Abstract
Study Objectives:
To investigate the efficacy of cognitive behavioral therapy for insomnia (CBTI) in adolescents.
Design:
A randomized controlled trial of CBTI in group therapy (GT), guided internet therapy (IT), and a waiting list (WL), with assessments at baseline, directly after treatment (post-test), and at 2 months follow-up.
Setting:
Diagnostic interviews were held at the laboratory of the Research Institute of Child Development and Education at the University of Amsterdam. Treatment for GT occurred at the mental health care center UvAMinds in Amsterdam, the Netherlands.
Participants:
One hundred sixteen adolescents (mean age = 15.6 y, SD = 1.6 y, 25% males) meeting DSM-IV criteria for insomnia, were randomized to IT, GT, or WL.
Interventions:
CBTI of 6 weekly sessions, consisted of psychoeducation, sleep hygiene, restriction of time in bed, stimulus control, cognitive therapy, and relaxation techniques. GT was conducted in groups of 6 to 8 adolescents, guided by 2 trained sleep therapists. IT was applied through an online guided self-help website with programmed instructions and written feedback from a trained sleep therapist.
Measurements and Results:
Sleep was measured with actigraphy and sleep logs for 7 consecutive days. Symptoms of insomnia and chronic sleep reduction were measured with questionnaires. Results showed that adolescents in both IT and GT, compared to WL, improved significantly on sleep efficiency, sleep onset latency, wake after sleep onset, and total sleep time at post-test, and improvements were maintained at follow-up. Most of these improvements were found in both objective and subjective measures. Furthermore, insomnia complaints and symptoms of chronic sleep reduction also decreased significantly in both treatment conditions compared to WL. Effect sizes for improvements ranged from medium to large. A greater proportion of participants from the treatment conditions showed high end-state functioning and clinically significant improvement after treatment and at follow-up compared to WL.
Conclusions:
This study is the first randomized controlled trial that provides evidence that cognitive behavioral therapy for insomnia is effective for the treatment of adolescents with insomnia, with medium to large effect sizes. There were small differences between internet and group therapy, but both treatments reached comparable endpoints.
Clinical Trial Registration:
This study was part of the clinical trial: Effectiveness of cognitive behavioral therapy for sleeplessness in adolescents; URL: http://www.isrctn.com/ISRCTN33922163; registration: ISRCTN33922163.
Citation:
de Bruin EJ, Bögels SM, Oort FJ, Meijer AM. Efficacy of cognitive behavioral therapy for insomnia in adolescents: a randomized controlled trial with internet therapy, group therapy and a waiting list condition. SLEEP 2015;38(12):1913–1926.
Keywords: sleep, insomnia, adolescents, cognitive behavioral therapy, internet treatment
INTRODUCTION
Insomnia is the most prevalent sleep disorder among adolescents.1,2 Reports of recent point prevalence according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)3 range from 7.8%, 7.9%, to 8.3%, and even up to 23.8% for older adolescents (16–18 years old).4–7 Furthermore, studies indicate that insomnia tends to persist over time,2,8 and that chronicity is high in adolescents, with up to 88% of adolescents with a history of insomnia reporting current insomnia.1 The differences in estimates of prevalence are partly due to the use of different definitions and criteria for insomnia.9,10
Insomnia and the chronic sleep deprivation it causes can lead to a wide range of problems. Studies show that adolescents with disturbed sleep report more depression and anxiety, inattention and conduct problems, drug and alcohol abuse, and impaired academic performance.11–16 Furthermore, adolescents with sleep disturbances report worse perceived health, more sleepiness, energy loss, and daytime fatigue. Also, sleep disturbances in children and adolescents may precede ADHD symptoms or exacerbate underlying ADHD later in development,17–20 suggesting that lack of sleep can actually cause ADHD or other mental disorders.
Effectiveness of cognitive behavioral therapy for insomnia (CBTI) in adults has been investigated extensively over the past 15 years in several modalities ranging from traditional face-to-face therapy and group therapy, to telephone delivered CBTI, internet delivered or mailed self-help, bibliotherapy, and online delivered self-help with support from an animated personal therapist.21–26 All these studies show CBTI to be highly effective for both short- and long-term outcomes, with medium to large effect sizes.
Despite the fact that CBTI has been established since over 10 years as the first choice in the treatment of insomnia in adults,21,27,28 and despite the high prevalence, high chronicity, and serious consequences of insomnia in adolescents, up-to-date research into effective cognitive behavioral treatment for insomnia in adolescents in a randomized controlled design is lacking.29 However, the studies on treatment of insomnia that were conducted with adolescents do indicate that CBTI could also be effective for this age group. In a study with 55 adolescents who had been treated for substance abuse, Bootzin and Stevens30 found improvements in several sleep variables after six sessions of cognitive behavioral group therapy for their sleep disturbances. This improvement, in turn, appeared to reduce substance abuse at follow-up 12 months later. In a single-subject study with three adolescents, Norell-Clarke et al.31 studied the effectiveness of cognitive therapy32 for insomnia and found reduced sleep onset latency (SOL), but no change in total sleep time (TST). In another single-subject study Hendricks et al. showed effectiveness of a multicomponent cognitive-behavioral intervention for problematic sleep in three female adolescents, with clinically meaningful improvements in TST, sleep efficiency (SE), and SOL.33 Finally, in a pilot RCT with 26 adolescents, De Bruin et al. showed improvements in most sleep variables with medium to large effect sizes for both internet and group delivered CBTI.34 Although none of these four studies were conducted in a randomized controlled design including a waiting list, and none investigated long-term results, they do indicate that CBTI can be effective for adolescents in the short term.
Since research indicates that adolescents, more than adults, are reluctant to seek help for psychological problems at public mental health services,35,36 effective treatments may be under-used by this group. Therefore it is important to develop low-threshold and easily accessible treatments that youngsters are more likely to utilize. Online therapy may serve as such a modality of treatment for adolescents,37,38 and over the last few years many studies have provided strong support for similar efficacy of online cognitive behavioral treatments compared to face-to-face treatment.39–41 These studies indicate that online applications of cognitive behavioral interventions are promising forms of treatment for adolescents.
To conclude, although there is a high prevalence of insomnia in adolescents and its consequences are severe for daily life, to date efficacy of cognitive behavioral interventions has not been investigated adequately. In addition, providing adolescents with the required help may be facilitated by online application of CBTI. Therefore, in this study we aim to investigate the efficacy of CBTI for adolescents, addressing the following questions: Is CBTI for adolescents effective in the short term and at 2 months follow-up? Are there differences in efficacy of different treatment modalities (i.e., internet therapy and group therapy)? We used a randomized controlled design with a face-to-face group therapy condition (GT), a guided internet therapy condition (IT), and a waiting list condition (WL). Treatment results are reported immediately after treatment and at 2 months follow-up. We hypothesized that group CBTI is effective compared to WL, and that internet CBTI is effective compared to WL. Because literature on differences between internet and face-to-face (group) therapy is inconclusive,39–42 we did not formulate hypotheses about differential effects of CBTI for IT or GT.
METHODS
Study Design
This study was a parallel-group, randomized controlled trial comprising 3 conditions (GT, IT, and WL) using simple randomization, with an equal allocation ratio, by referring to a table of random numbers. Participants who were randomized to the WL condition were offered to choose their preferred treatment (GT or IT) after follow-up measurements were completed. The trial followed CONSORT 2010 guidelines43 and recommendations on measuring outcomes in insomnia trials.44
Participants
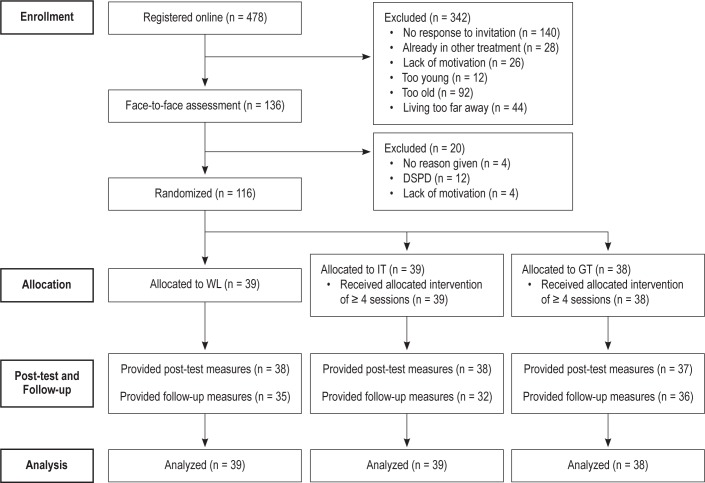
Based on information from the pilot study of this RCT34 we conducted an a priori power analysis with effect size Cohen's d = 0.70 between baseline and follow-up, and autocorrelation of 0.50 for objective SE. With the current sample size and α set to 0.05, we found a power of 0.86. Four hundred seventy-eight people registered for the study through a website. After screening, 342 potential participants were excluded or abandoned the study (Figure 1), and 136 were invited for further assessment in face-to-face interviews of one hour.
