partial sleep loss (4 h/night) or 5 nights in the rested condition (12 h/night). Remarkably, insulin sensitivity as well as disposition index, a marker of diabetes risk, were significantly decreased in the sleep loss condition. Impaired glucose metabolism has been observed in subsequent experimental studies with varying degrees of sleep loss.2,6–10 A recent paper revealed that sleep restriction has an impact on the molecular mechanisms in peripheral tissues, specifically adipocytes.8 In a randomized crossover design, subcutaneous fat biopsy samples were collected from 7 healthy lean participants following either 4 days of 4.5 h/night or 8 h/night of sleep in the laboratory. The isolated adipocytes were treated with increasing concentrations of insulin and measured for levels of phosphorylated Akt (pAkt), a crucial step in the insulin-signaling pathway. Following 4 nights of sleep deficiency, adipocytes exhibited a 30% reduction in insulin sensitivity as compared to those collected following 4 nights of normal sleep.8 Thus, to elicit the same pAkt response observed following normal sleep, higher concentrations of insulin were necessary in adipocytes following 4 nights of restricted sleep. The 30% reduction in insulin sensitivity observed in this study is similar to that seen in individuals with diabetes compared to controls11 and in obese individuals when compared to lean individuals.12 The direct action of sleep deficiency on peripheral adipose tissue may affect energy metabolism via alterations to insulin sensitivity within adipocytes.
Multiple studies have also recently been initiated to explore whether sleep extension and/or improvement in sleep quality will benefit glucose tolerance. Individuals who curtailed bedtimes on a regular basis were studied under habitual sleep conditions and following 6 weeks of sleep extension. On average, the participants extended sleep time by approximately 45 min during weekdays with no change on the weekends.9 As sleep time increased, particularly the amount of stage N2, the Homeostatic Model Assessment (HOMA) values (a marker of insulin resistance) decreased, while the Quantitative Insulin Sensitivity Check Index (QUICKI),13 a marker of insulin sensitivity, increased. Taken together, the current body of evidence indicates that sleep restriction has a detrimental effect on insulin sensitivity while sleep extension in restricted sleepers may actually improve glucose tolerance.
Circadian disturbance may also affect glucose metabolism. Low nocturnal melatonin secretion has been associated with increased diabetes risk and variants in the melatonin receptor that result in reduced function are associated with elevated glucose levels and increased risk of diabetes.14–16 In a study of 370 women, low melatonin secretion was independently associated with a higher risk of developing type 2 diabetes.17 When the circadian system is misaligned, desynchrony between the central and peripheral clocks occurs and glucose metabolism is adversely affected.18 Under a forced desynchrony protocol, when subjects slept and ate approximately 12 h out of phase relative to their endogenous circadian time conditions, an exaggerated postprandial glucose response was observed, which resulted in an overall increase in glucose levels.19 Most interestingly, glucose levels were elevated despite a concurrent rise in insulin levels, suggesting a decrease in insulin sensitivity resulting from circadian misalignment. In a recent laboratory study, when sleep restriction and circadian misalignment were combined, the reduction in insulin sensitivity in the male participants was almost twofold higher than that observed with nocturnal sleep restriction alone.20 Lastly, it has been reported that chronotype is associated with altered glycemic control in patients with type 2 diabetes.21 Those with a later chronotype, associated with a greater degree of circadian misalignment, had higher HbA1C levels after controlling for multiple confounders. Taken together, these data suggest that circadian misalignment adversely affects glucose metabolism, and furthermore that combining circadian misalignment with sleep restriction, a common occurrence during shift work, can exaggerate this effect.
In summary, there is a body of experimental evidence in support of a causal relationship between sleep and circadian disturbances and increased metabolic risk. Proof of concept studies examining whether optimizing sleep and/or circadian function can reduce the risk or severity of diabetes have only been recently initiated by a number of independent investigators. The next few years will see the findings from these efforts. Molecular mechanisms linking insufficient sleep, circadian dysfunction and metabolism remain largely unexplored. Of note, studies using animal models to examine metabolic consequences of circadian disruption rarely control for sleep duration or quality. Conversely, the vast majority of clinical diabetes research ignores sleep duration, sleep quality, presence of OSA and circadian phenotype as confounding predictors of diabetes risk and severity. Clinical trials for diabetes prevention or treatment should include assessment of habitual sleep duration and quality as well as exposure to circadian disruptors such as light at night, shift work and social jet lag. Individual differences in response to sleep restriction or/and sleep extension need to be explored. The feasibility and modalities for optimizing sleep and circadian function as novel behavioral interventions to prevent or treat diabetes are areas of opportunity.
OBSTRUCTIVE SLEEP APNEA AND DIABETES: CLINICAL STUDIES AND MECHANISMS
Dr. Allan Pack provided an overview of the data linking OSA and diabetes from both clinical and mechanistic perspectives. Obesity is a major risk factor that plays an important role in many proposed pathways. OSA is increasingly prevalent, as indicated by a recent report from the Wisconsin Sleep Cohort that estimated the current prevalence among those age 50–70 to be 17.4% in men and 9.1% in women, compared to previous data that suggested rates of 13.9% in men and 7.4% in women.22
Those with moderate to severe OSA have much higher levels of insulin resistance, even after controlling for the degree of adiposity.23,24 A recent meta-analysis showed that CPAP treatment improves insulin resistance in patients with sleep apnea without diabetes.25 This is consonant with a meta-analysis by Yang and colleagues that showed similar results in observational studies.26 Botros and colleagues showed that those with OSA are more likely to develop diabetes.27 This report was included in a subsequent meta-analysis that showed that OSA is associated with an overall odds ratio of 1.6 for incident diabetes. Further, use of CPAP may reduce the observed diabetes risk.27 This is in the context of studies that show that there is a very high prevalence of OSA in patients with type 2 diabetes28 and that OSA severity is associated with increasing HbA1c.29
OSA leads to both chronic intermittent hypoxia and disturbances in sleep continuity (sleep fragmentation and arousals). These, in turn, are associated with oxidative stress (and reactive oxygen species), increased activity in the nuclear factor κB (NF-κB) pathway, and sympathetic activation. These physiologic changes can interact and lead to a number of downstream changes, including HIF-1α activation,30 elevated adhesion molecules,31 elevated adipokines,32 and increased free fatty acids.33 Cyclical intermittent hypoxia (CIH), akin to that occurring in sleep apnea, can be simulated in animal models.34 This has been used to demonstrate that CIH impairs glucose homeostasis.35 Prabhakar and colleagues36 have described molecular pathways by which chronic intermittent hypoxia leads to increases in HIF-1α and pro-oxidants as well as decreases in HIF-2α and antioxidants by a calpase enzyme mechanism. The resulting activation of the carotid body results in increased catecholamines due to adrenal medullary and sympathetic neuronal activation. This is supported by data from Shin and colleagues, who showed that the effects of intermittent hypoxia on glucose tolerance are blocked by carotid sinus nerve sectioning.37 There are also data suggesting a link between cyclical intermittent hypoxia and β-cell function.38 An important caveat is that these studies largely use extreme hypoxia that is not relevant for the vast majority of patients. The effects of cyclical intermittent hypoxia in mice on lipids are only seen at very severe levels of hypoxia.39 Thus, there is a need for studies with cyclical intermittent hypoxia that study the effects of less extreme levels of hypoxia that are relevant to clinical patients.