Figure 1.
Participant flow through the recruitment procedure.
Inclusion criteria were (1) age between 12–19 years, (2) in secondary school or after (i.e., further education or work), (3) living within traveling distance from the treatment facilities, (4) meeting the diagnostic criteria of the DSM-IV for primary insomnia. These criteria concern difficulties falling asleep, difficulties staying asleep, or not feeling rested after getting up, presence of these problems for at least 1 month, and clinically significant consequences for daily life.3 Exclusion criteria were other sleep problems, other psychiatric problems (including suicidal plans), other physical problems that interfere with sleep, and drug or medication use (including melatonin) that affect sleep.
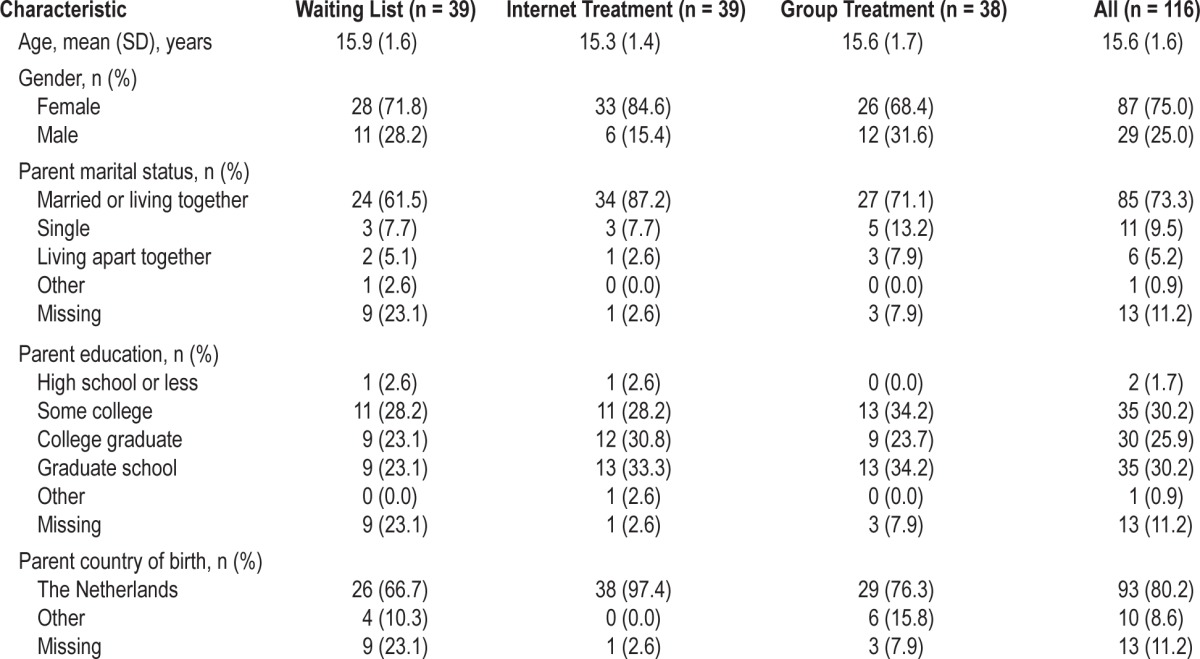
Of the remaining 136 participants, 20 left the study or were excluded based on inclusion and exclusion criteria (Figure 1). This resulted in a group of 116 adolescents between 12–19 years (mean = 15.6, SD = 1.6 years, 25% males) who met the diagnostic criteria for primary insomnia according to the DSM-IV. After randomization, 39 were assigned to the waiting list, 39 to internet treatment, and 38 to group treatment (see Table 1 for demographic characteristics).
Table 1.
Demographic characteristics of the participants.
Measures
Data were collected at baseline (2 weeks prior to the start of the treatment), directly after the treatment (post-test), and 2 months later (follow-up).
Actigraphy and Sleep Logs
Sleep was measured using wrist actigraphy (Actiwatch AW4; Cambridge Neurotechnology Ltd., Cambridge, England) and online sleep logs45 for 7 nights at all 3 measurement occasions. For both instruments sleep parameters were recorded or calculated for time in bed (TIB), TST, SOL, wake after sleep onset (WASO), and SE (percentage TST of TIB).
Actigraphy measures were recorded with 1-min epochs, and analyzed with Actiwatch Sleep Analysis 7 software. As is recommended by the manufacturer, the medium sensitivity algorithm was used. Based on estimates obtained from polysomnography this algorithm has the best sensitivity (0.96), specificity (0.42), and accuracy (0.79) for insomnia.46 Participants were instructed to wear the Actiwatch on their non-dominant wrist when they went to bed, remove it in the morning after getting up, and to press the event marker button at lights out and when getting up, which adds a marker that can be used to synchronize activity measures to actual bed times. As recommended in other studies,47,48 we visually examined all actigraphy data and corrected them where necessary. The following general rule was applied for data from the sleep logs or event markers from the Actiwatch that did not correspond with the visual inspection: If the sleep log indicated a bedtime at which it was obvious from the actigraphy data that the participant was already asleep, we set the bedtime to the first peak before the drop-off. If the reported time of getting up in the sleep log indicated a time at which it was obvious that the individual was still asleep, we corrected the data by changing the time of getting up to the first peak after the indicated time. We used multilevel regression analyses to analyze data from sleep logs and actigraphy. Because this method allows for missing data of participants at different measurement occasions, we did not impute missing data. See for a more detailed description of analyses the section below.
Participants were instructed to fill out the sleep logs online every day within 1 hour after getting up, and received a reminder text message on their mobile phone if the sleep log was not completed before 16:00. Sleep logs could be filled out up to midnight the following day at the latest, as retrospective data with a larger time span were considered unreliable. The sleep logs consisted of 8 questions registering bedtimes, time of lights out, SOL, WASO, wake up time, and get-up time. From the bedtimes of the sleep logs, we calculated TIB, TST, and SE. Furthermore, the sleep logs contained 2 questions measuring subjective sleep quality of the previous night for feeling rested at get-up time (5-point Likert scale, from “not at all rested” to “very well-rested”), and quality of sleep (5-point Likert scale, from “very poor” to “very good”).
Questionnaires
The Holland Sleep Disorder Questionnaire (HSDQ)49 consists of 40 items, scored on a 5-point rating scale, to diagnose common sleep disorders and is based on the 6 main categories of sleep disorders as described in the International Classification of Sleep Disorders, Second Edition.50 It contains 6 subscales for insomnia, sleep-related breathing disorders, hypersomnia, circadian rhythm sleep disorders, parasomnia, and restless legs syndrome or periodic limb movement disorder. Cronbach α in a Dutch sample of 1,269 patients and 412 participants without sleep complaints was 0.90 and ranged from 0.73 to 0.81 for the 6 subscales. The overall accuracy was 88%, and a score above the cutoff of 3.68 on a range of 1 to 5 on the Insomnia scale (HSDQi) is an indication of insomnia.
The Chronic Sleep Reduction Questionnaire (CSRQ)51 consists of 20 items of 3 ordinal response categories (1–3) that measure symptoms of chronic sleep reduction (i.e. shortage of sleep, irritation, loss of energy, and sleepiness) in the previous 2 weeks (e.g., “I am a person who does not get enough sleep,” “Others think that I am easily irritated,” “I am active during the day,” “Do you feel sleepy during the day?”). Higher scores indicate more chronic sleep reduction. Cronbach α in a pre-adolescent population was 0.84,52 0.85 in a Dutch adolescent population, and 0.87 in an Australian adolescent population.51
Treatments
Participants in both GT and IT received 6 weekly sessions of cognitive behavior therapy for adolescent insomnia (CBTI). The protocol was based on CBTI for adults21,28 and adapted for use with adolescents by the research team and an experienced CBTI sleep therapist. The protocol contained psychoeducation, sleep hygiene, restriction of time in bed, stimulus control, cognitive therapy, and relaxation techniques. All sessions started with a review of the sleep variables from the sleep logs. Bedtime advise based on these sleep variables was explained, after which specific exercises of stimulus control, sleep hygiene, relaxation, and cognitive therapy were introduced or continued from the previous session. See Table 2 for the structure of the protocol and the content of each session. Experienced and certified sleep psychotherapists supervised all therapists.
Table 2.
Content of group and internet cognitive behavioral therapy for insomnia.
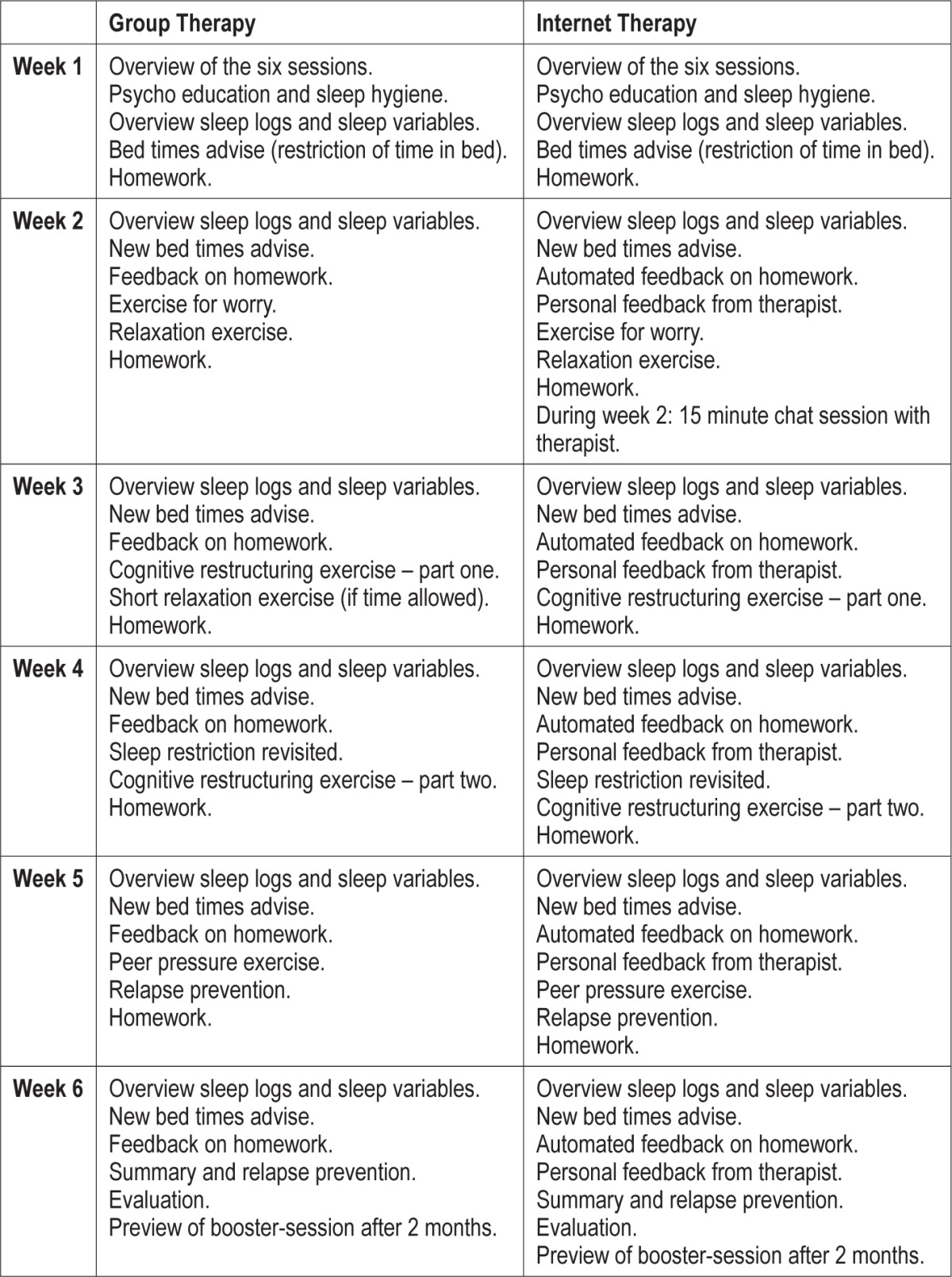
Participants in the GT condition came to a secondary youth mental health care center in Amsterdam and received a 1.5-h session in groups of 6 to 8 participants. Each group was guided by 3 therapists to ensure therapy integrity. Participants in the IT condition could log on to a website where at a fixed time of day, once every week, a consult was made available with exercises, movies, questionnaires, automated feedback, and personalized bedtime advice and written feedback from a sleep therapist. After the second internet session, participants had a 15-min chat session with their personal therapist. See for a more detailed description of both treatment modalities De Bruin et al.34 and Table 2.
Treatment Fidelity and Participant Attrition
The GT protocol was outlined in detail per session, and therapists, together with an independent supervising CBTI expert, met to discuss progress and small adjustments to the program for each session when necessary. The group sessions were recorded with a video camera, and rated by 2 independent researchers for integrity on a 5-point rating scale (1 = not addressed at all, to 5 = very well addressed) for each of the 32 elements from the total 6-week protocol. Mean overall integrity was 3.89 (SD = 1.47), suggesting good integrity. One-way ANOVA showed no significant differences between the groups for average rating of integrity (F4, 126 = 1.40, P = 0.24).
Each participant in IT was guided by a personal therapist, who spent a limited amount of time on preparing each session. Therapists for IT were also supervised by an independent CBTI expert, and met prior to the sessions to discuss treatment progress and guidelines to maintain high treatment integrity and intra-therapist concordance of bedtime advice. From a preliminary analysis of hours needed to complete 6 sessions of CBTI, based on timesheets for treatment of 18 participants in IT and 2 rounds of GT with a total of 15 participants, it appeared that for both treatment modalities about 4 h of therapist time per participant were required. One-way ANOVA showed that there were no significant differences between number of words used for feedback by the 5 therapists who guided IT participants (mean number of words = 373 [SD 79], F4, 34 = 1.23, P = 0.32). Furthermore, because IT consisted of preprogrammed sessions according to the same protocol as the group treatment, with the same order of exercises, questionnaires, movies, and modules for each participant, and therapist input was limited to one paragraph of personal advice, personalized bedtime advice embedded in the preprogrammed modules, and one 15-min chat session after the first session, there were no other differences in treatment content that could influence integrity for IT.
Of the 38 participants who were randomized to GT, one participant completed only 4 sessions, and 2 completed 5 sessions. All other participants completed all 6 sessions. Of the 39 participants in IT, one opened only 4 sessions and all others opened all 6 sessions.
Of 39 participants in WL 38 (97.4%) provided post-test measurements and 35 (90%) provided follow-up measurements. Of 39 participants in IT, 38 (97.4%) provided post-test measurements and 32 (82.1%) provided follow-up measurements. Of 38 participants in GT, 37 (97.4%) provided post-test measurements and 36 (94.7%) provided follow-up measurements. There were no differences in gender (Fisher exact test: χ2(1) = 0.11, P = 0.58) or age (t114 = 0.87, P = 0.39) between participants who did or did not provide post-test measures. There was also no difference in gender of participants who did or did not provide measures at follow-up (Fisher exact test: χ2(1) = 0.03, P = 0.58), but there was a significant difference in age, with participants who did not provide measures at follow-up being older (16.6, [SD 1.95 years]) than the participants who did provide measures (15.5, [SD 1.5 years]), (t114 = 1.17, P = 0.03).
Procedure
The study was approved by the medical ethics committee of the Academic Medical Center in Amsterdam and registered at http://www.isrctn.com/ISRCTN33922163. The study was advertised in relevant media, online newsletters, lectures at schools, and in leaflets that were mailed to healthcare professionals, schools, and institutions in and around Amsterdam, the Netherlands. Participants registered with a web-form and, after screening for eligibility, received an extensive information letter and logon data to fill out questionnaires on demographic information, socioeconomic status, school level, the HSDQ to screen for sleep problems, and the Youth Self Report (YSR)53 to screen for psychiatric problems. Parents also filled out a questionnaire on demographic information. After further screening of the participants based on inclusion and exclusion information from the questionnaires, participants were either excluded or invited with their parents for a face-to-face intake interview for diagnosis of sleep problems. If participants met inclusion criteria, informed consent was obtained from participants and their parents, and they were randomized to one of the 3 conditions and received information on the use of the actigraphy device and sleep logs. Participants who were randomized to WL could choose GT or IT after 2 month follow-up measurement was completed. Parents received a booklet with general information about the study and the content of the treatment. They were informed that their child could do the treatment independently, although for some exercises their support could be helpful (e.g., restriction of time in bed).