Intermediate pathways and other factors triggered by intermittent hypoxemia may contribute to insulin resistance, hypertension, and cardiovascular disease. Not only is obesity also associated with these same changes, but it is associated with sleep apnea as well. This represents a complex association whereby the role of CPAP in cardiometabolic risk may be moderated by obesity and/or weight loss.40–43
In addition to intermittent hypoxia, sleep fragmentation may itself lead to diabetes risk. Sleep fragmentation can be induced in mice with minimal effects on total sleep duration. Sleep loss induces the unfolded protein response in brain.44 Wang and colleagues found increases in food intake and body weight in mice over 8 weeks of sleep fragmentation.45 Sleep disruption leads to increased endoplasmic reticulum stress in the hypothalamus with an upregulation of expression of a number of molecular chaperones.46 There is also an increase in protein-tyrosine phosphatase 1B (PTP1B), which can lead to attenuation in leptin receptor signaling.46 As leptin is a key molecule in signaling pathways that control satiety,47,48 this could potentially explain a connection between sleep fragmentation and food intake. NADPH oxidase 2 (NOX2) enzyme, which results in production of reactive oxygen species, may be another potential pathway linking sleep fragmentation with diabetes.49 Using NOX2 KO mice, Zhang and colleagues found that alterations in glucose tolerance, induced by sleep fragmentation, were mediated by NOX2.50
Dr. Naresh Punjabi continued the discussion about OSA and diabetes risk. He reviewed and cited studies that reported an association between OSA and metabolic dysfunction. Following this, Dr. Punjabi discussed randomized controlled trials that have investigated the impact of CPAP treatment on metabolic outcomes in OSA patients. The majority of the reported studies are small, from clinic-based samples and vary with respect to the extent of CPAP adherence and the type of measures for assessing metabolic dysfunction (e.g., fasting glucose, HbA1c, oral glucose tolerance test). These methodological differences result in conflicting data on the efficacy of CPAP treatment for metabolic disorders in OSA patients. Dr. Punjabi also presented his data from animal models demonstrating that 7 days of intermittent hypoxia decreases glucose tolerance, β-cell function, and insulin sensitivity and increases pancreatic lipid peroxidation in mice.35 Exposing the mice subsequently to 7 days of normoxia did not reverse the effects of intermittent hypoxia on glucose tolerance, β-cell dysfunction or pancreatic lipid peroxidation. These results suggest that intermittent hypoxia may cause irreversible metabolic effects, underscoring the potential importance of early intervention in OSA. Dr. Punjabi concluded that much more research is needed to investigate the role OSA in metabolic dysfunction. Specifically, large, multi-centered clinical trials are needed to investigate the impact of OSA treatment on metabolic outcomes such as whether treatment of patients with OSA with CPAP therapy can induce clinically significant improvements in insulin sensitivity and glucose tolerance. A particular area of opportunity is to determine whether CPAP treatment in patients with OSA and pre-diabetes can reduce the incident rate of diabetes. Observational data27 suggest that this is so but a randomized trial is required to assess this definitively. A recent laboratory-based study has shown improvement in glucose metabolism with CPAP in OSA patients with pre-diabetes.51 In the context of such studies, it is important to examine the possible effects of OSA severity and other patient-related factors such as age on treatment-related improvements in metabolic function. Moreover, studies need to account for duration of metabolic dysfunction in the assessment of whether CPAP has less favorable effects in those with longstanding disease.
SESSION II: NEURAL REGULATION OF ENERGY BALANCE, SLEEP, AND CIRCADIAN RHYTHMS
The talks in this section focused on several interrelated themes and approaches that could be applied to the challenges in this area of research. Dr. Joel Elmquist focused on hypothalamic control of metabolism and feeding, particularly on the role of leptin and POMC neurons, and novel tools that can be used to probe these functions. Dr. Etienne Challet discussed the importance of incorporating diurnal species into the investigation of circadian effects on metabolic function, and also emphasized that many metabolic changes observed in circadian disruption are similar to those observed in aging. Finally, Dr. Paul Franken proposed the novel hypothesis that clock genes may be an important common pathway by which both circadian disruption and sleep disruption exert effects on metabolic processes.
The overall focus of Dr. Elmquist's talk was on the role of leptin signaling in chemically identified neurons thereby providing increased resolution of the neural circuits that mediate feeding and satiety. He discussed tools that can be used to dissect the hypothalamic feeding circuits that include neurons expressing neuropeptide-Y (NPY), agouti-related peptide (AgRP), and pro-opiomelanocortin (POMC).52 Dr. Elmquist presented the current prevalent model of energy balance by which AgRP stimulates food intake and alpha melanocyte stimulating hormone (α-MSH) decreases food intake by acting through second order neurons in the hypothalamus. He then explained how leptin can modulate this system by increasing the activity of α-MSH neurons and decreasing the activity of AgRP cells. Given the opposite effects of leptin (primarily produced by adipocytes) on food consumption through α-MSH and AgRP neurons, analysis of these circuits is difficult. Thus, Dr. Elmquist described efforts to modulate the activity of the leptin receptor (LepR) in specific sub-types of neurons using Cre-lox approaches, and the insights that have followed.53,54 Coupling this approach with inducible CRE recombination activated by a tamoxifen-specific estrogen receptor further provides temporal control. To emphasize the complexity of interactions at POMC neurons in the arcuate nucleus of the hypothalamus, Dr. Elmquist showed that serotonin (5-HT), acting through multiple receptors, can have differential effects on cells in the circuit, thus producing specific and separable responses.
Dr. Elmquist's elegant work illustrated that signaling within the hypothalamic feeding circuit is nuanced, and sophisticated approaches using genetic targeting of chemically identified cells, as well as temporal control using conditional repression, can be leveraged to better understand the system. Even when the phenotype of the neurons involved is identified, further complexity is caused by differential outcomes driven by specific receptor activations; in the case of arcuate POMC neurons, 5-HT signaling through one of many receptors can have different outcomes on both physiology and feeding behavior. Thus, both developing new tools to refine the targeting to specific neuron types, as well as targeting specific intracellular signaling cascades is an important approach to more fully dissect the role of the hypothalamus in feeding and metabolic function.