Statistical Analyses
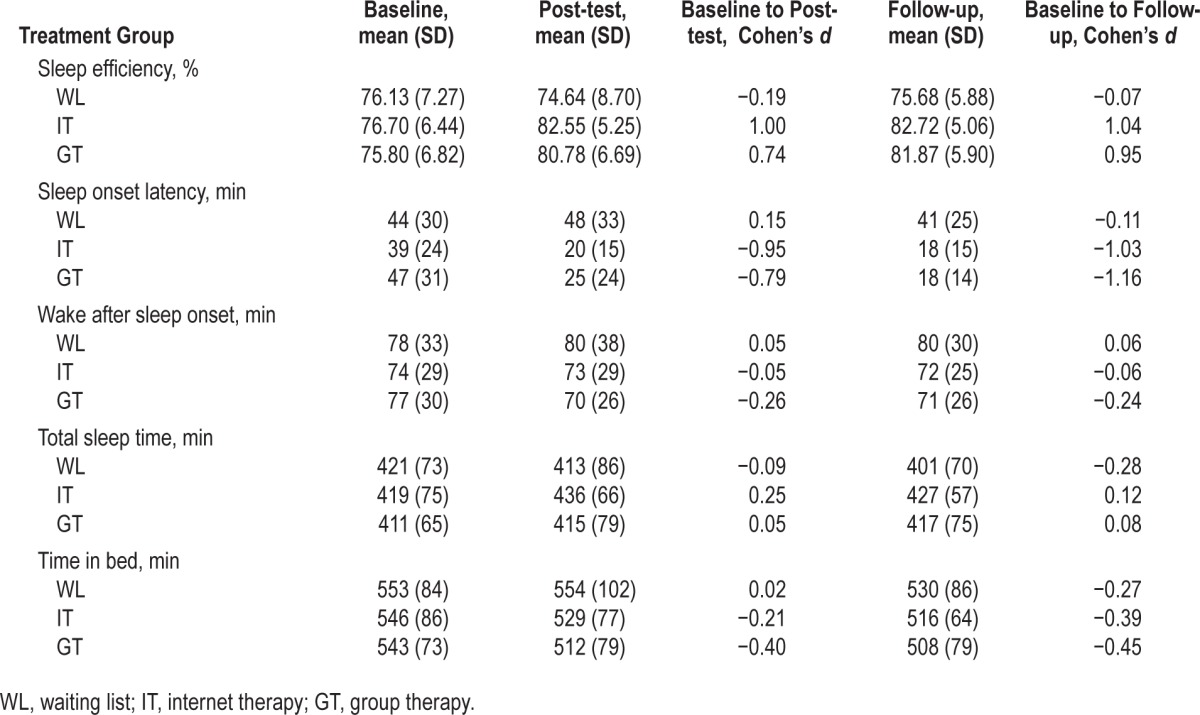
To compare demographic variables between the conditions one-way ANOVAs and χ2 tests were used. For the outcome variables (actigraphy, sleep logs, and questionnaires) within-group effect sizes were calculated to indicate the degree of change in response to treatment for each condition separately using Cohen's d (d = [M2 − M1] / SDpooled, where M1 = baseline mean, M2 = post-test mean or follow-up mean, and SD-pooled = pooled standard deviation, obtained by pooling the baseline SD across groups) (see Table 3.). To test significant differences in outcomes between the groups, data from the actigraphy, sleep logs, and questionnaires were analyzed using multilevel regression analysis (also known as linear mixed model analysis) in which repeated measures were considered as nested within participants. Multilevel regression analysis allows inclusion of participants with missing data at one or more measurement occasions,54 so all participants were included in the analyses. All models included an intercept representing the mean score of WL at baseline and regression coefficients representing differences between IT and GT compared to WL, differences between post-test and baseline, and differences between follow-up and post-test. To investigate the differential effects of condition (WL, IT, and GT) on sleep variables, we also included regression coefficients for interaction effects of time (post-test versus baseline, and follow-up versus post-test) * condition (IT versus WL, and GT versus WL). To investigate differences of treatment-effects for IT and GT, we ran separate analyses with models that included interaction effects for GT compared to IT with both time-lags of measurements from baseline to post-test and post-test to follow-up.
Table 3.
Means, standard deviations and within group effect sizes (Cohen's d) of sleep variables from actigraphy.
To control for effects of age and gender on the outcome variables, we first ran the models including the variables age and gender. There was a significant difference in nationality of parents between the conditions (see Results), so we included this variable in the models as well. Models with age, gender, and nationality of parent showed no significant differential effects on sleep outcomes from actigraphy, sleep logs, and questionnaires, so we repeated the analyses without these 3 variables.
All predictor and outcome variables were standardized, which allows for interpretation of the β coefficients as Cohen's d effect sizes (ESs), with 0.20, 0.50, and 0.80 indicating small, medium, and large ESs, respectively.54 As the independent variables were coded binary (0, 1), separate ESs for both conditions can be deduced from ESs for the main effects of IT and GT and ESs for interaction effects. When there is a significant interaction of time * condition (IT or GT), the ES for IT or GT is the addition of the ES of condition (IT or GT) and the ES of the interaction.
The primary outcome measure was objectively measured SE, as SE includes information on both difficulties falling asleep and staying asleep.44 To indicate clinical significance of improvements, measures from the HSDQi and CSRQ were chosen, as these indicate symptoms of insomnia in daily life (HSDQi), and daytime consequences of sleep reduction over a longer period of time (CSRQ). Since adolescent insomnia is for a large part characterized by (subjectively experienced) difficulties falling asleep,13 and SOL as registered with sleep logs was used to measure clinical significant improvements. We calculated and analyzed 2 indicators of clinical significance; the proportions of participants in each condition that reached a score below cutoff as a proxy for high end-state functioning, and the proportions of participants in each condition that attain clinically significant change. Scores below cutoff were defined as follows: HSDQi below 3.68, CSRQ below 40, and SOL from sleep logs below 30 minutes.44,49,51,56 To calculate clinically significant change of scores from the HSDQi and CSRQ we used the Reliable Change Index57 (RCI = (x2 − x1) / Sdiff, where x1 is the score at baseline and x2 is the score at post-test or follow-up, and Sdiff is the standard error of the difference, which can be calculated with Sdiff = √2(SE)2, where SE is the standard error of the measurement). To calculate SE reliability measures for the HSDQi and CSRQ were used from previous studies.49,51 Similar to research with adults,44,58 we considered a 50% decrease of SOL from sleep logs as clinically significant change. To compare the proportions of participants in the 3 conditions who reached a score below cutoff or exhibited clinically significant change, χ2 tests were used.
RESULTS
Participants
Table 1 shows demographic characteristics of all participants. Most of the participants were female (75.0%), and from parents who were married or living together (73.3%). There were no significant differences between the groups in age (F2,113 = 1.23, P = 0.30) or proportion of males and females (χ2(2) = 3.01, P = 0.22). In the IT condition there was a coincidental significantly smaller proportion of participants with parents born outside of the Netherlands compared to the waiting list (χ2(1) = 5.38, P = 0.03) and group treatment (χ2 (1) = 7.10, P = 0.01) conditions. There were, however, no baseline differences or differential effects over time for this variable, so further analyses were conducted without this variable.
Baseline Characteristics
According to actigraphy measures it took participants at baseline on average about 44 min to fall asleep (See Table 3). They spent 9 h in bed (TIB), they were awake after sleep onset (WASO) for 78 min, and slept for 7 h (TST). The proportion of sleep relative to the time spent in bed (SE) was 76%.
All these measures, except SOL, were somewhat better for self-reported sleep variables from sleep logs (see Table 4), which was mainly due to less reported WASO (about 20 min). Participants in IT reported a significantly lower amount of WASO at baseline compared to WL and GT (12 min compared to 25 and 22, respectively; see Table 5). Since no other significant differences in sleep log measures of sleep variables occurred, participants in IT started out with a somewhat higher SE (84.59%) than WL (80.41%) and GT (79.99%) (β = 0.33, P = 0.03). At baseline there was a small effect size difference (β = 0.25) for average sleep quality with a slightly higher score for IT compared to WL and GT, but this was not significant (P = 0.09). Questionnaires concerning insomnia complaints and symptoms of chronic sleep reduction at baseline, as measured with the HDSQi and CSRQ (Table 4), showed no significant differences between any of the conditions for either of the questionnaires.
Table 4.
Means, standard deviations, and within group effect sizes (Cohen's d) of sleep variables from sleep logs and questionnaires.
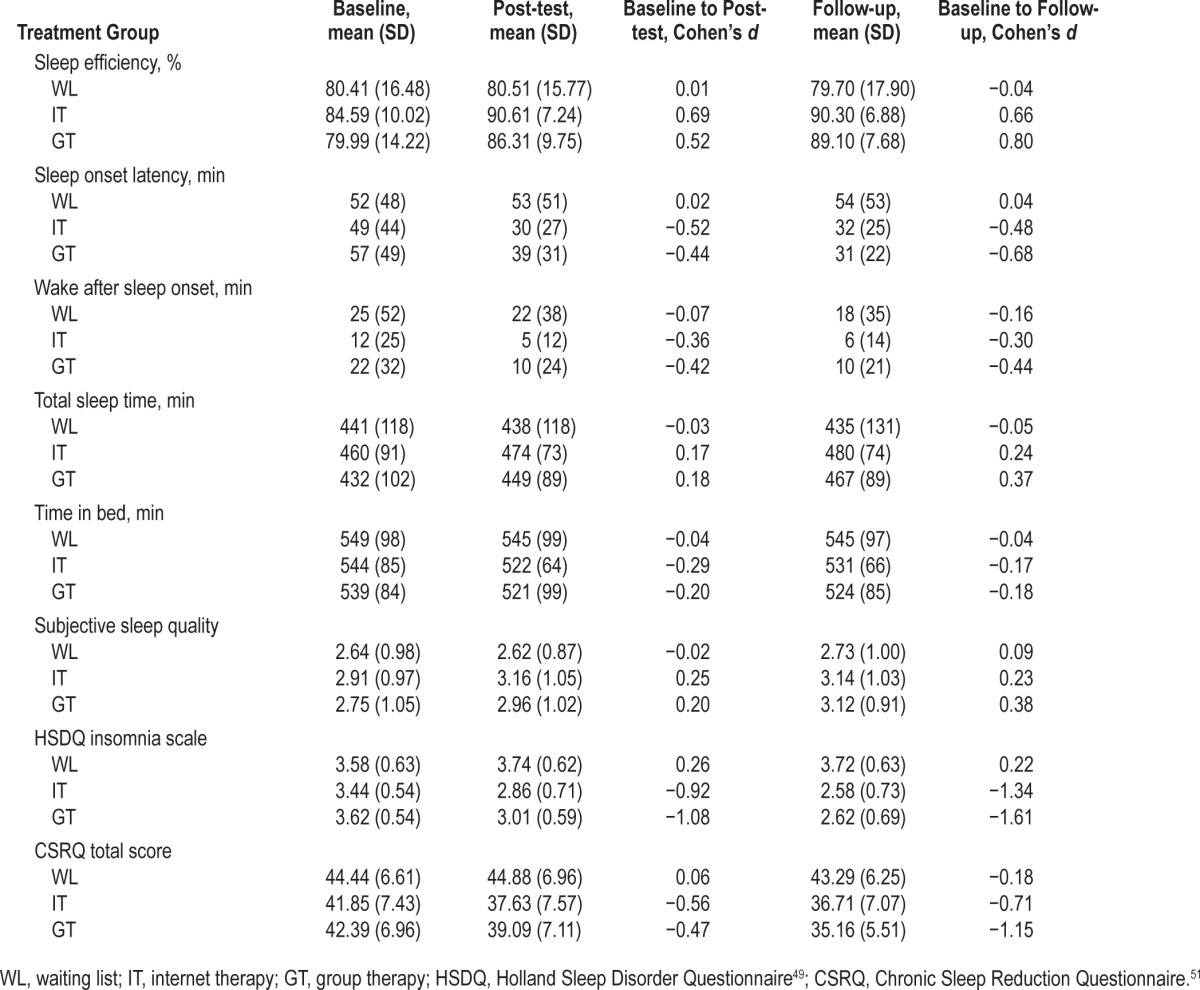
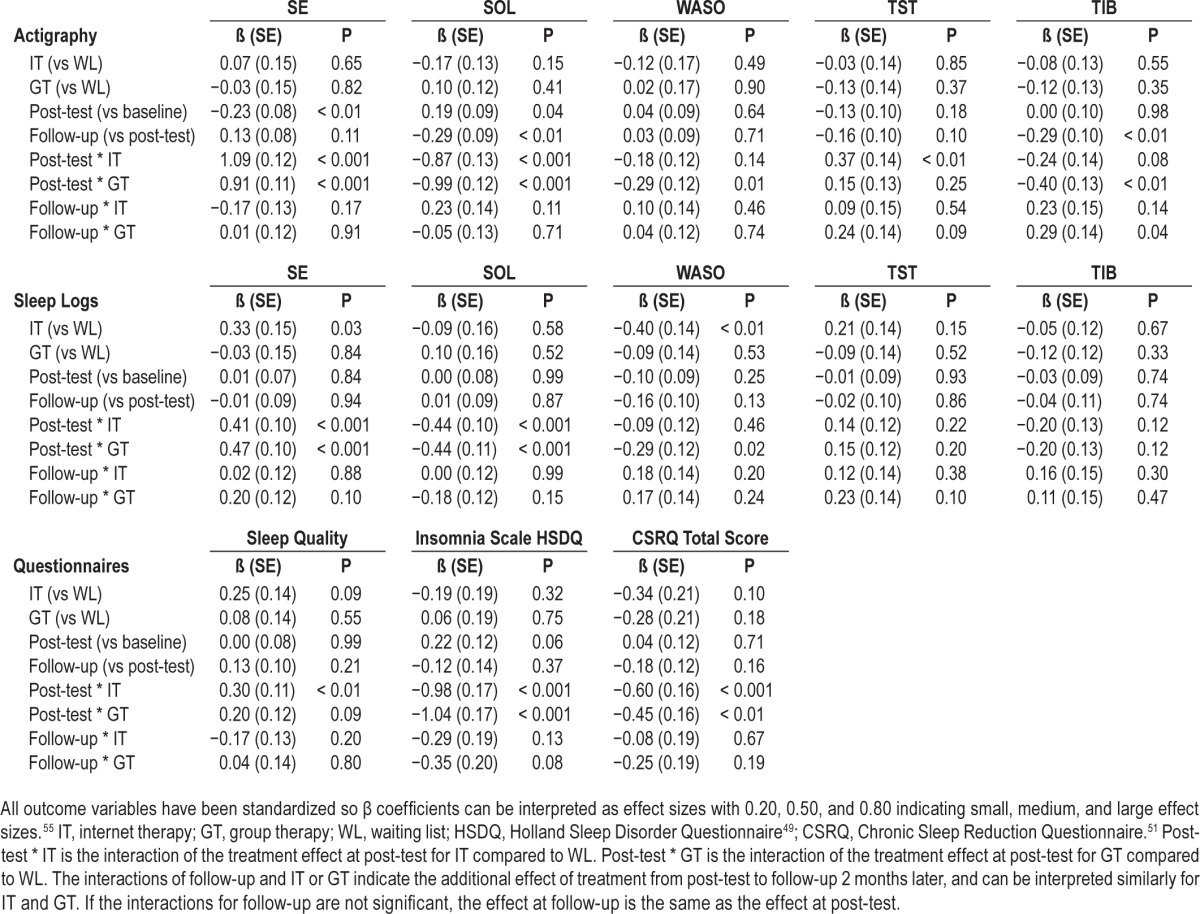
Table 5.
Parameter estimates for measurements at baseline, post-test and 2-month follow-up, and treatment condition for all variables from actigraphy, sleep logs, and questionnaires.
Post-test and Follow-up Effects for Actigraphy Measures
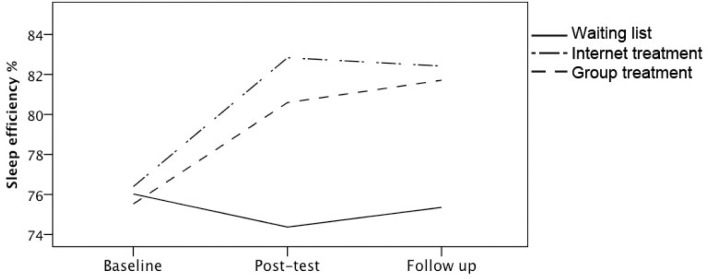
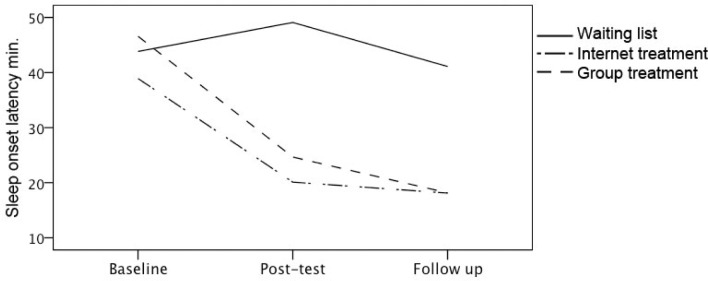
Actigraphy measures showed a significant interaction effect for SE at post-test for IT (β = 1.09, P < 0.001) and GT (β = 0.91, P < 0.001), compared to WL, indicating a significant increase of SE for the treatment conditions but not for WL (see Figure 2). There was no further change in SE at follow-up, indicating that the increase of SE for the treatment conditions was maintained 2 months after treatment was concluded. SOL also improved significantly for IT and GT compared to WL at post-test (β = −0.87, P < 0.001, and β = −0.99, P < 0.001, respectively). This was maintained at follow-up, as is indicated by the absence of an interaction at follow-up (Figure 3). WASO decreased significantly at post-test for GT compared to WL (β = −0.29, P = 0.01). There was also a decrease of WASO in IT, but this was not significant (β = −0.18, P = 0.14). There was a significant increase in TST for IT at post-test (β = 0.37, P < 0.01). The increase of TST for the GT was not significant at post-test, and although at follow-up the increase of TST in GT continued, as is indicated by the interaction, this did not reach significance (β = 0.24, P = 0.09). Finally, TIB decreased at post-test for GT and IT, but this was significant only for GT (β = −0.40, P < 0.01 for GT, and β = −0.24, P = 0.08 for IT). At follow-up, however, the TIB for GT reversed back to somewhat higher levels again (β = 0.29, P = 0.04), indicating that participants from GT restricted their TIB during treatment, but lengthened TIB after treatment was concluded.
Figure 2.
Estimated marginal means for sleep efficiency from actigraphy at baseline, post-test and at 2 months follow-up for the waiting list, internet treatment and group treatment conditions.
Figure 3.
Estimated marginal means for sleep onset latency from actigraphy at baseline, post-test and at 2 months follow-up for the waiting list, internet treatment and group treatment conditions.