Dr. Etienne Challet presented work on how diurnal and nocturnal species differ in their circadian organization and responses to metabolic challenges.55 Despite behavior being sequestered to different times, diurnal and nocturnal mammals share a conserved core oscillator. Peak Period1 expression in the SCN and circulating melatonin concentration, for example, occur at the same time of day in both groups. Other outputs of the clock are out of phase between diurnal and nocturnal organisms, including adrenal glucocorticoid secretion, leptin concentration, and behavioral state. Utilizing this dichotomy in central-peripheral phase angle between diurnal and nocturnal animals may contribute to understanding entrainment mechanisms. This could be particularly important in the study of metabolism, because in both diurnal and nocturnal species, light cues, food cues, and sleep debt all converge on peripheral organs but with differential effects.
Dr. Challet's work has focused on the inter-relationships among metabolic disturbances, circadian disruptions, and sleep debt. Dr. Challet's group looked at the effect of normo-caloric and hypocaloric ultradian feeding. Timed hypocaloric feeding shifts molecular circadian rhythms in the suprachiasmatic nucleus. In diurnal rodents, the effect is to phase delay the rhythm while in nocturnal animals it leads to the opposite—a phase advance.55 High fat diets disturb circadian rhythms, flattening the day-night pattern of food intake and lengthening the free-running period in constant darkness.56,57 Phase shifting and re-entrainment are also attenuated, implicating an effect on the SCN. Sleep debt also affects circadian rhythms and metabolic function and can have opposite effects in diurnal versus nocturnal rodents. For example, sleep deprivation and caffeine reduce phase shifts in nocturnal rodents and increase phase shifts in diurnal rodents.58,59 These manipulations also impair glucose tolerance in nocturnal rodents. Given these opposite effects in diurnal and nocturnal rodents, it is critically important to determine whether metabolic effects of sleep disturbances are the same or different between the two groups. Dr. Challet introduced the term “chronobesity” for the increased body weight and impaired insulin secretion commonly caused by circadian disruption.60,61
In closing, Dr. Challet addressed two important concepts: (1) diurnal and nocturnal animals share some features, but responses to diet and sleep differ. Diurnal animals are, therefore, important chronobiological tools with great translational potential62; and (2) impaired metabolism caused by circadian disruption may signal accelerated aging, and can be studied in diurnal species. For example, circadian-disrupted grass rats exhibit shorter telomeres and impaired glucose responses intermediate between the glucose tolerance found in young and old animals.63
Dr. Paul Franken stressed the independent and combinatorial contributions of the circadian clock and the sleep-wake cycle on physiological and genomic rhythms. This is an important complement to the investigation of these separate effects in humans under conditions of forced desynchrony (see Session V, below).
Physiology and behavior that remains rhythmic in constant conditions are often ascribed to an effect of the SCN clock; but other contributing factors such as sleep have been less well investigated. Twenty years ago, however, Dr. Franken showed that > 80% of the variation in daily rhythms of body temperature in the rat can be attributed to the sleep/wake cycle.64 In his talk, he presented his current systems biology approach to these questions. To determine the extent to which sleep and wake affect the transcriptional regulation of circadian rhythms, the transcriptome was examined in mice that were sleep deprived for 6 hours prior to sacrifice. Of ∼2,000 genes that cycle in whole brain, only ∼400 remained rhythmic in sleep deprived mice.65 Similar effects of sleep disruption have now been reported in human blood as well.66,67 This is not surprising given that the sleep/wake cycle affects temperature, metabolism, inflammatory function, redox state, stress, and even light exposure, all of which can directly alter gene expression.
Genes can therefore be classified as being regulated by the circadian cycle only (Bmal1), the sleep-wake cycle only (Homer1a)65, or by both (Per2).64,68 Per2 is of particular interest as a core clock gene that is also sensitive to sleep. In vivo imaging of Per2 using the Per2::Luciferase knockin mouse69 shows that sleep deprivation increases Per2 expression. The duration of the sleep deprivation effect depends on tissue; in liver, Per2 expression remains significantly elevated 6 hours after the intervention, even after expression has normalized in brain and kidney.70 Ultimately, the effects of sleep on gene expression rhythms depend on the gene, its location, and the time of the day.68,70–74
The change in expression across much of the genome may indicate that sleep alters the chromatin landscape. Bmal1 binding to E box elements varies with time of day in many tissues, and 6 hours of sleep deprivation is sufficient to reduce BMAL1 binding to the promoter regions of Per2 as well as other genes.74 This is an important observation, since Bmal1 has thousands of binding sites within the genome.75
Sleep is controlled by both a circadian process and a homeostatic process76 Given that sleep and waking have effects on core clock genes as part of their system-wide genetic effects, it may be that clock genes represent a common pathway by which both sleep disruptions and circadian disruptions are linked to their effects on energy metabolism.
SESSION III: MECHANISMS OF SLEEP AND CIRCADIAN DISRUPTION ON METABOLISM
Understanding how disrupting sleep and circadian behavior regulates metabolic function will require elucidating the molecular intersections between metabolic pathways and sleep and molecular clocks. In this section, three speakers described studies utilizing animal models to investigate the molecular links between the circadian clock and nutritional state. These studies highlight the utility of animal models ranging from fruit flies to rodents in discovery-oriented science, and the importance of tissue specific analysis to dissect the relationship between circadian pacemaker neurons and metabolic function.
While it has long been known that circadian clocks in the brain drive feeding rhythms and these in turn impact liver clocks, much less is understood about how the clock regulates the physiology of other peripheral tissues. Work from Dr. Joe Bass reveals that global disruption of the circadian system through a mutation in the canonical circadian transcription factor, CLOCK, display a loss of rhythmic feeding and a host of diabetes and obesity-related disorders, including increased visceral adiposity and reduced glucose tolerance in mice.77 To explore the peripheral effects of circadian rhythms in pancreatic β-cells, Dr. Bass' group performed adult-specific ablation of BMAL1, in β-cells in mice that results in hyperglycemia and hypoinsulinemia induced by reduced insulin exocytosis from β-cells.78 Thus, cell autonomous core clock function in β-cells affects insulin secretion from pancreatic β-cells. Loss of the clock in these cells results in hyperglycemia, phenocopying the increased glucose load observed in clinical studies. These findings provide strong evidence for tissue-specific function of local tissue clocks and highlight the utility of experimental genetic models for both in vivo and in vitro manipulation to dissect mechanistic actions of the clock within distinct cellular populations.
A number of studies indicate beneficial effects on life span and metabolism when feeding time is restricted to an animal's active period. One explanation is that restricted feeding time is capable of aligning food intake with circadian expression of the appropriate genes in central and peripheral tissues.79 Dr. Satchin Panda presented work examining the role of time-restricted feeding (TRF) on metabolic factors and gene expression profiles. Mice with food availability restricted to 8 h/day during their waking period exhibited an increase in the number of cycling transcripts as well as a larger amplitude in the diurnal rhythm of gene expression rhythms compared to that in ad libitum fed animals, indicating this feeding regimen increases the robustness of peripheral circadian clocks.80 Placing animals on a TRF regimen does not impact overall food intake, but improves a number of traits associated with metabolic pathology including reduction in body weight, serum cholesterol, hepatic triglyceride levels, and insulin level fluctuations in microflora that are protective against the development of obesity.81,82 Cardiac disease represents another common risk associated with sleep loss, circadian disruption and obesity. The effects of TRF on cardiac function are currently being studied in the fruit fly, Drosophila melanogaster. Flies provide a powerful system for genetic analysis of cardiac function, and flies display both age- and diet-dependent reduction in cardiac performance.83–85 The age-dependent reduction in cardiac performance is partially alleviated by TRF, and this improved function appears to be linked to TRF-dependent changes in mitochondrial function and the circadian clock.86 Therefore, TRF appears to function in the mammalian liver and microbiome, as well as the fruit fly heart to improve pathologies related to aging and metabolic function.