Post-test and Follow-up Effects for Sleep Log Measures and Questionnaires
SE and SOL improved for IT and GT at post-test (β = 0.41, β = 0.47, for SE, and β = −0.44, β = −0.44 for SOL, respectively, all P's < 0.001), and these improvements were maintained at follow-up as indicated by a lack of significant interactions for both treatment conditions and follow-up (Table 5). WASO improved for GT at post-test (β = −0.29, P = 0.02), but not for IT. Although for IT and GT TST from sleep logs increased, and TIB decreased at post-test, these improvements were not significant. Ratings of sleep quality improved for IT and GT but this was significant for IT only (β = 0.30, P < 0.01, and β = 0.20, P = 0.09, respectively), which was maintained at follow-up. Scores from the HSDQi improved significantly after treatment for IT and GT compared to WL (β = −0.98, P < 0.001, and β = −1.04, P < 0.001, respectively). Scores from the CSRQ showed a similar pattern of improvement at post-test for IT and GT compared to WL (β = −0.60, P < 0.001, and β = −0.45, P < 0.01, respectively). For both the HSDQi and CSRQ these improvements were maintained at follow-up.
Differences between IT and GT
Similar analyses with models with an alternative parameterization allowed for direct comparisons of GT versus IT. For GT, the results showed a slightly smaller increase between baseline and post-test of SE from actigraphy (β = −0.18). From sleep logs there appeared a slightly larger increase of SE from post-test to follow-up (β = 0.19), and a larger decrease of WASO from baseline to post-test (β = −0.22). However, none of these differences between the 2 treatment conditions were significant at the 0.05-level (all P's between 0.07 and 0.10).
Clinical Significance: High End-State Functioning and Clinically Significant Change
Numbers and percentages of participants with scores below the cutoff and their RCI's for each measurement occasion are presented in Table 6.
Table 6.
Number and percentage of participants with clinical scores on the HSDQi, CSRQ and sleep onset latency from actigraphy, in each condition at baseline, post-test, and follow-up.
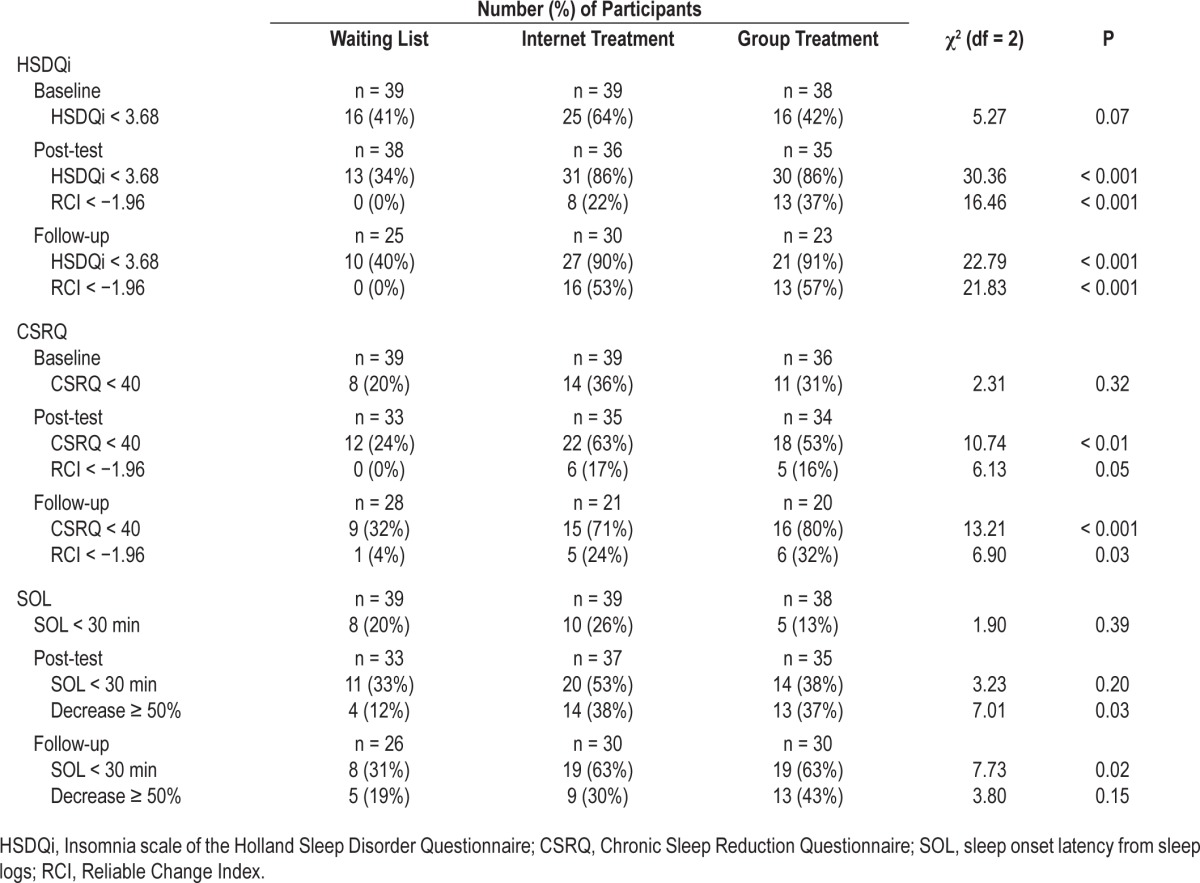
High end-state functioning was greatest for the HSDQi and CSRQ where significantly more participants in the treatment conditions reached a score below the cutoff49,51,44,56 at either post-test or follow-up (between 71% and 91% at follow-up, P's < 0.001), whereas these proportions remained at the level of baseline for WL (40% and 32%, respectively, at follow-up). There were also significantly larger proportions of participants with clinically significant change of scores of the HSDQi and CSRQ at post-test and follow-up in both treatment conditions (between 24% and 57% at follow-up, P < 0.001 and P = 0.03, respectively) compared to WL (0% and 4%, respectively, at follow-up). The proportion of participants in the treatment conditions compared to WL, who reached scores below cutoff for SOL from sleep logs, however, was significantly larger only at follow-up (63% for IT and GT, compared to 31% in WL, P = 0.02). A larger proportion of participants in the treatment conditions also reached a decrease of SOL larger than 50%, but this was only significant at post-test (38% for IT and 37% for GT versus 12% for WL, P = 0.03).
DISCUSSION
In this study we investigated efficacy of CBTI for adolescents with insomnia in a randomized controlled trial comparing guided internet therapy (IT), group therapy (GT), and a waiting list (WL) condition. The main results were that adolescents in both treatment conditions improved significantly and importantly on SE, SOL, WASO, and TST, compared to waiting list. Most of these improvements were found in both objective and subjective measures. There were small differences between IT and GT, with slightly more decrease of WASO in GT from both actigraphy and sleep log measures, and slightly more increase of TST in IT from actigraphy. Furthermore, insomnia complaints and symptoms of chronic sleep reduction decreased significantly for both the treatment conditions compared to waiting list. Effect sizes of improvements controlled for WL ranged from medium to large. These results were confirmed by the greater proportions of participants in IT and GT with high end-state functioning after treatment (63% to 91% for the treatment conditions, versus 31% to 40% in WL), and with clinically significant change (24% to 57% for the treatment conditions, versus 0% to 20% for WL).
With these results this RCT provides preliminary evidence that cognitive behavioral treatment for insomnia is also effective for adolescents in both short and longer term. Effect sizes for the primary outcome measure, objectively measured SE from actigraphy, were large for both treatment modalities (1.09 and 0.91 for IT and GT, respectively). There was a slightly larger increase in objective TST at post-test for IT. WASO, however, improved more for GT. Since TIB decreased more for GT at post-test, these small but significant differences might be due to a more stringent application of restriction of time in bed for GT, resulting in less opportunity to increase TST. This notion is further underlined by the larger decrease of WASO for GT, indicating a more stable sleep, which is one of the expected results of restriction of time in bed.59 The sleep logs also showed a larger decrease of WASO for GT, but IT started out with a coincidental better baseline according to these measures, with less WASO and a higher SE, compared to WL and GT. All results for daytime symptoms of insomnia and chronic sleep reduction from the questionnaires, and sleep quality from sleep logs, showed a similar pattern of improvement for both treatment conditions at post-test, which was maintained at follow-up. Notable for these improvements of daytime symptoms are the large within-group effect sizes, ranging from −0.71 to −1.61 for symptoms of chronic sleep reduction and insomnia, with the larger effect sizes attained in GT (although not statistically significant). Finally, of note is that most improvements at post-test were either maintained at follow-up or continued to improve in the time after post-test to follow-up (although not statistically significant if compared to the improvement at post-test). This indicates that the improvements after CBTI, including the clinically significant changes, are stable over time.
Since adolescents are reluctant to seek help for psychological problems,35,36 developing treatment modalities that cater to adolescents' characteristics is important. Compliance rates were high in both our treatment conditions (in GT only one participant completed four sessions, and two completed five sessions of CBTI; in IT only one opened four sessions of CBTI, all others completed all sessions of CBTI). This shows that both treatments were well accepted by adolescents. From the pilot study of this RCT,34 which investigated also feasibility, it appeared that especially the personalized bedtimes advice was appreciated by the participants, but all other techniques were favorably mentioned as well, in both IT and GT. Notwithstanding this finding, all except one of the participants from the waiting list, who could choose their preferred form of treatment (group or internet) after the 2 month follow-up, chose the internet treatment. This indicates that internet treatment may well be a low threshold and acceptable treatment form for adolescents, which accommodates adolescents' motivation for autonomy, competing psychosocial demands, and ambivalence about entering a psychological treatment setting.33
The treatment protocol we used was based on the CBTI components as described in the benchmark reports of Morgenthaler et al.28 and Morin et al.21 that are reference sources for most CBTI research. It included sleep/psychoeducation, sleep hygiene, relaxation techniques, restriction of time in bed, cognitive therapy, and stimulus control (Table 2). For stimulus control however, we did not apply the technique in which a patient is advised to get up if awake in bed for longer than 20 minutes. The adolescents in our study did not experience much (self-reported) WASO, and the exercise of restriction of time in bed mostly resulted in later bedtimes and stable get up time. Since opportunity to sleep was already restricted, we reserved the rule to get up if awake for longer than 20 minutes as a measure of last resort if other exercises would not produce any results, and the nature of the participant's sleep pattern fit the criteria to apply this technique. However, with none of the participants was this the case. Efficacy of both treatment forms was comparable to each other and to results from research on CBTI with adults.21–26,60–62 This shows that CBTI—although developed for treatment of adults—has similar effects on insomnia in adolescents.
Most of the outcomes in our study were also comparable to the study of Bootzin and Stevens30 on CBTI treatment of adolescents with a history of substance abuse and the single case study of Hendricks et al.33 In both these studies, however, participants also reported an increase in subjective TST of one hour or more, and a trend of increase in objective TST of about 23 minutes, whereas in our study we did find an increase in TST, but the size was much smaller (about 7 minutes in actigraphy measures and 23 minutes in sleep logs at follow-up). Both these studies used comparable CBTI protocols to our study. The smaller increase of TST in our study could be due to a more stringent restriction of time in bed, which is further confirmed by the somewhat larger decrease of SOL in our study. Besides, Bootzin and Stevens30 also used bright light therapy in addition to the CBTI, which may have had an additional effect, particularly for adolescents with a delayed sleep phase. We excluded adolescents with a primary delayed sleep phase syndrome, even though for most adolescents with insomnia the predominant complaint concerns SOL. Although bright light might aid adolescents with a delayed sleep phase, the primary concern of the present study is treatment for insomnia, which is distinctly different from delayed sleep phase syndrome. Differentiating between these two sleep disorders that affect adolescents is important for both treatment and research.
In our RCT, we did not find a relation between age and the effect of treatment. This shows that the effect of the cognitive behavioral treatment as was used in the current study is generalizable to adolescents of all ages. This is interesting, because it is known that there are distinct differences in bedtimes, sleep hygiene related behaviors, and sleep parameters for adolescents of different ages.63
All participants and their parents met face-to-face with a clinician before treatment—in some cases this would also be their personal sleep-therapist during treatment—so personal contact was established from the start. In the internet treatment, in which further contact with a therapist was limited to written feedback for each sessions and one 15-minute chat session, this personal contact and the continuous guidance at all sessions from a personal therapist may have enhanced the effectiveness, as has been reported in other research.25 However, in the group treatment all participants and therapists met face-to-face at every session. This might explain some of the (small) differences of treatment effects between the conditions immediately after treatment, but note that overall both treatment conditions reached similar efficacy at follow-up, both on an individual level (clinical significance) and on a group level.
The participants in our study were recruited and selected based on (among others) criteria for primary insomnia according to the DSM-IV. With the introduction of the DSM-5, which occurred after the initial design of our study, criteria to meet insomnia disorder now also include that the sleep problems have to occur at least 3 days per week and be present for at least 3 months.64 For the DSM-IV the problems had to be present for at least 1 month, and a minimum amount of days per week was not set, although in clinical practice a minimum of 3 days per week was conventional. From the diagnostic interviews, however, it appeared that all of the participants in our study suffered from self-reported insomnia at least 3 days a week and for 3 or more months and thus also met criteria for insomnia disorder according to the DSM-5.
Despite of its merits, such as using a randomized controlled design with two treatment groups and a control group, and subjective and objective measures, this study also has some limitations. The participants were recruited from the general population and participants with severe signs, diagnoses, or treatment for psychiatric disorders (including anxiety and depression) were excluded in our study. Therefore, generaliz-ability to populations in for instance clinical settings, where insomnia is often found after diagnosis for another psychological disorder already has been established, may be limited. However, the causal relation of insomnia and comorbid disorders has been shown to be reciprocal,2,65–67 and in adults CBTI improves subjective sleep experiences regardless of depressive symptom severity.67 This underlines the importance to provide diagnosis and treatment for insomnia in adolescents independent of other psychological disorders, in order to prevent not only the direct consequences of insomnia, but also the possible causation, maintenance, or amelioration of other psychological disorders.64 Another limitation of this study may be that the recruitment of participants from the general population could have biased the level of motivation, which may be higher in participants from the general population compared to patients who are referred to treatment centers. This can play an important role in the commitment to the treatment, especially with adolescents who are in the middle of age-related developmental tasks and feel ambivalent about entering psychological treatment.33 Therefore we recommend further research into the effects of such variables as motivation, commitment, parental support, and self-selection for treatment on effectiveness of CBTI in adolescents. Finally, although an a priori power analysis indicated that the sample sizes were large enough to detect efficacy of CBTI in IT and GT compared to WL; in a separate analysis comparing IT and GT directly, we found some small effect size differences between the two treatment modalities that did not reach significance. This indicates that power was not sufficient to detect small differences between IT and GT if they existed. We note that based on the same parameters as we used for the a priori power analysis, we would need sample sizes as large as 400 per group, to achieve a power of 0.80 to find differences between IT and GT with effect sizes as small as Cohen's d of 0.2. Nevertheless, in our study, both treatment groups reached similar end-points.
Three other recommendations for further research need to be mentioned here. First, participants in our study received six sessions of CBTI, while research has shown that with adults the dose-response effects of CBTI is highest with four biweekly sessions if compared to one, two, or eight sessions over the course of eight weeks.68 Further research into dose response specifically for adolescents is warranted. Second, as we reported in the pilot study of this RCT,34 there is a large discrepancy between objective and subjective WASO. This could be caused by sleep state misperception69 or increased sleep motor activity in adolescents.70 Further research is needed to clarify this issue. Finally, chronicity of insomnia is shown to be high in adolescents and to persist over time.1,2,8 The impact of certain cognitive processes like sleep-related worry on sleep parameters has been shown to be dependent on how long the person has been suffering from insomnia, with no impact on subjective sleep perception when this is shorter than 7 months, but a clear negative impact when people suffer from insomnia between 7–12 months.71 Furthermore, sleep related cognitions (e.g., unrealistic expectations, faulty beliefs and appraisals) play an important role in causing or perpetuating insomnia.72 We recommend further studies on duration of insomnia, sleep related cognitions, and their effects on CBTI in adolescents.
To conclude, this RCT is the first study that provides prove that CBTI is effective for the treatment of insomnia in adolescents in both group treatment and internet treatment fashion, in the short and longer term, with medium to large effect sizes, with minimal differences between these two modes of treatment, and with clinically significant improvements. Therefore we recommend CBTI as treatment for adolescent insomnia. To reach a large group of adolescents, internet treatment of adolescent insomnia should be promoted in mental health organizations.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a grant from The Netherlands Organization for Health Research and Development ZonMw. The authors have indicated no financial conflicts of interest. All authors contributed significantly to this manuscript.
Footnotes
A commentary on this article appears in this issue on page 1839.