The number of ways that metabolism, sleep, and circadian pathways are integrated continues to expand. An emerging body of evidence indicates intimate links between epigenetic modifications and broad metabolic changes in the animal. Metabolic status, including levels of NAD (nicotinamide adenine dinucleotide), SAM (s-adenosyl methionine), FAD (Flavin adenine dinucleotide), acetyl CoA, and potentially glucose levels, all result in differential histone modifications. These modifications impact the transcription of the nearby genes through DNA accessibility and thus serve as a potential gene regulatory system for key metabolic enzymes. Therefore, this system may connect diet to the transcript oscillations of metabolic genes.79 To understand this connection, Dr. Paolo Sassone-Corsi and colleagues have coupled a metabolic approach to quantify cycling metabolites under high fat and normal food conditions with an analysis of chromatin modulating enzymes. Previous work has shown that ∼50% of metabolites oscillate in the liver, suggesting they may be functionally downstream of the circadian clock.87 These data are openly shared on the web resource CircadiOmics, presenting a rich resource for the community. Feeding mice a high fat diet over 10 weeks both disrupts the cycling of one set of genes and metabolites in the liver but also induces the cycling of a second class of genes and metabolites that do not cycle under normal diet conditions.88 These data further suggest that levels of NAD+ may play a role in this switch. NAD+ is a cofactor for SIRT1 to activate CLOCK/ BMAL1 transcriptional activity. To further understand the connection between diet, metabolic enzymes and circadian cycling, the Irvine group compared knockouts of two proteins thought to modify the chromatin environment.89 SIRT1 and SIRT6 are chromatin deacetylases expressed in similar cells. Comparing gene expression and metabolic profiles suggests that they have different targets with only about 10% overlap in gene expression between the 2 knockout mice. Moreover, metabolic constituents such as lipids and carbohydrates are markedly different between the 2 knockout mice. Therefore, one role of the circadian clock could be to regulate specific deacetylases which then partition metabolic output of the liver. For example, SIRT1 controls chromatin changes that permit transcription of peptide and cofactor genes while the presence of SIRT6 permits transcription of lipid and carbohydrate related genes. Thus, chromatin modifications likely play a critical role in the rhythmicity and function of the circadian system.
Taken together, these studies further our understanding of the reciprocal interaction between conserved metabolic and circadian pathways. Metabolic input modulates clock dependent gene expression, while alterations in circadian clocks can alter critical metabolic functions that impact diverse physiological processes related to health and pathology. Understanding how diet and cellular metabolism regulate circadian function will require a better understanding of the mechanisms linking these processes. Model organisms will play a major role in investigating the molecular connections between conserved metabolic and timing pathways. From fruit flies to mice, these organisms permit genetic and biochemical approaches that expand our understanding of the molecular connections that will likely translate to an improved understanding of human health and potential treatments. The studies presented provide a strong rationale for the future use of genetically amenable model systems to provide a framework that will guide clinical studies.
SESSION IV: APPROACHES TO UNDERSTANDING THE MECHANISMS CONTRIBUTING TO GLUCOSE HOMEOSTASIS
Effective treatment of metabolic diseases, such as type 2 diabetes, requires circulating blood glucose to be maintained within a relatively narrow range. Both hyperglycemia and hypoglycemia are associated with specific risks posing a significant challenge for treatment of individuals with diabetes. In the fourth session, speakers focused on various approaches used to understand the physiological mechanisms contributing to regulation of glucose homeostasis.
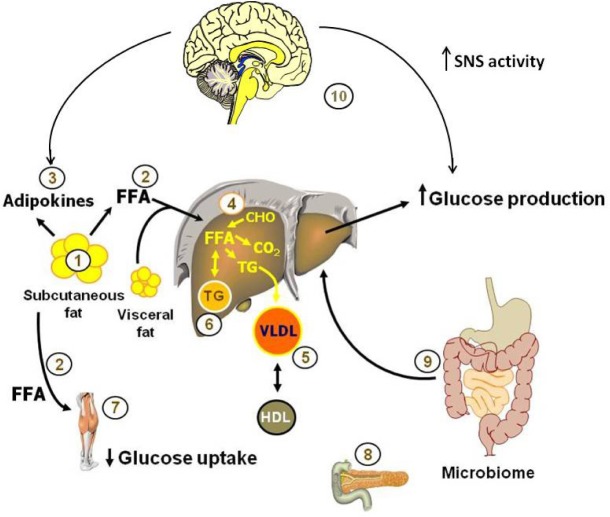
Dr. Sam Klein opened the session with a dataset highlighting the importance of looking beyond body mass index (BMI) as an indicator of metabolic health. Patients with similar BMIs, exhibit a wide range of insulin sensitivity as measured by using the hyperinsulinemic-euglycemic clamp procedure, indicating that BMI-independent physiological parameters contribute to differences in insulin sensitivity. Instead of BMI, Dr. Klein characterizes obese patients based on metabolic status, and in particular on the ability of insulin to stimulate glucose uptake and intrahepatic triglyceride content. People with normal levels of intrahepatic triglyceride are usually “metabolically normal” and those with high levels of intrahepatic triglyceride are usually “metabolically abnormal.”90 In fact, the amount of intrahepatic triglyceride is directly associated with the degree of insulin resistance, and has been used as a marker of metabolic dysfunction. Klein's findings demonstrate that metabolically abnormal obese subjects are more likely to exhibit adverse metabolic profiles including high blood pressure, plasma triglycerides, and very low-density lipoprotein (VLDL), and decreases in multi-organ insulin sensitivity in response to moderate (6%) weight gain compared to similar weight gain in metabolically normal obese subjects.90 Importantly, these data indicate that investigators must take into account the metabolic status of a patient, not just their body weight when investigating changes in metabolic health. A systems biology research approach in human subjects is needed to provide a comprehensive assessment of the effect of sleep and circadian disruption on metabolic function. These studies involve sophisticated metabolic testing, such as the hyperinsulinemic-euglycemic clamp procedure, in conjunction with isotope tracer infusion and tissue biopsies, to dissect the complex and interactive effects of sleep and circadian disruption on multiple organ systems simultaneously.91 The various components that need to be addressed to provide a comprehensive assessment are outlined in Figure 1.