REFERENCES
- 1.Johnson EO, Roth T, Schults L, Breslau N. Epidemiology of DSM- IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:E247–56. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RE, Roberts CR, Duong HT. Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study. J Adolesc Health. 2008;42:294–302. doi: 10.1016/j.jadohealth.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 4.Dohnt H, Gradisar M, Short MA. Insomnia and its symptoms in adolescents: comparing DSM-IV and ICSD-II diagnostic criteria. J Clin Sleep Med. 2012;8:295–9. doi: 10.5664/jcsm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung KF, Kan KK, Yeung WF. Insomnia in adolescents: prevalence, help-seeking behaviors, and types of interventions. Child Adolesc Ment Health. 2014;19:57–63. doi: 10.1111/camh.12009. [DOI] [PubMed] [Google Scholar]
- 6.Amaral MO, Pereira CM, Martins DI, de Serpa CR, Sakellarides CT. Prevalence and risk factors for insomnia among Portuguese adolescents. Eur J Pediatr. 2013;172:1305–11. doi: 10.1007/s00431-013-2037-0. [DOI] [PubMed] [Google Scholar]
- 7.Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. 2013;22:549–56. doi: 10.1111/jsr.12055. [DOI] [PubMed] [Google Scholar]
- 8.Thorleifsdottir B, Björnsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–38. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Roberts RE. Comparability of sleep disorders diagnoses using DSM-IV and ICSD classifications with adolescents. Sleep. 2001;24:920–5. doi: 10.1093/sleep/24.8.920. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon MM, Reynolds CF. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Med. 2009;10:952–60. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40:700–8. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66–71. doi: 10.1016/j.jad.2012.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–84. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 14.Meijer AM, Reitz E, Dekovic M, van den Wittenboer M, Stoel RD. Longitudinal relations between sleep quality, time in bed and adolescent problem behaviour. J Child Psychol Psychiatry. 2010;51:1278–86. doi: 10.1111/j.1469-7610.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- 15.Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10:323–37. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration, and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14:179–89. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Owens J, Gruber R, Brown T, et al. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17:550–64. doi: 10.1177/1087054712457992. [DOI] [PubMed] [Google Scholar]
- 18.Gruber R. Sleep characteristics of children and adolescents with attention deficit-hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2009;18:863–76. doi: 10.1016/j.chc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Millman RP. Working group on sleepiness in adolescents/young adults, & AAP Committee on Adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–86. doi: 10.1542/peds.2005-0772. [DOI] [PubMed] [Google Scholar]
- 20.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Hohagen F, Voderholzer U, Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251:35–41. doi: 10.1007/s004060170066. [DOI] [PubMed] [Google Scholar]
- 23.Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36:353–62. doi: 10.5665/sleep.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancee J, van den Bout J, van Straten A, Spoormaker VI. Internet-delivered or mailed self-help treatment for insomnia? A randomized waiting-list controlled trial. Behav Res Ther. 2012;50:22–9. doi: 10.1016/j.brat.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Jernelöv S, Lekander M, Blom K, et al. Efficacy of a behavioral self-help treatment with or without therapist guidance for co-morbid and primary insomnia - a randomized controlled trial. BMC Psychiatry. 2012;12:5. doi: 10.1186/1471-244X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espie CA, Kyle SD, Williams C, et al. A randomised, placebo-controlled trial of online cognitive behavioural therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–81. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesson AL, McDowell WA, Littner M, et al. Practice parameters for the non-pharmacological treatment of chronic insomnia. Sleep. 1999;22:28–33. doi: 10.1093/sleep/22.8.1128. [DOI] [PubMed] [Google Scholar]
- 28.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 29.Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Ped Psychol. 2014;39:932–48. doi: 10.1093/jpepsy/jsu041. [DOI] [PubMed] [Google Scholar]
- 30.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005;25:629–44. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Norell-Clarke A, Nyander E, Jansson-Fröjmark M. Sleepless in Sweden: a single subject study of effects of cognitive therapy for insomnia on three adolescents. Behav Cogn Psychother. 2011;39:367–74. doi: 10.1017/S1352465810000664. [DOI] [PubMed] [Google Scholar]
- 32.Harvey AG. A cognitive theory and therapy for chronic insomnia. J Cogn Psychother. 2005;19:41–59. [Google Scholar]
- 33.Hendricks MC, Ward CM, Grodin WL, Slifer KJ. Multicomponent cognitive-behavioural intervention to improve sleep in adolescents: a multiple baseline design. Behav Cogn Psychother. 2014;42:368–73. doi: 10.1017/S1352465813000623. [DOI] [PubMed] [Google Scholar]
- 34.De Bruin EJ, Oort FJ, Bögels SM, Meijer AM. Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: a pilot study. Behav Sleep Med. 2014;12:235–54. doi: 10.1080/15402002.2013.784703. [DOI] [PubMed] [Google Scholar]
- 35.Cheng TC. Factors related to adolescents' seeking help from social workers in mental health settings. Child Youth Serv Rev. 2009;31:807–12. [Google Scholar]
- 36.Logan DE, King CA. Parental facilitation of adolescent mental health service utilization: a conceptual and empirical review. Clin Psychol: Sci Pract. 2001;8:319–33. [Google Scholar]
- 37.Nelson TD, Nelson JM. Evidence-based practice and the culture of adolescence. Prof Psychology: Res Pract. 2010;41:305–11. [Google Scholar]
- 38.Havas J, De Nooijer J, Crutzen R, Feron F. Adolescents' views about an internet platform for adolescents with mental health problems. Health Ed. 2011;111:164–76. [Google Scholar]
- 39.Stinson J, Wilson R, Gill N, Yamada J. Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. J Ped Psychol. 2009;34:495–510. doi: 10.1093/jpepsy/jsn115. [DOI] [PubMed] [Google Scholar]
- 40.Palermo TM, Wilson AC. eHealth applications in pediatric psychology. In: Roberts MC, Steele RG, editors. Handbook of pediatric psychology. 4th ed. New York, NY: Guilford; 2009. pp. 227–37. [Google Scholar]
- 41.Cushing CC, Steele RG. A Meta-analytic review of ehealth interventions for pediatric health promoting and maintaining behaviors. J Pediatr Psychol. 2010;35:937–49. doi: 10.1093/jpepsy/jsq023. [DOI] [PubMed] [Google Scholar]
- 42.Andersson G, Carlbring P, Ljótsson B, Hedman E. Guided internet-based CBT for common mental disorders. J Contemp Psychother. 2013;43:223–233. [Google Scholar]
- 43.Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 44.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;7:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 45.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 47.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 48.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 49.Kerkhof GA, Geuke ME, Brouwer A, Rijsman RM, Schimsheimer RJ, Van Kasteel V. A new sleep disorders questionnaire based on the International Classification of Sleep Disorders-2. J Sleep Res. 2012;22:104–7. doi: 10.1111/j.1365-2869.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 50.American Academy of Sleep Medicine. Westchester IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed: Diagnostic and coding manual. [Google Scholar]
- 51.Dewald JF, Short MA, Gradisar M, Oort FJ, Meijer AM. The Chronic Sleep Reduction Questionnaire (CSRQ): a cross-cultural comparison and validation in Dutch and Australian adolescents. J Sleep Res. 2012;21:584–94. doi: 10.1111/j.1365-2869.2012.00999.x. [DOI] [PubMed] [Google Scholar]
- 52.Meijer A. Chronic sleep reduction, functioning at school and school performance in adolescents. J Sleep Res. 2008;17:395–405. doi: 10.1111/j.1365-2869.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- 53.Achenbach TM. Burlington: University of Vermont, Department of Psychiatry; 1991. Manual for the Youth Self-Report and 1991 Profile. [Google Scholar]
- 54.Snijders T, Bosker R. Multilevel analysis. London: Sage Publications; 1999. [Google Scholar]
- 55.Cohen J. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 56.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psych. 1991;59:12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 58.Harris J, Lack L, Kemp K, Wright H, Bootzin R. A randomized controlled trial of intensive sleep retraining (ISR): a brief conditioning treatment for chronic insomnia. Sleep. 2012;35:49–60. doi: 10.5665/sleep.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Dongen PA, Rogers NL, Dinges DF. Sleep debt: theoretical and empirical issues. Sleep Biol Rhythms. 2003;1:5–13. [Google Scholar]
- 60.Van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13:61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Strom L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol. 2004;72:113–20. doi: 10.1037/0022-006X.72.1.113. [DOI] [PubMed] [Google Scholar]
- 62.Van Straten A, Emmelkamp J, de Wit J, et al. Guided Internet-delivered cognitive behavioural treatment for insomnia: a randomized trial. Psychol Med. 2014;44:1521–32. doi: 10.1017/S0033291713002249. [DOI] [PubMed] [Google Scholar]
- 63.De Bruin EJ, Van Kampen RK, Van Kooten T, Meijer AM. Psychometric properties and clinical relevance of the adolescent sleep hygiene scale in Dutch adolescents. Sleep Med. 2014;15:789–97. doi: 10.1016/j.sleep.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 64.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 65.Pasch KE, Laska MN, Lytle, Moe SG. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? Am J Health Behav. 2011;34:237–48. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–56. [PMC free article] [PubMed] [Google Scholar]
- 67.Hamoen AB, Redlich EM, Weerd AW. Effectiveness of cognitive behavioral therapy for insomnia: influence of slight-to-moderate depressive symptom severity and worrying. Depress Anxiety. 2014;31:662–8. doi: 10.1002/da.22258. [DOI] [PubMed] [Google Scholar]
- 68.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007;30:203–12. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- 69.Mercer J, Bootzin RR, Lack LC. Insomniacs' perception of wake instead of sleep. Sleep. 2002;25:564–71. [PubMed] [Google Scholar]
- 70.Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13:378–84. doi: 10.1016/j.sleep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Jansson M, Linton SJ. The development of insomnia within the first year: a focus on worry. British J Health Psychology. 2006;11:501–11. doi: 10.1348/135910705X57412. [DOI] [PubMed] [Google Scholar]
- 72.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.