Figure 1.
Pathogenesis of obesity-related metabolic dysfunction. Studies of metabolic dysfunction in people need to provide a comprehensive approach and consider the complex changes that occur in multiple organs. Specifically, the following need to be assessed: (1) changes in adipose tissue biology that can be assessed by fat biopsies; (2) adipose tissue lipolytic activity and the release of free fatty acids (FFA) into the bloodstream; (3) adipokines; (4) metabolic changes in the liver, including intrahepatic triglyceride content; (5) very low density lipoprotein (VLDL) secretion rate; (6) de novo lipogenesis rates, fatty acid oxidation and liver insulin sensitivity; (7) changes in muscle insulin sensitivity and metabolite content, (8) β-cell function; (9) gut microbome; and (10) the brain plays an important role in glucose and lipid homeostasis, receiving information from periphery signals generated by food ingestion and subsequently contributing to the regulation of metabolism through innervation of metabolically active tissues such as muscle, pancreas, liver and adipose tissue. In obesity-related pathogenesis, increased sympathetic activation increases lipolysis and stimulates hepatic glucose production. Figure provided by Dr. Sam Klein.
Dr. Philip Kern spoke about adipose tissue inflammation and its role in insulin resistance. In a normal, healthy state, adipose tissue expands to meet lipid storage demands. Dysfunctional adipose expansion results in an increase in the secretion of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-6. Indeed, elevated TNF and IL-6 expression in human adipose tissue is associated with insulin resistance independent of obesity. Furthermore, unhealthy adipose tissue in insulin resistant subjects is typically characterized by increased levels of inflammatory macrophages, increased extracellular matrix materials and increased numbers of mast cells.92 Treatment with pioglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, improves insulin resistance, decreases inflammation, and changes adipose tissue physiology—leading to smaller adipose cells with paradoxically larger total adipose tissue mass fat storage potential.92 Facilitating adipose tissue expansion via healthier adipocytes may directly benefit glucose metabolism by reducing plasma FFA and adipokine secretion.93 In addition to adipose cell size, the processes of white adipose tissue changing to a more metabolically-active brown/beige fat, is also a key physiological component and may contribute to overall metabolic health.94,95 Again, investigators must examine the specific role of key metabolic tissues, such as adipose tissue, in determining a patient's metabolic health and not rely solely on BMI status.
Dr. Elizabeth Seaquist focused on peripheral and central nervous system responses to hypoglycemia and revealed some intriguing interactions with sleep. Hypoglycemia, which often occurs during insulin therapy for patients with either type 1 or type 2 diabetes, is the major limiting factor in reaching optimal glycemic target goals. Dr. Seaquist first reviewed the physiology that underlies the counter-regulatory responses to hypoglycemia and discussed that repeated episodes of hypoglycemia decrease the autonomic and adrenal medullary response to elicit an efficient, timely counter regulation to falling glucose levels. If this autonomic failure occurs during sleep, the plummeting glucose levels can lead to death. During recurrent hypoglycemia, the brain appears to use glycogen as an alternative fuel source and, as a result, has altered glucose sensing. She showed evidence that the thalamus of patients with type 1 diabetes displayed an impaired awareness of hypoglycemia and the thalamus did not show the pattern of neuronal activation during hypoglycemia as seen in healthy volunteers.96 Moreover, Seaquist discussed that hypoglycemia during sleep can affect other parameters, such as morning food intake and memory consolidation. Dr. Seaquist highlighted the importance of studying the brain in humans when investigating the effects of sleep on metabolism and novel imaging strategies can advance this area of science.
As discussed in the first session, sleep apnea is associated with dysregulated glucose metabolism. Dr. Michael Joyner continued the OSA discussion with a focus on the carotid bodies (CB). During a hypoxic event, the CBs sense the drop in oxygen saturation and activate the sympathetic nervous system (SNS) which in turn may contribute to increased endogenous glucose production as observed in patients with OSA who repeatedly suffer cyclical intermittent hypoxia. In support of this, Dr. Joyner presented findings demonstrating decreased counter-regulatory hormone responses and increased glucose infusion rates during a hypoglycemic clamp in humans under hyperoxic conditions that “turn off” CBs relative to normoxic conditions.97 Moreover, CBs sense more than just hypoxia and also respond to glucose, insulin, and pro-inflammatory cytokines.98–100 Narkiewicz et al.101 reported that obese patients without OSA have similar SNS activity relative to normal patients whereas obese patients with occult OSA have elevated SNS activity relative to normal patients and obese patients without OSA. These findings indicate OSA may be obligatory for elevated SNS activity in obese patients. In conclusion, Dr. Joyner's data suggest that CBs are involved in dysregulated glucose metabolism in OSA patients, highlighting that CBs sense more than just hypoxia and also respond to glucose, insulin, and pro-inflammatory cytokines.
Dr. Richa Saxena concluded session IV with a discussion about the role of genetics and potential interactions with sleep, circadian timing, and type 2 diabetes (T2D) risk. Common variants in the melatonin receptor gene, MTNR1B, are associated with increased type 2 diabetes risk.14,15 Specifically, individuals with the G allele have a reduced insulin response to glucose and reduced disposition index (a validated T2D risk marker). To further investigate these associations, Dr. Saxena in collaboration with Dr. Frank Scheer at Harvard's Division of Sleep Medicine has assessed MTNR1B variation in individuals with specific measures of sleep and circadian physiology from over 20 laboratory controlled studies. Preliminary findings suggest that individuals with the GG allele have later timing of their dim-light melatonin offset and a longer duration of elevated melatonin. Furthermore, carriers of this T2D MTNR1B risk allele have decreased glucose tolerance during an oral glucose tolerance test relative to non-carriers under conditions of exogenous melatonin administration. Based on these findings, Dr. Saxena proposed a model where individuals with the T2D MTNR1B risk allele have elevated melatonin levels later in the morning when they are normally consuming food and this interaction of elevated melatonin with concurrent food intake mediates dysregulated glucose metabolism and increased T2D risk. Importantly, findings from studies that use melatonin as a marker of circadian phase show that circadian misalignment results in dysregulated glucose metabolism.19,20 These previous studies do not assess a causal role of melatonin in mediating dysregulated glucose metabolism but show that circadian misalignment in general results in dysregulated glucose metabolism. Dr. Saxena proposes that melatonin may have a direct role in mediating dysregulated glucose metabolism in individuals with the T2D MTNR1B risk allele. Together with the previous speakers, these findings provide further evidence that we need to take a multi-faceted approach to investigating the impact of sleep and circadian disruption on dysregulated glucose metabolism and T2D risk, including the role of genetic variants that underlie individual differences in response to sleep disorders and circadian disruption.
SESSION V: CURRENT/ONGOING STUDIES ON SLEEP AND CIRCADIAN DISRUPTIONS IN HUMANS
Dr. Frank Scheer addressed the separate effects of the circadian system, the behavioral cycle and their interaction, i.e., circadian misalignment, on human metabolism. In a forced desynchrony protocol, circadian misalignment rapidly (after just 3 days) increased the postprandial responses of glucose and insulin, demonstrating reduced glucose tolerance and suggesting reduced insulin sensitivity.19 In the same study, circadian misalignment decreased levels of the satiety hormone leptin. These effects, if maintained chronically, could help explain why shift work is a risk factor for diabetes and obesity, respectively. In this study, these effects could not be fully explained by changes in sleep duration. This conclusion was supported by an elegant experimental study in which circadian misalignment reduced insulin sensitivity without changes in sleep duration.20 In a recent simulated night work study, the independent effects of circadian phase and circadian misalignment on glucose tolerance were investigated.102 Postprandial glucose levels were higher in the biological evening than in the biological morning independent of behavioral cycle effects (including sleep/wake and fasting/feeding cycle), and this circadian influence was much larger than the effect of the behavioral cycle, indicating a dominant role of the circadian system in the well-known daily rhythm in glucose tolerance. In addition, circadian misalignment resulting from a rapid 12-h shift of the behavioral cycle decreased glucose tolerance. This effect was sustained over 3 days of night work exposure, providing additional evidence for circadian misalignment as a mechanistic link between shift work and increased diabetes risk. Interestingly, these variations in glucose tolerance seemed to be explained, at least in part, by different mechanisms: decreased pancreatic β-cell function (lower early-phase insulin) during the biological evening and decreased insulin sensitivity (elevated postprandial glucose despite higher late-phase insulin) without change in early-phase insulin during circadian mis-alignment. Even the timing of meals and the distribution of caloric intake during the daytime has recently been found to influence energy balance in humans. In a large longitudinal weight loss study, overweight and obese individuals who ate their main meal early lost about 25% more weight than those who ate their main meal late, despite similar caloric intake and physical activity.103 In a subsequent experimental study, this general concept was confirmed, showing that a high-caloric breakfast rather than a high-caloric dinner during a weight loss diet resulted in improved weight-loss despite similar 24-hour caloric intake.104 Not only does the circadian system influence the physiological and metabolic response to meals, the circadian rhythm has also been shown to influence subjective hunger and appetite independent of the fasting/feeding cycle, with a peak in the biological evening and a trough in the biological morning.105 Finally, one of the factors that may be involved in the adverse effects of circadian misalignment on glucose tolerance is the elevated melatonin concentration during nighttime food intake. Dr. Scheer presented a study that showed that a pharmacological dose of melatonin decreased glucose tolerance assessed by an oral glucose tolerance test, regardless of whether melatonin was administered in the morning or evening.106 These results suggest caution when using melatonin close to meals.
Dr. Kenneth Wright addressed the impact of insufficient sleep and circadian misalignment on metabolism in humans. The effect of insufficient sleep on total daily energy expenditure (whole room calorimetry) and energy balance was examined in a 2-week long protocol.107 Subjects were allowed ad libitum food intake and were scheduled to 5 h (insufficient) and 9 h sleep opportunities. Findings demonstrated that although energy expenditure increased during the insufficient sleep condition, subjects also increased nutrient consumption, particularly carbohydrates eaten as post-dinner snacks. This resulted in a net positive energy balance and weight gain. Insufficient sleep led to a delay in the timing of melatonin onset and an increased duration between wake time and circadian melatonin offset phase, which likely contributed to changes in eating behaviors. Dr. Wright reviewed previous work demonstrating that eating during the typical sleep period contributes to weight gain in mice,108 and he suggested that eating at inappropriate circadian times may be a novel risk factor for weight gain and obesity. The effect of circadian misalignment on energy expenditure was examined in a 6 day protocol simulating a common night shift work schedule.109 Total daily energy expenditure decreased during the shift work schedule following the transition day to the night shift. Such a decrease in total daily energy expenditure implies that even if food in-take is maintained, a nightshift schedule could lead to weight gain; this effect would be exacerbated if physical activity was decreased and food intake increased under such conditions. Dr. Wright noted that findings from epidemiological studies suggest that there are long-term metabolic effects of night shift work, but controlled laboratory studies to assess these effects are necessarily limited in duration. This observation highlighted a recurrent discussion topic at the workshop relating to the need to design studies that could address precise physiological questions in the context of real-world situations. For example, how is energy expenditure altered in people who have been working a night shift for an extended period of time?
Dr. Charles Czeisler reviewed the effects of light exposure on sleep and circadian rhythms and discussed some of the implications for metabolism and metabolic diseases. To provide historical context, he cited studies demonstrating that average sleep durations have declined over the past 100 years.110 Both the phase of the clock and melatonin secretion, a marker of circadian phase, are known to be highly sensitive to light: even dim room light can affect plasma melatonin concentrations and the phase of the intrinsic pacemaker.111 Current practices, such as reading light-emitting eReaders instead of books, introduce additional phase-delays and acutely suppress melatonin secretion.112 Epidemiological studies have linked the short sleep durations and circadian disruption prevalent in modern society to a range of negative health outcomes including a higher risk of metabolic syndrome and diabetes. Dr. Czeisler presented his work showing that in humans 3 weeks of sleep restriction (5.6 h of sleep opportunity per 24 h) concurrent with circadian disruption increases postprandial glucose levels, possibly due to impaired β-cell function.113 These adverse alterations were abolished following 9 days of recovery sleep and circadian re-entrainment to a normal sleep/wake cycle. These results suggest that inter-individual differences in circadian phase may translate to differential metabolic risks. When intrinsic circadian periods were assessed in healthy adults, over 75% of subjects had periods longer than 24 h.114 However, intrinsic circadian periods tended to be shorter in women, consistent with results from studies in nonhuman animals.115,116 More work is needed to determine the relationships between intrinsic circadian period and susceptibility to circadian disruption and resulting metabolic effects.
Dr. Louis Ptacek discussed his research on heritable human circadian and sleep variants. Dr. Ptacek described a 3-process conceptual model that includes the clock, the homeostat, and behavioral drive. He cited several studies in which mutations in clock genes were linked to familial sleep-wake phenotypes including advanced and delayed sleep phase and natural short sleep.117–120 He provided several examples of cases in which genetic traits of the clock and sleep homeostat inform other phenotypes. For example, mutations in casein kinase 1δ cause familial advanced sleep phase and migraine with aura.121 Another family with a circadian gene mutation also has depression with a seasonal component. Interestingly, mice carrying this mutation also have measures of “depression-like behavior” that are sensitive to light cycles. He also reviewed animal studies in which these phenotypes were explored explicitly. For example, modulation of O-GlcNAcylation was identified in a chemical genetic screen of GSK3β (glycerol synthase kinase). O-GlucNAc transferase (OGT) and O-GlucNAse (OGA) were identified as GSK3β targets and novel circadian genes. Alterations of circadian period in cells, mice and Drosophila, resulted from manipulations of OGT and OGA. Reciprocal regulation of circadian clock and protein O-GlcNAcylation is therefore a direct connection between clock and metabolism.122 Dr. Ptacek also discussed results suggesting that these interactions are also impacted by glucose levels. Given the plethora of data linking chronic desynchrony and sleep deprivation with increased risks of many health consequences, including metabolic disorders, further exploration of subjects from advanced sleep phase and natural short sleep families is important to see whether these genetic forms also confer risk (or protection) to metabolic effects. In addition, mouse models of many of the identified human mutations are available for such investigations.
CONCLUSIONS
Several important themes emerged from this workshop. First, it is essential that we encourage joint principal investigators with investigators from the sleep/circadian research community on the one hand and investigators in metabolism, glucose control, etc. on the other. There is much to be gained from fostering interaction.
Sleep/circadian investigators in human studies need to move beyond studies of insulin and glucose and conduct studies at a molecular level assessing effects on key end organs such as fat and muscle that can be assessed in investigations in humans. The microbiome needs to be considered. Investigators in metabolism need, in turn, to consider the role of inadequate sleep, circadian mal-alignment and sleep disorders such as sleep apnea in their human studies. It is conceivable that they play a role in differentiating the metabolically impaired compared to not impaired obese subjects. The techniques to assess sleep, sleep-disordered breathing have markedly improved and assessments do not require in-laboratory studies thereby reducing protocol burden. New mobile approaches are developing and assessment of sleep, sleep-disordered breathing should be part of all ongoing cohort studies and clinical trials in diabetes, etc.
Another important theme was the concern that while acute studies in simulated inadequate sleep, as well as shift work, using in-laboratory studies in healthy volunteers had the advantage of control of many key variables, the results may not extrapolate to real-world chronic “cases” in the community since chronic adaptations may occur. There is a need for complementary studies in individuals with chronic inadequate sleep and shift workers using in-depth metabolic phenotyping.
Attention also needs to expand beyond the traditional metabolic systems—liver, pancreas, adipose tissue and muscle—but also include the carotid body and brain. Novel neuroimaging approaches in humans with inadequate sleep/shift work is an area of opportunity.
Studies also need to incorporate evaluation of genetic and epigenetic changes. There is a rapidly developing understanding of both rare and common variants and it is important to understand whether variants that affect sleep also affect metabolism and vice versa.
A number of specific areas for immediate translational research were identified. Current findings suggest that there is a need for the following: a) clinical trials to assess impact of OSA on metabolic health. A particular area of opportunity is likely to be in pre-diabetes, i.e., before β-cell failure has occurred. Given the very high prevalence of obstructive sleep apnea in obese individuals, identification and treatment of OSA could be a preventative strategy to reduce development of diabetes; b) more in-depth studies are also needed to investigate whether food timing can be used as a novel preventative or therapeutic strategy in patients with or at risk for type 2 diabetes,123 particularly in shift workers, in obese and overweight individuals.
We also need to explore the underlying neuroendocrine, cellular and molecular mechanisms that influence energy expenditure, physical activity, hunger and appetite control, and eating behaviors. A particular area of opportunity is to establish the interaction of food intake with melatonin profiles including understanding the role of genetic variations in circadian-related genes associated with diabetes risk, e.g., MTNR1B and CRY2.
Thus, this is an area with many opportunities for translational research and it is to be hoped that this workshop catalyzes the development of this important area of inquiry.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bass owns stock in Reset Therapeutics. Dr. Grandner has consulted for Bayer, Nexalin Technologies, and FitBit. Dr. Joyner has financial interests in Boston Scientific and Edwards Life Sciences. Dr. Kern has received research support from KinDex Pharmaceuticals. Dr. Klein has financial interests in AspireBariatrics, Human Longevity Inc., and MetroBiotech Midwest and has consulted for Takeda, Janssen, and Danone/Yakult. Dr. Pack has received royalties from McGraw Hill Inc. Dr. Punjabi has received research support form ResMed and Respironics. Dr. Saxena has financial interests in Surface Oncology and Astrazeneca. Dr. Seaquest has consulted for Novo Norodisk, Sanofi, and Locemia and has received research support from Eli Lilly. Dr. Wright has received research support from Philips, Inc., and is on the advisory board of Torvec Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the outstanding work by Dr. Corrine Silva and Dr. Karen Teff from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in organizing this workshop. We also acknowledge the support of the NIDDK in sponsoring the event. An unrestricted educational grant was given to the Sleep Research Society by Phillips Respironics to support travel awards for early-stage investigators.
Footnotes
A commentary on this article appears in this issue on page 1847.
REFERENCES
- 1.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–73. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 2.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–38. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 4.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. doi: 10.3389/fneur.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 6.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;38:707–15. doi: 10.5665/sleep.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds AC, Dorrian J, Liu PY, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS ONE. 2012;7:e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwagi A, Verso MA, Andrews J, Vasquez B, Reaven G, Foley JE. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983;72:1246–54. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornholm M, Al-Khalili L, Dicker A, et al. Insulin signal transduction and glucose transport in human adipocytes: effects of obesity and low calorie diet. Diabetologia. 2002;45:1128–35. doi: 10.1007/s00125-002-0875-9. [DOI] [PubMed] [Google Scholar]
- 13.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrino. Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 14.Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 17.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–96. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litinski M, Scheer FA, Shea SA. Influence of the Circadian System on Disease Severity. Sleep Med Clin. 2009;4:143–63. doi: 10.1016/j.jsmc.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reutrakul S, Hood MM, Crowley SJ, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36:2523–9. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 24.Punjabi NM. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009;69(Suppl 2):13–27. doi: 10.2165/11531150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc. 2013;10:115–20. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Liu Z, Yang H, Luo Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17:33–8. doi: 10.1007/s11325-012-0680-8. [DOI] [PubMed] [Google Scholar]
- 27.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanduri J, Peng YJ, Yuan G, Kumar GK, Prabhakar NR. Hypoxiainducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl) 2015;93:473–80. doi: 10.1007/s00109-015-1274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pak VM, Grandner MA, Pack AI. Circulating adhesion molecules in obstructive sleep apnea and cardiovascular disease. Sleep Med Rev. 2014;18:25–34. doi: 10.1016/j.smrv.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Araujo Freitas IG, de Bruin PF, Bittencourt L, de Bruin VM, Tufik S. What can blood biomarkers tell us about cardiovascular risk in obstructive sleep apnea? Sleep Breath. 2015;19:755–68. doi: 10.1007/s11325-015-1143-9. [DOI] [PubMed] [Google Scholar]
- 33.Barcelo A, Pierola J, de la Pena M, et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J. 2011;37:1418–23. doi: 10.1183/09031936.00050410. [DOI] [PubMed] [Google Scholar]
- 34.Lim DC, Brady DC, Po P, et al. Simulating obstructive sleep apnea patients' oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J Appl Physiol. 2015;118:544–57. doi: 10.1152/japplphysiol.00629.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polak J, Shimoda LA, Drager LF, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:1483–90. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–10. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin MK, Yao Q, Jun JC, et al. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol. 2014;117:765–76. doi: 10.1152/japplphysiol.01133.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic beta-cell function by chronic intermittent hypoxia. Exp Physiol. 2013;98:1376–85. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102:557–63. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Gurubhagavatula I, Teff K, et al. Continuous positive airway pressure, weight loss, or both for obstructive sleep apne. N Engl J Med. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pak VM, Keenan BT, Jackson N, et al. Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes (Lond) 2015;39:472–9. doi: 10.1038/ijo.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamidi S, Wroblewski K, Broussard J, et al. Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care. 2012;35:2384–9. doi: 10.2337/dc12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 44.Naidoo N. Cellular stress/the unfolded protein response: relevance to sleep and sleep disorders. Sleep Med Rev. 2009;13:195–204. doi: 10.1016/j.smrv.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Carreras A, Lee S, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring) 2014;22:758–62. doi: 10.1002/oby.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakim F, Wang Y, Carreras A, et al. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep. 2015;38:31–40. doi: 10.5665/sleep.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arble DM, Vitaterna MH, Turek FW. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS ONE. 2011;6:e25079. doi: 10.1371/journal.pone.0025079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2059–66. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang SX, Khalyfa A, Wang Y, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond) 2014;38:619–24. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elmquist JK, Marcus JN. Rethinking the central causes of diabetes. Nat Med. 2003;9:645–7. doi: 10.1038/nm0603-645. [DOI] [PubMed] [Google Scholar]
- 53.Coppari R, Ichinose M, Lee CE, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Berglund ED, Vianna CR, Donato J, Jr., et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–9. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendoza J, Gourmelen S, Dumont S, Sage-Ciocca D, Pevet P, Challet E. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J Physiol. 2012;590:3155–68. doi: 10.1113/jphysiol.2012.230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586:5901–10. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Challet E, Turek FW, Laute M, Van Reeth O. Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: role of serotonergic and metabolic signals. Brain Res. 2001;909:81–91. doi: 10.1016/s0006-8993(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 59.Vivanco P, Studholme KM, Morin LP. Drugs that prevent mouse sleep also block light-induced locomotor suppression, circadian rhythm phase shifts and the drop in core temperature. Neuroscience. 2013;254:98–109. doi: 10.1016/j.neuroscience.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai LL, Tsai YC, Hwang K, Huang YW, Tzeng JE. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am J Physiol Endocrinol Metab. 2005;289:E212–7. doi: 10.1152/ajpendo.00603.2004. [DOI] [PubMed] [Google Scholar]
- 61.Bartol-Munier I, Gourmelen S, Pevet P, Challet E. Combined effects of high-fat feeding and circadian desynchronization. Int J Obes (Lond) 2006;30:60–7. doi: 10.1038/sj.ijo.0803048. [DOI] [PubMed] [Google Scholar]
- 62.Kumar Jha P, Challet E, Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol Cell Endocrinol. 2015 Feb 7; doi: 10.1016/j.mce.2015.01.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Grosbellet E, Zahn S, Arrive M, et al. Circadian desynchronization triggers premature cellular aging in a diurnal rodent. FASEB J. 2015 Aug 10; doi: 10.1096/fj.14-266817. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Franken P. A role for clock genes in sleep homeostasis. Curr Opin Neurobiol. 2013;23:864–72. doi: 10.1016/j.conb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Maret S, Dorsaz S, Gurcel L, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U A. 2007;104:20090–5. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archer SN, Laing EE, Moller-Levet CS, et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014;111:E682–91. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–41. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curie T, Mongrain V, Dorsaz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–23. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curie T, Maret S, Emmenegger Y, Franken P. In vivo imaging of the central and peripheral effects of sleep deprivation and suprachiasmatic nuclei lesion on PERIOD-2 protein in mice. Sleep. 2015;38:1381–94. doi: 10.5665/sleep.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franken P, Dudley CA, Estill SJ, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci U S A. 2006;103:7118–23. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franken P, Thomason R, Heller HC, O'Hara BF. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wisor JP, Pasumarthi RK, Gerashchenko D, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci. 2008;28:7193–201. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mongrain V, La Spada F, Curie T, Franken P. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PLoS ONE. 2011;6:e26622. doi: 10.1371/journal.pone.0026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–54. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 77.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–8. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–17. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Na J, Musselman LP, Pendse J, et al. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013;9:e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ocorr K, Akasaka T, Bodmer R. Age-related cardiac disease model of Drosophila. Mech Ageing Dev. 2007;128:112–6. doi: 10.1016/j.mad.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birse RT, Choi J, Reardon K, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–44. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–9. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci U S A. 2012;109:5541–6. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–78. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masri S, Rigor P, Cervantes M, et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158:659–72. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fabbrini E, Yoshino J, Yoshino M, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–95. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshino J, Klein S. A novel link between circadian clocks and adipose tissue energy metabolism. Diabetes. 2013;62:2175–7. doi: 10.2337/db13-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spencer M, Yang L, Adu A, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS ONE. 2014;9:e102190. doi: 10.1371/journal.pone.0102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desouza C, Gilling L, Fonseca V. Management of the insulin resistance syndrome. Curr Diab Rep. 2001;1:140–7. doi: 10.1007/s11892-001-0026-6. [DOI] [PubMed] [Google Scholar]
- 94.Kern PA, Finlin BS, Zhu B, et al. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. J Clin Endocrinol Metab. 2014;99:E2772–9. doi: 10.1210/jc.2014-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mangia S, Tesfaye N, De Martino F, et al. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab. 2012;32:2084–90. doi: 10.1038/jcbfm.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol. 2010;588:4593–601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–8. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 99.Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556:255–66. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shu HF, Wang BR, Wang SR, et al. IL-1beta inhibits IK and increases [Ca2+]i in the carotid body glomus cells and increases carotid sinus nerve firings in the rat. Eur J Neurosci. 2007;25:3638–47. doi: 10.1111/j.1460-9568.2007.05586.x. [DOI] [PubMed] [Google Scholar]
- 101.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 102.Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112:E2225–34. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21:2504–12. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 105.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21:421–3. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–9. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014;111:17302–7. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16:203–11. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J Clin Endocrinol Metab. 2000;85:2189–96. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- 112.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112:1232–7. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schull J, Walker J, Fitzgerald K, et al. Effects of sex, thyroparathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–6. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- 116.Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- 117.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 118.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 119.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 120.He Y, Jones CR, Fujiki N, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–70. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5:183ra56, 1–11. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaasik K, Kivimae S, Allen JJ, et al. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 2013;17:291–302. doi: 10.1016/j.cmet.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jakubowicz D, Wainstein J, Ahren B, et al. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58:912–9. doi: 10.1007/s00125-015-3524-9. [DOI] [PubMed] [Google Scholar]